Abstract
A 59-year-old Portuguese Caucasian man with a history of heavy alcohol intake and no significant medical history presented with ascites, weight loss and general malaise. The ascitic fluid analysis showed 921 cells/mm3 with mononuclear predominance (93.6%), elevated total proteins and a slightly elevated serum-ascites albumin gradient. The abdominal ultrasound confirmed the presence of chronic liver disease with ascites, and additionally on CT there was evidence of peritoneal thickening. On repeat paracentesis, the ascitic fluid analysis showed elevated adenosine deaminase but it was negative for the presence of mycobacteria by Ziehl-Neelsen stain, Löwenstein-Jensen culture and PCR amplification. Due to the persistent suspicion of tuberculosis, a laparoscopy was performed showing multiple small white tubercles scattered over the peritoneum. Peritoneal biopsies showed the presence of necrotising granulomas and cultures were positive for Mycobacterium tuberculosis complex. After a 6-month course of tuberculostatics, the ascites resolved completely. The patient remained asymptomatic.
Keywords: Tb And Other Respiratory Infections, Liver Disease
Background
Far from being a disease of the past, tuberculosis (TB) is still a disease with a significant incidence in countries like Portugal (18. 6/100.000 inhabitants) and factors like migration or cuts in health budgets may be responsible for an increased number of cases in developed countries.1
Tuberculosis is reported to be the cause of ascites in only 2% of patients; however ascites constitutes the most common form of presentation of tuberculous peritonitis (TBP). The diagnosis of TBP as the aetiology of ascites may not be straightforward due to the low yield of direct microscopy and mycobacterial cultures obtained from the ascitic fluid. It has been described that the risk of TB is increased in patients with liver cirrhosis, particularly in those with alcohol-related liver disease.2 Since there are far more common causes for ascites, especially in this population, the correct diagnosis might never be established. So it is necessary to be aware of the risk of TB in patients with chronic liver disease and to maintain a high level of suspicion to perform tests with higher sensitivity and specificity, such as laparoscopy with peritoneal biopsies that can confirm the diagnosis.
Case presentation
A 59 year-old Portuguese Caucasian man presented in the emergency department with a 2-month history of progressive abdominal distension, weight loss (10 kg) and asthenia. The patient denied fever, loss of appetite, night sweats, change in bowel habit or any other symptoms on systemic enquiry. He had an alcohol intake of >80 g/day, with no known comorbidities or regular medication. There was no history of travelling or previous TB.
On physical examination, he was afebrile, non-icteric, with normal breath sounds and voluminous non-tense ascites.
Investigations
The initial blood tests showed normal full blood count and prothrombin time, with an aspartate transaminase (AST) of 84 IU/L (N 5-34 IU/L) and normal alanine transaminase (ALT), normal renal function, a total bilirubin of 1.7 mg/dL (N 0.2–1.2 mg/dL) and C reactive protein of 55 mg/L (n<5 mg/L). Hepatitis B surface antigen, hepatitis C antibody, hepatitis C virus antibody and HIV 1 and 2 antibodies were negative. The chest X-ray was normal. A diagnostic paracentesis showed increased cellularity (921 cells/mm3) with mononuclear predominance (93.6%), normal amylase level, elevated total proteins 5 g/dL (n<1 g/dL) and a serum to ascites albumin gradient of 1.17 g/dL. The abdominal ultrasound showed ascites and features consistent with chronic liver disease. An abdominal and pelvic CT scan was performed with evidence of peritoneal thickening (figure 1). On the oesophagogastroduodenoscopy, small oesophageal varices and portal hypertensive gastropathy were present. A repeat paracentesis was performed to rule out malignancy or TB as the cause of ascites. No malignant cells were found but adenosine deaminase (ADA) activity was 101 IU/L (n<40 UI/L); however Ziehl-Neelsen stain, Löwenstein-Jensen cultures and PCR amplification of the ascitic fluid were negative for the presence of mycobacteria. In the absence of a definitive diagnosis, a diagnostic laparoscopy was performed to obtain peritoneal tissue samples. Laparoscopy revealed multiple small whitish nodules in the peritoneum consistent with peritoneal TB (figure 2). Peritoneal biopsies were obtained and the histology showed necrotising granulomas (figure 3), suggestive of TB. Peritoneal samples were also sent for acid-fast bacilli cultures and, a few weeks later, Mycobacterium tuberculosis complex was isolated, sensitive to all first-line anti-TB medication.
Figure 1.
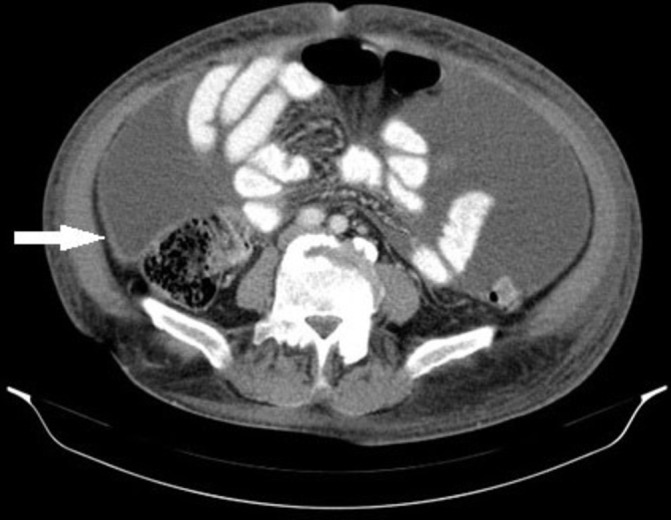
Abdominal CT showing peritoneal thickening (white arrow).
Figure 2.
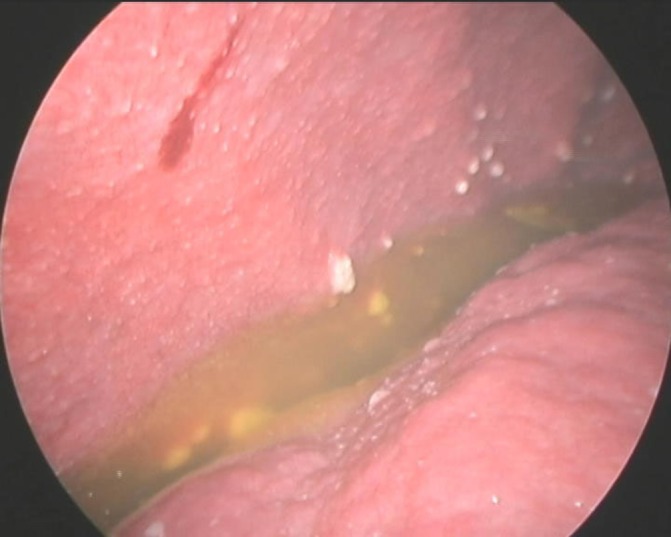
Laparoscopic view showing peritoneal tubercles and ascitic fluid.
Figure 3.
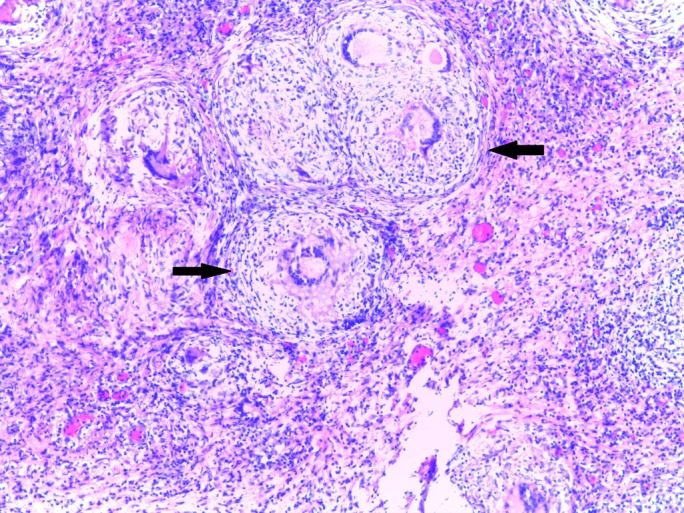
Peritoneal biopsies showing granulomas (black arrows) with multinucleated giant cells and central necrosis.
Differential diagnosis
In this patient with ascites and elevated cell count, the differential diagnosis included spontaneous or secondary bacterial peritonitis, pancreatic ascites, peritoneal carcinomatosis and tuberculous peritonitis. In spontaneous and secondary peritonitis, there is usually a predominance of polymorphonuclear leucocytes, which was not the case; furthermore the imaging studies did not show evidence of any surgically treatable intra-abdominal cause of infection. The amylase level in the ascitic fluid and pancreas imaging were normal, which excluded pancreatic ascites. So the differential diagnosis mainly included peritoneal carcinomatosis and peritoneal TB. ADA activity was elevated, but that can occur in both previous conditions. The cytology of the ascitic fluid was negative for malignant cells, and Ziehl-Neelsen stain and Löwenstein-Jensen cultures for mycobacteria in the ascitic fluid were also negative. To differentiate between these two entities, a laparoscopy was performed to collect peritoneal tissue samples for histology. The macroscopic appearance was very suggestive of TBP due to the presence of whitish peritoneal tubercles. The diagnosis of TBP was confirmed on histological analysis that showed necrotising granulomas and acid-fast bacilli cultures from peritoneal tissue that isolated mycobacteria.
Treatment
As soon as the results from the biopsy were known, the patient was started on tuberculostatics: isoniazid 300 mg/day for 6 months, rifampicin 600 mg/day for 6 months, ethambutol 1200 mg/day for 2 months and pyrazinamide 500 mg twice daily for 2 months.
Outcome and follow-up
The patient was followed up regularly in the outpatient clinic during the duration of treatment. There was a complete resolution of symptoms and an increase in weight. He did not report any side effects and there was no evidence of hepatic toxicity in the follow-up blood tests. After the end of treatment the abdominal ultrasound confirmed the absence of ascites. The patient remained asymptomatic.
Discussion
The peritoneum constitutes a common site for extrapulmonary TB, usually due to the reactivation of latent foci established after haematogenous dissemination from a primary lung infection. In our patient there was no previous history of TB. The clinical manifestations are usually non-specific with ascites, abdominal pain and weight loss being the most common symptoms.3 Patients with cirrhosis seem to have an increased risk of TB, especially those with alcohol-related liver disease but the reason for this remains unclear.2 In this particular population, where there are other more common causes for ascites than TB, the clinical suspicion is of paramount importance for the diagnosis. The analysis of the ascitic fluid usually shows elevated leucocyte count with predominance of lymphocytes and high protein levels (>2.5 mg/dL). However, it has been described that in cirrhotic patients, it is possible to have polymorphonuclear predominance in the ascitic fluid, which may lead to the erroneous interpretation of spontaneous bacterial peritonitis.4 The serum-ascites albumin gradient is normally <1.1 g/dL, except in patients where portal hypertension may contribute to the ascites, such as those with liver disease, as was the case with our patient. ADA is an enzyme involved in the proliferation and differentiation of T lymphocytes. An ADA activity in the ascitic fluid ≥30 U/L seems to have a good sensitivity for the diagnosis of TB, even in cirrhotic patients.5 6 However false positives may occur, for example in peritoneal carcinomatosis, and that is why other investigations such as ascites cytology and imaging studies should also be performed. Detection of mycobacteria in the ascitic fluid by Ziehl-Neelsen staining has a low diagnostic yield (positive only in 3% of cases) and culture of the fluid can be positive in as few as 35% of cases; amplification by PCR method has also low sensitivity.3 In our case report, all three methods were negative for mycobacteria in the ascitic fluid; this low sensitivity is most likely due to the low concentration of mycobacteria in the ascitic fluid in TBP.7 Regarding imaging methods, the presence of intra-abdominal fluid, lymphadenopathy and a thickened peritoneum, mesentery or omentum are among the most common features in abdominal ultrasound and CT.8 But once again, these radiological features are not specific for TBP and thus tissue samples are almost always needed.
Due to the poor performance of most less-invasive diagnostic methods, laparoscopy/laparotomy with peritoneal biopsies is regarded nowadays as the diagnostic method of choice. The visual aspects found can be very suggestive and are reported to reach 93% sensitivity for diagnosis: whitish peritoneal nodules (<5 mm), peritoneal thickening, ascites and adhesions are the most common.3 The peritoneal biopsies histology and mycobacterial culture can lead to the diagnosis.3 9 The case we described illustrates how well laparoscopy may enable the diagnosis of TBP in a patient where ascitic fluid analysis did not identify the presence of mycobacteria. Granulomas with caseation necrosis are the most characteristic histological finding and can be found in 85%–90% of biopsies. Mycobacterial cultures from biopsies have a higher diagnostic yield than those from ascitic fluid; both allow the performance of drug susceptibility tests to guide antimycobacterial therapy.
As for the treatment, the same 6-month regimen used for pulmonary TB is recommended for TBP. However, patients with previous liver disease might be more prone to hepatotoxic reactions caused by isoniazid, pyrazinamide or rifampicin. In this setting, a closer monitoring or even alternative regimes with less potentially hepatotoxic drugs might be considered depending on the severity of liver disease.10 In this case, we chose the standard 6-month regimen with close clinical and analytical surveillance of the patient, with no adverse effects noted.
The case presented shows how the diagnosis of TBP may be challenging. The awareness of the disease and the knowledge of the limitations of conventional diagnostic methods may avoid the delay in treatment which is associated with increased mortality in patients with this condition.4
Learning points.
Tuberculosis (TB) should always be considered in the differential diagnosis of ascites, especially in patients with chronic liver disease.
The sensitivity of direct microscopy and mycobacterial culture of the ascitic fluid is low.
Laparoscopy/laparotomy with biopsies are the most reliable methods of diagnosing peritoneal TB.
In patients with suggestive laparoscopic appearances and histology, microbiology results should not delay the anti-TB therapy, as this could have a negative impact on outcome and prognosis.
Footnotes
Contributors: AMV, BP and HG were involved in the patient's care. AMV and BP wrote the manuscript. RO and HG reviewed the manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Direcção-Geral da Saúde [Internet]. Programa Nacional para a infecção VIH/SIDA e Tuberculose. Direcção-Geral da Saúde. https://www.dgs.pt/em-destaque/dia-mundial-da-tuberculose-24-de-marco7 (accessed 20 Feb 2017).
- 2.Thulstrup AM, Mølle I, Svendsen N, et al. Incidence and prognosis of tuberculosis in patients with cirrhosis of the liver. A Danish nationwide population based study. Epidemiol Infect 2000;124:221–5. 10.1017/S0950268899003593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanai FM, Bzeizi KI. Systematic review: tuberculous peritonitis—presenting features, diagnostic strategies and treatment. Aliment Pharmacol Ther 2005;22:685–700. 10.1111/j.1365-2036.2005.02645.x [DOI] [PubMed] [Google Scholar]
- 4.Chow KM, Chow VC, Hung LC, et al. Tuberculous peritonitis-associated mortality is high among patients waiting for the results of mycobacterial cultures of ascitic fluid samples. Clin Infect Dis 2002;35:409–13. 10.1086/341898 [DOI] [PubMed] [Google Scholar]
- 5.Burgess LJ, Swanepoel CG, Taljaard JJ. The use of adenosine deaminase as a diagnostic tool for peritoneal tuberculosis. Tuberculosis 2001;81:243–8. 10.1054/tube.2001.0289 [DOI] [PubMed] [Google Scholar]
- 6.Liao YJ, Wu CY, Lee SW, et al. Adenosine deaminase activity in tuberculous peritonitis among patients with underlying liver cirrhosis. World J Gastroenterol 2012;18:5260–5. 10.3748/wjg.v18.i37.5260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Runyon B. Ascites and spontaneous bacterial peritonitis : Feldman M, Friedman LS, Brandt LJ, Sleisenger and Fordtran's gastrointestinal and liver disease: pathophysiology, diagnosis, management. 10th edition Philadelphia: USA. Elsevier, 2015:1553–75. [Google Scholar]
- 8.da Rocha EL, Pedrassa BC, Bormann RL, et al. Abdominal tuberculosis: a radiological review with emphasis on computed tomography and magnetic resonance imaging findings. Radiol Bras 2015;48:181–91. 10.1590/0100-3984.2013.1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavalli Z, Ader F, Valour F, et al. Clinical presentation, diagnosis, and bacterial epidemiology of peritoneal tuberculosis in two university hospitals in France. Infect Dis Ther 2016;5:193–9. 10.1007/s40121-016-0113-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Treatment of tuberculosis: guidelines. 4th edition Geneva: World Health Organization, 2010. [PubMed] [Google Scholar]


