Abstract
An unusual micelle was discovered in mixtures of the nonionic detergent octaethyleneglycol-mono-n-dodecylether with disaturated phospholipids such as 1,2-dimyristoyl-sn-glycero-3-phosphocholine or 1,2-dipalmitoyl-sn-glycero-3-phosphocholine in water. These mixtures undergo a structural transition upon cooling through the chain-melting temperatures of the respective phospholipids, resulting in the formation of mixed micelles. Structural features of the micellar particles were studied here by synchrotron x-ray scattering. The translucent micellar solutions showed characteristic wide-angle reflections that were attributed to ordered hydrocarbon chains, whereas the absence of small-angle x-ray reflections indicated that there is no long-range order in these mixtures. The presence of ordered phospholipid acyl chains was confirmed by differential scanning calorimetry and isothermal titration calorimetry. The endothermic differential scanning calorimetry signals observed in the up-scan mode were tentatively ascribed to chain melting and mixing of the components. Isothermal titration of the mixed-micellar solutions into an excess of the detergent octaethyleneglycol-mono-n-dodecylether resulted in sudden uptake of the latent heat by the gel-state phospholipids. The heat uptake per mol of phospholipid decreased with increasing detergent/phospholipid molar ratio. A simple geometric model is presented assuming that the dominating particle species in the mixtures is a discoidal phospholipid aggregate with ordered acyl chains, surrounded by a toroidal detergent hoop. The model implies that the fraction of ordered phospholipid chains decreases with increasing detergent/phospholipid molar ratio, in agreement with the calorimetric results and high-resolution NMR spectroscopy.
Membrane solubilization is a key technique in biochemistry. The intermediates encountered upon solubilization of biomembranes by nonionic detergents have been thoroughly studied (1), e.g., cryo-transmission electron microscopy revealed large irregular bilayer sheets and threadlike cylindrical structures (2–4). However, structural details of the small mixed micelles, arising after complete solubilization, are usually beyond direct visualization in the electron microscope. The properties of mixed detergent/lipid micelles recently came into focus when detergent insoluble regions (so-called “rafts”) were discovered in cell membranes (5). Improving the understanding of bilayer solubilization and mixed micelle formation is therefore a topic of considerable interest.
Detergents from the poly(oxyethylene) series have found widespread application in membrane biochemistry, owing to their inoffensiveness toward membrane proteins (6). Commonly used members of this class of nonionic amphiphiles are Triton X-100 ([p-(1,1,3,3-tetramethylbutyl)phenyl]poly(oxyethylene)) and C12E8 (octaethyleneglycol-mono-n-dodecyl ether). Biomembrane solubilization using poly(oxyethylene) detergents is remarkable for its temperature dependence (7–9), e.g., the differential solubilization of cell membranes by Triton X-100 far below room temperature has been used to verify the existence of raft-like membrane domains (5).
We previously have shown that mixtures of C12E8 with 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) or 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) undergo a reversible bilayer-micelle transition upon decreasing the temperature below the main-phase transition temperature tm of the respective pure phospholipid (7, 8). The detergent-phospholipid mixed micelles obtained after a sudden temperature change from above tm to ≈10°C below tm have not been fully characterized so far. Various techniques were combined here to explore the properties of these unusual micellar aggregates. X-ray scattering using synchrotron radiation as well as differential scanning calorimetry (DSC) and isothermal titration calorimetry (ITC) revealed the presence of ordered phospholipid acyl chains in the mixed micellar dispersions below tm. A model will be presented that accounts for the wide-angle x-ray reflections obtained from the micellar solution of mixtures of C12E8 with DMPC or DPPC, and for the decreasing heat consumption observed when mixtures with increasing detergent/phospholipid molar ratio were titrated into an aqueous solution of C12E8.
Materials and Methods
Sample Preparation.
The nonionic detergent C12E8 was obtained from Kouyoh Trading (Tokyo) or Fluka. Phospholipids were purchased from Sigma and Avanti Polar Lipids. DMPC with fully deuterated acyl chains (DMPC-d54) was also from Avanti. The required amounts of C12E8 and phospholipid were dissolved in chloroform and dried under a stream of nitrogen. Drying was completed on a vacuum line overnight. The mixtures were suspended in doubly distilled water by vortexing at temperatures above the main-phase transition temperatures tm of the phospholipids (24°C for DMPC and 41°C for DPPC). The samples were again homogenized immediately before the measurements by incubation for 2 min above tm and subsequent cooling on ice. This procedure was repeated until optically clear solutions were obtained below the respective phase transition temperatures tm of the phospholipid components. The pretreatment was particularly critical for the system C12E8/DPPC, which showed a propensity to precipitate after 24 h at 4°C when the molar ratio was ≤ 1:1.
X-Ray Scattering.
Synchrotron x-ray scattering patterns were recorded on the X13 double-focusing monochromator-mirror camera of the European Molecular Biology Laboratory Outstation at Deutsches Elektronen Synchrotron (10). The data acquisition system used allows for simultaneously recording reflections at different angular regimes (11); it consists of two linear detectors with delay line readout connected electronically in series. The entire system covers the range from (20 nm)−1 < s < (2 nm)−1 in the small-angle x-ray scattering (SAXS) regime with s = 1/d = (2/λ) sinΘ (d, lattice spacing; λ, wavelength of x-rays = 0.15 nm; Θ, scattering angle) and from (0.6 nm)−1 < s < (0.35 nm)−1 in the wide-angle x-ray scattering (WAXS) regime. The x-ray flux was measured with an ionization chamber close to the sample. Synchrotron x-ray patterns were obtained at different temperatures in a continuous scan mode at 1°C/min. The temperature was controlled by a water circulating bath. To minimize the x-ray dose on the sample during the heating and cooling scans a fast solenoid-driven shutter controlled by the data acquisition system was used to prevent irradiation of the sample in those periods where no diffraction data were taken. The raw data were normalized against the incident beam intensity using the signal of the ionization chamber. Samples were investigated in thin-walled glass capillaries mounted in a thermostat. Details are described elsewhere (12).
NMR Measurements.
NMR measurements were performed by using a Varian 400S spectrometer. 1H-, 13C- and 31P-NMR spectra were acquired at 400 MHz, 100 MHz, and 162 MHz, respectively, with a standard 5-mm high-resolution probe, whereas 2H spectra were obtained at 61.4 MHz in 5-mm i.d. Teflon tubes using a flat wire solenoid and a dedicated high-power amplifier. A phase cycled quadrupolar echo sequence (π/2)φ − τ1 − (π/2)φ±90 − τ2 acquisition was applied in the latter case for data acquisition.
Calorimetry.
A Microcal (Amherst, MA) VP-DSC was used for DSC. The sample cell (cell volume 0.513 ml) was filled with the equilibrated detergent/phospholipid mixtures at a constant phospholipid concentration of 2.5 mM. ITC was performed with a Microcal VP-ITC calorimeter equipped with a 250-μl syringe. Solutions containing 2.5 mM DMPC or 2.5 mM DPPC and variable amounts of C12E8 in water were titrated into a 10 mM aqueous C12E8 solution. The volume of the calorimeter cell containing the detergent solution was 1.41 μl. Typically 5-μl volumes of the detergent/phospholipid mixture were injected into the detergent solution, and 20 injections were executed. Baseline corrections and integration of the calorimeter signals were done by Microcal origin software. The first two data points were discarded, and an average of the next five data points were entered into the final data evaluation. Control titrations with 2.5 mM C12E8 in the syringe (without phospholipid) yielded a small exothermic heat of 0.08 kcal/mol, which was neglected in the data evaluation. The samples were degassed at 3 kPa under stirring before measurement.
Results
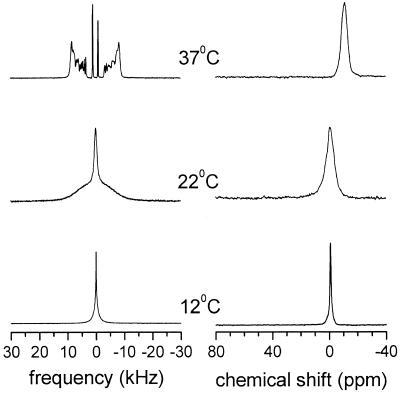
Fluid-state mixtures of the nonionic detergent C12E8 with DPPC or DMPC (molar ratio detergent/phospholipid 1:2) undergo a bilayer to micelle transition in the vicinity of the phase transition temperature tm of the pure phospholipids (7, 8). This process is illustrated in Fig. 1 where 2H- and 31P-NMR spectra of the system C12E8/DMPC-d54 are shown as a function of temperature. Well resolved quadrupolar splittings in the 2H-NMR spectrum and a single homogeneous signal at −13 ppm in the 31P-NMR spectrum were observed at 37°C, indicating that the bilayer aggregates are spontaneously oriented in the magnetic field with the normal to the bilayer surface perpendicular to the field direction. The macroscopic alignment is a result of the decreased elastic modulus of the aggregates, i.e., the torque arising from the anisotropic magnetic susceptibility of the alkyl chains overcomes the bending rigidity of the bilayers (13). As a result of micelle formation below 20°C the resolution of 2H quadrupolar splittings is lost, the entire spectral intensity eventually merges into a single line, and the chemical shift of the 31P-NMR signal moves toward 0 ppm. This transition is accompanied by a sudden decrease in light scattering and viscosity. The size of the aggregates above 30°C as determined by dynamic light scattering was ≥200 nm whereas an average size of ∼7 nm was found below 20°C. An analogous behavior was found for mixtures of C12E8 and DPPC where the bilayer-micelle transition occurred around 38°C (7).
Figure 1.
Temperature dependence of 2H-NMR spectra (Left) and 31P-NMR spectra (Right) of a C12E8/DMPC-d54 mixture at molar ratio 1:2.
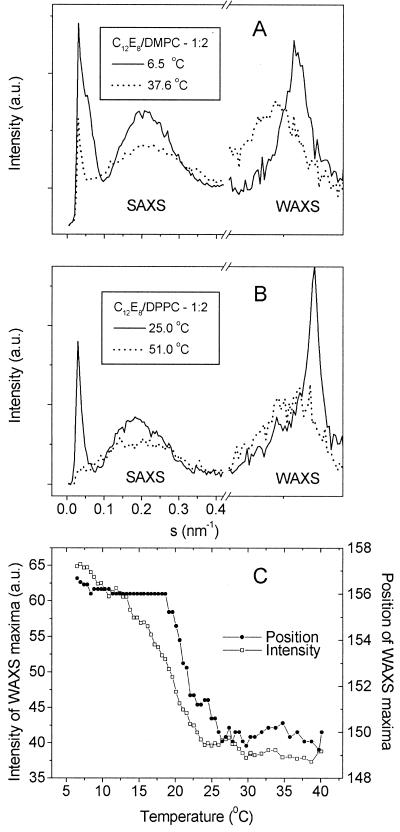
The mixed micelles obtained below the transition temperature were further characterized by x-ray scattering using synchrotron radiation. The experimental setup allowed measurements over a broad range of scattering angles comprising the small-angle and the wide-angle regimes (10–12). X-ray scattering patterns of C12E8/DMPC and C12E8/DPPC mixtures (1:2 molar ratio) at different temperatures are shown in Fig. 2 A and B. For both samples a broad scattering distribution centered around s = (0.2 nm)−1 is observed in SAXS at all temperatures, which is compatible with scattering from micelles. In the WAXS regime, which is indicative of the ordering of the aliphatic chains of the lipids, a strong reflection is observed in both samples below the main transition temperature tm. Above tm, a broad reflection centered at smaller angles remains. The behavior of the WAXS reflection in both samples is typical for what is known from pure lipids (14), i.e., the ordering of the aliphatic chains in the gel and ripple phase of lipids vanishes above the main transition temperature tm and the sharp x-ray reflection fades out into a broad maximum centered around (4.6 nm)−1. It is noteworthy that the WAXS reflection of the sample containing DPPC is sharper than the one with DMPC, which reflects the better ordering of the aliphatic chains in DPPC due to two additional CH2 groups in the chains. The temperature-dependent behavior of the WAXS reflections of the C12E8/DMPC sample is shown in Fig. 2C. Up to about 20°C the position of the reflection in the WAXS remains constant. Between 20°C and 25°C it shifts toward smaller angles. The intensity of the reflection is gradually reduced until about 25°C when it remains constant. It should be noted that in the DPPC sample the behavior is similar, indicating that the change in the micelle is associated with melting of the aliphatic chains.
Figure 2.
Synchrotron x-ray patterns of (A) C12E8/DMPC and (B) C12E8/DPPC mixtures at molar ratio 1:2 obtained below and above the respective phase transition temperatures of the pure phospholipids. (C) Temperature dependence of position (given as channel numbers) and intensity of the wide-angle reflections in the C12E8/DMPC system. Total concentration of the samples, 10% (wt/vol) in water.
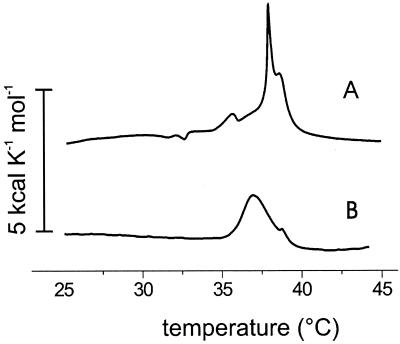
The presence of ordered phospholipid hydrocabon chains in the micelles obtained below tm of the respective phospholipids also was demonstrated by calorimetry. DSC was applied for a number of C12E8/DMPC and C12E8/DPPC molar ratios. The total phospholipid concentration was kept constant (2.5 mM) so as to enable a direct comparison with respect to the transition enthalpy of the lipids. A heating scan in the C12E8/DPPC system at a molar ratio 1:2 yielded endothermic peaks at 36°C, 38°C, and 38.7°C, respectively (Fig. 3A). The signal at 36°C shifted to 34.9°C in the corresponding down scan (not shown), whereas the other signals were completely reversible. With increasing detergent/phospholipid molar ratios the signals became considerably broader and the heat capacity maxima shifted toward lower temperatures, e.g., an up scan in an equimolar C12E8/DPPC mixture yielded a maximum at 37°C and a small peak at 38.8°C (Fig. 3B). In the C12E8/DMPC mixture (1:2 mol/mol) a narrow heat capacity maximum was found at 23°C that shifted to somewhat higher temperatures upon increasing the detergent/lipid molar ratio, in contrast to the C12E8/DPPC system (not shown). A quantitative determination of enthalpies from individual signals was not feasible, due to the broadening of the transitions. However, estimates obtained by integrating the entire DSC traces showed that the total enthalpy tended to zero with increasing molar ratio. Eventually, DSC signals were no longer detectable, which points at the formation of ordinary micelles with a disordered hydrocarbon core (15) when the detergent/phospholipid molar ratio became ≥4.
Figure 3.
DSC of C12E8/DPPC mixtures. DPPC concentration 2.5 mM. (A) Molar ratio 1:2. (B) Molar ratio 1:1. Scanning rate 6°C/h.
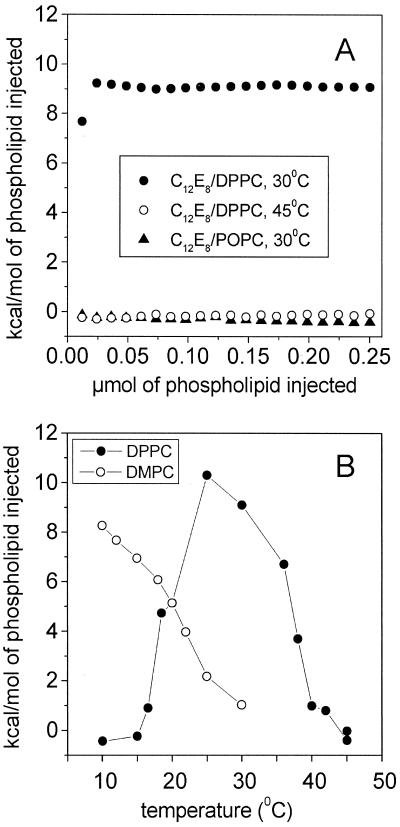
These observations prompted us to use ITC as an alternative for the determination of the overall transition enthalpy. It can be assumed that rapid dilution of the putative “nonclassical micelles” (with ordered rather than liquid phospholipid hydrocarbon chains) by injection into a large excess of the detergent alone results in the formation of ordinary micelles. The dilution will be accompanied by the sudden uptake of the latent heat associated with the order-disorder transition, providing the injections are performed well below the transition temperature tm of the respective phospholipid. The experiment shown in Fig. 4A for the C12E8/DPPC mixture is in excellent agreement with these expectations. Five-microliter portions of the mixed micellar solution (molar ratio detergent/phospholipid 1:2, phospholipid concentration 2.5 mM) were injected at 30°C into the sample cell of the ITC instrument containing 1.41 ml of an aqueous 10 mM C12E8 solution, which corresponds to an 1,120-fold molar excess of the detergent for the first injection. Virtually the same integrated heat values (in terms of kcal/mol of phospholipid injected) were consumed in each titration step when 20 injections were performed, except for the first injection, which usually gave artifactual results (compare Fig. 4A). This enthalpy (9.1 kcal/mol of DPPC) was close to literature values (16) of the molar chain-melting enthalpy of pure DPPC (8.7 kcal/mol). Analogous titrations were performed with the C12E8/DMPC mixture (not shown in Fig. 4A). Here the experiment was run at 13°C, which corresponds to 30°C in the C12E8/DPPC system if one considers reduced temperatures according to Tred = (T − Tm)/Tm where the experimental temperature T and the transition temperature Tm are given in Kelvin rather than in centigrade. The molar heat absorbed upon titration of a 1:2 mol/mol mixture was less (7.2 kcal/mol) than that observed for the C12E8/DPPC system at the same molar ratio, in agreement with the lower chain melting enthalpy of DMPC (16).
Figure 4.
(A) Integrated endothermic heat values obtained upon titration of C12E8/DPPC and C12E8/POPC mixtures into a 10 mM aqueous C12E8 solution by high-sensitivity ITC. The phospholipid concentration in the syringe was 2.5 mM and the detergent/phospholipid molar ratio 1:2. Twenty injections (5 μl each) were performed. (B) Temperature dependence of the heat uptake observed upon titration of C12E8/DPPC and C12E8/DMPC mixtures (1:2 mol/mol) into a 10 mM C12E8 solution. Ten injections (5 μl) were performed for each data point, and the integrated heat values of the following five injections were averaged. All heat values are uncorrected (no control titration subtracted).
Only small exothermic enthalpies were obtained when the titrations were carried out well above the respective chain- melting temperatures of the phospholipids as shown for the C12E8/DPPC mixture in Fig. 4A. The thermal behavior was further studied by performing titrations over a broad temperature range. As shown in Fig. 4B, there was no heat consumption below 15°C and above 39°C in the 1:2 mol/mol C12E8/DPPC mixture. Similarly, in the C12E8/DMPC system the heat consumption decreased going from 10°C to 30°C, although less abruptly than in the C12E8/DPPC system. Unfortunately, reliable measurements were not feasible below 10°C due to instrumental limitations. Therefore, a sudden drop of the heat consumption at lower temperatures could not be established here.
The notion that mixed micelles containing DMPC or DPPC undergo an order-disorder transition upon sudden dilution into an excess of the detergent was further confirmed when these lipids were replaced by 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), which has a chain-melting transition at tm ≈ −3°C (17). The only calorimetric response to be expected here is the heat of dilution of the detergent/phospholipid mixture into the detergent solution. It has been shown previously that this enthalpy is smaller than that of the chain-melting transition at least by an order of magnitude (18). Titration of a C12E8/POPC mixture (1:2 mol/mol) at 30°C into a 10 mM solution of C12E8 yielded a negligible enthalpy, indicating that the POPC dilution does not involve a chain-melting transition (Fig. 4A). Likewise, no thermal transitions were found by DSC performed in the temperature range from 20°C to 45°C. It must be noted that the 1:2 mol/mol C12E8/POPC mixture was slightly turbid at 30°C, in contrast to the corresponding micellar mixtures containing DMPC and DPPC at 13°C and 30°C, respectively.
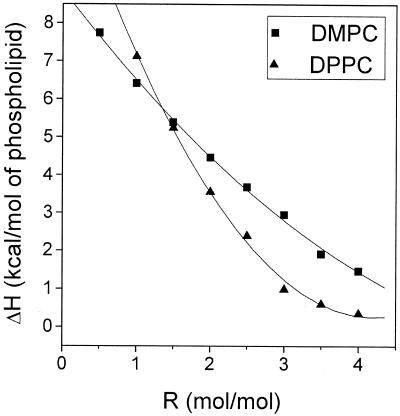
To explore the stability of the intramicellar chain ordering as a function of micelle composition, titrations were carried out for a broad range of detergent/phospholipid molar ratios. Samples were prepared so as to keep the total phospholipid concentration constant at 2.5 mM for all mixtures. Again, the titrations were performed at 30°C and 13°C for DPPC and DMPC, respectively. The results summarized in Fig. 5 indicate that the heat consumption per mol of phospholipid and, equivalently, the proportion of lipid in the ordered state decreases with increasing detergent/phospholipid ratios. Moreover, it can be recognized that the latent heat tends to zero as the ratio approaches a value of 4 mol/mol (see Discussion).
Figure 5.
Heat uptake as a function of the C12E8/phospholipid molar ratio R measured by ITC. ■, C12E8/DMPC, titrations performed at 13°C. ▴, C12E8/DPPC, titrations performed at 30°C. The phospholipid concentration in the syringe was kept constant at 2.5 mM. The solid curves are second-order polynomial fits to the data according to Eqs. 1-5 in Discussion.
The presence of ordered acyl chains in mixed micelles containing DMPC or DPPC but not POPC was also evident from high-resolution NMR spectroscopy. Motional restriction results in appreciable line broadening in 13C- and 1H-NMR spectra of mixed micelles containing the fully saturated phospholipids. Natural abundance 13C-NMR spectra of C12E8/DPPC and C12E8/POPC mixed micelles (molar ratio 2:1) were acquired at 13°C (see Fig. 7, which is published as supplemental data on the PNAS web site, www.pnas.org). Both samples were translucent and nonviscous at this temperature, in agreement with the absence of large aggregates. The signals of the acyl chain methyl and methylene carbons were severely broadened in the C12E8/DPPC sample, in contrast to the respective signals in the C12E8/POPC mixture. Similar observations were made by 1H-NMR spectroscopy, i.e., a strongly broadened ω-methyl signal appeared in an up-field position from the narrow triplet signal of the detergent terminal methyl resonance in the micellar detergent/phospholipid mixtures (spectra not shown).
Discussion
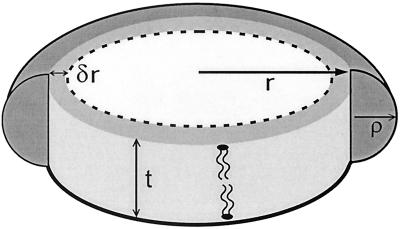
The presence of ordered hydrocarbon chains in a micellar detergent/phospholipid mixture and an unusually large transition enthalpy are the main findings of the present study. Altogether, these observations suggest that the mixed micelle under study is composed of a discoidal cluster of almost pure phospholipid in the gel state, the interfacial edge of which is shielded from the surrounding aqueous medium by a semitoroidal collar of detergent molecules (Fig. 6). The validity of this model has been tested by evaluating the ITC experiments summarized in Fig. 5. The broken circle in Fig. 6 depicts an outer phospholipid layer (average width δr) that is perturbed by contact with the surrounding detergent. This layer is assumed to be in the fluid state while the rest of the bilayer patch (radius r − δr) is in an ordered state. The radius ρ of the detergent semitorus is half the bilayer thickness t, i.e., ρ = t/2. The volumes of the entire phospholipid bilayer patch, the perturbed outer phospholipid layer alone, and the detergent semitorus will be denoted by Vb, Vp, and Vs, respectively. The ratios of these volumes are given by
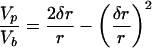 |
1 |
and
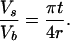 |
2 |
Assuming equal mass densities for the detergent and phospholipid, Eq. 2 yields a relation between 1/r and the total molar ratio R = nD/nL of detergent and phospholipid
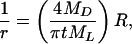 |
3 |
where MD and ML denote the respective molecular weights. The size of the circular bilayer patch (Fig. 6) may be calculated from r, e.g., one obtains ≈680 and 10 lipid molecules for the C12E8/DPPC system with R = 0.5 and R = 4, respectively, using published data for the phospholipid molecular area in the gel state (19). It is noteworthy that these numbers compare favorably with the size of the so-called cooperative unit as determined previously for DPPC multibilayers and sonicated vesicles (20).
Figure 6.
Model of the gel-state micelle. t, thickness of the phospholipid bilayer; r, radius of the bilayer patch; δr, width of perturbed lipid layer; ρ, radius of the C12E8 semitorus.
The total molar transition enthalpy obtained by detergent dilution (compare Fig. 5) is proportional to the fraction of phospholipid in the ordered state, namely, 1 − Vp/Vb,
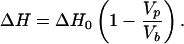 |
4 |
Combining Eqs. 1, 3, and 4 one obtains an approximate expression for the experimental molar enthalpy
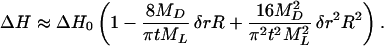 |
5 |
The plots in Fig. 5 of ΔH vs. R for DMPC and DPPC are indeed nonlinear as expected from Eq. 5. A second-order polynomial fit to the data (solid lines in Fig. 5) gives ΔH = 8.87–2.53 R + 0.17 R2 and ΔH = 12.18–5.87 R + 0.68 R2 for DMPC and DPPC, respectively. To test the consistency with Eq. 5 the dimensionless quantity 4MDδr/πtML can be calculated from the first- and second-order coefficients, which yield 0.14 and 0.14 for DMPC and 0.23 and 0.24 for DPPC. Thus, the width of the perturbed layer (Fig. 6) may be obtained, providing the thickness t of the micelle is known. A reasonable estimate yields t = 4.83 nm and t = 4.60 nm for the C12E8/DPPC and the C12E8/DMPC mixed micelles, respectively, using the distance of the electron density maxima obtained by x-ray diffraction for DMPC and DPPC bilayers at 30°C and 50°C, respectively (19) and adding 1 nm to account for the dimension of the headgroup region (21). The resulting perturbed layer thicknesses are δr = 0.69 nm and δr = 1.25 nm, which roughly accounts for two layers of the phospholipids in the liquid crystalline state.
This analysis only allows for “nonclassical” micelles as depicted in Fig. 6. Alternative models are not consistent with the data, however, e.g., a linear rather than a nonlinear relation between ΔH and R (compare Fig. 5) would be expected if one considers the possibility of ordinary (liquid) micelles in equilibrium with discoidal aggregates of constant average size. Accordingly, no indication was found in the 13C-NMR spectra for a subpopulation of fluid lipids that increases as a function of increasing molar ratio R. The model also neglects the heat of remixing upon dilution of the micelles into the detergent solution. An endothermic enthalpy of approximately 1.2 kcal/mol has previously been observed (18) upon dilution of small amounts (0.028 μmol) of POPC vesicles into a C12E8 solution (2.5 mM in 1.34 ml). The magnitude of ΔH0 as obtained by extrapolation from the nonliner fit to the data in Fig. 5 (12.2 and 8.9 kcal/mol of phospholipid for DPPC and DMPC, respectively) is indeed larger than literature values obtained by DSC (16) for the pure phospholipids (8.7 kcal/mol and 6.2 kcal/mol). After correcting for the mixing enthalpy the heat consumption measured by ITC will be still somewhat larger than the literature data. Hence, the concentration of disordered phospholipids solubilized in the semitoroidal detergent volume VS (which amounts to decreasing ΔH0) may be negligible when the titrations are performed ≥10°C below tm. The magnitude of the transition enthalpy also suggests that the components are almost completely segregated. This may be no longer true, however, when the temperature approaches tm where the heat consumption decreases with increasing temperature (Fig. 4B). There is also an abrupt decrease of the heat uptake when the micelles are added more than 15°C below tm, suggesting that there is a threshold temperature where the phospholipid core of the micelles becomes resistant against solubilization. This finding is in agreement with earlier observations on the low temperature insolubility of gel-state phospholpids in nonionic detergents (22, 23).
Considering the DSC results shown in Fig. 3 one arrives at the same qualitative conclusions as obtained by the ITC experiments, i.e., the heat absorbed in the up scans points at an order-disorder transition. Further, the decrease of this endothermic heat with increasing detergent/phospholipid ratio is in line with the model described above. However, there are considerable differences associated with the different experimental protocols that make a direct comparison difficult. First, the heat uptake obtained in the ITC experiment (which has been attributed to the chain-melting transition) occurs ≈8–11°C below the transition temperature of the DSC scans (compare Figs. 3 and 4), which may account for the large absolute values of the heat consumption obtained by titration. Second, in contrast to DSC, the sudden dilution of the phospholipid results in an ≈1,000-fold molar excess of the detergent in the ITC cell after the first injection, i.e., there are only ordinary micelles with highly disordered phospholipids according to the results shown in Fig. 5. This transition is certainly accompanied by a larger gain in configurational entropy than the chain melting transition during a DSC up scan, which largely leads to some sort of liquid crystalline state such as bilayer flakes and long cylindrical micelles (2). It also can be assumed that the up-scan DSC signals in Fig. 3 reflect different consecutive steps, e.g., the peaks at 38°C and 38.7°C (Fig. 3A) and at 37°C and 38.8°C (Fig. 3B) may be attributed to chain melting and component mixing, respectively.
A tentative understanding of the temperature-induced micelle formation described here may be achieved, taking account of previous work. It has been shown recently by deuterium NMR that the presence of C12E8 strongly affects the the elastomechanic properties of bilayer membranes on a mesoscopic length scale (13). The decrease of the elastic constants of large liquid crystalline aggregates certainly represents a premise for the final, entropy-driven breakdown into smaller mixed micelles. The increasing water binding of the poly(ethyleneoxide) moiety of the detergent also promotes micelle formation with decreasing temperature. This has been rationalized in terms of a critical packing parameter (24) defining the limiting conditions for the stability of an aggregated state, i.e., the average shape of the highly hydrated detergent molecule meets the criteria for a toroidal detergent layer (Fig. 6) with its positive surface curvature (7, 25). Repulsive forces between the hydrated detergent layers also prevent the micelles from aggregation. As mentioned above (see Materials and Methods) the micellar solutions tend to aggregate very slowly at 4°C. Visible aggregation and precipitation of gel-state DPPC was observed after 24 h whereas the onset of DMPC precipitation was noticed after 1 week, suggesting that the micellar aggregates obtained after rapid cooling of the mixtures through the phase transition temperature represent a kinetically trapped state the stability of which depends on the gel-state free energy of the phospholipids.
In conclusion, we have shown that the temperature-driven solubilization of saturated phospholipid bilayers by a nonionic detergent involves an ordering of the lipid acyl chains. A simple model of the ensuing mixed micelle is in good qualitative agreement with measurements of the residual latent heat. We believe that our results bear relevance to the issue of detergent solubilization of partially ordered microdomains in biological membranes.
Supplementary Material
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft, Sonderforschungsbereich 266, Teilprojekt B12.
Abbreviations
- DMPC
1,2-dimyristoyl-sn-glycero-3-phosphocholine
- DPPC
1,2-dipalmitoyl-sn-glycero-3-phosphocholine
- POPC
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
- C12E8
octaethyleneglycol-mono-n-dodecylether
- DSC
differential scanning calorimetry
- ITC
isothermal titration calorimetry
- SAXS
small-angle x-ray scattering
- WAXS
wide-angle x-ray scattering
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Almgren M. Biochim Biophys Acta. 2000;1508:146–163. doi: 10.1016/s0005-2736(00)00309-6. [DOI] [PubMed] [Google Scholar]
- 2.Edwards K, Almgren M. J Colloid Interface Sci. 1991;147:1–21. [Google Scholar]
- 3.Vinson P K, Talmon Y, Walter A. Biophys J. 1989;56:669–681. doi: 10.1016/S0006-3495(89)82714-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambert O, Levy D, Ranck J-L, Leblanc G, Rigaud J-L. Biophys J. 1998;74:918–930. doi: 10.1016/S0006-3495(98)74015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.London E, Brown D A. Biochim Biophys Acta. 2000;1508:182–195. doi: 10.1016/s0304-4157(00)00007-1. [DOI] [PubMed] [Google Scholar]
- 6.Silvius J R. Annu Rev Biophys Biomol Struct. 1992;21:323–348. doi: 10.1146/annurev.bb.21.060192.001543. [DOI] [PubMed] [Google Scholar]
- 7.Otten D, Löbbecke L, Beyer K. Biophys J. 1995;68:584–597. doi: 10.1016/S0006-3495(95)80220-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beyer K. Progr Coll Polym Sci. 1997;103:78–86. [Google Scholar]
- 9.Patra S K, Alonso A, Goni F M. Biochim Biophys Acta. 1998;1373:112–118. doi: 10.1016/s0005-2736(98)00095-9. [DOI] [PubMed] [Google Scholar]
- 10.Rapp G. Acta Phys Polonica A. 1992;82:103–120. [Google Scholar]
- 11.Rapp G, Gabriel A, Dosiere M, Koch M H J. Nucl Instrum Meth Phys Res A. 1995;357:178–182. [Google Scholar]
- 12.Rappolt M, Rapp G. Ber Bunsenges Phys Chem. 1996;100:1153–1162. [Google Scholar]
- 13.Otten D, Brown M F, Beyer K. J Phys Chem B. 2000;104:12119–12129. [Google Scholar]
- 14.Small D M. The Physical Chemistry of Lipids-From Alkanes to Phospholipids. New York: Plenum; 1986. [Google Scholar]
- 15.Zulauf M, Weckström K, Hayter J B, Degiorgio V, Corti M. J Phys Chem. 1985;89:3411–3417. [Google Scholar]
- 16.Cevc G, Marsh D. Phospholipid Bilayers, Physical Principles, and Models. New York: Wiley; 1987. [Google Scholar]
- 17.Koster K L, Webb M S, Bryant G, Lynch D V. Biochim Biophys Acta. 1994;1193:143–150. doi: 10.1016/0005-2736(94)90343-3. [DOI] [PubMed] [Google Scholar]
- 18.Heerklotz H, Lantzsch G, Binder H, Klose G. J Phys Chem. 1996;100:6764–6774. [Google Scholar]
- 19.Nagle J F, Tristram-Nagle S. Biochim Biophys Acta. 2000;1469:159–195. doi: 10.1016/s0304-4157(00)00016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marsh D, Watts A, Knowles P F. Biochim Biophys Acta. 1977;465:500–514. doi: 10.1016/0005-2736(77)90268-1. [DOI] [PubMed] [Google Scholar]
- 21.Sun W J, Tristram-Nagle S, Suter R M, Nagle J F. Biophys J. 1996;71:885–891. doi: 10.1016/S0006-3495(96)79290-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ribeiro A A, Dennis E A. Biochim Biophys Acta. 1973;332:26–35. [Google Scholar]
- 23.Robson R J, Dennis E A. Biochim Biophys Acta. 1979;573:489–500. doi: 10.1016/0005-2760(79)90223-6. [DOI] [PubMed] [Google Scholar]
- 24.Israelachvili J N, Narcelja S, Horn R G. Q Rev Biophys. 1980;13:121–200. doi: 10.1017/s0033583500001645. [DOI] [PubMed] [Google Scholar]
- 25.Thurmond R L, Otten D, Brown M F, Beyer K. J Phys Chem. 1994;98:972–983. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.