Abstract
Objective
To determine population-based incidence estimates of BCC and cSCC.
Patients and Methods
We reviewed the medical records of a population-based cohort diagnosed with nonmelanoma skin cancer between January 2, 2000 and December 31, 2010. Sex- and age-adjusted incidence rates were calculated and compared to estimates from previous periods.
Results
The age-adjusted BCC incidence per 100,000 persons was 360.0 (95% CI, 342.5–377.4) for men and 292.9 (95% CI, 278.6–307.1) for women. The age-adjusted cSCC incidence per 100,000 persons was 207.5 (95% CI, 193.9–221.1) for men and 128.8 (95% CI, 119.4–138.2) for women. From years 1976–1984 to 2000–2010, the age- and sex-adjusted BCC incidence per 100,000 persons increased from 222.0 (95% CI, 204.5–239.5) to 321.2 (95% CI, 310.3–332.2), and from 61.8 (95% CI, 52.3–71.4) to 162.5 (95% CI, 154.6–170.3) for cSCC. Over time, the anatomical distribution of BCC shifted from the head and neck to the torso, and cSCC shifted from the head and neck to the extremities.
Conclusions
The incidences of BCC and cSCC are increasing, with a disproportionate increase in cSCC relative to BCC. There is also a disproportionate increase in women of both tumors, and shifting of anatomical distributions.
Introduction
Nonmelanoma skin cancer (NMSC) has a greater prevalence in whites than all other cancers combined.1,2 The absence of an NMSC registry1,3–6 necessitates estimates of incidence rates that are discrepant. The World Health Organization estimates that 2 to 3 million NMSCs occur annually worldwide;7 others estimate that 5.4 million NMSCs occur annually in the United States alone.6 Recent estimates suggest that between 186,157 and 700,000 cutaneous squamous cell carcinomas (cSCCs) are diagnosed annually in the United States.2,8 Studies from around the world have described an increasing incidence of NMSC.3,8–17
The last population-based incidence studies in the US utilized 1976–1984 data for basal cell carcinoma (BCC) and 1984–1992 data for cSCC.18,19 The primary aim of our study was to determine the sex- and age-specific population-based incidence and trends of BCC and cSCC in Olmsted County, Minnesota, from 2000 through 2010.
Methods
Study Setting
In 2010, Olmsted County, MN, had a population of 144,248 (74% of which resided in Rochester, the county seat).20 Although the average socioeconomic status, proportion of college graduates, and proportion of non-Hispanic whites are higher than national averages, epidemiologic studies in Olmsted County have historically been consistent with national data.21
This study was approved by the Mayo Clinic and Olmsted Medical Center institutional review boards. A retrospective, population-based cohort was identified through the Rochester Epidemiology Project (REP), a research infrastructure (R01 AG034676) that captures health care information for virtually all residents of Olmsted County from 1966 to the present, with 93% of Olmsted County residents seeing a health care provider within the previous 3 years.22
Study Criteria
Using the REP, all medical records were identified for Olmsted County residents who received an International Classification of Diseases, Ninth Revision code diagnosis of 173.00–173.99 from January 2, 2000, to December 31, 2010. An NMSC was considered incident if it was a patient’s first BCC or cSCC and was diagnosed during the study period while the patient resided in Olmsted County. A patient could have an incident BCC or cSCC (or both) during the study period. Exclusion criteria included the following: 1) younger than 18 years; 2) cSCC in situ; 3) no BCC or invasive cSCC; 4) previous diagnosis of BCC or cSCC before January 2, 2000; 5) anogenital location; 6) not an Olmsted County resident at the time of incident tumor diagnosis; 7) genetic disorder predisposing to NMSC; 8) previous radiotherapy to the area of tumor formation; and 9) denial of medical record access for research purposes.
Data Collection
Medical records were reviewed by an abstractor (J.G.M. or A.R.S.). The following data were collected: age at diagnosis, sex, race, and previous diagnosis of melanoma. The number of incident tumors, location, size, and histologic subtype were documented for BCC and cSCC, and acantholysis and perineural invasion were documented for cSCC only. For patients with multiple incident tumors, one tumor was randomly selected for data collection with a web-based randomization program.23 Dates were collected for local recurrence, nodal recurrence, distant metastasis, and most recent relevant clinical follow-up with a dermatologist or primary care provider for a skin examination. All data were entered into REDCap (Research Electronic Data Capture) hosted at Mayo Clinic.
Statistical Analyses
Data for BCC and cSCC were analyzed separately. Age- and sex-specific incidence rates per 100,000 persons in Olmsted County were calculated, with the numerator being the number of persons who had an incident BCC or cSCC diagnosis and the denominator being the age- and sex-specific counts of the Olmsted County population (from decennial census data and linear interpolation for intercensal years). Rates were adjusted for age and sex according to 2010 US population data; a Poisson error distribution was assumed for 95% CIs. Generalized linear regression models were used to evaluate incidence rates in relation to sex and age (Poisson error distribution was assumed, with crude incidence counts for sex and age groups, offset by the natural logarithm of the number of people).
To facilitate the comparison of incidence estimates for the 2000–2010 period with those from earlier periods, previous incidence rates were recalculated after limiting the cases in the previous periods to patients aged 18 years or older and using the total US population structure in 2010 to obtain age- and sex-adjusted estimates. For BCC, incident cases from Rochester were available for the 1976–1984 period.18 For cSCC, incident cases from Rochester were available for the 1976–1984 and 1984–1992 periods and are reported for the 1976–1984 and 1985–1992 periods.19 In addition, BCC and cSCC incident cases from patients between the ages of 18 and 39 years were available for the 1976–1999 period for all of Olmsted County.24 Denominators for each cohort were obtained from the decennial census for Rochester and Olmsted County with linear interpolation between census years.
Associations between histologic subtype, tumor site, and sex were evaluated with χ2 tests. For the 2000–2010 cohort, duration of follow-up was calculated from the date of the incident BCC or cSCC diagnosis to the date of recurrence or last relevant clinical follow-up. The cumulative incidence of local recurrence was estimated with the Kaplan-Meier method.
All P values were 2-sided; P values less than .05 were considered statistically significant. Statistical analyses were performed with SAS version 9.3 software.
Results
Basal Cell Carcinoma
From 2000 to 2010, 3,621 incident BCCs were diagnosed in 3,325 patients (mean age at diagnosis, 63.4 years; 50.2% male) (Table 1). Age- and sex-specific incidence rates are shown in Table 2. Incidence rates increased with age for women and at a faster rate for men (P<.001 for sex by age group interaction), with a peak among patients aged 80–89 years (Figure 1). Men had a significantly higher age-adjusted incidence rate (360.0 [95% CI, 342.5–377.4] per 100,000 persons) compared to women (292.9 [95% CI, 278.6–307.1] per 100,000 persons) (P<.001). The incidence of BCC in patients younger than 40 years was higher among women than among men (Table 2). A previous diagnosis of malignant melanoma or malignant melanoma in situ was recorded for 79 patients (2.4%). The average age at melanoma diagnosis was 59.7 years; at subsequent BCC diagnosis, 65.2 years.
Table 1.
Characteristics of Patients With Incident Cases of BCC and cSCC Diagnosed from January 2, 2000, through December 31, 2010, in Olmsted County, Minnesota
| Characteristic | BCC (n=3,325)a |
cSCC (n=1,653)a |
|---|---|---|
| Sex | ||
| Male | 1,669 (50.2%) | 911 (55.1%) |
| Female | 1,656 (49.8%) | 742 (44.9%) |
| Age at incident diagnosis, y | ||
| Mean (SD) | 63.4 (15.3) | 70.5 (13.4) |
| Median (IQR) | 63.8 (51.8–75.6) | 72.0 (61.5–80.7) |
| Range | (20.3–101.9) | (24.8–102.7) |
| Smoking status at time of incident diagnosis | ||
| Never | 1,797 (54.0%) | 764 (46.2%) |
| Current | 340 (10.2%) | 193 (11.7%) |
| Former | 1,178 (35.4%) | 693 (41.9%) |
| Unknown | 10 (0.3%) | 3 (0.2%) |
| Prior PUVA therapy | 6 (0.2%) | 7 (0.4%) |
| Immunosuppression at time of incident diagnosis | 88 (2.6%) | 80 (4.8%) |
| Reason for immunosuppression (n=88 for BCC; n=80 for cSCC) | ||
| HIV infection | 2 (2.3%) | 1 (1.2%) |
| Transplant | 31 (35.2%) | 27 (33.8%) |
| CLL/NHL | 0 | 2 (2.5%) |
| Inflammatory disease | 49 (55.7%) | 34 (42.5%) |
| Other | 6 (6.8%) | 16 (20.0%) |
| No. of incident tumors | ||
| 1 | 3,103 (93.3%) | 1,600 (96.8%) |
| 2 | 168 (5.1%) | 48 (2.9%) |
| 3 | 40 (1.2%) | 5 (0.3%) |
| ≥4 | 14 (0.4%) | 0 |
| Side of body | ||
| Left | 1,553 (46.7%) | 790 (47.8%) |
| Right | 1,403 (42.2%) | 720 (43.6%) |
| Other (eg, midline, central) | 366 (11.0%) | 143 (8.7%) |
| Unknown | 3 (0.1%) | 0 |
| Primary site | ||
| Head and neck | 2,167 (65.2%) | 992 (60.0%) |
| Torso | 797 (24.0%) | 156 (9.4%) |
| Extremities | 361 (10.9%) | 505 (30.6%) |
| Maximum dimension of lesion, cm | ||
| Mean (SD) | 1.0 (0.6) | 1.1 (0.6) |
| Median (IQR) | 1.0 (0.6–1.3) | 1.0 (0.7–1.3) |
| Range | 0.1–8.8 | 0.2–8.0 |
| BCC histologic type | ||
| Nodular (solely) | 1,459 (43.9%) | … |
| Superficial (solely) | 569 (17.1%) | … |
| Aggressive (solely) | ||
| Infiltrating | 197 (5.9%) | … |
| Micronodular | 97 (2.9%) | … |
| Metatypical | 93 (2.8%) | … |
| Morpheaform | 54 (1.6%) | … |
| Fibroepithelioma of Pinkus | 10 (0.3%) | … |
| Mixed | 352 (10.6%) | … |
| Other BCC | 36 (1.1%) | … |
| Type not specified | 458 (13.8%) | … |
| BCC mixed type (n=352) | ||
| With a nodular component | 305 (86.6%) | … |
| With a superficial component | 110 (31.2%) | … |
| With an aggressive component | 245 (69.6%) | … |
| SCC histologic type | ||
| SCC, well differentiated | … | 1,430 (86.5%) |
| SCC, moderately differentiated | … | 204 (12.3%) |
| SCC, poorly differentiated | … | 15 (0.9%) |
| Other SCC | … | 4 (0.2%) |
| Acantholytic histologic pattern | … | 15 (0.9%) |
| Perineural invasion | … | 3 (0.2%) |
| Collision tumor | 10 (0.3%) | 13 (0.8%) |
| Any treatment | 3,220 (96.8%) | 1,590 (96.2%) |
| Mohs surgery | 1,408 (42.3%) | 745 (45.1%) |
| Excision | 1,029 (30.9%) | 596 (36.1%) |
| Chemotherapy (monotherapy) | 23 (0.7%) | 2 (0.1%) |
| Electrodessication and curettage | 202 (6.1%) | 4 (0.2%) |
| Cryotherapy and curettage | 471 (14.2%) | 92 (5.6%) |
| Liquid nitrogen | 5 (0.2%) | 124 (7.5%) |
| Other | 85 (2.6%) | 2 (0.1%) |
Abbreviations: BCC, basal cell carcinoma; CLL, chronic lymphocytic leukemia; cSCC, cutaneous squamous cell carcinoma; HIV, human immunodeficiency virus; IQR, interquartile range; NHL, non-Hodgkin lymphoma; PUVA, psoralen–UV-A.
Categorical data are presented as number of patients (percentage of sample).
Table 2.
Incidence of Basal Cell Carcinoma and Cutaneous Squamous Cell Carcinoma per 100,000 Persons in Olmsted County, Minnesota, 2000–2010
| Age Group, y | Women
|
Men
|
Both Sexes
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Rate | 95% CI | No. | Rate | 95% CI | No. | Rate | 95% CI | |
| Basal Cell Carcinoma | |||||||||
| 18–29 | 28 | 23.7 | 15.8–34.3 | 13 | 11.5 | 6.1–19.6 | 41 | 17.7 | 12.7–24.1 |
| 30–39 | 108 | 99.4 | 81.6–120.0 | 73 | 66.1 | 51.8–83.1 | 181 | 82.6 | 71.0–95.5 |
| 40–49 | 284 | 253.9 | 225.2–285.2 | 204 | 186.3 | 161.6–213.7 | 488 | 220.5 | 201.3–240.9 |
| 50–59 | 334 | 353.4 | 316.5–393.4 | 364 | 407.3 | 366.5–451.3 | 698 | 379.6 | 351.9–408.8 |
| 60–69 | 297 | 503.7 | 448.1–564.4 | 389 | 723.7 | 653.6–799.3 | 686 | 608.7 | 564.0–656.0 |
| 70–79 | 312 | 784.0 | 699.4–876.0 | 387 | 1,185.9 | 1,070.7–1,310.1 | 699 | 965.1 | 894.9–1,039.4 |
| 80–89 | 235 | 892.7 | 782.2–1,014.5 | 212 | 1,391.6 | 1,210.6–1,592.1 | 447 | 1,075.6 | 978.2–1,180.1 |
| 90–110 | 58 | 785.8 | 596.7–1,015.8 | 27 | 1,116.2 | 735.6–1,624.0 | 85 | 867.3 | 692.8–1,072.5 |
| Total | 1,656 | … | … | 1,669 | … | … | 3,325 | … | … |
| Age-adjusteda | … | 292.9 | 278.6–307.1 | … | 360.0 | 342.5–377.4 | … | … | … |
| Age- and sex-adjusteda | … | … | … | … | … | … | … | 321.2 | 310.3–332.2 |
|
Cutaneous Squamous Cell Carcinoma | |||||||||
| 18–29 | 3 | 2.5 | 0.5–7.4 | 0 | 0 | … | 3 | 1.3 | 0.3–3.8 |
| 30–39 | 13 | 12.0 | 6.4–20.5 | 8 | 7.2 | 3.1–14.3 | 21 | 9.6 | 5.9–14.6 |
| 40–49 | 73 | 65.3 | 51.2–82.1 | 55 | 50.2 | 37.8–65.4 | 128 | 57.8 | 48.2–68.8 |
| 50–59 | 97 | 102.6 | 83.2–125.2 | 119 | 133.1 | 110.3–159.3 | 216 | 117.5 | 102.3–134.2 |
| 60–69 | 138 | 234.1 | 196.6–276.5 | 223 | 414.9 | 362.2–473.1 | 361 | 320.3 | 288.1–355.1 |
| 70–79 | 197 | 495.0 | 428.3–569.2 | 288 | 882.5 | 783.5–990.6 | 485 | 669.6 | 611.4–732.0 |
| 80–89 | 174 | 661.0 | 566.4–766.8 | 192 | 1,260.3 | 1,088.4–1,451.8 | 366 | 880.7 | 792.8–975.7 |
| 90–110 | 47 | 636.8 | 467.9–846.8 | 26 | 1,074.8 | 702.1–1,574.9 | 73 | 744.9 | 583.9–936.6 |
| Total | 742 | … | … | 911 | … | … | 1,653 | … | … |
| Age- adjusteda | … | 128.8 | 119.4–138.2 | … | 207.5 | 193.9–221.1 | … | … | … |
| Age- and sex-adjusteda | … | … | … | … | … | … | … | 162.5 | 154.6–170.3 |
Adjusted to the population structure of the total US population in 2010
Figure 1.
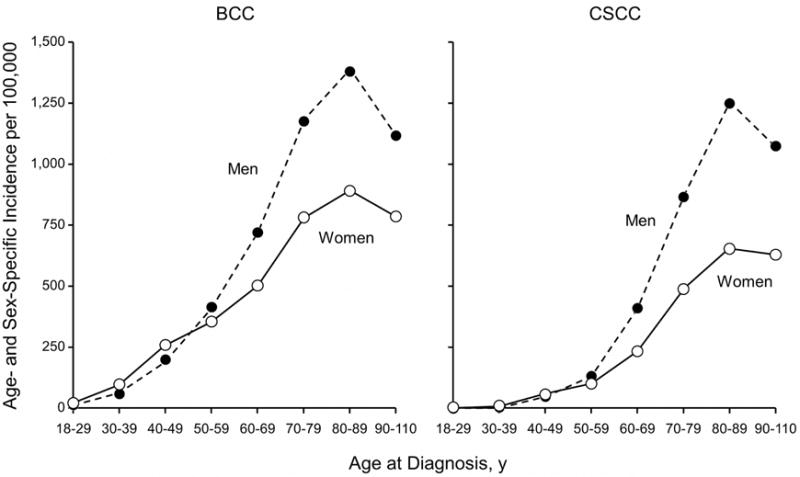
Age- and Sex-Specific Incidence of Basal Cell Carcinoma (BCC) and Cutaneous Squamous Cell Carcinoma (cSCC) in Olmsted County, Minnesota, 2000–2010.
The most common locations of BCCs for both sexes were the head and neck followed by the torso (Figure 2). The extremities were the least frequent site, but BCCs occurred in the extremities more commonly among women than men. The most common histologic subtype was nodular BCC (n=1,764; 53.1%), followed by superficial (n=679; 20.4%). Men had a statistically greater percentage of the nodular subtype (66.5%) compared to women (56.5%) (P<.001). Conversely, women had a statistically greater percentage of the superficial subtype (28.2%) compared to men (19.1%) (P<.001). A total of 686 tumors (20.6%) were an aggressive subtype or had an aggressive component (infiltrating, micronodular, metatypical, or morpheaform). Percentages of patients with an aggressive subtype did not differ between women (23.9%) and men (24.0%).
Figure 2.
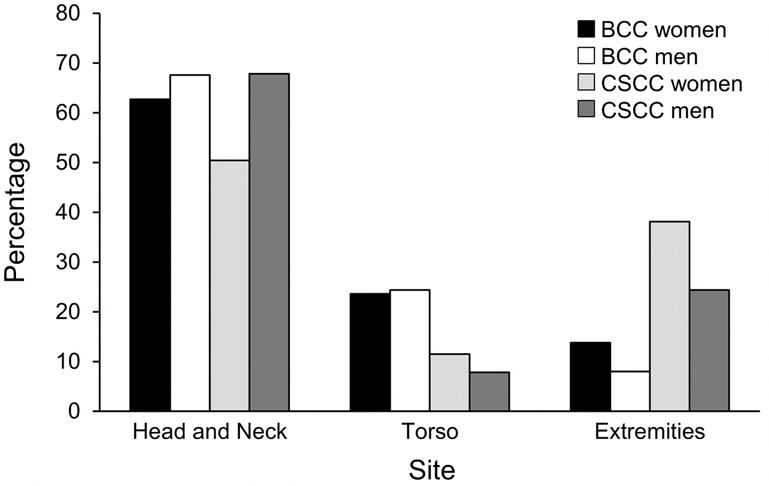
Sites of Incident Basal Cell Carcinoma (BCC) and Cutaneous Squamous Cell Carcinoma (cSCC) in Olmsted County, Minnesota, 2000–2010. Percentages are based on the number of patients in each subset.
Frequency distributions of BCC subtypes were significantly different depending on tumor location (P<.001). Nodular subtypes were most common on the head and neck (51.3%); superficial subtypes were most common on the extremities (43.2%) and torso (38.8%). Aggressive subtypes accounted for 17.4%, 6.1%, and 5.1% of tumors on the head and neck, extremities, and torso, respectively.
There were 68 recurrences (2.0%), with a median of 3.7 years (interquartile range [IQR], 1.7–5.8) between initial BCC diagnosis and recurrence. The median duration of relevant clinical follow-up among patients without a recurrence was 4.9 years (IQR, 1.6–7.9). The cumulative incidence of local recurrence of BCC was 0.3%, 1.1%, and 2.2% by 1, 3, and 5 years, respectively, after the incident diagnosis. No distant metastases were recorded.
The incidence of BCC increased among residents older than 18 years between the 1976–198418 and 2000–2010 periods. The age-adjusted incidence rates increased significantly (P<.001) among men from 263.2 (95% CI, 232.6–293.8) to 360.0 (95% CI, 342.5–377.4) per 100,000 persons, and among women, from 189.1 (95% CI, 168.7–209.5) to 292.9 (95% CI, 278.6–307.1) per 100,000 persons. The overall age- and sex-adjusted incidence rate increased significantly (P<.001) from 222.0 (95% CI, 204.5–239.5) to 321.2 (95% CI, 310.3–332.2) per 100,000 persons. The increasing incidence of BCC affected both sexes in virtually all age groups (Figure 3).
Figure 3.
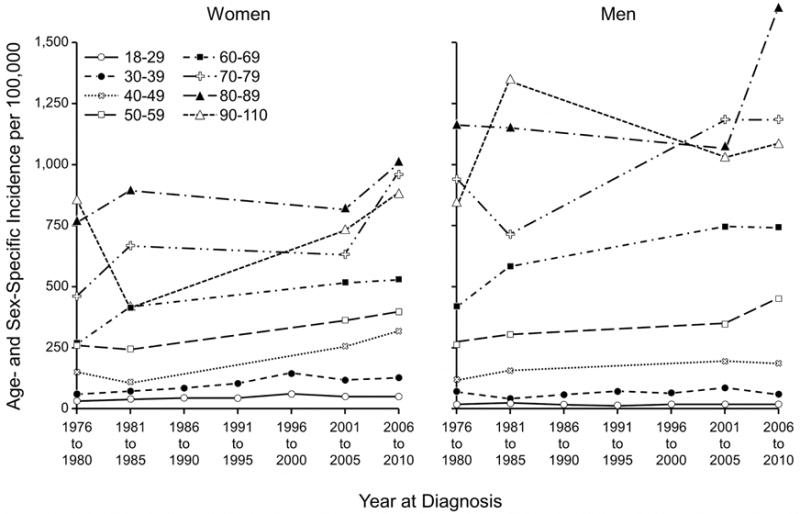
Age- and Sex-Specific Incidence of Basal Cell Carcinoma (BCC) Among Patients 18 Years or Older in Olmsted County, Minnesota, 1976–2010. Data were included for all 7 periods for age groups 18–29 and 30–39. For all other age groups, data were available for 4 periods: 1976–1980, 1981–1984, 2001–2005, and 2006–2010.
Cutaneous Squamous Cell Carcinoma
From 2000 through 2010, 1,711 incident cSCCs were diagnosed in 1,653 patients (mean age at diagnosis, 70.5 years; 55.1% male) (Table 1). Age- and sex-specific incidence rates are shown in Table 2. Incidence rates increased with age for women and at a faster rate for men (P<.001 for sex by age group interaction), with a peak among patients aged 80 to 89 years (Figure 1). Men had a significantly higher age-adjusted incidence rate (207.5 [95% CI, 193.9–221.1] per 100,000 persons) compared to women (128.8 [95% CI, 119.4–138.2] per 100,000 persons) (P<.001). A previous diagnosis of malignant melanoma or malignant melanoma in situ was recorded for 35 patients (2.1%), the majority of which were men (n=30; 85.7%). The average age at melanoma diagnosis was 64.8 years; at subsequent cSCC diagnosis, 73.1 years.
For men and women, the most common location of cSCC was the head and neck (Figure 2). The second most common location was the extremities, with women (38.1%) having a greater tendency than men (24.4%) to have tumors on the upper and lower extremities (P<.001). The torso was the least likely cSCC location.
There were 31 recurrences (1.9%), with a median of 3.1 years (IQR, 0.7–4.7) from cSCC diagnosis to recurrence. The median follow-up among those without a recurrence was 4.4 years (IQR, 1.3–7.5). The cumulative incidence of local recurrence after incident cSCC diagnosis was 0.8%, 1.2%, and 2.3% by 1, 3, and 5 years, respectively. Sentinel lymph node biopsy was performed in 2 patients; 1 patient had a positive biopsy result. Four patients had a distant metastasis, and 2 patients had a nodal recurrence; 1 of these patients had both distant metastasis and nodal recurrence.
Since the 1976–1984 and 1985–1992 periods, the incidence of cSCC has increased in persons older than 18 years.19 Age-adjusted incidence rates per 100,000 persons increased for men as follows: 96.2 (95% CI, 77.1–115.3) in 1976–1984, 222.7 (95% CI, 195.2–250.1) in 1985–1992, and 207.5 (95% CI, 193.9–221.1) in 2000–2010. For women, the age- adjusted incidence rates per 100,000 persons increased as follows: 35.3 (95% CI, 26.6–44.0) in 1976–1984, 101.9 (95% CI, 87.7–116.0) in 1985–1992, and 128.8 (95% CI, 119.4–138.2) in 2000–2010. The age- and sex-adjusted incidence rates per 100,000 persons increased as follows: 61.8 (95% CI, 52.3–71.4) in 1976–1984, 153.7 (95% CI, 139.6–167.7) in 1985–1992, and 162.5 (95% CI, 154.6–170.3) in 2000–2010. For women, the increase in cSCC incidence over time was statistically significant (P<.001). However, for men, the increase in cSCC incidence was significant between 1976–1984 and 1985–1992, with a gradual, nonsignificant decrease by 2000–2010.
Discussion
Basal Cell Carcinoma
The overall incidence of BCC increased 145% between 1976–1984 and 2000–2010. However, the increase was not uniform across age groups and gender. Women in the 40–49 age group had the greatest increase in incidence (2.46-fold); women in the 30–39 age group had the second greatest increase (1.91-fold). Among men, the incidence increased in all age groups except the 18–29 group, but the changes were smaller than those among women. A 2013 report of BCC incidence among 40–50 year-old US health care professionals showed that age-adjusted BCC incidence among women increased from 519 to 1,019 cases per 100,000 person-years during the 1986–1988 and 2004–2006 periods, respectively, and the incidence for men increased from 606 cases to 1,488 cases per 100,000 person-years during the 1988–1990 and 2004–2006 periods, respectively.25 While these incidence rates are markedly higher—and of greater magnitude for men than for women—compared to the results of our study when restricted to this age range, they are not derived from a population-based cohort.
In our study, the anatomical distribution of tumors changed over time. A significantly lower proportion of BCC tumors were observed on the head and neck during the 2000–2010 period (men, 67.6%; women, 62.7%) compared to the 1976–1984 period (men, 85.9%; women, 83.5%) (P<.001). A significantly larger proportion of BCCs were diagnosed on the torso during the 2000–2010 period (men, 24.4%; women, 23.6%) compared to the 1976–1984 period (men, 10.7%; women, 10.6%) (P<.001). This striking trend is consistent with more recent studies.24,26–31 The trends in incidence, anatomical distribution, and tumor subtype may reflect an increase in intermittent, recreational UV exposure.31
Cutaneous Squamous Cell Carcinoma
The overall incidence of cSCC increased 263% between 1976–1984 and 2000–2010, which was disproportionately higher than the increase in BCC. Among men, cSCC incidence decreased between the 1985–1992 and 2000–2010 periods, but among women incidences increased in many age groups. Women ages 50–59 had the greatest increase in incidence (1.55-fold); the next greatest increases were in the 70–79 (1.52-fold) and 40–49 age groups (1.51-fold). The increasing incidence of BCC at younger ages and of cSCC in older women may reflect tanning habits, which increase the intermittently intense and cumulative UV exposures. A 2012 US study estimated the cSCC incidence at 2 different latitudes.8 Age-adjusted incidence estimates for 2012 in the northern latitude group—most comparable to our cohort—ranged from 46.3 to 134.4 per 100,000 persons for men and from 15.7 to 42.9 per 100,000 persons for women. These estimated incidence rates are significantly lower than the incidence rates in our study, illustrating the challenge in ascertaining accurate epidemiologic data for a relatively common malignancy in the absence of a robust, unified data capture system.
A significant change occurred in the anatomical distribution of cSCC. The proportion of tumors on the extremities increased for men (24.4% in 2000–2010 vs 12.5% in 1976–1984; P=.007) and for women (38.1% in 2000–2010 vs 17.1% in 1976–1984; P<.001). As with BCC, these changes in anatomical distribution were observed in other recent studies10,11,32 and may be explained by increased cumulative sun exposure to these anatomical locations.
Younger Populations
For patients younger than 40 years, the increasing incidence of NMSC presents a worrisome trend.24,33 Incidence rates for both BCC and cSCC were higher for younger women than for younger men in our cohort. These results differ from those of previous studies, in which incidence rates of cSCC were higher among younger men than younger women.
Limitations
This study has several limitations. First, in this retrospective review, data were derived from documentation of confirmed NMSC, excluding NMSC treated without histologic confirmation. Second, Olmsted County’s location near the 44th parallel and its relatively high proportion of white residents influence the generalizability of the data. Third, the county’s relatively high proportion of college graduates and health care workers may positively influence incidence detection because of increased access to health care.
Conclusion
This study offers robust, comprehensive incidence data on BCC and cSCC from a well-defined population. The incidence of BCC and cSCC in Olmsted County, Minnesota, increased from 2000 to 2010 compared to results of prior population-based studies. The increase in cSCC incidence was disproportionately larger than that of BCC. Women had the greatest increase in incidence rates for both BCC and cSCC, and the anatomical distribution of tumors shifted to the torso for BCC and to the extremities for cSCC. As NMSC incidence rates increase, an emphasis on education, prevention, and surveillance strategies is imperative, and an accurate, accessible national database is needed.
Acknowledgments
This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01 AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
IRB Approval: This study was approved by the Mayo Clinic and Olmsted Medical Center institutional review boards.
Funding Sources: None.
Abbreviations
- BCC
basal cell carcinoma
- cSCC
cutaneous squamous cell carcinoma
- IQR
interquartile range
- NMSC
nonmelanoma skin cancer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data access and responsibility: Drs. Baum and Muzic had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Authors’ contributions: Study concept and design (Drs. Baum, Wright, and Muzic; Ms. Weaver); Acquisition of data (Drs. Muzic, Schmitt, and Sosa Seda; Ms. Olazagasti Lourido, Alniemi; Mr. Zubair); Analysis and interpretation of data (Drs. Baum, Muzic, and Schmitt; Ms. Weaver; Drafting of the manuscript (Dr. Muzic); Critical revision of the manuscript for important intellectual content (Drs. Baum, Schmitt, and Wright; Ms. Weaver); Statistical analysis (Ms. Weaver); Study supervision (Dr. Baum)
Dr. Baum takes responsibility for the integrity of the work as a whole, from inception to published article.
Conflict of Interest Disclosure: None declared.
Contributor Information
Dr. John G. Muzic, Department of Dermatology, Mayo Clinic, Rochester, Minnesota.
Dr. Adam R. Schmitt, Department of Dermatology, Mayo Clinic, Rochester, Minnesota.
Dr. Adam C. Wright, Dermatologic surgeon at Anderson and Rahman Dermatology, Knoxville, Tennessee.
Dr. Dema T. Alniemi, Transitional resident at MacNeal Hospital, Chicago, Illinois.
Dr. Adeel S. Zubair, Resident, Yale University, New Haven, Connecticut.
Dr. Jeannette M. Olazagasti Lourido, Resident, University of Texas Southwestern, Dallas, Texas.
Dr. Ivette M. Sosa Seda, Department of Dermatology, Mayo Clinic, Rochester, Minnesota.
Ms. Amy L. Weaver, Division of Biomedical Statistics and Informatics, Mayo Clinic, Rochester, Minnesota.
Dr. Christian L. Baum, Department of Dermatology, Mayo Clinic, Rochester, Minnesota.
References
- 1.Diepgen TL, Mahler V. The epidemiology of skin cancer. Br J Dermatol. 2002;146(Suppl 61):1–6. doi: 10.1046/j.1365-2133.146.s61.2.x. [DOI] [PubMed] [Google Scholar]
- 2.Stern RS. Prevalence of a history of skin cancer in 2007: results of an incidence-based model. Arch Dermatol. 2010;146(3):279–282. doi: 10.1001/archdermatol.2010.4. [DOI] [PubMed] [Google Scholar]
- 3.Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012;166(5):1069–1080. doi: 10.1111/j.1365-2133.2012.10830.x. [DOI] [PubMed] [Google Scholar]
- 4.Goodwin RG, Holme SA, Roberts DL. Variations in registration of skin cancer in the United Kingdom. Clin Exp Dermatol. 2004;29(3):328–330. doi: 10.1111/j.1365-2230.2004.01523.x. [DOI] [PubMed] [Google Scholar]
- 5.Geller AC, Swetter SM. Reporting and registering nonmelanoma skin cancers: a compelling public health need. Br J Dermatol. 2012;166(5):913–915. doi: 10.1111/j.1365-2133.2012.10911.x. [DOI] [PubMed] [Google Scholar]
- 6.Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the U.S. Population, 2012. JAMA Dermatol. 2015;151(10):1081–1086. doi: 10.1001/jamadermatol.2015.1187. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Skin Cancers. www.who.int/uv/faq/skincancer/en/index1.html.
- 8.Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68(6):957–966. doi: 10.1016/j.jaad.2012.11.037. [DOI] [PubMed] [Google Scholar]
- 9.Birch-Johansen F, Jensen A, Mortensen L, Olesen AB, Kjær SK. Trends in the incidence of nonmelanoma skin cancer in Denmark 1978–2007: rapid incidence increase among young Danish women. Int J Cancer. 2010;127(9):2190–2198. doi: 10.1002/ijc.25411. [DOI] [PubMed] [Google Scholar]
- 10.Hollestein LM, de Vries E, Nijsten T. Trends of cutaneous squamous cell carcinoma in the Netherlands: increased incidence rates, but stable relative survival and mortality 1989–2008. Eur J Cancer. 2012;48(13):2046–2053. doi: 10.1016/j.ejca.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Brewster DH, Bhatti LA, Inglis JH, Nairn ER, Doherty VR. Recent trends in incidence of nonmelanoma skin cancers in the East of Scotland, 1992–2003. Br J Dermatol. 2007;156(6):1295–1300. doi: 10.1111/j.1365-2133.2007.07892.x. [DOI] [PubMed] [Google Scholar]
- 12.Zamanian A, Farshchian M, Meheralian A. A 10-year study of squamous cell carcinoma in Hamedan in the west of Iran (1993–2002) Int J Dermatol. 2006;45(1):37–39. doi: 10.1111/j.1365-4632.2005.02336.x. [DOI] [PubMed] [Google Scholar]
- 13.Sella T, Goren I, Shalev V, et al. Incidence trends of keratinocytic skin cancers and melanoma in Israel 2006–11. Br J Dermatol. 2015;172(1):202–207. doi: 10.1111/bjd.13213. [DOI] [PubMed] [Google Scholar]
- 14.Perera E, Gnaneswaran N, Staines C, Win AK, Sinclair R. Incidence and prevalence of non-melanoma skin cancer in Australia: A systematic review. Australas J Dermatol. 2015;56(4):258–267. doi: 10.1111/ajd.12282. [DOI] [PubMed] [Google Scholar]
- 15.Demers AA, Nugent Z, Mihalcioiu C, Wiseman MC, Kliewer EV. Trends of nonmelanoma skin cancer from 1960 through 2000 in a Canadian population. J Am Acad Dermatol. 2005;53(2):320–328. doi: 10.1016/j.jaad.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 16.Athas WF, Hunt WC, Key CR. Changes in nonmelanoma skin cancer incidence between 1977–1978 and 1998–1999 in Northcentral New Mexico. Cancer Epidemiol Biomarkers Prev. 2003;12(10):1105–1108. [PubMed] [Google Scholar]
- 17.Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001;344(13):975–983. doi: 10.1056/NEJM200103293441306. [DOI] [PubMed] [Google Scholar]
- 18.Chuang TY, Popescu A, Su WP, Chute CG. Basal cell carcinoma: a populationbased incidence study in Rochester, Minnesota. J Am Acad Dermatol. 1990;22(3):413–417. doi: 10.1016/0190-9622(90)70056-n. [DOI] [PubMed] [Google Scholar]
- 19.Gray DT, Suman VJ, Su WP, Clay RP, Harmsen WS, Roenigk RK. Trends in the population-based incidence of squamous cell carcinoma of the skin first diagnosed between 1984 and 1992. Arch Dermatol. 1997;133(6):735–740. [PubMed] [Google Scholar]
- 20.US Census Bureau: Olmsted County, Minnesota. http://quickfacts.census.gov/qfd/states/27/27109.html.
- 21.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87(2):151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41(6):1614–1624. doi: 10.1093/ije/dys195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.GraphPad Software: Random Numbers. http://www.graphpad.com/quickcalcs/
- 24.Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294(6):681–690. doi: 10.1001/jama.294.6.681. [DOI] [PubMed] [Google Scholar]
- 25.Wu S, Han J, Li WQ, Li T, Qureshi AA. Basal-cell carcinoma incidence and associated risk factors in U.S. women and men. Am J Epidemiol. 2013;178(6):890–897. doi: 10.1093/aje/kwt073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flohil SC, Seubring I, van Rossum MM, Coebergh JW, de Vries E, Nijsten T. Trends in basal cell carcinoma incidence rates: a 37-year Dutch observational study. J Invest Dermatol. 2013;133(4):913–918. doi: 10.1038/jid.2012.431. [DOI] [PubMed] [Google Scholar]
- 27.Jurciukonyte R, Vincerzevskiene I, Krilaviciute A, Bylaite M, Smailyte G. Epidemiology of basal cell carcinoma in Lithuania, 1996–2010. Br J Dermatol. 2013;169(5):1100–1105. doi: 10.1111/bjd.12485. [DOI] [PubMed] [Google Scholar]
- 28.Richmond-Sinclair NM, Pandeya N, Ware RS, et al. Incidence of basal cell carcinoma multiplicity and detailed anatomic distribution: longitudinal study of an Australian population. J Invest Dermatol. 2009;129(2):323–328. doi: 10.1038/jid.2008.234. [DOI] [PubMed] [Google Scholar]
- 29.Bastiaens MT, Hoefnagel JJ, Bruijn JA, Westendorp RG, Vermeer BJ, Bouwes Bavinck JN. Differences in age, site distribution, and sex between nodular and superficial basal cell carcinoma indicate different types of tumors. J Invest Dermatol. 1998;110(6):880–884. doi: 10.1046/j.1523-1747.1998.00217.x. [DOI] [PubMed] [Google Scholar]
- 30.Arits AH, Schlangen MH, Nelemans PJ, Kelleners-Smeets NW. Trends in the incidence of basal cell carcinoma by histopathological subtype. J Eur Acad Dermatol Venereol. 2011;25(5):565–569. doi: 10.1111/j.1468-3083.2010.03839.x. [DOI] [PubMed] [Google Scholar]
- 31.Lovatt TJ, Lear JT, Bastrilles J, Wong C, Griffiths CE, Ramachandran S, et al. Associations between UVR exposure and basal cell carcinoma site and histology. Cancer Lett. 2004;216(2):191–197. doi: 10.1016/j.canlet.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Jung GW, Metelitsa AI, Dover DC, Salopek TG. Trends in incidence of nonmelanoma skin cancers in Alberta, Canada, 1988–2007. Br J Dermatol. 2010;163(1):146–154. doi: 10.1111/j.1365-2133.2010.09809.x. [DOI] [PubMed] [Google Scholar]
- 33.Evans SS, Jih MH, Goldberg LH, Kimyai-Asadi A. Increased burden of melanoma and nonmelanoma skin cancer in young women. Dermatol Surg. 2014;40(12):1385–1389. doi: 10.1097/DSS.0000000000000188. [DOI] [PubMed] [Google Scholar]


