Abstract
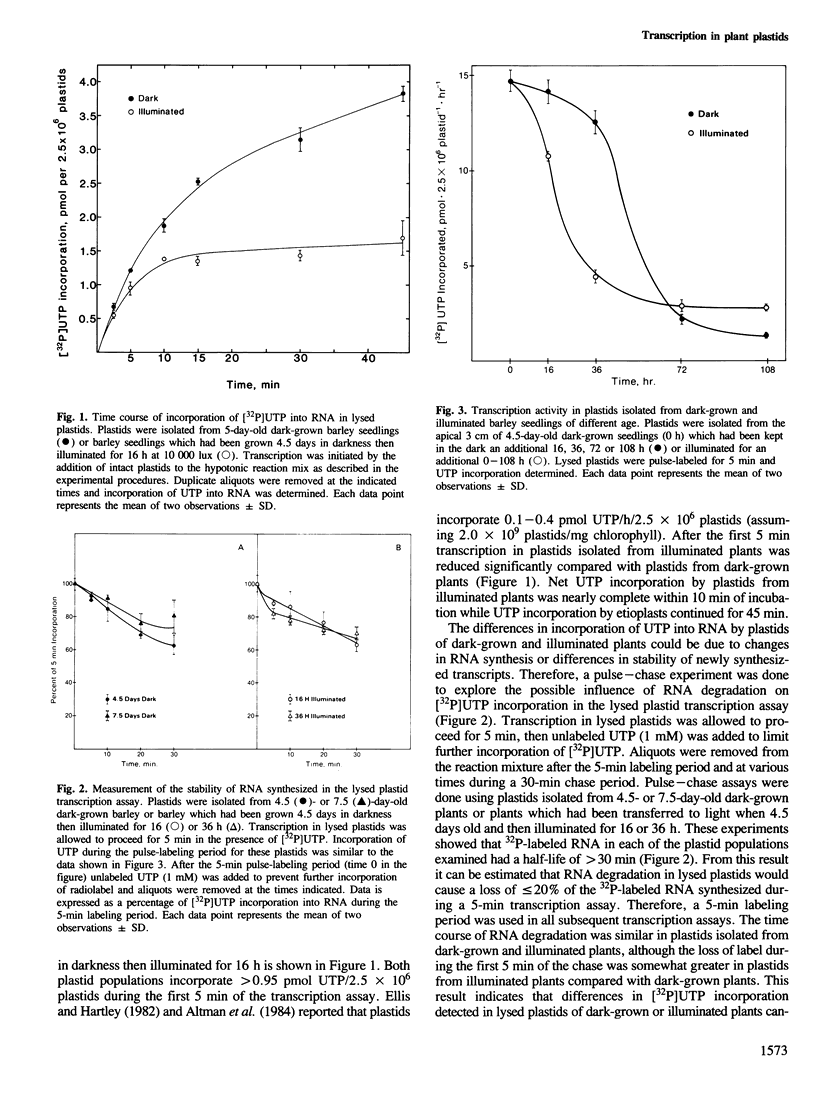
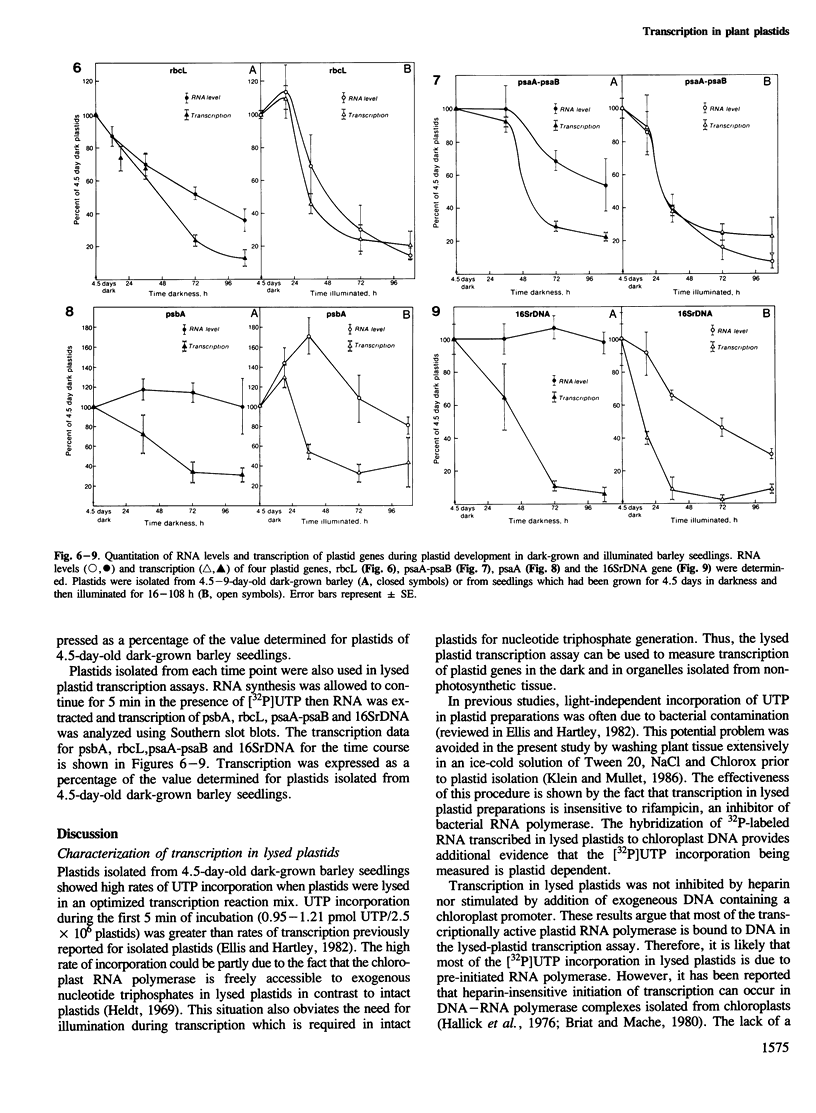
Transcription in lysed barley plastids and Northern slot blot analyses were used to determine the relationship between changes in RNA levels and transcription during plastid development. Transcription in plastids of 4.5–9-day-old dark-grown or illuminated barley seedlings declined up to 10-fold as a function of plant age. Decreased transcription of some plastid genes (rbcL, psaA-psaB) was paralleled by decreased levels of mRNA. In other cases (16SrDNA, psbA) the changes in transcription were not followed by proportional changes in RNA levels indicating that RNA stability is important in establishing the amount of plastid RNA for these genes. Further analysis showed that transcription of the plastid rRNA transcription unit is regulated differently than the transcription of protein coding genes such as psbA or rbcL.
Keywords: barley, chloroplast, development, RNA, transcription
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman A., Cohen B. N., Weissbach H., Brot N. Transcriptional activity of isolated maize chloroplasts. Arch Biochem Biophys. 1984 Nov 15;235(1):26–33. doi: 10.1016/0003-9861(84)90251-0. [DOI] [PubMed] [Google Scholar]
- Bedbrook J. R., Link G., Coen D. M., Bogorad L. Maize plastid gene expressed during photoregulated development. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3060–3064. doi: 10.1073/pnas.75.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J., Jenkins G. I., Hartley M. R. Differential regulation of the accumulation of the light-harvesting chlorophyll a/b complex and ribulose bisphosphate carboxylase/oxygenase in greening pea leaves. J Cell Biochem. 1984;25(1):1–13. doi: 10.1002/jcb.240250102. [DOI] [PubMed] [Google Scholar]
- Bradley D., Gatenby A. A. Mutational analysis of the maize chloroplast ATPase-beta subunit gene promoter: the isolation of promoter mutants in E. coli and their characterization in a chloroplast in vitro transcription system. EMBO J. 1985 Dec 30;4(13B):3641–3648. doi: 10.1002/j.1460-2075.1985.tb04129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briat J. F., Laulhere J. P., Mache R. Transcription activity of a DNA-protein complex isolated from spinach plastids. Eur J Biochem. 1979 Jul;98(1):285–292. doi: 10.1111/j.1432-1033.1979.tb13187.x. [DOI] [PubMed] [Google Scholar]
- Briat J. F., Mache R. Properties and characterization of a spinach chloroplast RNA polymerase isolated from a tanscriptionally active DNA-protein complex. Eur J Biochem. 1980 Oct;111(2):503–509. doi: 10.1111/j.1432-1033.1980.tb04966.x. [DOI] [PubMed] [Google Scholar]
- Dean C., Leech R. M. Genome Expression during Normal Leaf Development : I. CELLULAR AND CHLOROPLAST NUMBERS AND DNA, RNA, AND PROTEIN LEVELS IN TISSUES OF DIFFERENT AGES WITHIN A SEVEN-DAY-OLD WHEAT LEAF. Plant Physiol. 1982 Apr;69(4):904–910. doi: 10.1104/pp.69.4.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish L. E., Jagendorf A. T. Light-induced increase in the number and activity of ribosomes bound to pea chloroplast thylakoids in vivo. Plant Physiol. 1982 Apr;69(4):814–824. doi: 10.1104/pp.69.4.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish L. E., Kück U., Bogorad L. Two partially homologous adjacent light-inducible maize chloroplast genes encoding polypeptides of the P700 chlorophyll a-protein complex of photosystem I. J Biol Chem. 1985 Feb 10;260(3):1413–1421. [PubMed] [Google Scholar]
- Gallagher T. F., Ellis R. J. Light-stimulated transcription of genes for two chloroplast polypeptides in isolated pea leaf nuclei. EMBO J. 1982;1(12):1493–1498. doi: 10.1002/j.1460-2075.1982.tb01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant J. A. Stringent control in E. coli. Annu Rev Genet. 1979;13:393–415. doi: 10.1146/annurev.ge.13.120179.002141. [DOI] [PubMed] [Google Scholar]
- Gausing K. Regulation of ribosome production in Escherichia coli: synthesis and stability of ribosomal RNA and of ribosomal protein messenger RNA at different growth rates. J Mol Biol. 1977 Sep 25;115(3):335–354. doi: 10.1016/0022-2836(77)90158-9. [DOI] [PubMed] [Google Scholar]
- Greenberg B. M., Narita J. O., DeLuca-Flaherty C., Gruissem W., Rushlow K. A., Hallick R. B. Evidence for two RNA polymerase activities in Euglena gracilis chloroplasts. J Biol Chem. 1984 Dec 10;259(23):14880–14887. [PubMed] [Google Scholar]
- Gruissem W., Greenberg B. M., Zurawski G., Prescott D. M., Hallick R. B. Biosynthesis of chloroplast transfer RNA in a spinach chloroplast transcription system. Cell. 1983 Dec;35(3 Pt 2):815–828. doi: 10.1016/0092-8674(83)90114-9. [DOI] [PubMed] [Google Scholar]
- Gruissem W., Zurawski G. Analysis of promoter regions for the spinach chloroplast rbcL, atpB and psbA genes. EMBO J. 1985 Dec 16;4(13A):3375–3383. doi: 10.1002/j.1460-2075.1985.tb04093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen G., Guilfoyle T. J. Rapid induction of selective transcription by auxins. Mol Cell Biol. 1985 Jun;5(6):1197–1203. doi: 10.1128/mcb.5.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallick R. B., Lipper C., Richards O. C., Rutter W. J. Isolation of a transcriptionally active chromosome from chloroplasts of Euglena gracilis. Biochemistry. 1976 Jul 13;15(14):3039–3045. doi: 10.1021/bi00659a016. [DOI] [PubMed] [Google Scholar]
- Hartley M. R., Ellis R. J. Ribonucleic acid synthesis in chloroplasts. Biochem J. 1973 May;134(1):249–262. doi: 10.1042/bj1340249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt H. W. Adenine nucleotide translocation in spinach chloroplasts. FEBS Lett. 1969 Sep;5(1):11–14. doi: 10.1016/0014-5793(69)80280-2. [DOI] [PubMed] [Google Scholar]
- Jolly S. O., Bogorad L. Preferential transcription of cloned maize chloroplast DNA sequences by maize chloroplast RNA polymerase. Proc Natl Acad Sci U S A. 1980 Feb;77(2):822–826. doi: 10.1073/pnas.77.2.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly S. O., McIntosh L., Link G., Bogorad L. Differential transcription in vivo and in vitro of two adjacent maize chloroplast genes: The large subunit of ribulosebisphosphate carboxylase and the 2.2-kilobase gene. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6821–6825. doi: 10.1073/pnas.78.11.6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. R., Mullet J. E. Control of gene expression during higher plant chloroplast biogenesis. Protein synthesis and transcript levels of psbA, psaA-psaB, and rbcL in dark-grown and illuminated barley seedlings. J Biol Chem. 1987 Mar 25;262(9):4341–4348. [PubMed] [Google Scholar]
- Klein R. R., Mullet J. E. Regulation of chloroplast-encoded chlorophyll-binding protein translation during higher plant chloroplast biogenesis. J Biol Chem. 1986 Aug 25;261(24):11138–11145. [PubMed] [Google Scholar]
- Kleinkopf G. E., Huffaker R. C., Matheson A. Light-induced de Novo Synthesis of Ribulose 1,5-Diphosphate Carboxylase in Greening Leaves of Barley. Plant Physiol. 1970 Sep;46(3):416–418. doi: 10.1104/pp.46.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuz K., Dehesh K., Apel K. The light-dependent accumulation of the P700 chlorophyll a protein of the photosystem I reaction center in barley. Evidence for translational control. Eur J Biochem. 1986 Sep 15;159(3):459–467. doi: 10.1111/j.1432-1033.1986.tb09908.x. [DOI] [PubMed] [Google Scholar]
- Link D. P., Lantz B. M. Vasodilator response in the lower extremity induced by contrast medium. I Canine model. Acta Radiol Diagn (Stockh) 1982;23(2):81–86. doi: 10.1177/028418518202300201. [DOI] [PubMed] [Google Scholar]
- Link G., Bogorad L. Sizes, locations, and directions of transcription of two genes on a cloned maize chloroplast DNA sequence. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1832–1836. doi: 10.1073/pnas.77.4.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link G., Coen D. M., Bogorad L. Differential expression of the gene for the large subunit of ribulose bisphosphate carboxylase in maize leaf cell types. Cell. 1978 Nov;15(3):725–731. doi: 10.1016/0092-8674(78)90258-1. [DOI] [PubMed] [Google Scholar]
- Link G. DNA sequence requirements for the accurate transcription of a protein-coding plastid gene in a plastid in vitro system from mustard (Sinapis alba L.). EMBO J. 1984 Aug;3(8):1697–1704. doi: 10.1002/j.1460-2075.1984.tb02034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita J. O., Rushlow K. E., Hallick R. B. Characterization of a Euglena gracilis chloroplast RNA polymerase specific for ribosomal RNA genes. J Biol Chem. 1985 Sep 15;260(20):11194–11199. [PubMed] [Google Scholar]
- Orozco E. M., Jr, Mullet J. E., Chua N. H. An in vitro system for accurate transcription initiation of chloroplast protein genes. Nucleic Acids Res. 1985 Feb 25;13(4):1283–1302. doi: 10.1093/nar/13.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss T., Link G. Characterization of transcriptionally active DNA-protein complexes from chloroplasts and etioplasts of mustard (Sinapis alba L.). Eur J Biochem. 1985 Apr 15;148(2):207–212. doi: 10.1111/j.1432-1033.1985.tb08826.x. [DOI] [PubMed] [Google Scholar]
- Robertson D., Laetsch W. M. Structure and function of developing barley plastids. Plant Physiol. 1974 Aug;54(2):148–159. doi: 10.1104/pp.54.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodermel S. R., Bogorad L. Maize plastid photogenes: mapping and photoregulation of transcript levels during light-induced development. J Cell Biol. 1985 Feb;100(2):463–476. doi: 10.1083/jcb.100.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushlow K. E., Orozco E. M., Jr, Lipper C., Hallick R. B. Selective in vitro transcription of Euglena chloroplast ribosomal RNA genes by a transcriptionally active chromosome. J Biol Chem. 1980 Apr 25;255(8):3786–3792. [PubMed] [Google Scholar]
- Shinozaki K., Ohme M., Tanaka M., Wakasugi T., Hayashida N., Matsubayashi T., Zaita N., Chunwongse J., Obokata J., Yamaguchi-Shinozaki K. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986 Sep;5(9):2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer D., Whitfeld P. R. Ribonucleic acid synthesizing activity of spinach chloroplasts and nuclei. Arch Biochem Biophys. 1967 Aug;121(2):336–345. doi: 10.1016/0003-9861(67)90085-9. [DOI] [PubMed] [Google Scholar]
- Stirdivant S. M., Crossland L. D., Bogorad L. DNA supercoiling affects in vitro transcription of two maize chloroplast genes differently. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4886–4890. doi: 10.1073/pnas.82.15.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y. S., Kung S. D., Bogorad L. Phytochrome control of levels of mRNA complementary to plastid and nuclear genes of maize. Plant Physiol. 1985 Oct;79(2):371–376. doi: 10.1104/pp.79.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Clegg M. T., Brown A. H. The Nature of Nucleotide Sequence Divergence between Barley and Maize Chloroplast DNA. Genetics. 1984 Apr;106(4):735–749. doi: 10.1093/genetics/106.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]