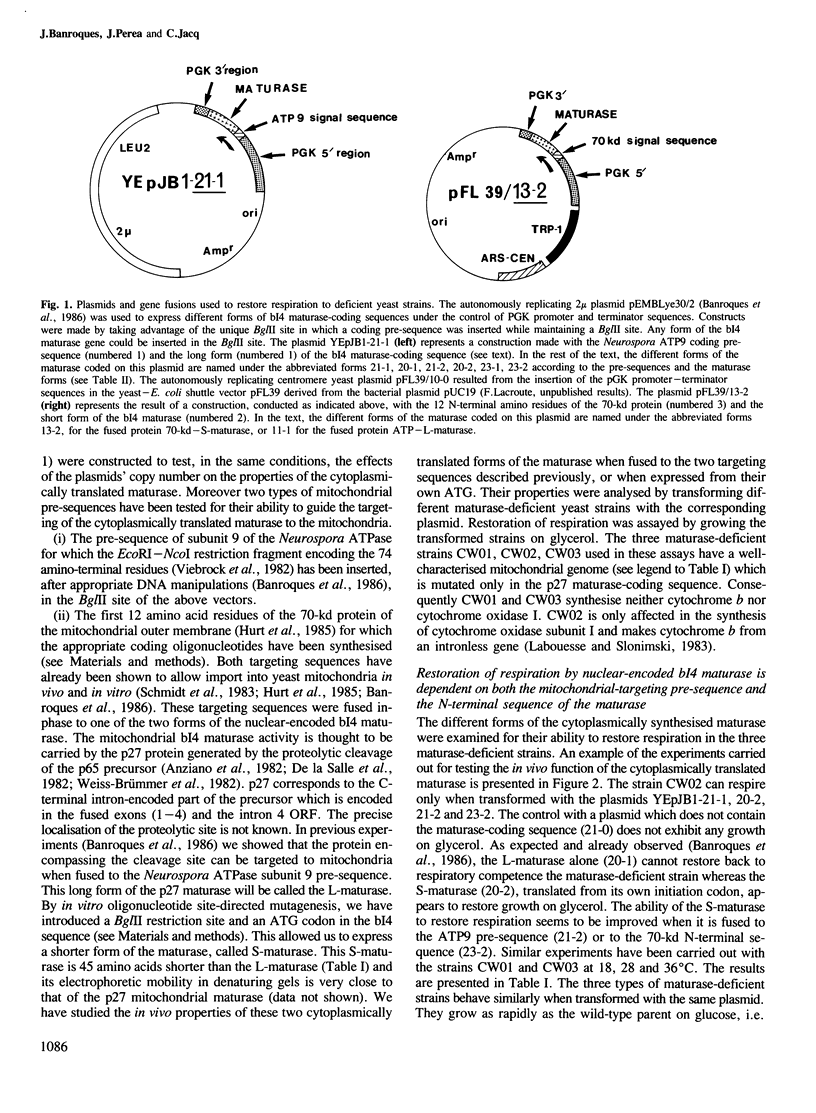
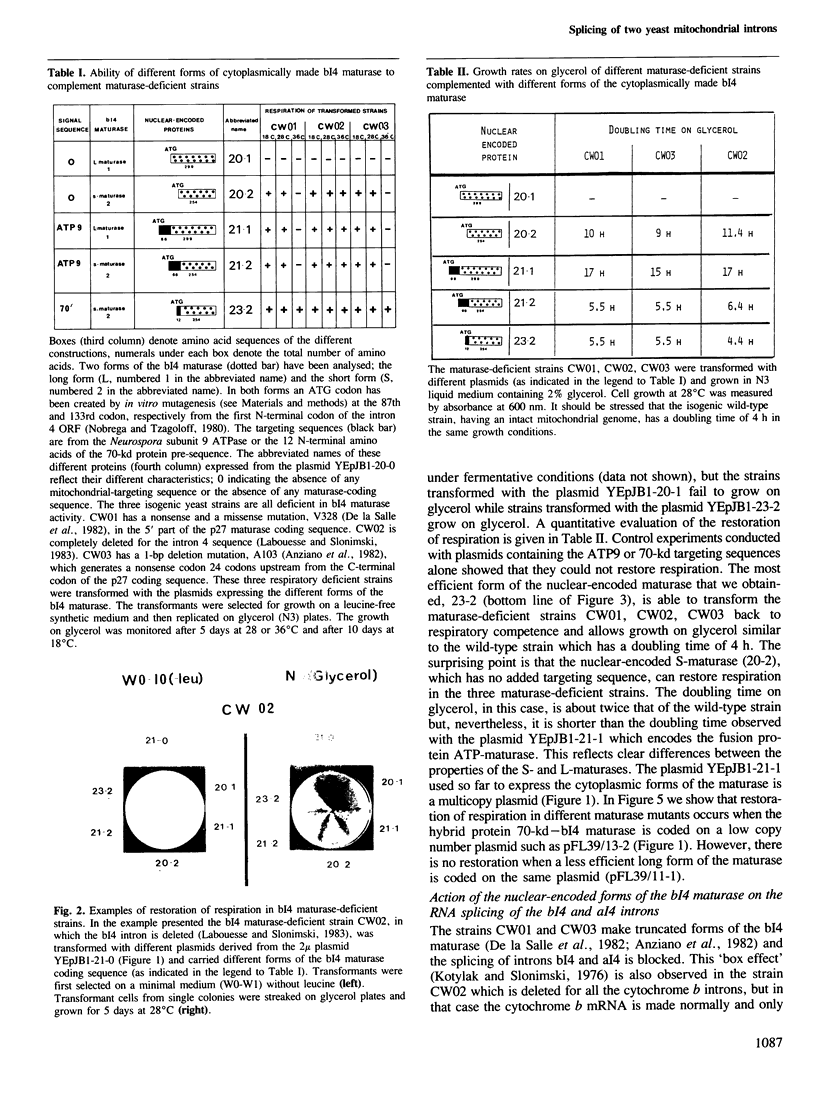
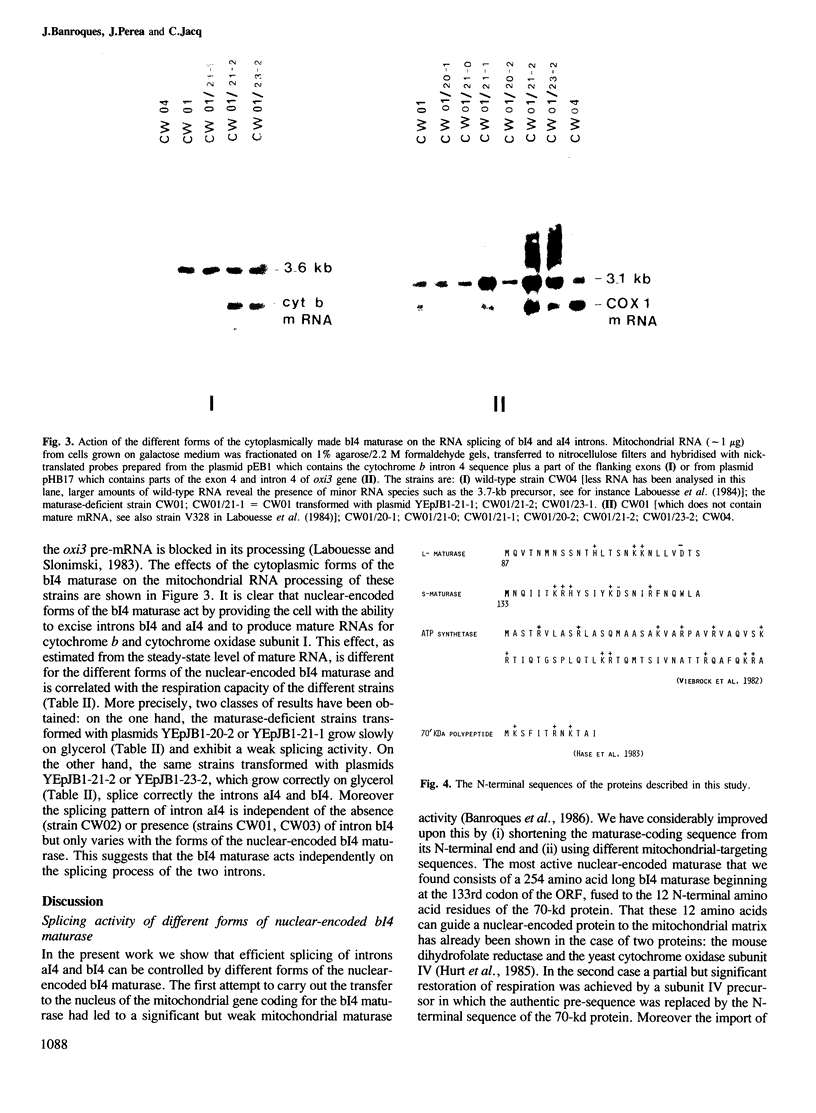
Abstract
bI4 maturase encoded by the fourth intron of the yeast mitochondrial cytochrome b gene, controls the splicing of both the fourth intron of the cytochrome b gene and the fourth intron of the gene encoding subunit I of cytochrome oxidase. It has been shown previously that a cytoplasmically translated hybrid protein composed of the pre-sequence of subunit 9 of Neurospora ATPase fused to a part of the bI4 maturase can be guided to mitochondria where it could compensate maturase deficiencies. This in vivo complementation of maturase mutants can be easily estimated by restoration of respiration. This work examines the efficiency of different bI4 maturase constructions to restore respiration in different yeast maturase-deficient strains. It is shown that the N-terminal end of the bI4 maturase plays a crucial role in the maturase activity. Moreover, the 12 N-terminal amino acids of the mitochondrial outer membrane protein constitute the most efficient mitochondrial targeting sequence in this system. Surprisingly enough, it was found that the cytoplasmically translated bI4 maturase containing the 254 C-terminal amino acid coded by the intron open reading frame can complement maturase mutations without any added mitochondrial-targeting sequence.
Full text
PDF
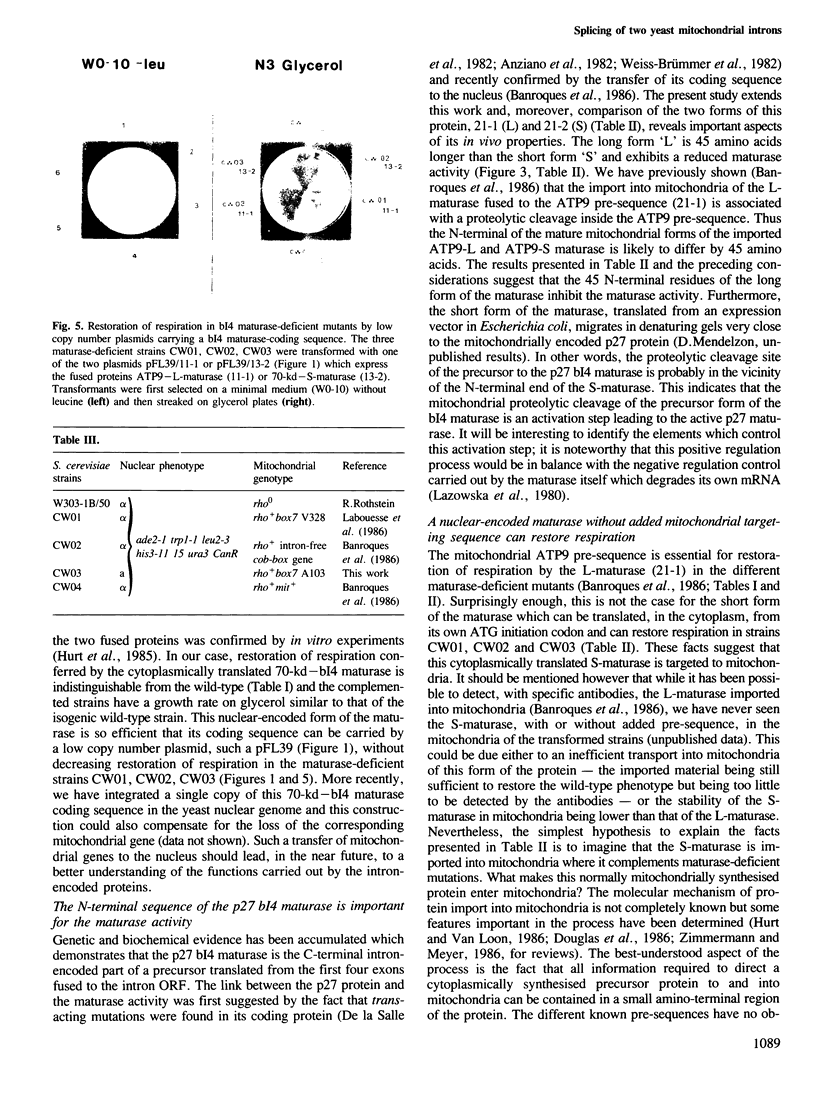


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anziano P. Q., Hanson D. K., Mahler H. R., Perlman P. S. Functional domains in introns: trans-acting and cis-acting regions of intron 4 of the cob gene. Cell. 1982 Oct;30(3):925–932. doi: 10.1016/0092-8674(82)90297-5. [DOI] [PubMed] [Google Scholar]
- Banroques J., Delahodde A., Jacq C. A mitochondrial RNA maturase gene transferred to the yeast nucleus can control mitochondrial mRNA splicing. Cell. 1986 Sep 12;46(6):837–844. doi: 10.1016/0092-8674(86)90065-6. [DOI] [PubMed] [Google Scholar]
- Carignani G., Groudinsky O., Frezza D., Schiavon E., Bergantino E., Slonimski P. P. An mRNA maturase is encoded by the first intron of the mitochondrial gene for the subunit I of cytochrome oxidase in S. cerevisiae. Cell. 1983 Dec;35(3 Pt 2):733–742. doi: 10.1016/0092-8674(83)90106-x. [DOI] [PubMed] [Google Scholar]
- Carter P., Bedouelle H., Winter G. Improved oligonucleotide site-directed mutagenesis using M13 vectors. Nucleic Acids Res. 1985 Jun 25;13(12):4431–4443. doi: 10.1093/nar/13.12.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech T. R., Bass B. L. Biological catalysis by RNA. Annu Rev Biochem. 1986;55:599–629. doi: 10.1146/annurev.bi.55.070186.003123. [DOI] [PubMed] [Google Scholar]
- De La Salle H., Jacq C., Slonimski P. P. Critical sequences within mitochondrial introns: pleiotropic mRNA maturase and cis-dominant signals of the box intron controlling reductase and oxidase. Cell. 1982 Apr;28(4):721–732. doi: 10.1016/0092-8674(82)90051-4. [DOI] [PubMed] [Google Scholar]
- Douglas M. G., Geller B. L., Emr S. D. Intracellular targeting and import of an F1-ATPase beta-subunit-beta-galactosidase hybrid protein into yeast mitochondria. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3983–3987. doi: 10.1073/pnas.81.13.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas M. G., McCammon M. T., Vassarotti A. Targeting proteins into mitochondria. Microbiol Rev. 1986 Jun;50(2):166–178. doi: 10.1128/mr.50.2.166-178.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujardin G., Jacq C., Slonimski P. P. Single base substitution in an intron of oxidase gene compensates splicing defects of the cytochrome b gene. Nature. 1982 Aug 12;298(5875):628–632. doi: 10.1038/298628a0. [DOI] [PubMed] [Google Scholar]
- Garriga G., Lambowitz A. M., Inoue T., Cech T. R. Mechanism of recognition of the 5' splice site in self-splicing group I introns. Nature. 1986 Jul 3;322(6074):86–89. doi: 10.1038/322086a0. [DOI] [PubMed] [Google Scholar]
- Garriga G., Lambowitz A. M. Protein-dependent splicing of a group I intron in ribonucleoprotein particles and soluble fractions. Cell. 1986 Aug 29;46(5):669–680. doi: 10.1016/0092-8674(86)90342-9. [DOI] [PubMed] [Google Scholar]
- Guiso N., Dreyfus M., Siffert O., Danchin A., Spyridakis A., Gargouri A., Claisse M., Slonimski P. P. Antibodies against synthetic oligopeptides allow identification of the mRNA-maturase encoded by the second intron of the yeast cob-box gene. EMBO J. 1984 Aug;3(8):1769–1772. doi: 10.1002/j.1460-2075.1984.tb02044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt E. C., Müller U., Schatz G. The first twelve amino acids of a yeast mitochondrial outer membrane protein can direct a nuclear-coded cytochrome oxidase subunit to the mitochondrial inner membrane. EMBO J. 1985 Dec 16;4(13A):3509–3518. doi: 10.1002/j.1460-2075.1985.tb04110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacq C., Banroques J., Becam A. M., Slonimski P. P., Guiso N., Danchin A. Antibodies against a fused 'lacZ-yeast mitochondrial intron' gene product allow identification of the mRNA maturase encoded by the fourth intron of the yeast cob-box gene. EMBO J. 1984 Jul;3(7):1567–1572. doi: 10.1002/j.1460-2075.1984.tb02012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labouesse M., Dujardin G., Slonimski P. P. The yeast nuclear gene NAM2 is essential for mitochondrial DNA integrity and can cure a mitochondrial RNA-maturase deficiency. Cell. 1985 May;41(1):133–143. doi: 10.1016/0092-8674(85)90068-6. [DOI] [PubMed] [Google Scholar]
- Labouesse M., Herbert C. J., Dujardin G., Slonimski P. P. Three suppressor mutations which cure a mitochondrial RNA maturase deficiency occur at the same codon in the open reading frame of the nuclear NAM2 gene. EMBO J. 1987 Mar;6(3):713–721. doi: 10.1002/j.1460-2075.1987.tb04812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labouesse M., Netter P., Schroeder R. Molecular basis of the 'box effect', A maturase deficiency leading to the absence of splicing of two introns located in two split genes of yeast mitochondrial DNA. Eur J Biochem. 1984 Oct 1;144(1):85–93. doi: 10.1111/j.1432-1033.1984.tb08434.x. [DOI] [PubMed] [Google Scholar]
- Labouesse M., Slonimski P. P. Construction of novel cytochrome b genes in yeast mitochondria by subtraction or addition of introns. EMBO J. 1983;2(2):269–276. doi: 10.1002/j.1460-2075.1983.tb01416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazowska J., Jacq C., Slonimski P. P. Sequence of introns and flanking exons in wild-type and box3 mutants of cytochrome b reveals an interlaced splicing protein coded by an intron. Cell. 1980 Nov;22(2 Pt 2):333–348. doi: 10.1016/0092-8674(80)90344-x. [DOI] [PubMed] [Google Scholar]
- Macreadie I. G., Scott R. M., Zinn A. R., Butow R. A. Transposition of an intron in yeast mitochondria requires a protein encoded by that intron. Cell. 1985 Jun;41(2):395–402. doi: 10.1016/s0092-8674(85)80012-x. [DOI] [PubMed] [Google Scholar]
- Mellor J., Dobson M. J., Roberts N. A., Tuite M. F., Emtage J. S., White S., Lowe P. A., Patel T., Kingsman A. J., Kingsman S. M. Efficient synthesis of enzymatically active calf chymosin in Saccharomyces cerevisiae. Gene. 1983 Sep;24(1):1–14. doi: 10.1016/0378-1119(83)90126-9. [DOI] [PubMed] [Google Scholar]
- Michel F., Lang B. F. Mitochondrial class II introns encode proteins related to the reverse transcriptases of retroviruses. Nature. 1985 Aug 15;316(6029):641–643. doi: 10.1038/316641a0. [DOI] [PubMed] [Google Scholar]
- Nobrega F. G., Tzagoloff A. Assembly of the mitochondrial membrane system. DNA sequence and organization of the cytochrome b gene in Saccharomyces cerevisiae D273-10B. J Biol Chem. 1980 Oct 25;255(20):9828–9837. [PubMed] [Google Scholar]
- Perea J., Jacq C. Role of the 5' hairpin structure in the splicing accuracy of the fourth intron of the yeast cob-box gene. EMBO J. 1985 Dec 1;4(12):3281–3288. doi: 10.1002/j.1460-2075.1985.tb04078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer M., Edmundson A. B. Use of helical wheels to represent the structures of proteins and to identify segments with helical potential. Biophys J. 1967 Mar;7(2):121–135. doi: 10.1016/S0006-3495(67)86579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt B., Hennig B., Köhler H., Neupert W. Transport of the precursor to neurospora ATPase subunit 9 into yeast mitochondria. Implications on the diversity of the transport mechanism. J Biol Chem. 1983 Apr 25;258(8):4687–4689. [PubMed] [Google Scholar]
- Viebrock A., Perz A., Sebald W. The imported preprotein of the proteolipid subunit of the mitochondrial ATP synthase from Neurospora crassa. Molecular cloning and sequencing of the mRNA. EMBO J. 1982;1(5):565–571. doi: 10.1002/j.1460-2075.1982.tb01209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss-Brummer B., Rödel G., Schweyen R. J., Kaudewitz F. Expression of the split gene cob in yeast: evidence for a precursor of a "maturase" protein translated from intron 4 and preceding exons. Cell. 1982 Jun;29(2):527–536. doi: 10.1016/0092-8674(82)90169-6. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]
- van Loon A. P., Brändli A. W., Schatz G. The presequences of two imported mitochondrial proteins contain information for intracellular and intramitochondrial sorting. Cell. 1986 Mar 14;44(5):801–812. doi: 10.1016/0092-8674(86)90846-9. [DOI] [PubMed] [Google Scholar]
- van der Horst G., Tabak H. F. Self-splicing of yeast mitochondrial ribosomal and messenger RNA precursors. Cell. 1985 Apr;40(4):759–766. doi: 10.1016/0092-8674(85)90335-6. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Mitochondrial targeting sequences may form amphiphilic helices. EMBO J. 1986 Jun;5(6):1335–1342. doi: 10.1002/j.1460-2075.1986.tb04364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]