Abstract
Inflammasomes are multiprotein complexes that sense pathogen-associated and danger-associated molecular patterns and induce inflammation in cells. The NALP3 inflammasome is tightly regulated by recently discovered control mechanisms, but other modulators still remain to be characterized. NLR family CARD-containing 3 (NLRC3) protein, a caspase recruitment domain (CARD)–containing member of the nucleotide oligomerization domain–like receptor (NLR) family, was found to down-regulate the NF-κB pathway and stimulator of interferon genes (STING)–dependent cytokine secretion. However, the effect of NLRC3 on the NALP3 inflammasome or other inflammasomes is still unknown. We hypothesized that NLRC3 might inhibit NALP3 inflammasome complex assembly. Toward this end, we tested whether NLRC3 overexpression or knockdown influences NALP3 activity in human monocyte and HEK293FT cells when the complex is ectopically reconstituted. We found that NLRC3 indeed decreases NALP3-induced IL-1β maturation and secretion, pro-caspase-1 cleavage, and speck formation by apoptosis-associated speck-like protein containing a CARD (ASC) protein in response to NALP3 activators. We also show that endogenous NLRC3 interacts with both ASC and pro-caspase-1 but not with NALP3, disrupts ASC speck formation through its CARD, and impairs the ASC and pro-caspase-1 interaction. Moreover, the NLRC3 CARD alone could dampen IL-1β secretion and ASC speck formation induced by NALP3 mutants associated with autoinflammatory diseases. In conclusion, we show here that, besides its role in the inhibition of the NF-κB pathway, NLRC3 interferes with the assembly and activity of the NALP3 inflammasome complex by competing with ASC for pro-caspase-1 binding.
Keywords: autoimmune disease, caspase 1 (CASP1), inflammasome, NLRP3, pathogen-associated molecular pattern (PAMP), ASC, NLRC3, inhibition, sterile inflammation
Introduction
Inflammasomes are multiprotein complexes responsible for the sensing of pathogen-associated molecular patterns and danger-associated molecular patterns and the induction of inflammation (1). Different types of inflammasomes have been identified, and most of them have in common the adaptor protein ASC, 2 the effector pro-caspase-1, and the downstream inflammatory mediator cytokine pro-IL-1β (2). The assembly of the inflammasome is mediated by the homotypic interaction of ASC and pro-caspase-1 through their CARDs (3) and the formation of ASC foci called “specks” (4). This interaction results in the cleavage of pro-caspase-1 and the maturation and secretion of IL-1β (4). The NALP3 (also called NLRP3 or cryopyrin) inflammasome is specifically activated by ionophores such as ATP and nigericin (5), crystals such as monosodium urate (MSU) (6) and many others, whereas the NLRC4 (or IPAF) inflammasome is stimulated by Pseudomonas aeruginosa infection (7). A two-step process is required to promote the secretion of IL-1β by the NALP3 inflammasome: the induction of the NFκB pathway through Toll-like receptors (called the “priming step”) (8) and the complex assembly (called the “activation step”) mediated by potassium efflux (9, 10), lysosomal rupture (11), and reactive oxygen species production (12).
Mutations in the NALP3 gene are associated with autoinflammatory syndromes known as cryopyrin-associated periodic syndromes (or CAPS) (13), and among these mutations, the NALP3 p.R260W, and p.D303N mutants enhance the ectopic assembly of ASC specks (14, 15) and result in an increase in pro-caspase-1 cleavage (14) and IL-1β secretion (16, 17). IL-1β blockers such as anakinra are used to treat CAPS (18, 19), and identification of novel inhibitors of the NALP3 inflammasome that act upstream of IL-1β, on the NALP3 mRNA level (20), on ASC oligomerization (21), or on caspase-1 inhibition (22), is presently ongoing, and these inhibitors may be used in therapy of autoinflammatory diseases in the future.
Although the most studied NOD-like receptor (NLR) family members belong to the pro-inflammatory group, more recently, NLR proteins with anti-inflammatory roles have been characterized. Especially the members of the NLRC subgroup, NLRC5, NLRX1, and NLRC3, were shown to be negative regulators of inflammation. NLRX1 inhibited the NFκB pathway by binding to the active IκB kinase in response to LPS stimulation (23, 24). Furthermore, increased IFN-β secretion was observed in immune cells from NLRC5 knock-out mice when stimulated with vesicular stomatitis virus or poly(I:C) (24).
NLRC3, a less studied member of the NLR family, contains a C-terminal leucine-rich repeat (LRR) domain, a central NACHT domain and a N-terminal CARD (25). NLRC3 was shown to inhibit the NFκB pathway (25, 26) through the ubiquitination of TRAF6 (26). NLRC3 also inhibited stimulator of interferon genes–dependent IFNβ, TNFα, and IL-6 secretions in response to cytoplasmic DNA stimulation. NLRC3 altered stimulator of interferon genes and TBK1 interaction by directly binding to these proteins, and NLRC3 KO mice responded more efficiently to HSV-1 virus infection because IFN-β levels were higher in the sera of these mice compared with WT NLRC3–expressing counterparts (27). We have also shown previously that the overexpressed NLRC3 protein co-localizes with the inflammasome components and decreases IL-1β processing and ASC speck assembly in HEK293FT cells (28).
In this study, the suppressive effect of the NLRC3 protein on inflammasomes is confirmed, and the molecular mechanism of this inhibition is elucidated. We determined that, besides its inhibitory role on the NFκB pathway described previously, NLRC3 also acts on the activation step of the inflammasome by decreasing the efficiency of the complex assembly by competing with ASC for pro-caspase-1 binding via its N-terminal CARD domain. We propose to use the CARD domain of NLRC3 as a novel inhibitor of the NALP3 inflammasome.
Results
Overexpression of NLRC3 inhibits NALP3-induced IL-1β secretion
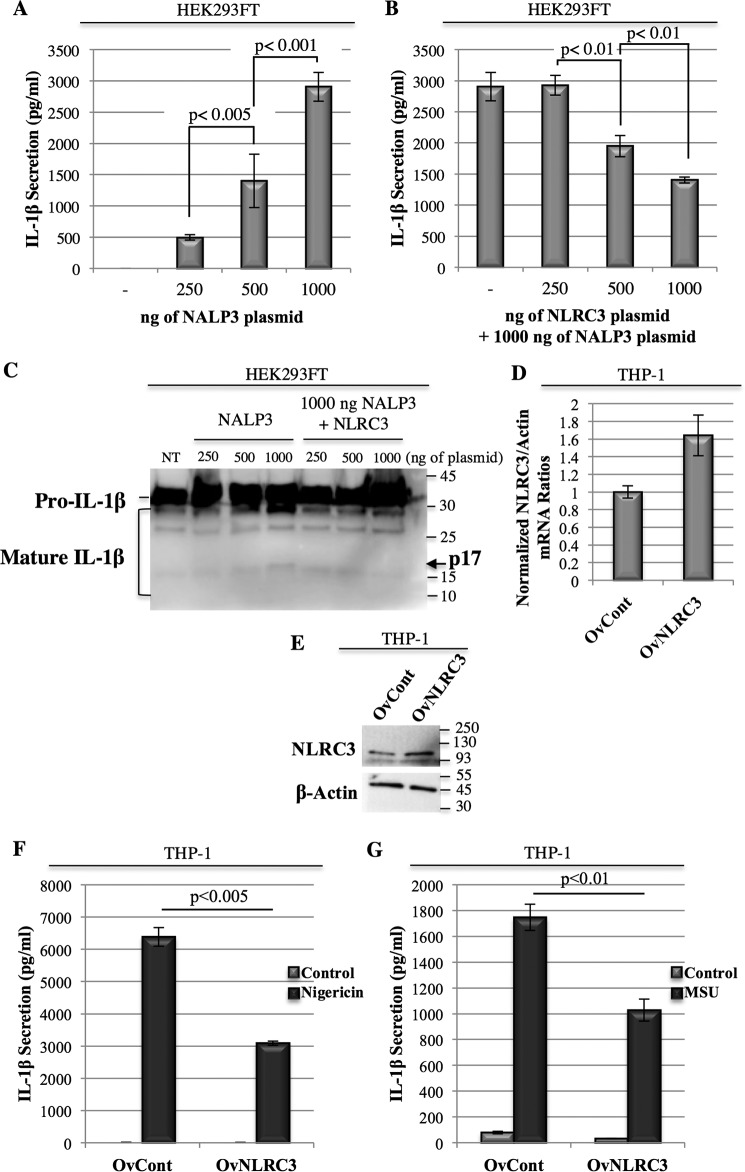
Previous studies showed that overexpression of the inflammasome components was sufficient to induce activation of the complex and maturation and secretion of caspase-1 and IL-1β in a NALP3- and ASC-dependent manner. To assess whether NLRC3 has an effect on the activation of the complex, we ectopically expressed NALP3, ASC, pro-caspase-1, and pro-IL-1β in HEK293FT cells in the presence or the absence of NLRC3 and measured the resulting IL-1β secretion. Although negligible levels of IL-1β were secreted from ASC, pro-caspase-1 and pro-IL-1β co-transfected HEK293FT cells, co-expression of NALP3 with these proteins induced a dose-dependent increase in IL-1β secretion (Fig. 1A; 496 ± 42.7 pg/ml for 250 ng, 1400 ± 427.5 pg/ml for 500 ng, and 2903.7 ± 229.81 pg/ml for 1000 ng of NALP3 plasmid), thus confirming the specificity of the experimental setting. Interestingly, when NLRC3 was co-expressed with NALP3 and the other inflammasome-forming proteins, a significant reduction in IL-1β secretion was observed (Fig. 1B; 2903.7 ± 229.81 pg/ml for 1000 ng of NALP3 plasmid alone versus 1403.5 ± 48.1 pg/ml for 1000 ng of NALP3 and 1000 ng of NLRC3 plasmids, p = 0.0001). Moreover, the more NLRC3 was expressed, the higher decrease in IL-1β release that was obtained (2925.5 ± 151.2 pg/ml for 250 ng, 1949.3 ± 171.9 pg/ml for 500 ng, and 1403.5 ± 48.1 pg/ml for 1000 ng of NLRC3 plasmid co-transfected with 1000 ng of NALP3 plasmid). NLRC3 not only dampened NALP3-induced IL-1β secretion (Fig. 1, A and B) but also inhibited pro-IL-1β maturation (Fig. 1C) when ectopically expressed in HEK293FT cells.
Figure 1.
Overexpression of NLRC3 inhibits NALP3-induced IL-1β secretion. A and B, IL-1β secretion in ectopically assembled NALP3 inflammasomes in HEK293FT cells transfected with 500 ng of pro-IL-1β, 250 ng of ASC, and 250 ng of pro-caspase-1 plasmids and the indicated amount of NALP3 (A) or 1 μg of NALP3 and the indicated amount of NLRC3 (B). Each condition was duplicated. Secreted IL-1β was quantified 24 h after transfection in cell-free supernatants by ELISA. C, IL-1β cleavage in HEK293FT cells with ectopically assembled NALP3 inflammasomes. NT, non-transfected. D, NLRC3 mRNA levels in the THP-1 OvCont and OvNLRC3 cell lines. Quantitative PCR results of relative mRNA of NLRC3/β-actin are given. The NLRC3/β-actin ratio of OvCont cells was assigned as 1 arbitrary unit. E, NLRC3 protein levels in THP-1 OvControl and OvNLRC3 stable lines. F and G, IL-1β secretion in THP-1 OvNLRC3 and THP-1 OvControl stable lines in response to nigericin (F) and MSU (G) treatment.
To further confirm our results, we generated THP-1 stable lines that overexpressed the NLRC3 protein (named OvNLRC3) and confirmed its expression both at the mRNA (Fig. 1D) and the protein levels (Fig. 1E). Induction of the NALP3 inflammasome by stimulating stable THP-1 cells with nigericin (Fig. 1F) or MSU (Fig. 1G) triggered a significantly lower IL-1β release from THP-1 OvNLRC3 cells compared with their OvControl counterpart (3084.8 ± 64.1 pg/ml for OvNLRC3 versus 6385.7 ± 287.2 pg/ml for OvCont when treated with nigericin, p = 0.0039), suggesting that NLRC3 acts as an inhibitor of NALP3-induced IL-1β secretion in both cell types.
Knockdown of NLRC3 in THP-1 cells results in an increase in IL-1β secretion and pro-IL-1β and pro-caspase-1 cleavage
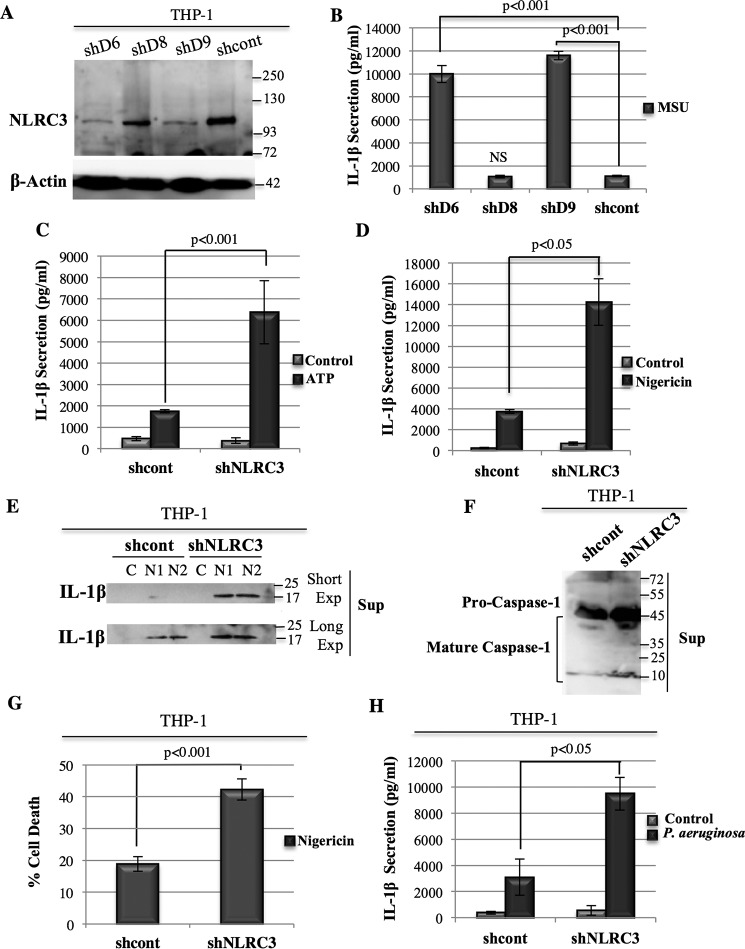
To eliminate possible overexpression artifacts, we used human monocytic THP-1 cells that endogenously express NALP3 and inflammasome components and generated stable NLRC3 knockdown THP-1 cell lines by using three different shRNAs (shD6, shD8, and shD9) (Fig. 2A). We obtained a significant decrease in NLRC3 levels with shD9- and shD6-infected cells, whereas no change was observed with the shD8 cell line (Fig. 2A). When stimulated with MSU, the IL-1β secretion observed correlated with the NLRC3 levels (Fig. 2B; 1118.13 ± 60.7 pg/ml for shcont versus 1067.11 ± 110.4 pg/ml for shD8, p = 0.45; 9991.68 ± 740.6 pg/ml for shD6, p = 10−7; 11591.68 ± 372.8 pg/ml for shD9, p = 10−9).
Figure 2.
Knockdown of NLRC3 in THP-1 cells results in an increase in IL-1β secretion and pro-IL-1β and pro-caspase-1 cleavage. A, NLRC3 protein levels in three different stable THP-1 NLRC3 KD cell lines (shD6, shD8, and shD9) and their control line (shcont). B, IL-1β secretion in shD6, shD8, shD9, and shcont THP-1 stable cell lines in response to MSU stimulation (representative result of four independent sets of experiments). NS, not significant. C and D, IL-1β secretion in shNLRC3 THP-1 cell lines in response to ATP (C) (representative result of three independent sets of experiments) and nigericin (D) (combined results of two independent sets of experiments) stimulation. E, pro-IL-1β cleavage in the cell lysate and supernatant of the shcont and shNLRC3 cell lines in response to nigericin stimulation. Control (C) samples represent cells not treated with nigericin. Two independent samples were treated with nigericin (N1 and N2). Exp, exposure. F, pro-caspase-1 cleavage in the supernatant (Sup) of nigericin-treated shcont and shNLRC3 cells. G, LDH results of nigericin-treated shcontrol and shNLRC3 THP-1 cells. Shown are representative result of three sets of independent experiment. H, IL-1β secretion in response to P. aeruginosa infection of the shcont and shNLRC3 cell lines. Two-tailed Student's t test was performed.
Because the shD9 cell line gave the more significant decrease in NLRC3 level, we used it in the rest of our experiments and named it shNLRC3. Similarly, although basal IL-1β secretion was not significantly different between shNLRC3 and shcont cells, stimulation of the NALP3 inflammasome with ATP (Fig. 2C; 6376.7 ± 1473.5 pg/ml for shNLRC3 versus 1746 ± 71.3 pg/ml for shcont, p = 0.0008; supplemental Fig. 1A) or nigericin (Fig. 2D, 14253.4 ± 2239.9 pg/ml for shNLRC3 versus 3760.1 ± 182.4 pg/ml for shcont, p = 0.022) resulted in higher IL-1β release in cells where NLRC3 was knocked down. Moreover, an increase in pro-IL-1β cleavage in the supernatant (Fig. 2E and supplemental Fig. 1B) and higher pro-caspase-1 processing (Fig. 2F) were observed in the shNLRC3 line compared with shcont in response to nigericin treatment. Also, the shNLRC3 line induced higher cell death compared with its control shcont counterpart when stimulated with nigericin (Fig. 2G; 42.2 ± 3.3% versus 18.9 ± 2.3% of cell death, p = 10−5, and supplemental Fig. 2). Thus, we concluded that the endogenous NLRC3 protein suppresses the activation of the NALP3 inflammasome induced by different types of ligand and the resulting pyroptotic cell death.
NLRC3 is also an inhibitor of the NLRC4 (IPAF) inflammasome
To determine whether the inhibitory effect of NLRC3 is specific to the NALP3 complex or whether it could also modulate the activity of other inflammasomes, the NLRC4 (or IPAF) inflammasome was stimulated by live P. aeruginosa infection. Knockdown of the NLRC3 protein in THP-1 cells significantly increased IL-1β secretion in response to P. aeruginosa infection compared with NLRC3-expressing cells (Fig. 2H, 9493.9 ± 1256.8 pg/ml for shNLRC3 versus 3099.96 ± 1256.7 pg/ml for shcont, p = 0.04). Because NLRC3 inhibits both the NALP3 and NLRC4 inflammasomes, we concluded that it may be acting downstream of the receptor proteins.
NLRC3 does not regulate the protein levels of the inflammasome components
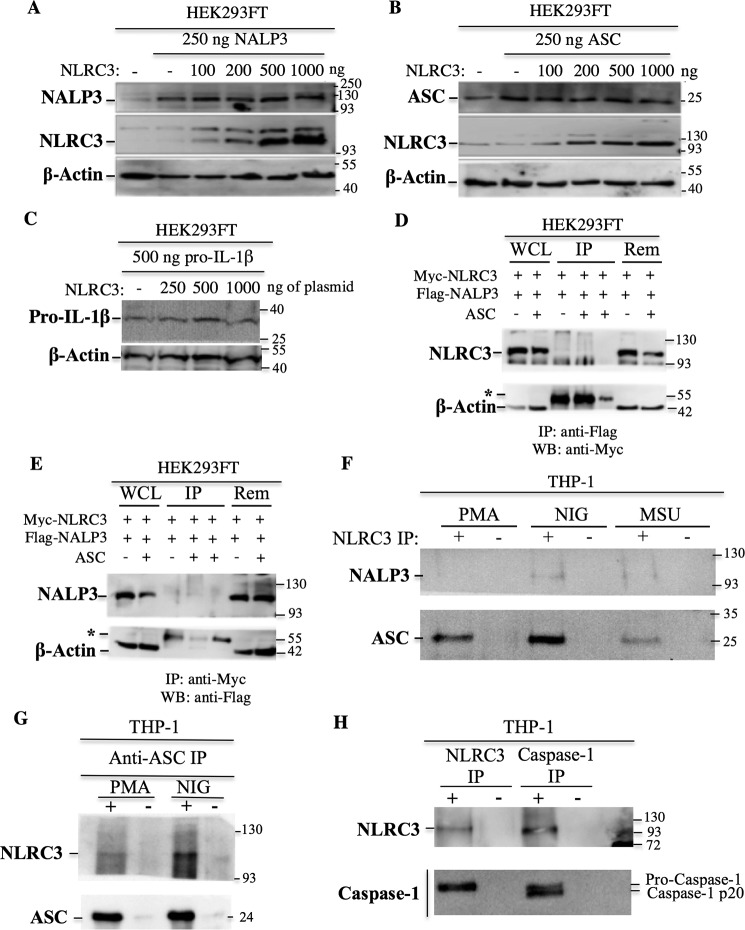
NLRC3 was shown to inhibit the NFκB pathway by inducing the ubiquitination of TRAF6 and targeting it for proteasomal degradation (26). We investigated whether NLRC3 exert its inhibitory effect through induction of proteasomal degradation of the inflammasome components by co-transfecting constant NALP3, ASC, and pro-IL-1β concentrations and increasing the amount of NLRC3. No difference was observed in transfected NALP3, ASC, or pro-IL-1β protein levels at different NLRC3 concentrations (Fig. 3, A–C), suggesting that NLRC3 inhibits the inflammasome by a mechanism independent of protein degradation or synthesis.
Figure 3.
NLRC3 interacts endogenously with pro-caspase-1 via its CARD and with ASC but not with NALP3. A–C, NLRC3 does not regulate the protein levels of the inflammasome components. Equal amounts of the NALP3 (A), ASC (B), and pro-IL-1β (C) proteins were transfected into HEK293FT cells in the presence of increasing concentrations of NLRC3. Protein levels were analyzed by Western blotting. D and E, 5 × 106 HEK293FT cells were transfected with 6 μg of ASC, Myc-NLRC3, and FLAG-NALP3 plasmids. An irrelevant anti-HA antibody was used as a negative IP control. WCL, whole-cell lysates; Rem, remaining flow-through fraction after IP; asterisks, IgG heavy chains (55 kDa); WB, Western blot. Results from anti-FLAG (D) and anti-Myc (E) are shown. F, interaction of endogenous NLRC3 with endogenous NALP3 and endogenous ASC. IP was performed with anti-NLRC3 antibody in PMA-differentiated THP-1 cells and nigericin (NIG)- and MSU-treated THP-1 cells. G, interaction of endogenous NLRC3 with endogenous ASC. IP was performed with anti-ASC antibody in control and nigericin-treated THP-1 cells. H, interaction of endogenous NLRC3 with endogenous caspase-1 in THP-1 cells. +, IP with anti-NLRC3 or anti–caspase-1 antibody; −, IP with anti-rabbit IgG antibody.
NLRC3 interacts endogenously with pro-caspase-1 via its CARD and with ASC but not with NALP3
We have shown previously that NLRC3 co-localizes and interacts with ASC and caspase-1 when overexpressed in HEK293FT cells (28). Here we investigated whether NLRC3 could interact with NALP3. When the two proteins were overexpressed in HEK293FT cells, immunoprecipitation of either NALP3 (Fig. 3D) or NLRC3 (Fig. 3E) did not pull down NLRC3 or NALP3, respectively. Furthermore, the endogenous NLRC3 and NALP3 proteins did not interact with each other in any of the untreated or nigericin- and MSU treated THP-1 cells (Fig. 3F).
As a next step, to verify that the interactions that were seen previously between NLRC3 and ASC or caspase-1 are also occurring physiologically, the endogenous proteins were immunoprecipitated from THP-1 cells. Endogenous NLRC3 interacted with both endogenous ASC (Fig. 3, F and G) and pro-caspase-1 (Fig. 3H). In this study, we used a commercial anti–caspase-1 antibody that is directed against the p10 subunit of caspase-1. Although immunoprecipitation with the anti–caspase-1 p10 antibody pulled down both pro-caspase-1 and the mature, CARD-lacking caspase-1 p20 peptide, the anti-NLRC3 antibody immunoprecipitated only the CARD-containing pro form of caspase-1 (Fig. 3H). Thus, we concluded that NLRC3 interacts with the CARD of caspase-1.
NLRC3 prevents the assembly of ASC specks through its CARD and LRR domain
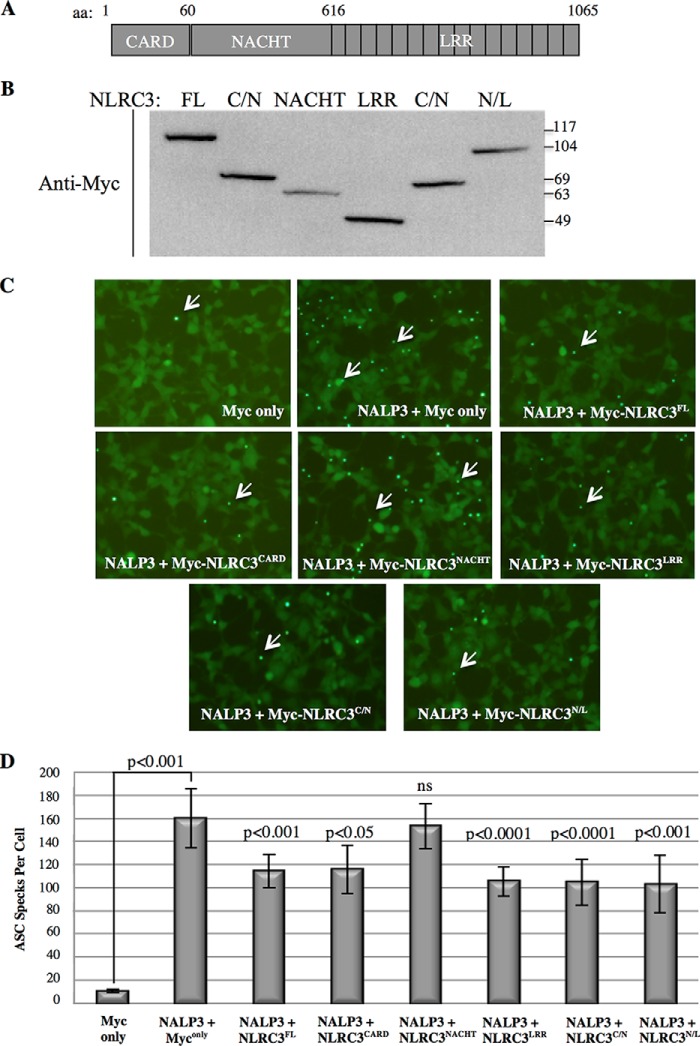
Another informative way of measuring inflammasome activity is to count the number of ASC specks that are formed. To characterize which domain of NLRC3 disrupts ASC speck formation, we cloned its NLRC3CARD (aa 1–60), NLRC3NACHT (aa 61–616), and NLRC3LRR (aa 617–1065) domains separately or two of its domains, NLRC3CARD/NACHT(aa 1–616) and NLRC3NACHT/LRR (aa 617–1065), together (Fig. 4A) and verified their expression by Western blotting (Fig. 4B).
Figure 4.
NLRC3 prevents the assembly of ASC specks through its CARD and LRR domain. A, NLRC3 protein structure. Different domains and amino acid numbers are shown. B, Western blot result showing the expression of Myc-tagged, full-length NLRC3 and separate domains. Anti-Myc antibody was used. C/N, CARD/NACHT; N/L, NACHT/LRR. Predicted molecular weights: FL, 117 kDa; NACHT, 63 kDa; LRR, 49 kDa; CARD/NACHT, 69 kDa; NACHT/LRR, 110 kDa. C, representative pictures of three sets of independent experiments of the effect of the domains of NLRC3 on ASC speck formation induced by NALP3. D, quantification of ASC specks formed in the presence of the domains of NLRC3 (the p values shown are the indicated condition compared with the NALP3+ Myc only condition, Student's t test).
Although transfection of NALP3 into HEK293FT-ASC-EGFP cells triggered ASC speck assembly (Figs. 4 and 6), full-length NLRC3 (NLRC3FL) itself or its domains had no effect on ASC speck formation on their own (data not shown). However, when NALP3 was co-expressed with NLRC3FL, NLRC3CARD, NLRC3LRR, NLRC3CARD/NACHT, and NLRC3NACHT/LRR, the number of ASC specks formed was significantly lower than the number of specks formed in the presence of NALP3 alone (Fig. 4, C and D). Thus, we concluded that the CARD and LRR domain of NLRC3 were responsible for the disruption of the NALP3-induced complex assembly and that the central NACHT domain had no effect.
Figure 6.

The CARD of NLRC3 is necessary and sufficient to inhibit inflammasome activation triggered by the NALP3 mutants associated with autoinflammatory syndromes. A, IL-1β secretion induced by WT NALP3 or NALP3 p.R260W and p.D303N mutants in the absence (black) or presence of full-length NLRC3 (gray) and NLRC3CARD (light gray). B and C, ASC speck formation induced by WT NALP3 or NALP3 p.R260W and NALP3 p.D303N mutants in the presence or absence of NLRC3 and NLRC3CARD. Shown are quantification of ASC specks (B) and representative images of three sets of independent experiments (C). Samples were compared with Student's t test.
NLRC3 co-localizes with diffused ASC proteins and competes with ASC for pro-caspase-1 binding
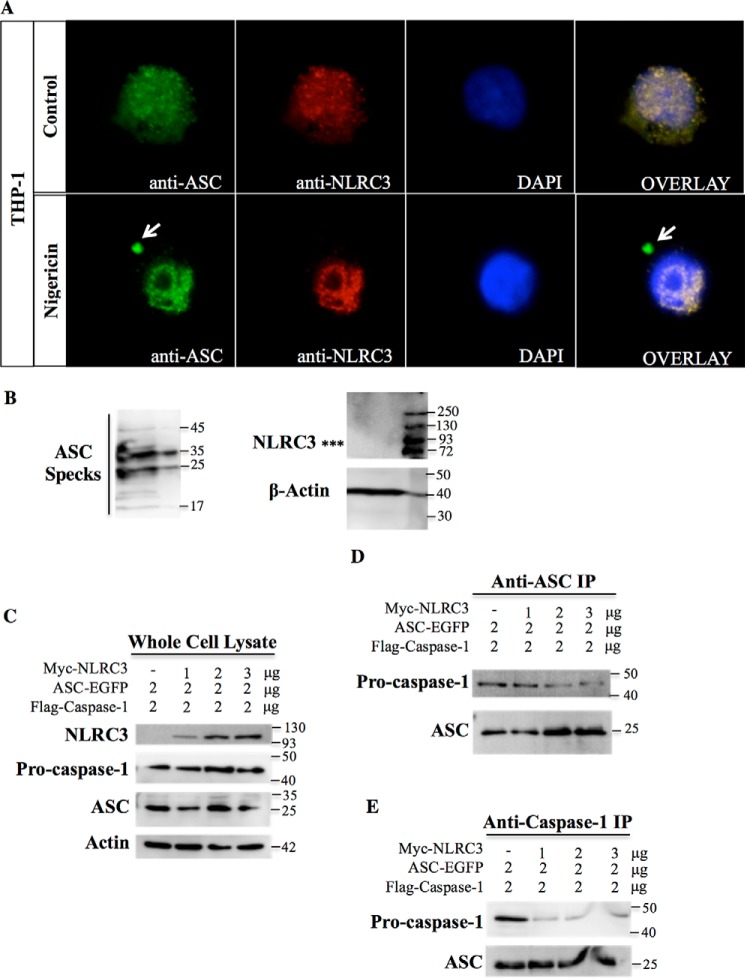
To confirm that NLRC3 co-localizes with ASC endogenously, we stained PMA-differentiated and nigericin-treated THP-1 macrophages with anti-NLRC3 and anti-ASC antibodies. We found that NLRC3 and diffuse ASC stainings overlapped each other in the cytosol; however, an NLRC3 signal was not observed on ASC specks (Fig. 5A). Furthermore, we could not detect the NLRC3 protein by Western blotting of the lysate containing ASC specks isolated from ASC- and NLRC3-co-transfected cells (Fig. 5B). Taken together, these results demonstrate that NLRC3 interacts with diffused ASC proteins but is not embedded into ASC specks after inflammasome activation.
Figure 5.
NLRC3 co-localizes with diffused ASC proteins and competes with ASC for pro-caspase-1 binding. A, endogenous ASC (green) and endogenous NLRC3 (red) co-localization in MSU-treated THP-1 cells. Arrows show ASC specks. B, NLRC3 expression in ASC specks isolated from mCherry-ASC–transfected HEK293FT cells. 12 μl of pure ASC specks were loaded on a gel. Left panel, ASC mono- and oligomers. Right panel, NLRC3 and actin blot. Asterisks, position where the NLRC3 band is expected to be (117 kDa). C–E, transfection of ASC and FLAG-pro-caspase-1 with increasing concentrations of Myc-NLRC3. Shown is protein expression in whole-cell lysate (C), anti-ASC IP (D), and anti-caspase-1 IP (E) lysates.
Because NLRC3 interacts with both ASC and pro-caspase-1, we determined whether NLRC3 might affect the interaction between these two proteins. Although ASC and pro-caspase-1 interacted with each other when they were overexpressed in HEK293FT cells, addition of NLRC3 resulted in a weaker interaction between ASC and pro-caspase-1 in both samples immunoprecipitated with anti-ASC (Fig. 5D) or anti–caspase-1 (Fig. 5E) antibodies. Moreover, increasing the concentration of NLRC3 by keeping a constant amount of ASC and pro-caspase-1 proteins (Fig. 5C) decreased the interaction between ASC and pro-caspase-1 even more. Thus, NLRC3 may exert its inhibitory effect before complex formation by sequestering the pro-caspase-1 proteins and by blocking the assembly of pro-caspase-1 and ASC required for inflammasome activity.
The CARD of NLRC3 is necessary and sufficient to inhibit inflammasome activation triggered by NALP3 mutants associated with autoinflammatory syndromes
Because mutations in the NALP3 gene are known to induce the constitutive activation of the NALP3 inflammasome, we tested whether full-length NLRC3 and NLRC3CARD are able to suppress this aberrant inflammatory response. We found that both full-length-NLRC3 (269.2 ± 5.8 pg/ml versus 216 ± 13.7 pg/ml for WT NALP3, p = 0.0004; 841.4 ± 26.6 pg/ml versus 311.2 ± 9.2 pg/ml for p.R260W NALP3, p = 10−8; 1150 ± 47.3 pg/ml versus 195.1 ± 32.1 pg/ml for p.D303N NALP3, p = 10−7) and NLRC3CARD (109.3 ± 21.8 pg/ml for WT NALP3, p = 10−6; 122 ± 14.8 pg/ml for p.R260W NALP3, p = 10−9; 45.2 ± 13.3 pg/ml for p.D303N NALP3, p = 10−8; all compared with the “no NLRC3” conditions) inhibit IL-1β secretion induced by the ectopically assembled inflammasome complex in HEK293FT cells, and NLRC3CARD had an even more significant effect than the full-length protein (Fig. 6A, p = 0.0002 for WT NALP3, p = 10−7 for NALP3 p.R260W, and p = 0.0001 for NALP3 p.D303N). Furthermore, NLRC3FL and NLRC3CARD also impaired the ASC speck formation triggered by WT and NALP3 p.R260W proteins (Fig. 6, B and C). On the other hand, no effect was observed on the number of ASC specks formed by NALP3 p.D303N proteins, probably because the concentration of transfected NLRC3 was not high enough to block the excessive effect of the NALP3 p.D303N mutant. These data demonstrate that the CARD of NLRC3 is able and sufficient to repress the NALP3 inflammasome activity under normal or pathologic conditions.
Discussion
We investigated, in this paper, the potential effect of the NLRC3 protein on the activation of the NALP3 inflammasome by using human THP-1 monocytes and HEK293FT cells when we had to ectopically express an exogenous protein. We found that NLRC3 decreases the number of ASC specks formed, the maturation of pro-IL-1β and pro-caspase-1 proteins, and the secretion of IL-1β in both overexpression and endogenous experimental settings. The suppressive effect of NLRC3 on the NALP3 inflammasome in THP-1 cells was stimulant-independent because complex activation in response to all ligands used (ATP, nigericin, and MSU) was affected by NLRC3.
Moreover, the CARD of NLRC3 was necessary to exert this inhibition. NLRC3 had no effect on protein levels, and its suppressive effect was independent of its role on the NFκB pathway because, with the use of HEK293FT cells, overexpression of these proteins bypassed transcriptional regulation of the inflammasome components. Another advantage of HEK293FT cells that do not express the ASC adaptor protein at all and negligible levels of the other inflammasome components was the possibility to introduce mutant NALP3 proteins or different NLRC3 domains without possible interference of the WT NALP3 or full-length NLRC3 proteins, respectively.
Recruitment of pro-caspase-1 proteins by the ASC adaptor to foci is a very critical step in the activation of inflammasomes. The CARD–CARD interaction between ASC and pro-caspase-1 was required for complex formation because mutagenesis of some residues in these domains abolished ASC/pro-caspase-1 binding and inflammasome activation (29). Similarly, the self-oligomerization of the CARD of caspase-1 was also shown to prevent its interaction with ASC (30). Here we showed that the presence of NLRC3, which also contains a CARD, decreases the interaction between ASC and pro-caspase-1. The absence of an NLRC3 and NALP3 interaction and the ability of NLRC3 to inhibit both NALP3 and NLRC4 inflammasomes and different NALP3 mutant–induced IL-1β secretion suggest that NLRC3 acts on the ASC/pro-caspase-1 level. A similar phenomenon is observed in NFκB pathway activation, where the ASC protein interferes with RIP2 and caspase-1 assembly through its CARD (31). More recently, an antibody generated against the CARD of ASC was sufficient to inhibit inflammasome activation (32), again suggesting how important the interaction of ASC and caspase-1 through their CARD domain is.
Overall, we propose in this paper that, besides its previously defined suppressive role in the NFκB pathway, NLRC3 is also a novel inhibitor of activation of the NALP3 and NLRC4 inflammasomes through disruption of ASC speck assembly by competing with the ASC protein for binding to the CARD of pro-caspase-1.
Experimental procedures
Plasmids used in this study
The pcDNA3-ASC, pcDNA3-FLAG-pro-caspase-1, pro-IL-1β-IRES-EGFP, pcDNA3-FLAG-WT_NALP3, pcDNA3-FLAG-R260W_NALP3, and pcDNA3-FLAG-D303N_NALP3 plasmids were kind gifts from Prof. Gabriel Nunez (University of Michigan). Full-length NLRC3 was cloned in our laboratory as described previously (28). The domains of NLRC3 were amplified from the pcDNA3-Myc-NLRC3 template with NLRC3CARD (aa 1–60) ForwCARD-XbaI (TAAGTCTAGAAGGAAGC-AAGAGGTGCGG) and RevCARD-Stop-NotI (TATGCGGCCGCTCAGTCATTGCTGCA), NLRC3NACHT (aa 61–616) ForwNACHT-NheI (GC-GGGCTAGCTCAAGGATACAGAGG) and RevNACHT-Stop-NotI (TAATGCGGCCGCTC-ACTGGGCACAG), NLRC3LRR (aa 617–1065) ForwLRR-XbaI (ATGGTCTAGTGAGGCCAAC-CTGTCC) and RevLRR-Stop-NotI (TTGCGGCC-GCTCAATCACATTTCAACAG), NLRC3CARD/NACHT (aa 1–616) ForwCARD-XbaI and RevNACHT-Stop-NotI, and NLRC3NACHT/LRR (aa 61–1065) ForwNACHT-NheI and RevLRR-Stop-NotI primers, digested with the indicated enzymes, and ligated into XbaI-PspOMI–digested and purified empty pcDNA3-Myc vector. To generate the pLenti-CMV-Blast-NLRC3 vector, full-length NLRC3 was extracted from the pET30a-NLRC3 vector by BglII and XhoI digestions and inserted in a 3:1 ratio into the BamHI- and XhoI-digested pure pLenti-CMV-Blast-Empty vector (kindly provided by Assist. Prof. Tolga Emre, Boğaziçi University, Istanbul, Turkey).
Ectopic reconstruction of the NALP3 inflammasome in HEK293FT cells for the measurement of the IL-1β secretion
750,000 HEK293FT cells were transfected using the calcium phosphate protocol with 250 ng of pcDNA3-ASC, 250 ng of pcDNA3-FLAG-pro-caspase-1, and 500 ng of pro-IL-1β-IRES-EGFP plasmids and the indicated amount of pcDNA3-FLAG-WT NALP3 or pcDNA3-MYC-NLRC3. Equal amounts of DNA were transfected under each condition by adding the empty pcDNA3 vector to compensate for differences. Cell supernatants were harvested 24 h after transfection and spun down to discard cells, and the IL-1β levels in 10% FBS containing DMEM were measured by ELISA as explained later. For analysis of CAPS mutants, besides the amount of ASC, pro-caspase-1, and pro-IL-1β indicated above, 1 μg of WT, p.R260W, or p.D303N NALP3 was transfected with 1 μg of empty vector, NLRC3FL, or NLRC3CARD.
Generation of the NLRC3 knockdown and NLRC3-overexpressing stable THP-1 cell lines
Viruses were produced in HEK293FT cells by transfection of pLKO.1-shNLRC3 and control pLKO.1-shGFP vectors (kindly provided by Assist. Prof. Ömer Yilmaz, Broad Institute, Massachusetts Institute of Technology, Boston, MA) or newly generated pLenti-CMV-Blast-NLRC3 (OvNLRC3) vectors and their control pLenti-CMV-Blast (named OvCont) together with the helper plasmids pMD2G and psPAX2. THP-1 cells were spinoculated for 30 min at 32 °C, incubated with the virus for 4 h at 37 °C and 5% CO2, and selected with 1 μg/ml puromycin or 1 μg/ml blasticidin. Four different shRNAs targeting different parts of NLRC3 cloned into the pLKO.1 vector (shNLRC3-D6 (TRCN0000168401), shNLRC3-D8 (TRCN0000168587), shNLRC3-D9 (TRCN0000168194), and mouse NLRC3-specific shmNLRC3-D7 (TRCN0000168383)) were used to confirm the reproducibility of the NLRC3 knockdown phenotype.
Activation of the NALP3 and IPAF/NLRC4 inflammasomes in THP-1 cells
106 shcont or shNLRC3 or OvNLRC3 and OvControl THP-1 stable lines were seeded into a 6-well plate and differentiated into macrophages with 3 h of 0.5 μm PMA treatment. The next day, cells were primed for 2 or 4 h with 100 ng/ml LPS and treated with 20 μm nigericin, 5 mm ATP, or 150 μg/ml MSU for 4 or 6 h, respectively, for NALP3 inflammasome activation and treated with 75 multiplicity of infection live P. aeruginosa for 4 h for IPAF/NLRC4 inflammasome activation. Supernatants were harvested and cleared from cells, and IL-1β levels were measured by ELISA.
Measurement of secreted IL-1β levels by ELISA
IL-1β levels were determined in 1 ml of cell supernatant by using the R&D Systems human IL-1β IL-1F2 kit according to the protocol of the manufacturer. Each treatment was performed in duplicate or triplicate, and the supernatants were measured in duplicate. Significance between samples was determined by two-tailed Student's t test.
Co-immunoprecipitation of the endogenous and overexpressed inflammasome components
Commercially available anti-NLRC3 (Abcam, ab77817), anti–caspase-1 (Santa Cruz Biotechnology, sc-515), anti–ASC hybridoma supernatant (a kind gift from Assist. Prof. Junya Masumoto, Ehime University, Ehime, Japan), and anti-NALP3 (Abcam, ab17267) antibodies were used. For co-immunoprecipitation in the overexpression system, anti-Myc (Cell Signaling Technology, 2272) and anti-FLAG (Cell Signaling Technology, 2368) antibodies were used.
ASC speck formation assay
The HEK293FT-ASC-EFGP stable cell lines generated in our laboratory were transfected with NALP3, full-length NLRC3, or the different domains of NLRC3 in a 6-well plate with 500 ng of NALP3, 500 ng of NLRC3, or 500 ng of the different domains of NLRC3 (Fig. 4) or 1 μg of WT NALP3, NALP3 pR260W, or NALP3 p.D303N and 1 μg of NLRC3 (Fig. 6). An equal amount of DNA was transfected by using the pcDNA3-Myc empty vector. The number of ASC specks was counted 24 or 48 h after transfection under a fluorescence microscope. Each condition was transfected into two wells, and eight randomly chosen fields were counted for each well.
Isolation of the ASC specks
ASC specks were isolated from four 100-mm plates of 5 × 106 HEK293FT cells each and transfected with 4 μg of mCherry-ASC plasmid. 24 h after transfection, samples were sonicated, vortexed until filaments were formed, and spun down at 200 × g for 1 h. The pellet was dissolved in 30 ml of PBS, passed through a 5-μm filter, and spun down at 2400 × g for 1 h. The final pellet containing ASC specks was dissolved in 500 μl of PBS. The purity of isolated ASC specks was verified under the microscope and by Western blotting.
Competition assay for the binding of NLRC3 to its interaction partners
5 × 106 HEK293FT cells were transfected with constant pcDNA3-ASC (2 μg) and pcDNA3-FLAG-pro-caspase-1 (2 μg) and increasing concentrations of pcDNA3-Myc-NLRC3 (1, 2, and 3 μg). Cells were harvested 24 h after transfection, and immunoprecipitation was performed.
LDH assay
An LDH cytotoxicity detection kit from Biovision was used according to the protocol of the manufacturer. The percentage of cell death was determined with the formula (OD sample − OD LDH−) × 100 / (OD LDH+ − OD LDH−). Untreated controls were used as LDH− samples and Triton X–treated control cells as LDH+ samples.
Author contributions
E. E. designed and performed the experiments and wrote the manuscript. M. B. contributed to the realization of some experiments. N. Ö. designed the experiments, wrote the manuscript, and provided financial resources.
Supplementary Material
Acknowledgments
We thank the persons cited in the text for generously sharing the indicated materials with us. We also thank Dr. Stefan A. Köstler for taking the confocal images.
This work was supported by EMBO-SDIG 1468 and Boğaziçi University Research Fund Projects BAP 6526 (11B01D10) and BAP 7360 (13B01M3) (to N. Ö.). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains supplemental Figs. 1 and 2.
- ASC
- apoptosis-associated speck-like protein containing a CARD
- CARD
- caspase recruitment domain
- MSU
- monosodium urate
- CAPS
- cryopyrin-associated periodic syndrome(s)
- NLR
- nucleotide oligomerization domain–like receptor
- LRR
- leucine-rich repeat
- aa
- amino acids
- PMA
- phorbol 12-myristate 13-acetate
- LDH
- lactate dehydrogenase
- OD
- optical density
- IP
- immunoprecipitation
- FL
- full-length
- IPAF
- interleukin-1 converting enzyme protease-activating factor.
References
- 1. Martinon F., Burns K., and Tschopp J. (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell 10, 417–426 [DOI] [PubMed] [Google Scholar]
- 2. Schroder K., and Tschopp J. (2010) The inflammasomes. Cell 140, 821–832 [DOI] [PubMed] [Google Scholar]
- 3. Srinivasula S. M., Poyet J. L., Razmara M., Datta P., Zhang Z., and Alnemri E. S. (2002) The PYRIN-CARD protein ASC is an activating adaptor for caspase-1. J. Biol. Chem. 277, 21119–21122 [DOI] [PubMed] [Google Scholar]
- 4. Fernandes-Alnemri T., Wu J., Yu J. W., Datta P., Miller B., Jankowski W., Rosenberg S., Zhang J., and Alnemri E. S. (2007) The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 14, 1590–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mariathasan S., Weiss D. S., Newton K., McBride J., O'Rourke K., Roose-Girma M., Lee W. P., Weinrauch Y., Monack D. M., and Dixit V. M. (2006) Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440, 228–232 [DOI] [PubMed] [Google Scholar]
- 6. Martinon F., Pétrilli V., Mayor A., Tardivel A., and Tschopp J. (2006) Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 [DOI] [PubMed] [Google Scholar]
- 7. Sutterwala F. S., Mijares L. A., Li L., Ogura Y., Kazmierczak B. I., and Flavell R. A. (2007) Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J. Exp. Med. 204, 3235–3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bauernfeind F. G., Horvath G., Stutz A., Alnemri E. S., MacDonald K., Speert D., Fernandes-Alnemri T., Wu J., Monks B. G., Fitzgerald K. A., Hornung V., and Latz E. (2009) Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183, 787–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pétrilli V., Papin S., Dostert C., Mayor A., Martinon F., and Tschopp J. (2007) Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 14, 1583–1589 [DOI] [PubMed] [Google Scholar]
- 10. Muñoz-Planillo R., Kuffa P., Martínez-Colón G., Smith B. L., Rajendiran T. M., and Núñez G. (2013) K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38, 1142–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hornung V., Bauernfeind F., Halle A., Samstad E. O., Kono H., Rock K. L., Fitzgerald K. A., and Latz E. (2008) Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 9, 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou R., Yazdi A. S., Menu P., and Tschopp J. (2011) A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225 [DOI] [PubMed] [Google Scholar]
- 13. Hoffman H. M., Mueller J. L., Broide D. H., Wanderer A. A., and Kolodner R. D. (2001) Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat. Genet. 29, 301–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu J. W., Wu J., Zhang Z., Datta P., Ibrahimi I., Taniguchi S., Sagara J., Fernandes-Alnemri T., and Alnemri E. S. (2006) Cryopyrin and pyrin activate caspase-1, but not NF-κB, via ASC oligomerization. Cell Death Differ. 13, 236–249 [DOI] [PubMed] [Google Scholar]
- 15. Baroja-Mazo A., Martín-Sánchez F., Gomez A. I., Martínez C. M., Amores-Iniesta J., Compan V., Barberà-Cremades M., Yagüe J., Ruiz-Ortiz E., Antón J., Buján S., Couillin I., Brough D., Arostegui J. I., and Pelegrín P. (2014) The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat. Immunol. 15, 738–748 [DOI] [PubMed] [Google Scholar]
- 16. Agostini L., Martinon F., Burns K., McDermott M. F., Hawkins P. N., and Tschopp J. (2004) NALP3 forms an IL-1β-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 20, 319–325 [DOI] [PubMed] [Google Scholar]
- 17. Dowds T. A., Masumoto J., Zhu L., Inohara N., and Núñez G. (2004) Cryopyrin-induced interleukin 1β secretion in monocytic cells: enhanced activity of disease-associated mutants and requirement for ASC. J. Biol. Chem. 279, 21924–21928 [DOI] [PubMed] [Google Scholar]
- 18. Hoffman H. M., Rosengren S., Boyle D. L., Cho J. Y., Nayar J., Mueller J. L., Anderson J. P., Wanderer A. A., and Firestein G. S. (2004) Prevention of cold-associated acute inflammation in familial cold autoinflammatory syndrome by interleukin-1 receptor antagonist. Lancet 364, 1779–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hawkins P. N., Lachmann H. J., and McDermott M. F. (2003) Interleukin-1-receptor antagonist in the Muckle-Wells syndrome. N. Engl. J. Med. 348, 2583–2584 [DOI] [PubMed] [Google Scholar]
- 20. Bauernfeind F., Rieger A., Schildberg F. A., Knolle P. A., Schmid-Burgk J. L., and Hornung V. (2012) NLRP3 inflammasome activity is negatively controlled by miR-223. J. Immunol. 189, 4175–4181 [DOI] [PubMed] [Google Scholar]
- 21. Coll R. C., Robertson A. A., Chae J. J., Higgins S. C., Muñoz-Planillo R., Inserra M. C., Vetter I., Dungan L. S., Monks B. G., Stutz A., Croker D. E., Butler M. S., Haneklaus M., Sutton C. E., Núñez G., et al. (2015) A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 21, 248–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stack J. H., Beaumont K., Larsen P. D., Straley K. S., Henkel G. W., Randle J. C., and Hoffman H. M. (2005) IL-converting enzyme/caspase-1 inhibitor VX-765 blocks the hypersensitive response to an inflammatory stimulus in monocytes from familial cold autoinflammatory syndrome patients. J. Immunol. 175, 2630–2634 [DOI] [PubMed] [Google Scholar]
- 23. Xia X., Cui J., Wang H. Y., Zhu L., Matsueda S., Wang Q., Yang X., Hong J., Songyang Z., Chen Z. J., and Wang R. F. (2011) NLRX1 negatively regulates TLR-induced NF-κB signaling by targeting TRAF6 and IKK. Immunity 34, 843–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tong Y., Cui J., Li Q., Zou J., Wang H. Y., and Wang R. F. (2012) Enhanced TLR-induced NF-κB signaling and type I interferon responses in NLRC5 deficient mice. Cell Res. 22, 822–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Conti B. J., Davis B. K., Zhang J., O'connor W. Jr, Williams K. L., and Ting J. P. (2005) CATERPILLER 16.2 (CLR16.2), a novel NBD/LRR family member that negatively regulates T cell function. J. Biol. Chem. 280, 18375–18385 [DOI] [PubMed] [Google Scholar]
- 26. Schneider M., Zimmermann A. G., Roberts R. A., Zhang L., Swanson K. V., Wen H., Davis B. K., Allen I. C., Holl E. K., Ye Z., Rahman A. H., Conti B. J., Eitas T. K., Koller B. H., and Ting J. P. (2012) The innate immune sensor NLRC3 attenuates Toll-like receptor signaling via modification of the signaling adaptor TRAF6 and transcription factor NF-κB. Nat. Immunol. 13, 823–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang L., Mo J., Swanson K. V., Wen H., Petrucelli A., Gregory S. M., Zhang Z., Schneider M., Jiang Y., Fitzgerald K. A., Ouyang S., Liu Z. J., Damania B., Shu H. B., Duncan J. A., et al. (2014) NLRC3, a member of the NLR family of proteins, is a negative regulator of innate immune signaling induced by the DNA sensor STING. Immunity 40, 329–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gültekin Y., Eren E., and Özören N. (2015) Overexpressed NLRC3 acts as an anti-inflammatory cytosolic protein. J. Innate Immun. 7, 25–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Proell M., Gerlic M., Mace P. D., Reed J. C., and Riedl S. J. (2013) The CARD plays a critical role in ASC foci formation and inflammasome signaling. Biochem. J. 449, 613–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Narayanan K. B., and Park H. H. (2015) Purification and analysis of the interactions of caspase-1 and ASC for assembly of the inflammasome. Appl. Biochem. Biotechnol. 175, 2883–2894 [DOI] [PubMed] [Google Scholar]
- 31. Sarkar A., Duncan M., Hart J., Hertlein E., Guttridge D. C., and Wewers M. D. (2006) ASC directs NF-κB activation by regulating receptor interacting protein-2 (RIP2) caspase-1 interactions. J. Immunol. 176, 4979–4986 [DOI] [PubMed] [Google Scholar]
- 32. Schmidt F. I., Lu A., Chen J. W., Ruan J., Tang C., Wu H., and Ploegh H. L. (2016) A single domain antibody fragment that recognizes the adaptor ASC defines the role of ASC domains in inflammasome assembly. J. Exp. Med. 213, 771–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.