Abstract
Background
Sepsis remains a leading cause of death in most ICUs. Many deaths in sepsis are due to nosocomial infections in patients who have entered the immunosuppressive phase of the disorder. One cause of immunosuppression in sepsis is T-cell exhaustion mediated by programmed cell death-1 (PD-1) interaction with its ligand (PD-L1). Studies demonstrated that blocking the interaction of PD-1 with PD-L1 with knockout mice or inhibitory antibodies reversed T cell dysfunction and improved sepsis survival. This study assessed the efficacy of a novel short-acting peptide (Compound 8) that inhibits PD-1/PD-L1 signaling in a clinically-relevant second-hit fungal sepsis model.
Methods
Mice underwent cecal ligation and puncture to induce peritonitis. Three days later, mice received intravenous injection of Candida albicans. Forty-eight hours following Candida infection, mice were treated with Compound 8 or inactive peptide. The effect of Candida infection on expression of co-inhibitory molecules, PD-1 and PD-L1 were quantitated by flow cytometry on CD4+, CD8+, natural killer (NK) cells, and NKT cells. The effect of Compound 8 on survival was also examined.
Results
Four days after fungal infection, PD-1 and/or PD-L1 expressions were markedly increased on CD4+, NK, and NKT cells in septic versus sham-operated mice (%PD-1 on CD4+, 11.9% vs 2.8%; and %PD-L1 on NKT, 14.8% vs 0.5%). Compared to control, Compound 8 caused a two-fold increase in survival from 30% to 60%, p < 0.05.
Conclusions
Compound 8 significantly improved survival in a clinically-relevant immunosuppressive model of sepsis. These results support immuno-adjuvant therapy targeting T-cell exhaustion in this lethal disease.
Keywords: therapeutic peptide, immunomodulatory drug, programmed cell death 1, immunosuppression, sepsis
Subject category: Shock, Sepsis, Trauma, Critical Care
1. Introduction
Sepsis is defined as life-threatening organ dysfunction that occurs due to a dysregulated host response to infection (1). Sepsis affects millions of people around the world each year and the incidence is increasing (2, 3). In the United States, sepsis contributes to more than 250,000 deaths annually and is the tenth leading cause of death (4, 5). Patients with sepsis often present with high fevers, shock, and some degree of organ dysfunction. For many years, the prevailing concept of sepsis was that it represented an unbridled cytokine-mediated inflammatory response (6–9). Consequently, most novel therapeutic approaches to sepsis focused on blocking the initial hyper-inflammatory, cytokine-mediated phase of disorder. Recently, the prevailing concept of sepsis has changed (7, 10–12), and evidence supporting the development of a progressive immunosuppressive phase of the disorder has accumulated. Apoptosis-induced depletion of immune effector cells with loss of CD4, CD8, B, and dendritic cells has been demonstrated in patients with sepsis (13, 14). Blood and autopsy studies from patients with sepsis showed decreased production of proinflammatory cytokines, decreased monocyte HLA-DR and T cell CD28 expressions, increased number of T regulatory cells, and increased expression of PD-1 and/or PD-L1 on innate and adaptive immune cells (15–21). Thus, patients with sepsis have phenotypic and function evidence of impaired immunity. This new recognition of the importance of the immunosuppressive phase of sepsis has led to studies of various immune-adjuvant agents to boost host immunity with the aim to improve outcome (7, 11, 12).
Among immunomodulatory therapy, blockade of PD-1/PD-L1 signaling is one of the new promising approaches to reverse immunosuppression in sepsis (12, 22, 23). Anti-PD-1 and anti-PD-L1 blocking antibodies have had remarkable success in improving survival in patients with cancer (24, 25), a disorder which shares many immunosuppressive mechanisms with sepsis (26). Clinically-relevant models of bacterial sepsis showed that anti-PD-1 and anti-PD-L1 antibodies improved survival, reduced lymphocyte apoptosis, and improved cytokine production (27–29). Therefore, anti-PD-1 and anti-PD-L1 antibodies may be effective not only in cancer but in sepsis as well (24, 26).
A number of approaches are available for therapeutic blocking of harmful receptor:ligand interaction such as may occur with PD-1:PD-L1. Although competitive, i.e., blocking antibodies generally have high avidity and high target specificity, they may have limitations of long circulating half-life (preventing titration of effect), precipitation of immunogenicity, high production costs, and expense (29). Alternatively therapeutic peptides, whose molecular weight is lower than antibodies’, may be one of effective clinical applications for treatment because of the negligible antigenicity, lower production cost, and increased product reproducibility (30). Another potential advantage of therapeutic peptides as opposed to antibodies is their shorter half-life, i.e, duration of action, than most antibodies. Therefore, any potential harmful side effects might be ameliorated more quickly following drug discontinuation. Finally, in disorders like sepsis, it might be easier to titrate the degree of inhibition of the PD-1:PD-L1 pathway using peptides as opposed to antibodies. Therefore, we conducted this study to assess the effect of Compound 8, short acting peptide that inhibits PD-1 interaction with PD-L1 on survival in a clinically-relevant two-hit model of sepsis.
2. Materials and Methods
2.1. Mice
Six- to eight-week-old male CD1 mice were purchased from Charles River Laboratories (Wilmington, MA, USA). Procedures were approved by the Animal Studies Committee at Washington University School of Medicine.
2.2. Anti-PD-L1 peptide
The peptide, Compound 8, was developed at Aurigene Discovery Technologies Limited (Bangalore, Karnataka, India) (US patent #: 8907053) (31). Compound 8 is a 29 amino acid peptide that potently blocks PD-L1 signaling as demonstrated by an EC50 <50 nM in rescue of PD-L1 mediated inhibition of lymphocyte proliferation and effector functions in both human and mouse systems (US patent #: 8907053).
2.3. Flow cytometry antibodies
The fluorescently labeled antibodies used for flow cytometry were purchased from the following companies, and the company protocols were followed in all applications: BioLegend (San Diego, CA, USA): anti-CD3-FITC (Cat. # 100306), anti-CD4-PerCP/Cy5.5 (Cat. # 100540), anti-B220-PerCP/Cy5.5 (Cat. # 103236), anti-CD3-PerCP/Cy5.5 (Cat. # 100328), anti-CD8-PerCP/Cy5.5 (Cat. # 100734), anti-CD8-APC (Cat. # 100712), and anti-CD279-APC (Cat. # 135209); eBioscience (San Diego, CA, USA): anti-DX5-FITC (Cat. # 11-5971-85), anti-CD274-PE (Cat. # 551892), and anti-F4/80-APC (Cat. # 17-4801-80); and BD Biosciences (San Jose, CA, USA): anti-CD 279-PE (Cat. # 551892).
2.4. Two-hit model of sepsis
The two-hit model of Candida albicans sepsis was used as previously described (32). The cecal ligation and puncture (CLP) model was used to induce a sublethal polymicrobial sepsis (28). Mice were anesthetized with isoflurane and a midline abdominal incision was performed. The cecum was ligated (at ~50%) and was punctured twice with a 27 gauge needle. The abdomen was closed in two layers and 1 ml of 0.9% normal saline mixed with 0.05 mg/kg bodyweight buprenorphine (PharmaForce., Columbus, OH, USA) was administered subcutaneously in order to ensure hydration and provide pain control. A single dose of imipenem (25 mg/kg) was given subcutaneously 4 hours post CLP surgery. This level of injury combined with limited antibiotic therapy was utilized to create a protracted infection due to a contained intra-abdominal abscess with low mortality (33). Sham-operated mice were treated identically except there was no cecum ligation nor puncture.
Three days post-CLP, surviving mice received 50 μl of the 0.3 A600 Candida albicans suspension intravenously. This two-hit sepsis model of CLP followed by Candida albicans was developed because it reflected the impaired immune status of patients with protracted sepsis who have secondary nosocomial fungal infection (32). The time point to inject Candida albicans and the dose was determined on the basis of previous studies (28, 32, 33). Candida suspension was not administered to sham-operated mice.
2.5. PD-1 and PD-L1 expressions in splenic immune cells following Candida infection
Spleens were harvested from sham and septic animals at Day 7 post-CLP (4 days post Candida infection) and splenocytes were examined for surface expression of PD-1 and PD-L1. Total cell count per spleen was performed using a ViCell Counter (Beckman Coulter, Brea, CA, USA). Splenocytes were prepared and were stained with the following combinations of fluorochrome-conjugated antibodies: CD3-FITC, CD279 (PD-1)-PE, CD4-PerCP-Cy5.5, and CD8-APC; DX5-FITC, CD274 (PD-L1)-PE, B220-PerCP-Cy5.5, and F4/80-APC; CD3-FITC, CD274-PE, CD4-PerCP-Cy5.5, and CD8-APC; and DX5-FITC, CD274-PE, CD3-PerCP-Cy5.5, and CD279-APC. Flow cytometric analysis was performed on FACScan (Becton Dickinson, San Jose, CA, USA) and Cell Quest Pro software (BD Pharmigen, San Diego, CA, USA) was utilized to analyze the data.
2.6. Survival study
Septic mice were divided into two groups: one group was treated with active peptide (Compound 8) and another group was treated with an inactive scrambled peptide. Peptides were diluted with sterile phosphate buffered saline and 3 mg/kg peptide or scrambled control peptide was subcutaneously administered, in a blinded fashion, three times daily from Day 5 through Day 13 post-CLP (Fig. 3B). Mice were observed for 14 days after CLP (27).
Figure 3. Kaplan-Meier survival curves of septic mice treated with anti-PD-L1 peptide versus inactive peptide.
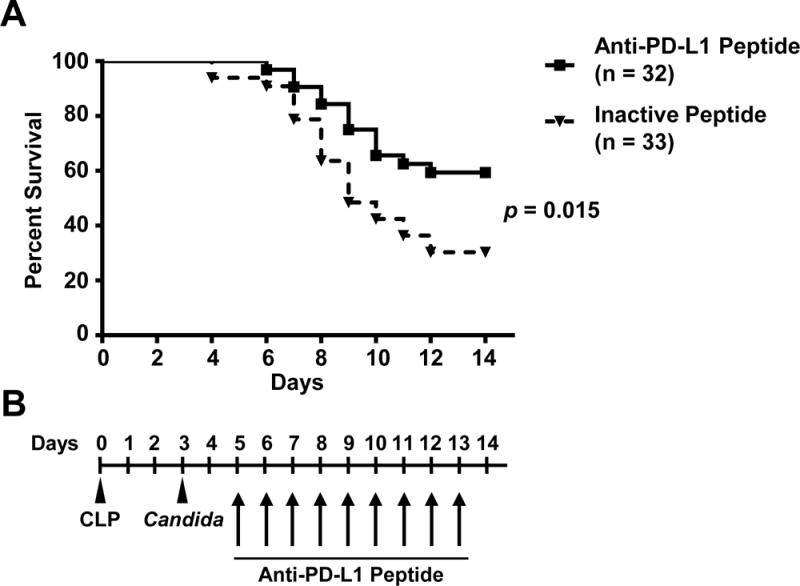
(A) Solid line shows the Kaplan-Meier curve of 32 sepsis model mice treated with anti-PD-L1 peptide, Compound 8; and dotted line shows the curve of 33 sepsis model mice treated with inactive peptide. The results represent the combined results from three independent survival studies. 19 of 32 (59.4%) mice survived in the anti-PD-L1 peptide treated group. However, only 10 of 33 (30.3%) mice survived in the inactive peptide treated group.
(B) Treatment schedule. Sepsis was induced by CLP surgery and Candida albicans was injected at Day 3 post-CLP. 3 mg/kg anti-PD-L1 peptide or inactive peptide was administered three times per day from Day 5 through Day 13.
2.7. Statistical analysis
Data were analyzed using the statistical software Prism (GraphPad, San Diego, CA, USA). For analyses for PD-1 and PD-L1 expressions on splenocytes, data were described in scatter plots and mean values were presented. Mann-Whitney U test was used for analyzing continuous variables between two groups. For survival study, 14-day survivals between the Compound 8 and the inactive peptide groups were shown by Kaplan-Meier survival curves, and a log rank test was performed for comparison. A p value less than 0.05 was considered statistically significant.
3. Results
3.1. PD-1 surface levels are higher on splenocytes from septic animals
PD-1 and PD-L1 surface expression were examined by comparing splenocytes from sham-operated mice to those of CLP mice with second-hit Candida infection 7 days after surgery (Figs. 1 and 2). These results are the data from two separate experiments with a total of 6 sham-operated mice and 12 mice that received CLP and Candida infection. CD4 T cells, natural killer (NK), and natural killer T-cells (NKT) had increases in both the percent of PD-1 positivity (p < 0.001, p = 0.008, and p = 0.010, respectively) and the PD-1 mean fluorescence intensity (MFI), (p = 0.010, p = 0.009 and p = 0.002, respectively) compared to comparable cells from sham-operated animals (Figs. 1A, 1B, and 1C). There was no difference in PD-1 surface expression on CD8+ T-cells (Fig. 1C)
Figure 1. Sepsis increases PD-1 expression in splenocytes in the two-hit sepsis model.
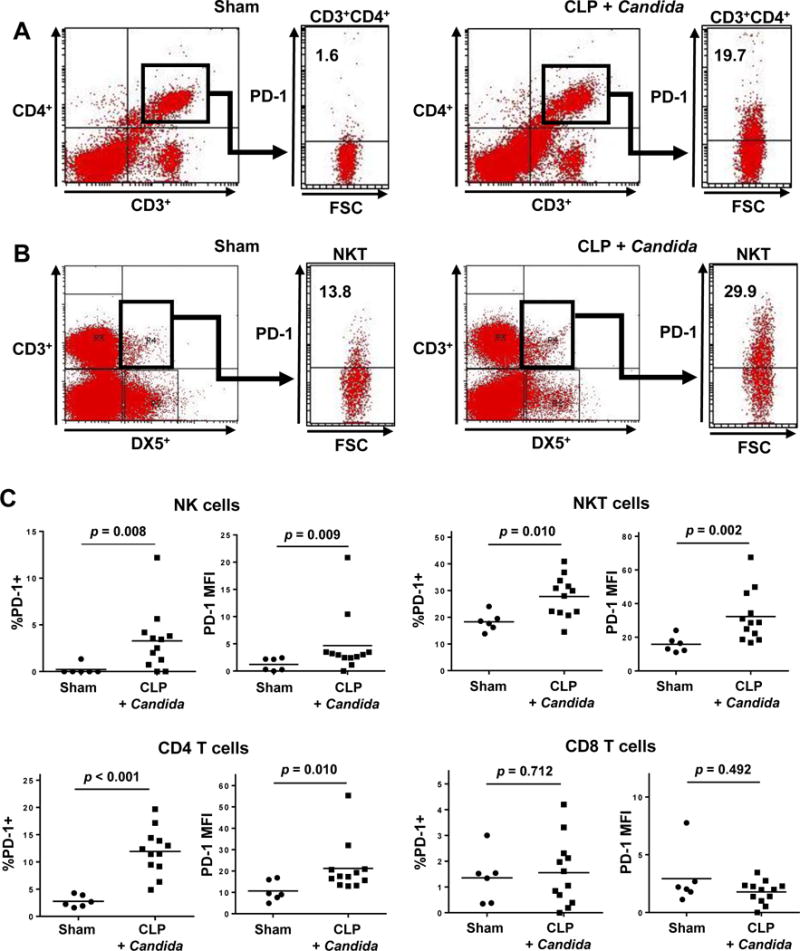
FSC denotes forward scatter of cells on flow cytometry; MFI, mean fluorescent intensity. Mice underwent sham or CLP surgery (Day 0). Candida albicans was intravenously injected to CLP mice at 3 days post-CLP; sham-operated mice did not receive Candida infection. PD-1 expression on splenocytes was quantitated via flow cytometry at 7 days post-surgery (4 days after Candida infection). Results of two independent experiments were combined and the total numbers of mice in sham-operated mice and CLP mice with Candida infection were 6 and 12, respectively. CD4 T cells were identified as CD3+CD4+; CD8 T cells as CD3+CD8+; NK cells as DX5+CD3−; and NKT cells as DX5+CD3+. PD-1+ cells were identified as CD279+.
(A) Representative flow diagrams of CD4 T cells are shown. The positivity of PD-1 expression on CD4 T cells was evaluated.
(B) The representative flow diagrams of NKT cells are shown. The positivity of PD-1 expression on NKT cells was evaluated.
(C) The percentages of PD-1+ cells and the MFI in CD4 T, CD8 T, NK, and NKT cells are shown.
Figure 2. Increased PD-L1 expression in splenocytes in the two-hit sepsis model.
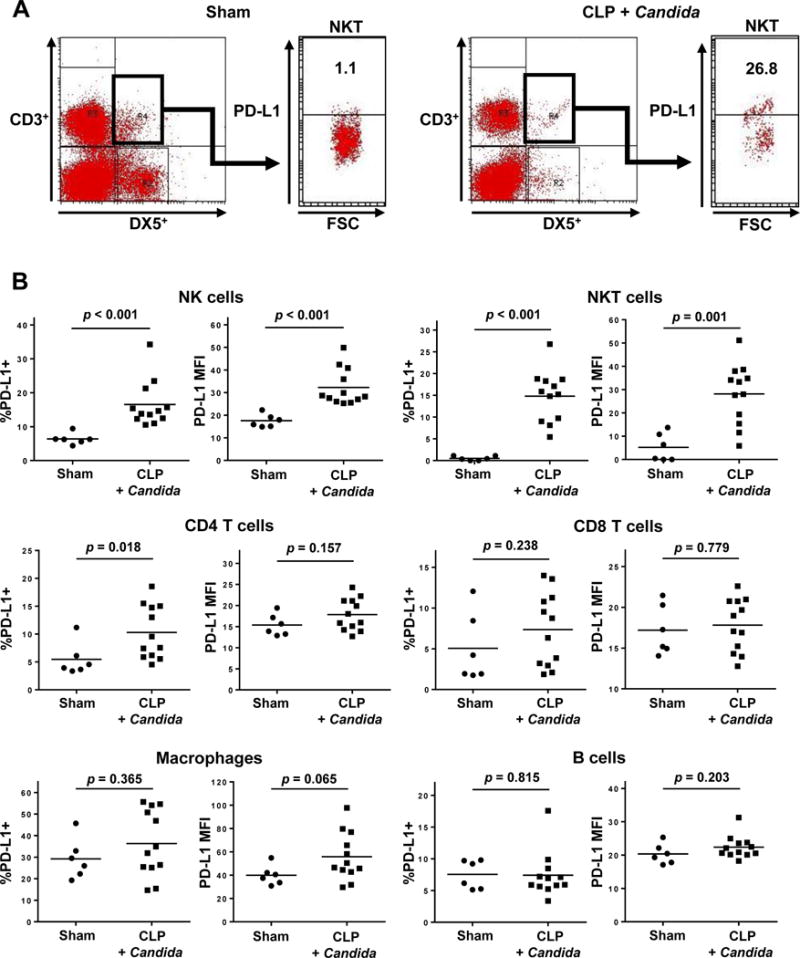
FSC denotes forward scatter of cells examined by flow cytometry; and MFI, mean fluorescent intensity.
Mice underwent sham or CLP surgery (Day 0). Candida albicans was intravenously injected to CLP mice at 3 days post-CLP, and sham-operated mice did not receive Candida infection. PD-1 expression on splenocytes was quantitated via flow cytometry at 7 days post-surgery (4 days after Candida infection). Results of two independent experiments were combined and the total numbers of mice in sham-operated mice and CLP mice with Candida infection were 6 and 12, respectively. T cells and NK cells were gated as indicated in Figure 2. Macrophages were identified as F4/80+; and B cells as B220+. PD-L1+ cells were identified as CD274+.
(A) Representative flow diagrams of NKT cells are shown. The positivity of PD-L1 expression on NKT cells was evaluated.
(B) The percentages of PD-L1+ cells and the MFI in NK, NKT, B, CD4 T, and CD8 T cells, and macrophages are shown.
3.2. PD-L1 surface levels are higher on splenocytes from septic animals
Like PD-1, PD-L1 exhibited higher surface levels on several cell types in septic animal spleens 7-days after surgery (Fig. 2). An increase in the percentage of NK, NKT, and CD4 T cells displaying PD-L1 was observed in septic animals compared to sham (p < 0.001, p < 0.001 and p = 0.018, respectively); there was a concurrent increase of PD-L1 MFI on NK and NKT populations (p < 0.001 and p = 0.001, respectively, Figs. 2A and 2B). The increase in the PD-L1 MFI of macrophages in the septic mice compared to sham-operated mice approached statistical significance (p = 0.065) (Fig. 2B).
3.3. Compound 8 peptide improves survival in the two-hit sepsis model
The results presented are the combined results of three independent studies. Administration of the Compound 8 peptide which targets PD-L1 conferred a survival advantage on septic animals (p = 0.015, Fig. 3). The groups begin to separate within a few days of the initiation of treatment, with the anti-PD-L1 peptide treated group having a survival rate roughly double the survival of the group that was treated with the scrambled peptide, i.e., 59.4% (19/32) versus 30.3% (10/33) of surviving, respectively.
4. Discussion
This study demonstrated that Compound 8, a peptide inhibitor that inhibits PD-1/PD-L1 signaling caused a two-fold improvement in survival in a clinically-relevant two-hit model of fungal sepsis. The current results are consistent with previous studies by multiple independent laboratories showing that anti-PD-1 and anti-PD-L1 antibodies can improve survival in a variety of clinically-relevant animal models of sepsis (27–29). The present model studied fungal infections. Candida infections are presently the third or fourth most common cause of bloodstream infections in many ICUs with a mortality of 30–40% (34). Thus, they represent a serious health problem. We used murine sepsis model in which mice were infected with Candida three days after a sub-lethal peritonitis infection. This fungal infection model reflects a more prolonged model of sepsis that allows for immunosuppressive mechanisms to evolve (28, 32). The fact that mortality from invasive fungal infection remains stubbornly high despite use of antimicrobial agents that are highly active against fungal organisms, suggests that defects in host immunity contribute to the high lethality.
Flow cytometric analysis demonstrated increased expression of the negative co-stimulatory molecules PD-1 and/or PD-L1 on various immune effector cells including CD4 T cells, NK cells, and NKT cells. PD-1 and PD-L1 mediate immunosuppression by recruiting the tyrosine phosphatase SHP-2 which dephosphorylates and inactivates proximal effector molecules including AKT and ZAP70 (35–37). Compound 8 is a highly potent antagonist of the PD-1 signaling, which disrupts the interaction of PD-1 with PD-L1, and shows an EC50 of <50 nM in rescue of PD-L1 mediated inhibition of lymphocyte proliferation and effector functions in both human and mouse systems (US Patent #: 8907053) (31). Compound 8 has several theoretical advantages over anti-PD-1 or anti-PD-L1 antibodies. Compared to antibodies, peptides will be less likely to induce immunogenicity and, because of their smaller size, may have a better distribution within the tissues. Finally, should it be desirable, it will likely be easier to titrate the amount of inhibition of the PD-1:PD-L1 pathway in sepsis using peptides as opposed to antibodies.
5. Conclusions
In conclusion, the present study demonstrates that, compared to cells from sham-operated mice, the co-inhibitory surface molecules PD-1 and PD-L1 are broadly expressed on immune effector cells following protracted fungal sepsis. The potent anti-PD-L1 peptide, Compound 8 caused an approximately two-fold improvement in survival in this clinically-relevant model of sepsis. Given its favorable properties, Compound 8 should be considered as another potential immunoadjuvant in sepsis.
Acknowledgments
We thank Dale Osborne and Meghan Wallace for assistance with cell preparation and bacterial preparation, and Andrew Walton for assistance with manuscript preparation.
M.R. and P.G.S. are full-time employees of Aurigene Discovery Technologies Limited. R.S.H received research funding from Aurigene Discovery Technologies Limited to support the studies testing Compound 8 in sepsis model. R.S.H. also received research funding from Bristol Meyers Squibb, Glaxo Smith Klein, and from Medimmune, LLC, and he is also an advisor to Glaxo Smith Klein plc. and MSD. Merck & Co., Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contribution:
M.R. and P.G.S. developed Compound 8 peptide. R.S.H. designed the survival study. J.S.M. assisted the survival study. Y.S., K.C.C., and R.S.H. designed the experiments. Y.S. and K.C.C. performed the experiments and analyzed the data. Y.S., K.C.C., and R.S.H. discussed the results and contributed to data interpretation. Y.S. wrote the first draft of the manuscript. K.C.C. assisted manuscript preparation. M.R., P.G.S., and R.S.H. contributed to the critical revision of the manuscript. All authors approved the final draft.
Disclosure
Other authors declare no conflict of interest.
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 3.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R, Surviving Sepsis Campaign Guidelines Committee including the Pediatric, S Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 4.Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med. 2006;34:15–21. doi: 10.1097/01.ccm.0000194535.82812.ba. [DOI] [PubMed] [Google Scholar]
- 5.Kung HC, Hoyert DL, Xu J, Murphy SL. Deaths: final data for 2005. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2008;56:1–120. [PubMed] [Google Scholar]
- 6.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 7.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13:260–268. doi: 10.1016/S1473-3099(13)70001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deitch EA. Animal models of sepsis and shock: a review and lessons learned. Shock. 1998;9:1–11. doi: 10.1097/00024382-199801000-00001. [DOI] [PubMed] [Google Scholar]
- 9.O’Reilly M, Newcomb DE, Remick D. Endotoxin, sepsis, and the primrose path. Shock. 1999;12:411–420. doi: 10.1097/00024382-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Hutchins NA, Unsinger J, Hotchkiss RS, Ayala A. The new normal: immunomodulatory agents against sepsis immune suppression. Trends Mol Med. 2014;20:224–233. doi: 10.1016/j.molmed.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 12.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13:862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hotchkiss RS, Tinsley KW, Swanson PE, Grayson MH, Osborne DF, Wagner TH, Cobb JP, Coopersmith C, Karl IE. Depletion of dendritic cells, but not macrophages, in patients with sepsis. J Immunol. 2002;168:2493–2500. doi: 10.4049/jimmunol.168.5.2493. [DOI] [PubMed] [Google Scholar]
- 14.Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE, Jr, Hui JJ, Chang KC, Osborne DF, Freeman BD, Cobb JP, Buchman TG, Karl IE. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 2001;166:6952–6963. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 15.van Dissel JT, van Langevelde P, Westendorp RG, Kwappenberg K, Frolich M. Anti-inflammatory cytokine profile and mortality in febrile patients. Lancet. 1998;351:950–953. doi: 10.1016/S0140-6736(05)60606-X. [DOI] [PubMed] [Google Scholar]
- 16.Ertel W, Kremer JP, Kenney J, Steckholzer U, Jarrar D, Trentz O, Schildberg FW. Downregulation of proinflammatory cytokine release in whole blood from septic patients. Blood. 1995;85:1341–1347. [PubMed] [Google Scholar]
- 17.Munoz C, Carlet J, Fitting C, Misset B, Bleriot JP, Cavaillon JM. Dysregulation of in vitro cytokine production by monocytes during sepsis. J Clin Invest. 1991;88:1747–1754. doi: 10.1172/JCI115493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rigato O, Salomao R. Impaired production of interferon-gamma and tumor necrosis factor-alpha but not of interleukin 10 in whole blood of patients with sepsis. Shock. 2003;19:113–116. doi: 10.1097/00024382-200302000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Sinistro A, Almerighi C, Ciaprini C, Natoli S, Sussarello E, Di Fino S, Calo-Carducci F, Rocchi G, Bergamini A. Downregulation of CD40 ligand response in monocytes from sepsis patients. Clin Vaccine Immunol. 2008;15:1851–1858. doi: 10.1128/CVI.00184-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang K, Svabek C, Vazquez-Guillamet C, Sato B, Rasche D, Wilson S, Robbins P, Ulbrandt N, Suzich J, Green J, Patera AC, Blair W, Krishnan S, Hotchkiss R. Targeting the programmed cell death 1: programmed cell death ligand 1 pathway reverses T cell exhaustion in patients with sepsis. Crit Care. 2014;18:R3. doi: 10.1186/cc13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, Bricker TL, Jarman SD, 2nd, Kreisel D, Krupnick AS, Srivastava A, Swanson PE, Green JM, Hotchkiss RS. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang X, Venet F, Wang YL, Lepape A, Yuan Z, Chen Y, Swan R, Kherouf H, Monneret G, Chung CS, Ayala A. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci U S A. 2009;106:6303–6308. doi: 10.1073/pnas.0809422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang X, Chen Y, Chung CS, Yuan Z, Monaghan SF, Wang F, Ayala A. Identification of B7-H1 as a novel mediator of the innate immune/proinflammatory response as well as a possible myeloid cell prognostic biomarker in sepsis. J Immunol. 2014;192:1091–1099. doi: 10.4049/jimmunol.1302252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hotchkiss RS, Moldawer LL. Parallels between cancer and infectious disease. N Engl J Med. 2014;371:380–383. doi: 10.1056/NEJMcibr1404664. [DOI] [PubMed] [Google Scholar]
- 27.Brahmamdam P, Inoue S, Unsinger J, Chang KC, McDunn JE, Hotchkiss RS. Delayed administration of anti-PD-1 antibody reverses immune dysfunction and improves survival during sepsis. J Leukoc Biol. 2010;88:233–240. doi: 10.1189/jlb.0110037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang KC, Burnham CA, Compton SM, Rasche DP, Mazuski R, Smcdonough J, Unsinger J, Korman AJ, Green JM, Hotchkiss RS. Blockade ofthe negative co-stimulatory molecules PD-1 and CTLA-4 improves survival in primary and secondary fungal sepsis. Crit Care. 2013;17:R85. doi: 10.1186/cc12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Zhou Y, Lou J, Li J, Bo L, Zhu K, Wan X, Deng X, Cai Z. PD-L1 blockade improves survival in experimental sepsis by inhibiting lymphocyte apoptosis and reversing monocyte dysfunction. Crit Care. 2010;14:R220. doi: 10.1186/cc9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Firer MA, Gellerman G. Targeted drug delivery for cancer therapy: the other side of antibodies. J Hematol Oncol. 2012;5:70. doi: 10.1186/1756-8722-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sasikumar PG, Ramachandra M. Immunosuppression modulating compounds. Aurigene Discovery Technologies Limited, Bangalore (IN), United State patent US 8,907,053 B2. 2014 [Google Scholar]
- 32.Davis CG, Chang K, Osborne D, Walton AH, Dunne WM, Muenzer JT. Increased susceptibility to Candida infection following cecal ligation and puncture. Biochem Biophys Res Commun. 2011;414:37–43. doi: 10.1016/j.bbrc.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Unsinger J, Burnham CA, McDonough J, Morre M, Prakash PS, Caldwell CC, Dunne WM, Jr, Hotchkiss RS. Interleukin-7 ameliorates immune dysfunction and improves survival in a 2-hit model of fungal sepsis. J Infect Dis. 2012;206:606–616. doi: 10.1093/infdis/jis383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mikulska M, Del Bono V, Ratto S, Viscoli C. Occurrence, presentation and treatment of candidemia. Expert Rev Clin Immunol. 2012;8:755–765. doi: 10.1586/eci.12.52. [DOI] [PubMed] [Google Scholar]
- 35.Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol. 2013;14:1212–1218. doi: 10.1038/ni.2762. [DOI] [PubMed] [Google Scholar]
- 36.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173:945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 37.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]


