Abstract
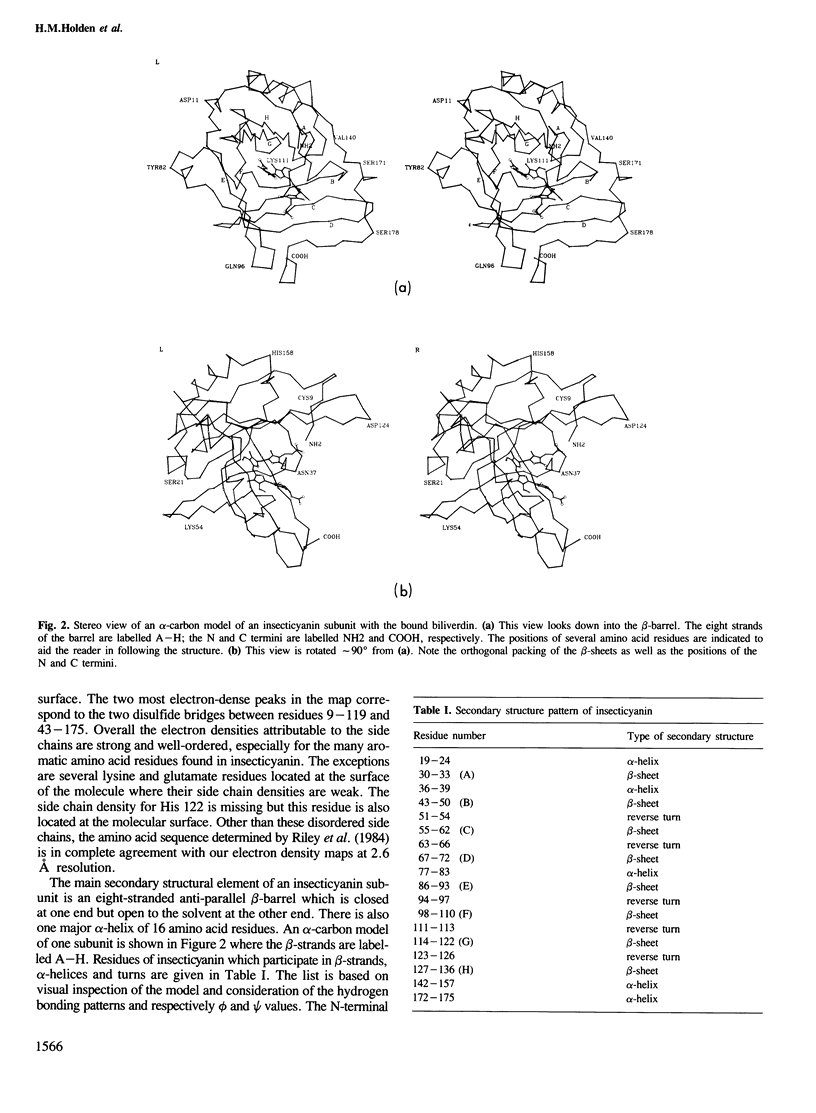
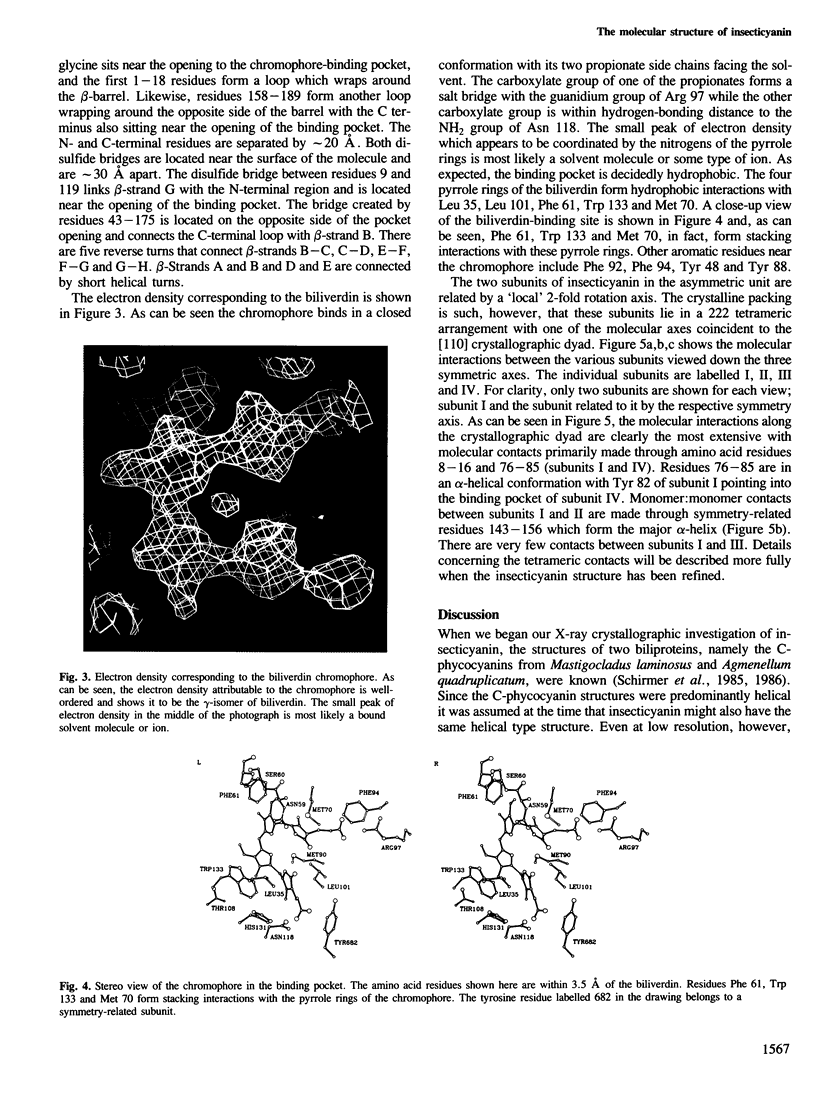
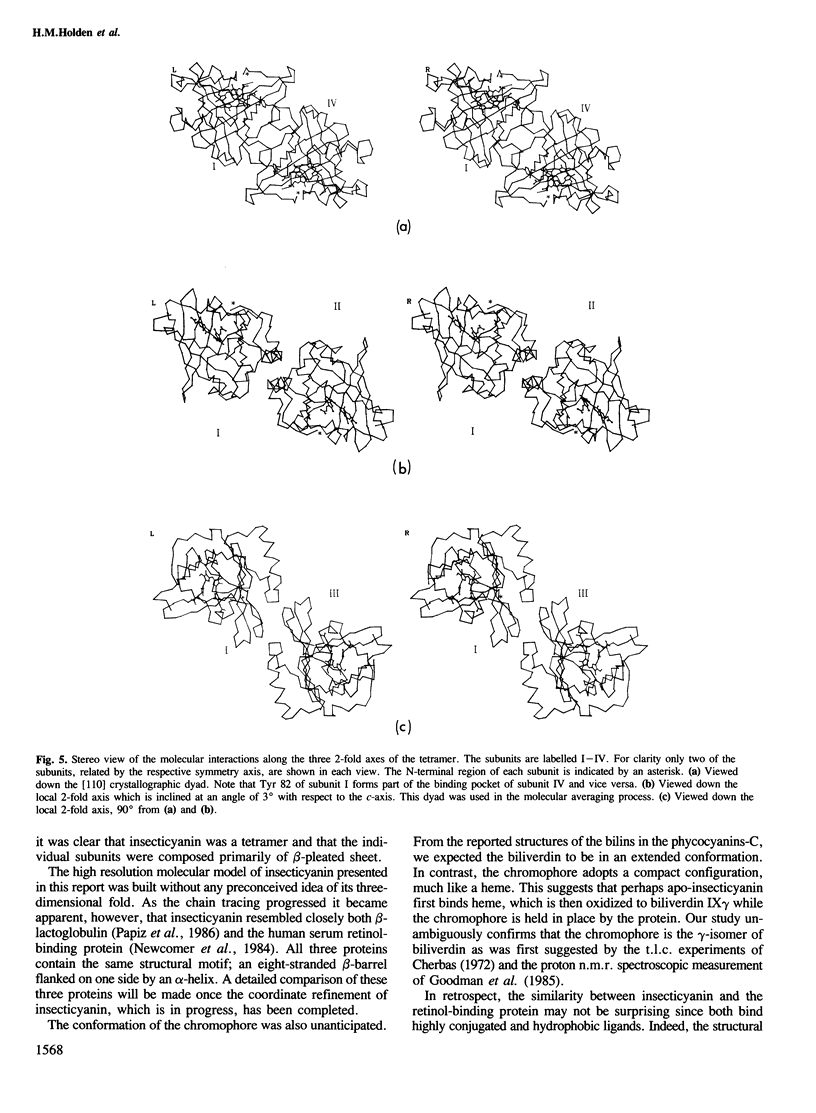
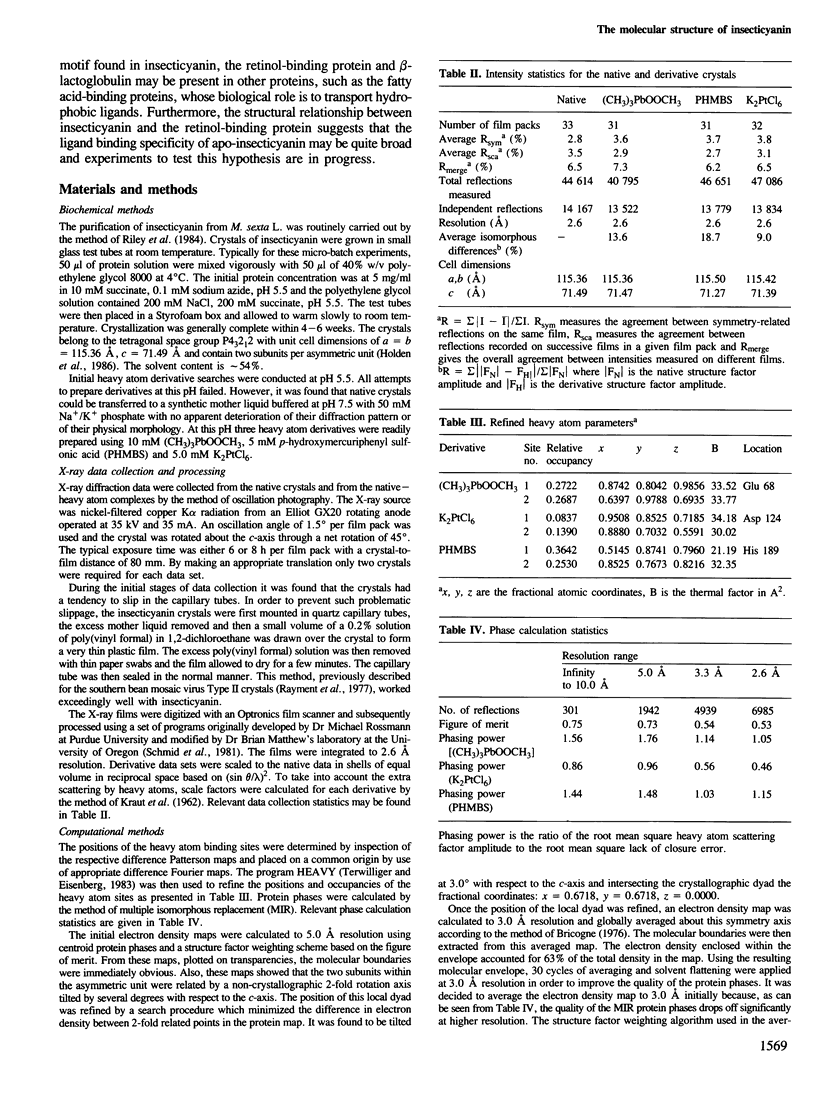
Insecticyanin, a blue biliprotein isolated from the tobacco hornworm Manduca sexta L., is involved in insect camouflage. Its three-dimensional structure has now been solved to 2.6 A resolution using the techniques of multiple isomorphous replacement, non-crystallographic symmetry averaging about a local 2-fold rotation axis and solvent flattening. All 189 amino acids have been fitted to the electron density map. The map clearly shows that insecticyanin is a tetramer with one of its molecular 2-fold axes coincident to a crystallographic dyad. The individual subunits have overall dimensions of 44 A X 37 A X 40 A and consist primarily of an eight-stranded anti-parallel beta-barrel flanked on one side by a 4.5-turn alpha-helix. Interestingly the overall three-dimensional fold of the insecticyanin subunit shows remarkable similarity to the structural motifs of bovine beta-lactoglobulin and the human serum retinol-binding protein. The electron density attributable to the chromophore is unambiguous and shows that it is indeed the gamma-isomer of biliverdin. The biliverdin lies towards the open end of the beta-barrel with its two propionate side chains pointing towards the solvent and it adopts a rather folded conformation, much like a heme.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Goodman W. G., Adams B., Trost J. T. Purification and characterization of a biliverdin-associated protein from the hemolymph of Manduca sexta. Biochemistry. 1985 Feb 26;24(5):1168–1175. doi: 10.1021/bi00326a017. [DOI] [PubMed] [Google Scholar]
- Holden H. M., Law J. H., Rayment I. Crystallization of insecticyanin from the hemolymph of the tobacco hornworm Manduca sexta L. in a form suitable for a high resolution structure determination. J Biol Chem. 1986 Mar 25;261(9):4217–4218. [PubMed] [Google Scholar]
- Jones T. A. Diffraction methods for biological macromolecules. Interactive computer graphics: FRODO. Methods Enzymol. 1985;115:157–171. doi: 10.1016/0076-6879(85)15014-7. [DOI] [PubMed] [Google Scholar]
- KRAUT J., SIEKER L. C., HIGH D. F., FREER S. T. Chymotrypsinogen: a three-dimensional fourier synthesis at 5 angstrom resolution. Proc Natl Acad Sci U S A. 1962 Aug;48:1417–1424. doi: 10.1073/pnas.48.8.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomer M. E., Jones T. A., Aqvist J., Sundelin J., Eriksson U., Rask L., Peterson P. A. The three-dimensional structure of retinol-binding protein. EMBO J. 1984 Jul;3(7):1451–1454. doi: 10.1002/j.1460-2075.1984.tb01995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papiz M. Z., Sawyer L., Eliopoulos E. E., North A. C., Findlay J. B., Sivaprasadarao R., Jones T. A., Newcomer M. E., Kraulis P. J. The structure of beta-lactoglobulin and its similarity to plasma retinol-binding protein. 1986 Nov 27-Dec 3Nature. 324(6095):383–385. doi: 10.1038/324383a0. [DOI] [PubMed] [Google Scholar]
- Riddiford L. M. Changes in translatable mRNAs during the larval-pupal transformation of the epidermis of the tobacco hornworm. Dev Biol. 1982 Aug;92(2):330–342. doi: 10.1016/0012-1606(82)90179-8. [DOI] [PubMed] [Google Scholar]
- Riley C. T., Barbeau B. K., Keim P. S., Kézdy F. J., Heinrikson R. L., Law J. H. The covalent protein structure of insecticyanin, a blue biliprotein from the hemolymph of the tobacco hornworm, Manduca sexta L. J Biol Chem. 1984 Nov 10;259(21):13159–13165. [PubMed] [Google Scholar]
- Schirmer T., Bode W., Huber R., Sidler W., Zuber H. X-ray crystallographic structure of the light-harvesting biliprotein C-phycocyanin from the thermophilic cyanobacterium Mastigocladus laminosus and its resemblance to globin structures. J Mol Biol. 1985 Jul 20;184(2):257–277. doi: 10.1016/0022-2836(85)90379-1. [DOI] [PubMed] [Google Scholar]
- Schirmer T., Huber R., Schneider M., Bode W., Miller M., Hackert M. L. Crystal structure analysis and refinement at 2.5 A of hexameric C-phycocyanin from the cyanobacterium Agmenellum quadruplicatum. The molecular model and its implications for light-harvesting. J Mol Biol. 1986 Apr 20;188(4):651–676. doi: 10.1016/s0022-2836(86)80013-4. [DOI] [PubMed] [Google Scholar]





