Abstract
Early screening for sleep apnea (SA) is rarely considered in patients with acute cerebral ischemia. We aimed to evaluate the feasibility of early SA screening on a stroke unit, its impact on post-discharge SA care and the relation of SA to clinical features. Patients with acute ischemic stroke (AIS) and transient ischemic attack (TIA) prospectively underwent overnight cardiorespiratory polygraphy within 3 ± 2 days of symptom-onset. Feasibility was defined as analyzable polygraphy in 90 % of studied patients. We enrolled 61 patients (84 % AIS, 16 % TIA): mean age 66 ± 8 years, 44 % men, median NIHSS 1 (0–15), median ESS 5 (0–13). Analyzability was given in 56/61 (91.8 %; one-sided 95 % CI, lower-bound 86.0 %) patients indicating excellent feasibility of early SA screening with no significant differences in stroke severity (100 % in TIA, 91 % minor stroke, 83 % major stroke, p = 0.474). Ninety-one percent (51/56) had an apnea–hypopnea index ≥5/h (median: 20/h [0–79]); 32 % (18/ 56) mild, 30 % (17/56) moderate, and 29 % (16/56) severe SA. When comparing sleep-related ischemic stroke (SIS) and non-SIS patients, no differences were found regarding the presence (95 vs. 89 %, p = 0.49) or severity (e.g., severe SA: 32 vs. 27 %, p = 0.69) of SA. After 12 months, 27/38 (71 %) patients given specific recommendations completed in-laboratory sleep work-up and 7/27 (25 %) were prescribed for non-invasive ventilatory correction. In conclusion, early SA screening is feasible in patients with acute cerebral ischemia and may have a positive impact on post-discharge SA care. Given the high frequency and atypical presentation of SA, early screening for SA should be considered in all acute cerebral ischemia patients.
Keywords: Stroke, Sleep apnea, Secondary prevention
Introduction
Sleep apnea (SA) is increasingly recognized by the stroke community as an independent stroke risk factor and serious disorder that contributes to early neurological deterioration, worse outcome and increased mortality in acute ischemic stroke (AIS) patients [1–7]. However, little has been done yet to incorporate facilitation of early evaluation and management of these patients into stroke guidelines [8–10]. Experts advocate that the suspicion of SA should trigger a comprehensive sleep evaluation including sleep-oriented history and objective sleep testing by means of attended, in-laboratory polysomnography (PSG) [11]. The latter approach may be reasonable in the ambulatory setting but in the hospital setting, only a few stroke centers have access to sleep medicine for in-patient populations and even this access may not be continuously available. Furthermore, AIS patients need continuous monitoring as well as constant nursing care and it may be hazardous to move them for in-laboratory sleep testing during the acute phase of stroke.
Identification of stroke etiology and associated vascular risk factors constitutes an integral part of organized inpatient stroke unit care, and its benefit on long-term functional outcome and survival of stroke patients has been clearly demonstrated [12]. Cardiorespiratory polygraphy, a portable SA screening device, reliably allows detection of SA when compared with the gold-standard PSG [13, 14]. Nonetheless, available data on the use of such devices in hospitalized patients, not to mention stroke patients are limited [15]. Thus, it is of particular interest, whether SA monitoring using such devices can be implemented during the early stage of stroke as part of organized stroke unit care.
We aimed to evaluate the feasibility of early bedside SA screening in patients with acute cerebral ischemia on a stroke unit and its impact on post-discharge SA care. As secondary aims we investigated the clinical presentation of SA in this patient population as well as its relation to diurnal variations in stroke occurrence.
Methods
Study population
We prospectively enrolled consecutive patients with AIS and transient ischemic attack (TIA) age 18–75 years who were admitted to our stroke unit at the Dresden University Stroke Center from November 2009 to May 2011 (convenience sampling). Exclusion criteria were an unfavorable premorbid functional condition defined by a modified Rankin Scale (mRS) score >3 points, pre-existing SA and severe comorbidity (i.e., congestive heart failure, chronic lung disorder, any actual malignant disease, dementia) that would compromise therapeutic consequences. Patients who were not able to give written consent (e.g., severe aphasia) were excluded from this study. Our study was approved by the local Institutional Review Board and written informed consent was obtained from each patient.
Clinical evaluation at baseline
Demographic and anthropometric (weight, height, body mass index [BMI]) data, baseline National Institutes of Health Stroke Scale (NIHSS) scores (categorized as minor <6 and major stroke ≥6 points), vascular risk factors, stroke etiology (classified by Trial of Org 10172 in Acute Stroke Treatment [TOAST] criteria) [16] and brain imaging findings were assessed prospectively. Presumptive onset of stroke symptoms was obtained from each patient and/or their relatives. Sleep-related ischemic stroke (SIS) was defined as the presence of stroke symptoms on awakening (wake-up stroke, WUS) or symptom-onset within 1 h of awakening from sleep. All other patients were considered non-SIS.
Evaluation for sleep apnea
We interviewed patients for the presence of excessive daytime sleepiness using the Epworth Sleepiness Scale (ESS). The ESS was considered abnormal when the score was ≥10 points [17].
All patients underwent overnight SA screening within 3 ± 2 days from symptom-onset while admitted to the stroke unit using a 6-channel portable cardiorespiratory polygraphy device (SOMNOcheck effort®, Weinmann Medical Technology, Hamburg, Germany). With this cardiorespiratory polygraphy device, nasal airflow was measured by using a thermistor, body position and respiratory movements by a piezoelectric sensor located in chest and abdomen fixing straps, and capillary oxygen saturation and heart rate were measured by a pulse oximeter finger clip. The patients were monitored from 11 pm to 7 am without interfering with conventional stroke care. Stroke fellows were instructed to attach the device properly by 10:30 pm and the device was detached by a nurse the next morning.
Polygraphy data were transmitted to a PC and SOMNOcheck analysis software (Weinmann Medical Technology, Hamburg, Germany) automatically generated the sleep polygram. The sleep polygrams were analyzed manually by a sleep neurologist (JK, WS) according to the American Academy of Sleep (AASM) guidelines (AASM Manual for Scoring of Sleep and Associated Events) [18]. An apnea during sleep was defined as cessation of airflow (>90 % fall in the amplitude of airflow signal compared to the baseline airflow) lasting at least 10 s. Apnea was further classified as obstructive with continued respiratory effort, as central with absent respiratory effort and as mixed with absent respiratory effort followed by resumption of respiratory effort during apnea. Hypopnea was defined as a 50 % or greater fall in airflow lasting ten or more seconds associated with a 3 % or greater fall in oxygen saturation from baseline. The apnea–hypopnea index (AHI) was calculated using the total number of respiratory events (apneas and hypopneas) per hour sleep, and categorized in any (AHI ≥ 5/h), mild (AHI 5–14/h), moderate (AHI 15–29/h) and severe (AHI ≥ 30/h) SA as previously reported [11].
Feasibility of overnight cardiorespiratory polygraphy was defined as an analyzable polygraphy in 90 % of studied patients. Analyzability was mainly based on completeness of polygraphy (i.e., all parameters were captured for at least 6 h allowing proper calculation of the AHI), but eventually at the discretion of the sleep neurologist (particularly for borderline findings). Reasons for non-analyzable polygraphies were recorded.
Post-discharge observation
According to polygraphy findings, each patient with moderate and severe SA was given a specific recommendation by a sleep neurologist (JK, WS) in order to undergo further in-laboratory sleep work-up (i.e., nocturnal PSG). In patients with mild SA, recommendations were given only when concurrent sleep-related symptoms were present as suggested by the AASM guidelines [11]. Further diagnostic and therapeutic sleep studies were performed outside this study. A follow-up at 6 and 12 months was performed by telephone interview including assessment of the mRS score and recurrent cardio- or cerebrovascular events. Results of further sleep laboratory work-up and corresponding SA therapy (if prescribed) were retrieved from sleep laboratory reports.
Statistical analysis
We assumed an analyzable polygraphy in 90 % of the study population. With a one-sided confidence interval (CI) of 95 % and a lower confidence limit of 82.5 %, a minimum of 44 analyzable patients would be needed to proof feasibility of polygraphy in our study population. Categorical variables were assessed using Chi-square tests or Fisher's exact test while continuous variables were assessed using Student's t test and Wilcoxon rank sum test, where appropriate. Spearman's correlation was used to assess correlations between the AHI and clinical variables of interest. Logistic regression was used to determine if AHI was a significant independent predictor of SIS or WUS. Since the latter was an exploratory analysis, no adjustments for multiple comparisons were made. Adjusted models were not used when the crude model was not significant. Sensitivity with corresponding 95 % CI's of cardiorespiratory polygraphy for detection of SA was calculated after computation of true positive and false negative values according to follow-up PSG results. Missing data for mRS were imputated using the last-observation-carried-forward method. A p value of <0.05 was considered to be statistically significant.
Results
Of approximately 800 patients with acute cerebral ischemia admitted to our tertiary stroke center during the study period, we enrolled a convenience sample of 61 patients: mean age was 65.6 ± 7.5 years, 44 % were men, median baseline NIHSS score was 1 (range 0–15) point, median ESS was 5 (0–13) points. Fifty-one out of 61 (84 %) patients had an AIS, 10/61 (16 %) had a TIA. Baseline characteristics and clinical data of the study population are presented in Table 1 .
Table 1. Baseline characteristics and clinical data of the study population.
| Total (n = 61) | Analyzable (n = 56) | Non-analyzable (n = 5) | p value | |
|---|---|---|---|---|
| Demographics | ||||
| Mean age ± SD, years | 64.6 ± 7.5 | 64.3 ± 7.7 | 67.6 ± 3.5 | 0.359 |
| Gender, male, n (%) | 27 (44) | 26 (46) | 1 (20) | 0.254 |
| Risk factors | ||||
| Hypertension, n (%) | 54 (89) | 49 (88) | 5 (100) | 0.401 |
| Diabetes, n (%) | 21 (34) | 18 (32) | 3 (60) | 0.209 |
| Dyslipidemia, n (%) | 35 (57) | 33 (59) | 2 (40) | 0.412 |
| Smoking (past 5 years), n (%) | 13 (21) | 12 (21) | 1 (20) | 0.940 |
| Coronary artery disease, n (%) | 4 (7) | 4 (7) | 0 | 0.536 |
| Atrial fibrillation, n (%) | 10 (16) | 8 (14) | 2 (40) | 0.137 |
| Previous ischemic stroke/TIA, n (%) | 22 (36) | 20 (36) | 2 (40) | 0.426 |
| Alcohol daily, n (%) | 8 (13) | 7 (12.5) | 1 (20) | 0.634 |
| Clinical data | ||||
| Mean BMI ± SD | 27.2 ± 3.8 | 27.1 ± 3.9 | 27.9 ± 1.6 | 0.708 |
| Median ESS, range | 5 (0–13) | 5 (0–13) | 5 (2–7) | 0.559 |
| Median NIHSS, range | 1 (0–15) | 1 (0–15) | 0 (0–7) | 0.191 |
| Stroke severity | 0.544* | |||
| TIA, n (%) | 10 (16) | 10 (18) | 0 | |
| Minor stroke, n (%) | 45 (74) | 41 (73) | 4 (80) | |
| Major stroke, n (%) | 6 (10) | 5 (9) | 1 (20) | |
| TOAST | 0.529* | |||
| Large artery, n (%) | 37 (61) | 34 (61) | 3 (60) | |
| Cardioembolism, n (%) | 12 (20) | 10 (18) | 2 (40) | |
| Small vessel, n (%) | 7 (11) | 7 (12.5) | 0 | |
| Other, n (%) | 0 | 0 | 0 | |
| Undetermined, n (%) | 5 (8) | 5 (9) | 0 | |
| Median mRS 6 months, range | 1 (0–6) | 1 (0–6) | 1 (0–4) | 0.794 |
| Median mRS 12 months, range | 1 (0–6) | 1 (0–6) | 1 (1–2) | 0.469 |
TIA transient ischemic attack, BMI body mass index, ESS Epworth Sleepiness Scale, NIHSS National Institutes of Health Stroke Scale, TOAST Trial of Org 10172 in Acute Stroke Treatment, mRS modified Rankin scale
Overall p value for all categories
Cardiorespiratory polygraphy was performed 2.1 ± 1.1 days after stroke onset. Analyzability was given in 56/61 (91.8 %; one-sided 95 % CI, lower-bound 86.0 %) patients indicating excellent feasibility of overnight SA monitoring in patients with acute cerebral ischemia. There were no significant differences in terms of analyzability according to stroke severity (100 % in TIA, 91 % minor stroke, 83 % major stroke, p = 0.474). Cardiorespiratory polygraphy was not analyzable due to missing data for nasal airflow in 1/61 (1.6 %) patient, for oxygen saturation in 2/61 (3.3 %), and due to patients' adherence in 2/61 (3.3 %) patients.
Median AHI was 20/h (0–79/h). Fifty-one out of 56 (91 %, 95 % CI, 80.7–96.1 %) analyzable patients had an AHI ≥ 5/h: 32 % (18/56) mild, 30 % (17/56) moderate and 29 % (16/56) severe SA. The predominant type of SA was obstructive (44/51, 86 %), whereas only 2/51 (4 %) patients had central and 5/51 (10 %) mixed SA. There was no significant difference in the prevalence of SA in TIAs, minor strokes, and major strokes (100, 88, and 100 %, respectively). According to stroke etiology (as indicated by TOAST classification), the prevalence of SA seemed higher in patients with large artery atherosclerosis, cardioembolism and small artery occlusion, as compared to those with undetermined cause (LAA 94 % [32/34] vs. CE 100 % [10/ 10] vs. SAO 100 % [7/7] vs. UND 40 % [2/5]; p < 0.001, respectively). In addition, the magnitude of AHI differed significantly across stroke etiologies with undetermined cause the lowest and small vessel disease as well as cardioembolism the highest (mean AHI: 6.2/h vs. 31.4/h vs. 33.4/h; p = 0.039). In terms of clinical presentation of SA, no significant correlation was found between excessive daytime sleepiness (ESS) and AHI ≥ 5/h (r = 0.123, p = 0.366). The moderate strength positive correlation seen between BMI and AHI ≥ 5/h (r = 0.250, p = 0.063) appeared slightly stronger in the AIS subgroup (r = 0.276, p = 0.063).
At discharge, recommendations for further in-laboratory sleep work-up were given to 38/51 (75 %) patients (Fig. 1). Mean elapsed time from recommendation to sleep laboratory work-up was 136 ± 62 days. After 6 months, 23 of 38 (61 %) patients completed in-laboratory PSG, of which 2/23 (9 %) started treatment with non-invasive ventilatory correction. One out of 51 (2 %) patients expired due to a recurrent ischemic stroke. After 12 months, 27/38 (71 %) patients completed in-laboratory PSG. Of these patients, 19/27 (70 %) had moderate-to-severe SA (i.e., clinically relevant) and 7/27 (25 %) were prescribed non-invasive ventilatory correction. The remaining 12/27 (75 %) patients refused non-invasive ventilatory correction or favored conservative treatment (i.e., sleep postural changes, weight loss, oral appliances). As compared with the follow-up complete PSG, cardiorespiratory polygraphy yielded sensitivity of 60 % (95 % CI 31.3–83.2 %) for detection of moderate and 77.8 % (95 % CI 45.3–93.7 %) for severe SA; however, in combined moderate-to-severe SA, sensitivity of early polygraphy reached 94.7 % (95 % CI 75.4–99.1 %). Two out of 51 (4 %) patients had a recurrent ischemic stroke, one of which expired. These two patients were diagnosed with severe SA, but noninvasive ventilatory therapy was not started yet. No cardiovascular events were reported during the post-discharge observation period. Functional outcome is presented in Table 1.
Fig. 1.
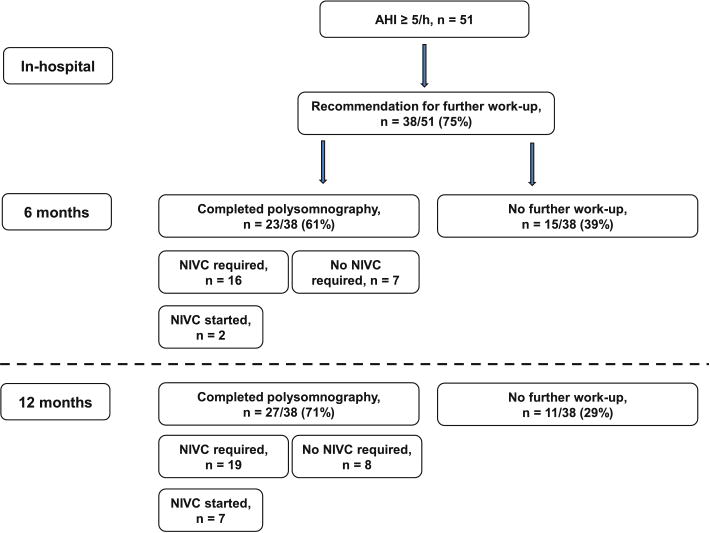
Post-discharge flow chart. AHI apnea–hypopnea index, NIVC non-invasive ventilatory correction
Diurnal variations of stroke
Sleep-related ischemic stroke was found in 19/56 (34 %) patients including 14/56 (25 %) patients with WUS. Fewer men were among SIS patients (26 vs. 57 %, p = 0.03) and SIS patients had a higher prevalence of atrial fibrillation (32 vs. 5 %, p = 0.008). There were no statistically significant differences in the remainder of baseline characteristics (Table 2). When comparing SIS and non-SIS patients, no differences were found regarding the presence (95 vs. 89 %, p = 0.49) or severity (e.g., severe SA: 32 vs. 27 %, p = 0.69) of SA. Similarly, there were no differences in the presence (100 vs. 88 %, p = 0.17) or severity (e.g., severe SA: 32 vs. 29 %, p = 0.66) of SA when comparing WUS and non-WUS patients. The AHI did not prove to be a significant independent predictor of SIS (p = 0.45) or WUS (p = 0.56).
Table 2. Baseline characteristics and clinical data of the study population (separated by sleep-related- and non-sleep-related stroke onset).
| SIS (n = 19) | Non-SIS (n = 37) | p value | |
|---|---|---|---|
| Demographics | |||
| Mean age ± SD, years | 65.8 ± 7.0 | 63.6 ± 8.1 | 0.304 |
| Gender, male, n (%) | 5 (26) | 21 (57) | 0.031 |
| Risk factors | |||
| Hypertension, n (%) | 17 (89) | 32 (88) | 0.749 |
| Diabetes, n (%) | 6 (32) | 12 (32) | 0.948 |
| Dyslipidemia, n (%) | 9 (47) | 24 (41) | 0.208 |
| Smoking (past 5 years), n (%) | 2 (11) | 10 (65) | 0.154 |
| Coronary artery disease, n (%) | 1 (5) | 3 (8) | 0.696 |
| Atrial fibrillation, n (%) | 6 (32) | 2 (5) | 0.008 |
| Previous ischemic stroke/TIA, n (%) | 4 (21) | 16 (43) | 0.183 |
| Alcohol daily, n (%) | 2 (11) | 5 (13.5) | 0.749 |
| Clinical Data | |||
| Mean BMI ± SD | 27.5 ± 2.9 | 27.0 ± 4.4 | 0.706 |
| Median ESS, range | 5 (0–13) | 5 (0–11) | 0.701 |
| Median NIHSS, range | 1 (0–15) | 1 (0–7) | 0.177 |
| Stroke Severity | 0.078* | ||
| TIA, n (%) | 1 (5) | 9 (24) | |
| Minor stroke, n (%) | 16 (84) | 25 (68) | |
| Major stroke, n (%) | 2 (11) | 3 (8) | |
| TOAST | 0.051* | ||
| Large artery, n (%) | 10 (53) | 24 (65) | |
| Cardioembolism, n (%) | 7 (37) | 3 (8) | |
| Small vessel, n (%) | 1 (5) | 6 (16) | |
| Other, n (%) | 0 | 0 | |
| Undetermined, n (%) | 1 (5) | 4 (11) | |
| Sleep apnea, n (%) | 18 (95) | 33 (89) | 0.801* |
| Mild | 5 (26) | 13 (35) | |
| Moderate | 7 (37) | 10 (27) | |
| Severe | 6 (32) | 10 (27) | |
SIS sleep-related ischemic stroke, TIA transient ischemic attack, BMI body mass index, ESS Epworth Sleepiness Scale, NIHSS National Institutes of Health Stroke Scale, TOAST Trial of Org 10172 in Acute Stroke Treatment
Overall p value for all categories
Discussion
Our study showed that early sleep apnea screening is feasible in the acute phase of stroke when a portable cardio-respiratory polygraphy device is used. High frequency of SA in our study population with no significant relation to clinical features highlights the importance of SA screening in all acute stroke patients. This implies a first step toward its routine implementation in organized stroke unit care.
Untreated SA of variable degree constitutes an independent risk factor for recurrent ischemic stroke [19–21]. As part of secondary prevention in stroke survivors with SA, initiation of non-invasive ventilatory correction reduces the risk of recurrent ischemic stroke and mortality [21–23]. Moreover, continuous positive airway pressure improves short-term functional outcome in stroke survivors with SA undergoing rehabilitation [24]. Apart from that, fewer publications showed feasibility and safety of early non-invasive ventilatory correction in the acute phase of stroke addressing the important association of SA and early neurological deterioration [10, 25–27]. However, timely detection of vascular risk factors remains a crucial key issue when goals for secondary prevention of stroke are defined [12]. Although awareness of SA and its sequelae in stroke survivors have increased among stroke-neurologists [28], post-stroke care commonly lacks accessible resources compromising proper screening and treatment of SA in these patients [29, 30]. A recently conducted survey revealed that ischemic stroke survivors perceived their risk of SA lower than it was and only a few of those who were at high risk of SA underwent corresponding screening or treatment after being discharged from the hospital [31]. Organized stroke unit care, which has a positive impact on outcome and recurrent stroke risk, may overcome this shortcoming [12], and so it is potentially worth devoting further in-hospital resources.
To the best of our knowledge, fewer studies assessed feasibility of early SA screening applying a portable easy-to-operate device in the acute phase of stroke as most studies utilized complete nocturnal PSG for SA testing in stroke patients and were primarily conducted to pursue epidemiological rather than feasibility objectives [3, 32, 33]. Furthermore, in previous studies, diagnostic testing for SA was mostly performed outside the acute phase and rather in a general neurology or rehabilitation ward [34, 35]. Furthermore, even though PSG is considered the gold-standard for diagnosis of SA [11], it requires considerable experience and constant access to sleep medicine that only a few stroke centers provide. Also, in the acute phase of stroke this approach may interfere with basic stroke care.
Aside from an association between the prevalence and severity of SA and presumed stroke etiology in our patient population (which should be interpreted with caution due to small absolute numbers in certain TOAST subtypes), we did not find further links and, therefore, no certain subgroup of stroke patients that preferably should be screened for SA. This might be due to the overall high frequency (91 %) of SA in our study population with no relevant differences according to stroke severity. Although some debate exists in this matter, our findings are in line with most previous studies [36, 37]. As demonstrated by Chan and colleagues [34] the majority of stroke patients with SA does not present with typical SA-related clinical features such as obesity and excessive daytime sleepiness. In our population, excessive daytime sleepiness did not prove to be associated with SA severity confirming the aforesaid results, whereas BMI was linked to severity of SA though. Beyond that, there is an ongoing controversy whether sleep-related stroke onset is associated with SA [33, 34, 38]. However, we did not find any relevant relation between SA and diurnal variations of stroke onset, not even when solely focusing on wake-up strokes as opposed to recent findings by Hsieh et al. [39]. Nonetheless, the question arises whether clinical features are necessary to select patients for SA screening, or selection criteria can be omitted and all stroke patients should be screened as recently suggested [37]. The overall excellent feasibility of SA screening in patients with TIA and AIS observed in our study aids this approach.
Within one year of discharge from our hospital, nearly three out of four patients with findings suggestive of treatable SA underwent comprehensive in-laboratory sleep work-up and therewith followed our recommendations. This is in contrast to a recent survey study in ischemic stroke survivors which showed that only 26 % of respondents were asked at least one SA symptom screening question by a healthcare provider after being discharged from the hospital [31]. Moreover, <20 % of those who responded to the survey underwent any SA screening. As with other modifiable stroke risk factors, SA requires timely detection, and early implementation of SA screening in acute stroke patients may have a positive impact on post-stroke care. However, elapsed time between initial SA screening and in-laboratory work-up appears rather long (on average 5 months). On the one hand, this was partly intended by our sleep neurologists who gave specific recommendations to stroke survivors as SA may slightly improve during the post-stroke phase [37]. On the other hand, stroke survivors' motivation for further SA work-up was rather driven by these recommendations than self-determination as SA-related symptoms were widely not present in these patients (e.g., most of the patients did not complain of excessive daytime sleepiness as assessed by the ESS). This might also explain that only a minority of patients who were found to have treatable SA eventually received non-invasive ventilatory correction, which was mostly due to patients' refusal of treatment. This emphasizes the need for constant medical attendance and continuing education provided to stroke survivors after discharge [40].
Our study has some limitations: First of all, our study population may reflect a sampling bias since very few had major strokes (median NIHSS was 1 point) and severe comorbidities were largely not present. In these patients early SA screening may be less feasible due to agitation and non-compliance. Thus, our feasibility rate might be overestimated and not entirely generalizable. Although we enrolled patients consecutively, we did not apply a screening log and therefore cannot provide the exact number of patients who did not fulfill inclusion and exclusion criteria. Secondly, we used a conservative definition for SA (which does not necessarily require treatment) and therefore might have overestimated the frequency of SA in our patient population. However, we strictly complied with the AASM guidelines as opposed to a less conservative cut-off frequently used in other trials [41]. Also, certain subanalyses may have been affected by the overall high frequency of SA in our study population. Lastly, we were not able to evaluate sleep stages during SA monitoring since the polygraphy device does not allow electroencephalography recording. However, cardiorespiratory polygraphy has been shown to reliably detect SA when compared with the gold-standard PSG [13].
In conclusion, early SA screening is feasible in patients with acute cerebral ischemia and may have a positive impact on post-discharge SA care. Given the high frequency and atypical presentation of SA in this patient population, early screening for SA should be considered in all acute cerebral ischemia patients.
Acknowledgments
Weinmann Medical Technology, Hamburg, Germany provided the SOMNO Check effort® device free of charge for this study.
Footnotes
Conflicts of interest: Dr Albright: The project described was supported by Award Numbers 5 T32 HS013852-10 from The Agency for Healthcare Research and Quality (AHRQ) and 3 P60 MD000502-08S1 from The National Institute on Minority Health and Health Disparities (NIMHD), National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the AHRQ or the NIH. The authors declare that they have no conflict of interest
Contributor Information
Jessica Kepplinger, Department of Neurology, Dresden University Stroke Center, University of Technology Dresden, Fetscherstrasse 74, 01307 Dresden, Germany.
Kristian Barlinn, Department of Neurology, Dresden University Stroke Center, University of Technology Dresden, Fetscherstrasse 74, 01307 Dresden, Germany; Department of Neurology, Comprehensive Stroke Center, University of Alabama Hospital, Birmingham, AL, USA.
Karen C. Albright, Department of Neurology, Comprehensive Stroke Center, University of Alabama Hospital, Birmingham, AL, USA; Department of Epidemiology, School of Public Health, University of Alabama Hospital, Birmingham, AL, USA; Health Services and Outcomes Research Center for Outcome and Effectiveness Research and Education (COERE), University of Alabama Hospital, Birmingham, AL, USA; Center of Excellence in Comparative Effectiveness Research for Eliminating Disparities (CERED) Minority Health and Health Disparities Research Center (MHRC), University of Alabama Hospital, Birmingham, AL, USA
Wiebke Schrempf, Division of Neurodegenerative Diseases, Department of Neurology, Dresden University of Technology, Dresden, Germany.
Amelia K. Boehme, Department of Neurology, Comprehensive Stroke Center, University of Alabama Hospital, Birmingham, AL, USA; Department of Epidemiology, School of Public Health, University of Alabama Hospital, Birmingham, AL, USA
Lars-Peder Pallesen, Department of Neurology, Dresden University Stroke Center, University of Technology Dresden, Fetscherstrasse 74, 01307 Dresden, Germany.
Uta Schwanebeck, Coordination Center for Clinical Studies, University of Technology Dresden, Dresden, Germany.
Xina Graehlert, Coordination Center for Clinical Studies, University of Technology Dresden, Dresden, Germany.
Alexander Storch, Division of Neurodegenerative Diseases, Department of Neurology, Dresden University of Technology, Dresden, Germany.
Heinz Reichmann, Department of Neurology, Dresden University Stroke Center, University of Technology Dresden, Fetscherstrasse 74, 01307 Dresden, Germany.
Andrei V. Alexandrov, Department of Neurology, Comprehensive Stroke Center, University of Alabama Hospital, Birmingham, AL, USA
Ulf Bodechtel, Department of Neurology, Dresden University Stroke Center, University of Technology Dresden, Fetscherstrasse 74, 01307 Dresden, Germany.
References
- 1.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 2.Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172:1447–1451. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iranzo A, Santamaría J, Berenguer J, Sanchez M, Chamorro A. Prevalence and clinical importance of sleep apnea in the first night after cerebral infarction. Neurology. 2002;58:911–916. doi: 10.1212/wnl.58.6.911. [DOI] [PubMed] [Google Scholar]
- 4.Alexandrov AV, Nguyen HT, Rubiera M, Alexandrov AW, Zhao L, Heliopoulos I, et al. Prevalence and risk factors associated with reversed Robin Hood syndrome in acute ischemic stroke. Stroke. 2009;40:2738–2742. doi: 10.1161/STROKEAHA.109.547950. [DOI] [PubMed] [Google Scholar]
- 5.Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O'Connor GT, Resnick HE, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182:269–277. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaneko Y, Hajek VE, Zivanovic V, Raboud J, Bradley TD. Relationship of sleep apnea to functional capacity and length of hospitalization following stroke. Sleep. 2003;26:293–297. doi: 10.1093/sleep/26.3.293. [DOI] [PubMed] [Google Scholar]
- 7.Parra O, Arboix A, Montserrat JM, Quintó L, Bechich S, Garcìa-Eroles L. Sleep-related breathing disorders: impact on mortality of cerebrovascular disease. Eur Respir J. 2004;24:267–272. doi: 10.1183/09031936.04.00061503. [DOI] [PubMed] [Google Scholar]
- 8.European Stroke Organisation (ESO) Executive Committee; ESO Writing Committee. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008;25:457–507. doi: 10.1159/000131083. [DOI] [PubMed] [Google Scholar]
- 9.Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/ American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:577–617. doi: 10.1161/01.STR.0000199147.30016.74. [DOI] [PubMed] [Google Scholar]
- 10.Barlinn K, Alexandrov AV. Sleep-disordered breathing and arterial blood flow steal represent linked therapeutic targets in cerebral ischaemia. Int J Stroke. 2011;6:40–41. doi: 10.1111/j.1747-4949.2010.00551.x. [DOI] [PubMed] [Google Scholar]
- 11.Epstein LJ, Kristo D, Strollo PJ, Jr, Friedman N, Malhotra A, Patil SP, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–276. [PMC free article] [PubMed] [Google Scholar]
- 12.Stroke Unit Trialists' Collaboration. Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev. 2007;(4):CD000197. doi: 10.1002/14651858.CD000197.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Collop NA, Anderson WM, Boehlecke B, Claman D, Goldberg R, Gottlieb DJ, Portable Monitoring Task Force of the American Academy of Sleep Medicine et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3:737–747. [PMC free article] [PubMed] [Google Scholar]
- 14.Masa JF, Corral J, Pereira R, Duran-Cantolla J, Cabello M, Herna´ndez-Blasco L, et al. Effectiveness of home respiratory polygraphy for the diagnosis of sleep apnoea and hypopnoea syndrome. Thorax. 2011;66:567–573. doi: 10.1136/thx.2010.152272. [DOI] [PubMed] [Google Scholar]
- 15.Venkateshiah SB, Collop NA. Sleep and sleep disorders in the hospital. Chest. 2012;141:1337–1345. doi: 10.1378/chest.11-2591. [DOI] [PubMed] [Google Scholar]
- 16.Adams H, Bendixen B, Kappelle L, Biller J, Love B, Gordon D, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 17.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 18.Iber C, Ancoli-Israel S, Chesson A, Quan SF, for the American Academy of Sleep Medicine . The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 1st. American Academy of Sleep Medicine; Westchester: 2007. [Google Scholar]
- 19.Dziewas R, Humpert M, Hopmann B, Kloska SP, Lu¨demann P, Ritter M, et al. Increased prevalence of sleep apnea in patients with recurring ischemic stroke compared with first stroke victims. J Neurol. 2005;252:1394–1398. doi: 10.1007/s00415-005-0888-7. [DOI] [PubMed] [Google Scholar]
- 20.Martìnez-Garcìa MA, Campos-Rodrìguez F, Soler-Catalun~a JJ, Catala´n-Serra P, Roma´n-Sa´nchez P, Montserrat JM. Increased incidence of nonfatal cardiovascular events in stroke patients with sleep apnoea: effect of CPAP treatment. Eur Respir J. 2012;39:906–912. doi: 10.1183/09031936.00011311. [DOI] [PubMed] [Google Scholar]
- 21.Martinez-Garcia MA, Galiano-Blancart R, Roman-Sanchez P, Soler-Cataluna JJ, Cabero-Salt L, Salcedo-Maiques E. Continuous positive airway pressure treatment in sleep apnea prevents new vascular events after ischemic stroke. Chest. 2005;128:2123–2129. doi: 10.1378/chest.128.4.2123. [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Garcia MA, Soler-Cataluna JJ, Ejarque-Martinez L, Soriano Y, Roma´n-Sa´nchez P, Illa FB, et al. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med. 2009;180:36–41. doi: 10.1164/rccm.200808-1341OC. [DOI] [PubMed] [Google Scholar]
- 23.Parra O, Sa´nchez-Armengol A, Bonnin M, Arboix A, Campos-Rodrìguez F, Pe´rez-Ronchel J, et al. Early treatment of obstructive apnoea and stroke outcome: a randomised controlled trial. Eur Respir J. 2011;37:1128–1136. doi: 10.1183/09031936.00034410. [DOI] [PubMed] [Google Scholar]
- 24.Ryan CM, Bayley M, Green R, Murray BJ, Bradley TD. Influence of continuous positive airway pressure on outcomes of rehabilitation in stroke patients with obstructive sleep apnea. Stroke. 2011;42:1062–1067. doi: 10.1161/STROKEAHA.110.597468. [DOI] [PubMed] [Google Scholar]
- 25.Tsivgoulis G, Zhang Y, Alexandrov AW, Harrigan MR, Sisson A, Zhao L, et al. Safety and tolerability of early noninva-sive ventilatory correction using bilevel positive airway pressure in acute ischemic stroke. Stroke. 2011;42:1030–1034. doi: 10.1161/STROKEAHA.110.600221. [DOI] [PubMed] [Google Scholar]
- 26.Minnerup J, Ritter MA, Wersching H, Kemmling A, Okegwo A, Schmidt A, et al. Continuous positive airway pressure ventilation for acute ischemic stroke: a randomized feasibility study. Stroke. 2012;43:1137–1139. doi: 10.1161/STROKEAHA.111.637611. [DOI] [PubMed] [Google Scholar]
- 27.Barlinn K, Balucani C, Palazzo P, Zhao L, Sisson A, Alexandrov AV. Noninvasive ventilatory correction as an adjunct to an experimental systemic reperfusion therapy in acute ischemic stroke. Stroke Res Treat. 2010;31:108253. doi: 10.4061/2010/108253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallace DM, Ramos AR, Rundek T. Sleep disorders and stroke. Int J Stroke. 2012;7:231–242. doi: 10.1111/j.1747-4949.2011.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skjodt NM. Approach to outpatient management of adult sleep apnea. Can Fam Physician. 2008;54:1408–1412. [PMC free article] [PubMed] [Google Scholar]
- 30.Ball EM, Simon RD, Jr, Tall AA, Banks MB, Nino-Murcia G, Dement WC. Diagnosis and treatment of sleep apnea within the community. The Walla Walla Project. Arch Intern Med. 1997;157:419–424. [PubMed] [Google Scholar]
- 31.Skolarus LE, Lisabeth LD, Morgenstern LB, Burgin W, Brown DL. Sleep apnea risk among mexican american and non-Hispanic white stroke survivors. Stroke. 2012;43:1143–1145. doi: 10.1161/STROKEAHA.111.638387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Broadley SA, Jørgensen L, Cheek A, Salonikis S, Taylor J, Thompson PD, et al. Early investigation and treatment of obstructive sleep apnoea after acute stroke. J Clin Neurosci. 2007;14:328–333. doi: 10.1016/j.jocn.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 33.Bassetti CL, Milanova M, Gugger M. Sleep-disordered breathing and acute ischemic stroke: diagnosis, risk factors, treatment, evolution, and long-term clinical outcome. Stroke. 2006;37:967–972. doi: 10.1161/01.STR.0000208215.49243.c3. [DOI] [PubMed] [Google Scholar]
- 34.Chan W, Coutts SB, Hanly P. Sleep apnea in patients with transient ischemic attack and minor stroke: opportunity for risk reduction of recurrent stroke? Stroke. 2010;41:2973–2975. doi: 10.1161/STROKEAHA.110.596759. [DOI] [PubMed] [Google Scholar]
- 35.Disler P, Hansford A, Skelton J, Wright P, Kerr J, O'Reilly J, et al. Diagnosis and treatment of obstructive sleep apnea in a stroke rehabilitation unit: a feasibility study. Am J Phys Med Rehabil. 2002;81:622–625. doi: 10.1097/00002060-200208000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Johnson KG, Johnson DC. Frequency of sleep apnea in stroke and TIA patients: a meta-analysis. J Clin Sleep Med. 2010;6:131–137. [PMC free article] [PubMed] [Google Scholar]
- 37.Hermann DM, Bassetti CL. Sleep-related breathing and sleep–wake disturbances in ischemic stroke. Neurology. 2009;73:1313–1322. doi: 10.1212/WNL.0b013e3181bd137c. [DOI] [PubMed] [Google Scholar]
- 38.Bassetti C, Aldrich M. Night time versus daytime transient ischaemic attack and ischaemic stroke: a prospective study of 110 patients. J Neurol Neurosurg Psychiatry. 1999;67:463–467. doi: 10.1136/jnnp.67.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsieh SW, Lai CL, Liu CK, Hsieh CF, Hsu CY. Obstructive sleep apnea linked to wake-up strokes. J Neurol. 2012;259:1433–1439. doi: 10.1007/s00415-011-6370-9. [DOI] [PubMed] [Google Scholar]
- 40.Ostwald SK, Davis S, Hersch G, Kelley C, Godwin KM. Evidence-based educational guidelines for stroke survivors after discharge home. J Neurosci Nurs. 2008;40:173–179. doi: 10.1097/01376517-200806000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown DL. Sleep disorders and stroke. Semin Neurol. 2006;26:117–122. doi: 10.1055/s-2006-933315. [DOI] [PubMed] [Google Scholar]


