Abstract
The use of auditory reaction time is a reliable measure of loudness perception in both animals and humans with reaction times (RT) decreasing with increasing stimulus intensity. Since abnormal loudness perception is a common feature of hyperacusis, a potentially debilitating auditory disorder in which moderate-intensity sounds are perceived as uncomfortable or painfully loud, we used RT measures to assess rats for salicylate-induced hyperacusis. A previous study using an operant conditioning RT procedure found that high-dose sodium salicylate (SS) induced hyperacusis-like behavior, i.e., faster than normal RTs to moderate and high level sounds, when rats were tested with broadband noise stimuli. However, it was not clear from that study if salicylate induces hyperacusis-like behavior in a dose- or frequency-dependent manner. Therefore, the goals of the current study were to determine how RT-intensity functions were altered by different doses of salicylate, and, using tone bursts, to determine if salicylate induces hyperacusis-like behavior across the entire frequency spectrum or only at certain frequencies. Similar to previous physiological studies, we began to see faster than normal RTs for sounds 60 dB SPL and greater with salicylate doses of 150 mg/kg and higher; indicating the rats were experiencing hyperacusis at high salicylate doses. In addition, high-dose salicylate significantly reduced RTs across all stimulus frequencies tested which suggests that a central neural excitability mechanism may be a potential driver of salicylate-induced changes in loudness perception and hyperacusis.
Keywords: Hyperacusis, Sodium salicylate, Reaction time, Operant conditioning, Loudness perception
1. Introduction
Most people find that sounds around 100 dB HL are uncomfortably loud (Sherlock and Formby, 2005) while sound intensities above 140 dB SPL evoke the sensation of pain and discomfort (Ades et al., 1959). However, about 6% of adults suffer from hyperacusis, a debilitating condition in which sounds of moderate intensity are perceived as intolerably loud or even painful, leading to negative emotions, a dependence on hearing protective devices, and avoidance of social situations (Anari et al., 1999; Katzenell and Segal, 2001; Andersson et al., 2002; Tyler et al., 2014; Chen et al., 2015). Among patients with a primary complaint of hyperacusis, more than 80% also suffer from tinnitus (Anari et al., 1999; Andersson et al., 2002; Dauman and Bouscau-Faure, 2005; Tyler et al., 2014) whereas among those with a primary complaint of tinnitus, about 40% also experience hyperacusis (Baguley, 2003). However, the prevalence of hyperacusis is likely much higher since most tinnitus patients are unaware of their loudness intolerance condition unless explicitly tested (Gu et al., 2010).
Given the frequent co-occurrence of tinnitus and hyperacusis, it is conceivable that these auditory disorders arise from a common mechanism within the auditory pathway (Gu et al., 2010; Hébert et al., 2013; Chen et al., 2015). Experimentally, sodium salicylate (SS), the active ingredient in aspirin, has long been known to induce temporary hearing loss and acute tinnitus in both humans and animals and it has served as an extremely useful model to investigate the neural and biological mechanisms underlying tinnitus and hearing loss (Myers and Bernstein, 1965; Mongan et al., 1973; McFadden et al., 1984; Brennan et al., 1996; Cazals, 2000; Stolzberg et al., 2012b; Sheppard et al., 2014; Chen et al., 2015). While high doses of salicylate are known to induce tinnitus in animal models, more recent studies indicate that salicylate also enhances the amplitude of the acoustic startle reflex, results interpreted as evidence of hyperacusis but may also be related to stress (Ison et al., 2007; Sun et al., 2009; Chen et al., 2014). There are, however, a number of limitations to using the acoustic startle reflex to assess loudness growth and hyperacusis. The reflex can only be elicited at relatively high sound levels (>80 dB SPL) and has a limited dynamic range since the startle response saturates at approximately 115 dB SPL (Lobarinas et al., 2013), however lower sound levels may be used to assess hyperacusis with prepulse inhibition (Turner and Parrish, 2008; Hickox and Liberman, 2014).
Previous studies in both humans and animals have established that reaction time (RT), the time between the onset of a stimulus and the response by a listener, is highly correlated with loudness perception across a wide range of stimulus levels and frequencies (Arieh and Marks, 2003; Wagner et al., 2004) for both normal-hearing (Marshall and Brandt, 1980; Leibold and Werner, 2002) and hearing-impaired listeners (Pfingst et al., 1975). In both humans and animals, there is an inverse relationship between RT and stimulus intensity, i.e., RT decreases with increasing stimulus intensity (Stebbins and Miller, 1964; Stebbins, 1966; Moody, 1973; Marshall and Brandt, 1980; Leibold and Werner, 2002; Lauer and Dooling, 2007; May et al., 2009). In a standard reaction time experiment, a listener responds to a sound, typically a pure tone, and the time it takes the listener to make a given response is recorded by the researcher. For adult humans, the response is typically a hand raise or button press (Marshall and Brandt, 1980; Arieh and Marks, 2003; Wagner et al., 2004). Researchers have also used a head turn as the response to measure auditory reaction time in infants (Leibold and Werner, 2002). Similar to humans, auditory reaction time is assessed in animals by measuring the time it takes an animal to make a response, i.e., a lever press (Stebbins and Miller, 1964; May et al., 2009), key peck (Lauer and Dooling, 2007), or nose poke (Chen et al., 2014), in response to a sound stimulus. The biggest procedural difference between human and animal reaction time studies is that humans can be instructed how to perform the task whereas animals have to be trained to perform the task.
In animals with noise-induced hearing loss, RT-intensity functions showed evidence of loudness recruitment in regions of hearing loss, i.e., at low stimulus intensities, RTs were slower than normal, but as intensity increased RT rapidly decreased and became equal to pre-exposure RT values (Moody, 1973; May et al., 2009). While RT studies have found evidence of loudness recruitment in hearing-impaired mammals, one study reported that canaries with high-frequency hereditary hearing loss had faster than normal RTs at high sound intensities. These results suggest that certain types of hearing loss might induce hyperacusis (Lauer and Dooling, 2007).
Since salicylate causes cochlear hearing loss, induces tinnitus, and enhances sound-evoked neural activity in the central auditory pathway, we measured RT-intensity functions to noise bursts before and after treating rats with a high dose of salicylate (Chen et al., 2014). In these preliminary studies, RTs were slower than normal at low intensities due to the salicylate-induced hearing loss, but as intensity increased there was a striking reduction of RT at moderate-to-high intensities (Chen et al., 2014, 2015). These results suggested that the rats were not only experiencing tinnitus, but also hyperacusis at sound levels above 50 dB SPL. Since salicylate is known to induce tinnitus at high doses (≥150 mg/kg) (Lobarinas et al., 2004), the goal of the current study was to extend our earlier findings to: (1) Using noise bursts, determine how RT-intensity functions were altered by different doses of salicylate (i.e., which doses of salicylate induced hyperacusis and over what intensity range does hyperacusis occur?), and (2) Using tone bursts, determine if salicylate induces hyperacusis-like behavior across the entire frequency spectrum or only at certain frequencies (i.e., hyperacusis spectral profile).
2. Materials and methods
2.1. Subjects
Seven Sprague-Dawley rats (2 male, 5 female) were obtained from Charles River Laboratories and were used in the operant conditioning task. All rats were individually housed and kept on a 12 h day/night cycle, lights on at 6 a.m. and off at 6 p.m. The rats started training at approximately 3–4 months of age. The rats were food restricted during the course of the experiment but were kept at or above 85% of their free-feeding weight. Rats had unrestricted access to water, except during testing. The test sessions ran for approximately 1 h a day, 6–7 days a week. All the procedures were approved by the University at Buffalo, SUNY's Institutional Animal Care and Use Committee.
2.2. Apparatus
Rats were tested in an acoustically transparent acrylic cage (28 × 30 × 38 cm) located inside a sound attenuating chamber (76 × 71 × 71 cm) lined with 5 cm thick sound attenuating foam (Illbruck, Inc., Minneapolis, MN, USA). The behavior of the animals during test sessions was monitored by a digital camera (Fire-i Digital Camera, Unibrain, San Ramon, CA, USA). The test cage was equipped with a speaker (FT28D Dome Tweeter, Fostex, Tokyo, Japan), feeder (Med Associates Model ENV-203M, St. Albans, VT, USA), and nose-poke hole equipped with infrared sensors (Vulintus, Dallas, TX, USA).
The experiment was conducted using custom behavioral software running on a personal computer (Microsoft Windows XP) described in a previous experiment (Stolzberg et al., 2013). The custom software controlled Tucker-Davis Technologies (TDT, Gainesville, FL) system-3 equipment. Sound stimuli were generated with TDT hardware and software (TDT RX6 processor, D/A converter, ~100 kHz sampling rate); digital inputs to and outputs from the testing cages were controlled by the TDT RX6 processor interfaced to a pellet feeder (Med Associates Model SG-501, St. Albans, VT, USA) and infrared sensors (Vulintus, Dallas, TX, USA). TDT RPvds software and custom MATLAB software (MathWorks, Nattick, MA, USA) were used to control all aspects of the experiment (Radziwon et al., 2015). Sound pressure levels were calibrated using a sound level meter (Larson Davis System 824; Fast setting; Flat weighting applied) equipped with a microphone (1/2″ free field microphone, model 2520, Larson-Davis, Depew, NY, USA) placed at the position where the animal's head would be when its nose is inside the nose poke hole.
2.3. Stimuli
The stimuli used in the reaction time experiments were broadband (BBN) noise bursts (1–42 kHz bandwidth, 300 ms duration, 5 ms cosine rise/fall times) and tone bursts (4, 8, 16, and 20 kHz, 300 ms duration, 5 ms cosine rise/fall times). The stimuli were presented at 30, 40, 50, 60, 70, 80, and 90 dB SPL. The rats were trained to detect the noise bursts and tone bursts in a quiet background using a go/no-go operant conditioning procedure.
2.4. Procedure
The first phase of the training process consisted of shaping the rat to poke its nose into the nose-poke hole and then going to the feeder trough for a food reward. Once the rats were reliably nose-poking, the auditory stimuli were introduced. As the rats continued their training, the waiting interval was systematically increased from 1 to 4 s, and catch trials were added.
To begin a trial, a rat was trained to hold its nose in the nose-poke hole, initiating a 1–4 s variable waiting interval. If the rat removed its nose from the nose-poke during the waiting interval, the trial would be aborted. However, if the rat held its nose in the nose-poke hole through the waiting interval, a single noise/tone burst was presented. The target stimuli were presented in a quasi-random order according to the psychophysical Method of Constant Stimuli (MOCS) (Dooling and Okanoya, 1995). Within each block of 10 trials, the stimuli would be presented randomly, one presentation per sound level, along with 3 catch trials. The trials in which a noiseburst or toneburst was presented were considered the “go” condition. If the rat removed its nose from the nose-poke within 2 s after the presentation of the stimulus, a food pellet (45 mg dustless rodent grain pellets, Bio-Serv, Frenchtown, NJ, USA) was delivered into the feeder trough and a “hit” was recorded. If the rat failed to remove its nose following the stimulus, a “miss” was recorded and no food pellet was given.
30% of the trials were catch trials, in which no stimulus was presented. These trials constituted the “no-go” condition and were used to ensure that the rat was under stimulus control. If the rat continued to hold its nose in the nose-poke during a catch trial, a “correct rejection”was recorded. The rat would not get a pellet for a correct rejection, but the next trial would begin immediately. However, if the rat removed its nose during a catch trial, a “false alarm” was recorded and the rat received a timeout (4–8 s), in which the house light would turn off and the rat could not initiate another trial. These catch trials were used to calculate false alarm rates and determine response biases.
2.5. Data analysis
RTs were taken from the onset of the noise/tone burst to the time the rat removed its nose from the nose-poke hole. Only RTs for hits (when the animal correctly detected the stimulus) were included in our analysis. In addition, data were excluded from our analyses if an animal's false alarm rate was greater than 30% during a given session.
For the dose-response experiment, differences between baseline and the salicylate RTs were analyzed using a 3-way repeated measures ANOVA with sound level (30–90 dB SPL in 10 dB steps), drug treatment (Baseline, Saline, or Salicylate), and dose (50, 100, 150, 200, 250, and 300 mg/kg) as factors. For the frequency response experiment using tone bursts, a 3-way repeated measures ANOVA with sound level (30–90 dB SPL in 10 dB steps), drug treatment (Baseline, Saline, and Salicylate), and tone frequency (4, 8, 16, 20 kHz) as factors was used to determine differences between baseline and salicylate RTs across frequency. Post-hoc analyses (Tukey pairwise comparisons) were carried out within each frequency to determine any significant differences among the three conditions (baseline, saline control, salicylate) at each sound level.
2.6. Salicylate and saline administration
After baseline data was collected, the rats were injected once per week with either a single injection of sodium salicylate, at varying doses (50, 100, 150, 200, 250, and 300 mg/kg for the broadband noise burst task; 200 and 250 mg/kg for the tone burst task), dissolved in saline (50 mg/mL), or an equivalent volume of saline (control condition). The injections were always administered 2 h prior to testing (Stolzberg et al., 2013; Chen et al., 2014, 2015). To control for any serial effects of the injections, the dosing schedule was randomized and a different random dosing schedule was administered for each rat. Therefore, the highest salicylate dose was not necessarily given last, and not all of the rats received salicylate on the same day—half of the rats received salicylate while the others received saline.
3. Results
3.1. Salicylate dose-response experiment
In a previous study (Chen et al., 2014), we found that 200 mg/kg of sodium salicylate altered the RT-intensity function to broadband noise bursts such that RTs were slower than normal at low intensities, but were much faster than normal at high intensities. However, it was unclear what effects lower or higher doses of salicylate would have on the RT-intensity function. To address this issue, we measured RT-intensity functions before and immediately after treating rats with salicylate doses ranging from 50 mg/kg to 300 mg/kg in 50 mg steps. For each condition, a saline injection of equivalent volume was also given to control for possible effects of the injection.
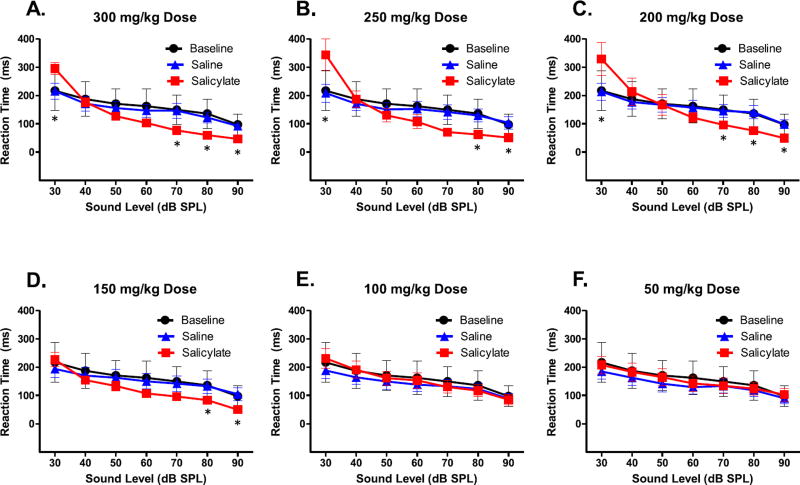
Fig. 1A–F shows the mean (N = 7, +/− SEM) RT-intensity functions for the six salicylate doses. For ease of comparison, all the panels were plotted with the same scale and an asterisk denoting statistically significant differences between saline and salicylate RTs (p < 0.05). Overall, we found a significant main effect of stimulus intensity (F(6,36) = 53.73, p = 0.001) showing that reaction times decreased with increasing stimulus intensity. In addition, we found a significant effect of Salicylate treatment (F(12,72) = 35.04, p = 0.001) for high-level doses (≥150 mg/kg) but no change in RTs following the 50 and 100 mg/kg salicylate treatment compared to baseline and Saline levels.
Fig. 1.
A–F. Mean reaction times of rats (N = 7) to broadband noise of varying intensity (30–90 dB), under different doses of either saline or salicylate (50–300 mg/kg) compared to baseline reaction times. Error bars represent SEM. *p < 0.05 Salicylate vs. saline.
Specifically, Fig. 1A shows the RT-intensity functions for the three conditions, pre-treatment baseline measures, the saline control injection, and the 300 mg/kg dose of salicylate. Importantly, the RTs for saline were similar to baseline measures indicating that the volume of fluid injected and the stress from the injection itself did not alter the RT-intensity function. The 300 mg/kg dose of SS, by contrast, had two effects. First, the RT at the lowest intensity, 30 dB SPL, was slower than normal (p = 0.002). This increase in RT is likely caused by salicylate-induced hearing loss (McFadden et al., 1984; Cazals, 2000; Radziwon et al., 2015). As intensity increased to 40 and 50 dB SPL, RTs were equal to baseline whereas for all intensities from 60 to 90 dB SPL, RTs were faster than normal particularly at the highest sound level (p = 0.003). This shortening of RT at high intensities cannot be due to a generalized salicylate-induced hyperactivity since it was not observed at the two lowest intensities. Rather than generalized hyperactivity, it appears that the change in RT following salicylate is directly linked to a change in loudness perception.
3.2. Salicylate frequency-response experiment
The purpose of this experiment was to identify the spectral profile of salicylate-induced hyperacusis. Based on previous electrophysiological data showing frequency-dependent changes in distortion product otoacoustic emissions (DPOAE) and the compound action potential (CAP) following salicylate treatment, and behavioral studies suggesting that the pitch of salicylate-induced tinnitus closely matches mid-frequency stimuli (~16 kHz) (Sheppard et al., 2014), it is possible that high-dose salicylate might show frequency-specific changes in the RT-intensity functions. On the other hand, if salicylate-induced hyperacusis relies on a more centralized, cortical mechanism, then we would expect uniform changes in RT across the range of stimulus frequencies tested (Stolzberg et al., 2012a; Sheppard et al., 2014).
Fig. 2A–D shows the mean (N = 7, +/− SEM) RT-intensity functions for the two salicylate doses across the four tone burst frequencies. For ease of comparison, all the panels were plotted with the same scale and an asterisk denoting statistically significant differences between saline and salicylate RTs (p < 0.05). The analysis showed a main effect of stimulus intensity, (F(6,36) = 59.34, p < 0.001). In other words, as the intensity of the tone increases, RTs decrease (Fig. 2A–D).
Fig. 2.
A–D. Mean reaction times of rats (N = 7) to four pure tones (4, 8, 16 & 20 kHz) of varying intensity (30–90 dB), under different doses of either saline or salicylate (200 or 250 mg/kg) compared to baseline reaction times. Error bars represent SEM. *p < 0.05 Salicylate 250 mg/kg vs. saline.
In addition, there were no significant main effects of drug treatment, (F(4,24) = 1.18, p = 0.34) or tone frequency, (F(3,18) = 1.24, p = 0.33); however, there was a significant interaction between drug treatment and tone intensity, (F(24,144) = 6.96, p < 0.001). The significant interaction between drug treatment and stimulus intensity indicates that RTs decrease faster at higher sound intensities (60–90 dB) during Salicylate treatment compared to baseline and Saline treatments (Fig. 2A–D).
The interaction between tone frequency and tone intensity was also significant, (F(18,108) = 5.57, p < 0.001), indicating that as stimulus intensity increases, RTs decrease faster at lower sound frequencies (4 and 8 kHz) compared to higher frequencies (16 and 20 kHz). However, there was no significant interaction between drug treatment and tone frequency, (F(12,72) = 0.68, p = 0.77), suggesting that salicylate-induced changes in RT occurred at all frequencies tested. The three-way interaction was also not significant, (F(72,432) = 1.09, p = 0.30). Taken together, these results suggest that a centralized gain control mechanism may be responsible for salicylate-induced hyperacusis (Formby et al., 2003; Stolzberg et al., 2012a).
4. Discussion
4.1. Salicylate dose-response experiment
To determine whether sodium salicylate would affect reaction times in a dose-dependent manner, we tested rats in our operant RT paradigm following 6 doses of salicylate ranging from 50 to 300 mg/kg. Consistent with previous behavioral studies measuring salicylate-induced tinnitus (Lobarinas et al., 2004; Stolzberg et al., 2013; Jones and May 2017), we began seeing evidence of hyperacusis, i.e. faster than normal RTs for sounds 60 dB SPL and greater, at salicylate doses of 150 mg/kg and greater but no change in RTs following low-level treatment. In addition to previous tinnitus studies, our RT loudness growth functions are similar to those seen in previous behavioral studies using the acoustic startle reflex to assess salicylate-induced hyperacusis where the amplitude of the startle reflex temporarily increased following high-dose salicylate treatment (Sun et al., 2009).
Our behavioral results are also consistent with physiological data showing sound-evoked hyperactivity in the auditory cortex and inferior colliculus beginning at approximately 60 dB SPL following salicylate administration (Sun et al., 2009; Stolzberg et al., 2012a). More importantly, our behavioral results also match physiological data showing enhanced sound-evoked activity in the auditory cortex following intense noise exposure (Salvi et al., 1990, 2000; Syka et al., 1994; Sun et al., 2012) and cochlear ablation (McAlpine et al., 1997; Mossop et al., 2000), despite reduced neural input from the cochlea (Auerbach et al., 2014). Our results suggest that salicylate-induced changes in RT are not only relevant to changes in loudness perception, but may also correlate with the physiological changes seen in the central auditory system following high-dose salicylate, cochlear ablation, and even noise exposure.
4.2. Salicylate frequency-response experiment
Human perceptual data suggest that hyperacusis patients with either little or moderate hearing loss (Noreña and Chery-Croze, 2007; Sheldrake et al., 2015), and normal-hearing listeners following a 2-week ear-plugging treatment (Formby et al., 2003), tend to experience reduced loudness discomfort levels across a broad range of stimulus frequencies suggesting that, together with previous animal physiological data, hyperacusis may be the result of an aberrant central gain mechanism (Salvi et al., 2000; Chen et al., 2013; Zeng, 2013; Auerbach et al., 2014; Hickox and Liberman, 2014). Since high-dose salicylate in the current study significantly reduced RTs at high sound levels across all stimulus frequencies tested and salicylate-induced tinnitus in rats appears to be atonal (Turner and Parrish, 2008; Jones and May 2017), the results from our frequency-response experiment, along with animal tinnitus experiments, provide additional evidence of a central neural excitability mechanism as an explanation of salicylate-induced changes in loudness perception. Given the reliability of the RT paradigm to measure salicylate-induced loudness hyperacusis, future studies are planned to determine if a similar pattern of results occurs following noise exposure.
5. Conclusions
We found that salicylate causes a reduction in behavioral reaction time in a dose-dependent manner and across a range of stimulus frequencies, suggesting that both the reaction time paradigm and the salicylate model are useful tools in the broader study of hyperacusis. Furthermore, our behavioral results appear highly correlated with the enhanced sound-evoked responses in the inferior colliculus and auditory cortex seen by previous researchers following salicylate treatment (Sun et al., 2009, 2014) and noise exposure (Salvi et al., 1990, 2000; Syka et al., 1994; Sun et al., 2012) pointing to a common central mechanism in the generation of hyperacusis. Although humans with hyperacusis rarely attribute their hyperacusis to aspirin, the use of the salicylate model of hyperacusis in animals provides the necessary groundwork for future studies of noise-induced hyperacusis and loudness recruitment.
Acknowledgments
This research was supported in part by grants from NIH (R01DC014452 and R01DC014693) and the Hearing Health Foundation (14-02607).
References
- Ades HW, Morrill SN, Graybiel A, Tolhurst GC. Threshold of aural pain to high-intensity sound. Aerosp. Med. 1959;30:678. [PubMed] [Google Scholar]
- Anari M, Axelsson A, Eliasson A, Magnusson L. Hypersensitivity to sound: questionnaire data, audiometry and classification. Scand. Audiol. 1999;28:219–230. doi: 10.1080/010503999424653. [DOI] [PubMed] [Google Scholar]
- Andersson G, Lindvall N, Hursti T, Carlbring P. Hypersensitivity to sound (hyperacusis): a prevalence study conducted via the internet and post. Int. J. Audiol. 2002;41:545–554. doi: 10.3109/14992020209056075. [DOI] [PubMed] [Google Scholar]
- Arieh Y, Marks LE. Recalibrating the auditory system: a speed-accuracy analysis of intensity perception. J. Exp. Psychol. Hum. Percept. Perform. 2003;29:523–536. doi: 10.1037/0096-1523.29.3.523. [DOI] [PubMed] [Google Scholar]
- Auerbach BD, Rodrigues PV, Salvi RJ. Central gain control in tinnitus and hyperacusis. Front. Neurol. 2014;5 doi: 10.3389/fneur.2014.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baguley DM. Hyperacusis. J. R. Soc. Med. 2003;96:582–585. doi: 10.1258/jrsm.96.12.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan JF, Brown CA, Jastreboff PJ. Salicylate-induced changes in auditory thresholds of adolescent and adult rats. Dev. Psychobiol. 1996;29:69–86. doi: 10.1002/(SICI)1098-2302(199601)29:1<69::AID-DEV4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Cazals Y. Auditory sensori-neural alterations induced by salicylate. Prog. Neurobiol. 2000;62:583–631. doi: 10.1016/s0301-0082(00)00027-7. [DOI] [PubMed] [Google Scholar]
- Chen G-D, Radziwon KE, Kashanian N, Manohar S, Salvi R. Salicylate-induced auditory perceptual disorders and plastic changes in non-classical auditory centers in rats. Neural Plast. 2014;2014 doi: 10.1155/2014/658741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GD, Stolzberg D, Lobarinas E, Sun W, Ding D, Salvi R. Salicylate-induced cochlear impairments, cortical hyperactivity and re-tuning, and tinnitus. Hear. Res. 2013;295:100–113. doi: 10.1016/j.heares.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-C, Li X, Liu L, Wang J, Lu C-Q, Yang M, Jiao Y, Zang F-C, Radziwon K, Chen G-D, Sun W, Muthaiah VPK, Salvi R, Teng G-J. Tinnitus and hyperacusis involve hyperactivity and enhanced connectivity in auditory-limbic-arousal-cerebellar network. eLife. 2015;4:e06576. doi: 10.7554/eLife.06576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauman R, Bouscau-Faure F. Assessment and amelioration of hyperacusis in tinnitus patients. Acta Oto-Laryngologica. 2005;125:503–509. doi: 10.1080/00016480510027565. [DOI] [PubMed] [Google Scholar]
- Dooling RJ, Okanoya K. The method of constant stimuli in testing auditory sensitivity in small birds. In: Klump GM, et al., editors. Methods in Comparative Psychoacoustics. Birkhäuser Basel; 1995. pp. 161–169. [Google Scholar]
- Formby C, Sherlock LP, Gold SL. Adaptive plasticity of loudness induced by chronic attenuation and enhancement of the acoustic background. J. Acoust. Soc. Am. 2003;114:55–58. doi: 10.1121/1.1582860. [DOI] [PubMed] [Google Scholar]
- Gu JW, Halpin CF, Nam E-C, Levine RA, Melcher JR. Tinnitus, diminished sound-level tolerance, and elevated auditory activity in humans with clinically normal hearing sensitivity. J. Neurophysiol. 2010;104:3361–3370. doi: 10.1152/jn.00226.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hébert S, Fournier P, Noreña A. The auditory sensitivity is increased in tinnitus ears. J. Neurosci. 2013;33:2356–2364. doi: 10.1523/JNEUROSCI.3461-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickox AE, Liberman MC. Is noise-induced cochlear neuropathy key to the generation of hyperacusis or tinnitus? J. Neurophysiol. 2014;111:552–564. doi: 10.1152/jn.00184.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ison JR, Allen PD, O'Neill WE. Age-related hearing loss in C57BL/6J mice has both frequency-specific and non-frequency-specific components that produce a hyperacusis-like exaggeration of the acoustic startle reflex. J. Assoc. Res. Otolaryngol. 2007;8:539–550. doi: 10.1007/s10162-007-0098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A, May BJ. Improving the reliability of tinnitus screening in laboratory animals. J. Assoc. Res. Otolaryngol. 2017;18:183–195. doi: 10.1007/s10162-016-0597-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenell U, Segal S. Hyperacusis: review and clinical guidelines. Otol. Neurotol. 2001;22:321–327. doi: 10.1097/00129492-200105000-00009. [DOI] [PubMed] [Google Scholar]
- Lauer AM, Dooling RJ. Evidence of hyperacusis in canaries with permanent hereditary high-frequency hearing loss. Seminars Hear. 2007;28:319–326. [Google Scholar]
- Leibold LJ, Werner LA. Relationship between intensity and reaction time in normal-hearing infants and adults. Ear Hear. 2002;23:92–97. doi: 10.1097/00003446-200204000-00002. [DOI] [PubMed] [Google Scholar]
- Lobarinas E, Hayes SH, Allman BL. The gap-startle paradigm for tinnitus screening in animal models: limitations and optimization. Hear. Res. 2013;295:150–160. doi: 10.1016/j.heares.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobarinas E, Sun W, Cushing R, Salvi R. A novel behavioral paradigm for assessing tinnitus using schedule-induced polydipsia avoidance conditioning (SIP-AC) Hear. Res. 2004;190:109–114. doi: 10.1016/S0378-5955(04)00019-X. [DOI] [PubMed] [Google Scholar]
- Marshall L, Brandt JF. The relationship between loudness and reaction time in normal hearing listeners. Acta Oto-Laryngologica. 1980;90:244–249. doi: 10.3109/00016488009131721. [DOI] [PubMed] [Google Scholar]
- May BJ, Little N, Saylor S. Loudness perception in the domestic cat: reaction time estimates of equal loudness contours and recruitment effects. J. Assoc. Res. Otolaryngol. 2009;10:295–308. doi: 10.1007/s10162-009-0157-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlpine D, Martin RL, Mossop JE, Moore DR. Response properties of neurons in the inferior colliculus of the monaurally deafened ferret to acoustic stimulation of the intact ear. J. Neurophysiol. 1997;78:767–779. doi: 10.1152/jn.1997.78.2.767. [DOI] [PubMed] [Google Scholar]
- McFadden D, Plattsmier HS, Pasanen EG. Aspirin-induced hearing loss as a model of sensorineural hearing loss. Hear. Res. 1984;16:251–260. doi: 10.1016/0378-5955(84)90114-x. [DOI] [PubMed] [Google Scholar]
- Mongan E, Kelly P, Nies K, Porter WW, Paulus HE. Tinnitus as an indication of therapeutic serum salicylate levels. J. Am. Med. Assoc. 1973;226:142–145. [PubMed] [Google Scholar]
- Moody DB. Behavioral studies of noise-induced hearing loss in primates: loudness recruitment. Adv. Oto-Rhino-Laryngology. 1973;20:82–101. doi: 10.1159/000393090. [DOI] [PubMed] [Google Scholar]
- Mossop JE, Wilson MJ, Caspary DM, Moore DR. Down-regulation of inhibition following unilateral deafening. Hear. Res. 2000;147:183–187. doi: 10.1016/s0378-5955(00)00054-x. [DOI] [PubMed] [Google Scholar]
- Myers EN, Bernstein JM. Salicylate ototoxicity: a clinical and experimental study. Arch. Otolaryngol. 1965;82:483–493. doi: 10.1001/archotol.1965.00760010485006. [DOI] [PubMed] [Google Scholar]
- Noreña AJ, Chery-Croze S. Enriched acoustic environment rescales auditory sensitivity. NeuroReport. 2007;18:1251–1255. doi: 10.1097/WNR.0b013e3282202c35. [DOI] [PubMed] [Google Scholar]
- Pfingst BE, Hienz R, Kimm J, Miller J. Reaction-time procedure for measurement of hearing. I. Suprathreshold functions. J. Acoust. Soc. Am. 1975;57:421–430. doi: 10.1121/1.380465. [DOI] [PubMed] [Google Scholar]
- Radziwon KE, Stolzberg DJ, Urban ME, Bowler RA, Salvi RJ. Salicylate-induced hearing loss and gap detection deficits in rats. Front. Neurol. 2015;6 doi: 10.3389/fneur.2015.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi RJ, Saunders SS, Gratton MA, Arehole S, Powers N. Enhanced evoked response amplitudes in the inferior colliculus of the chinchilla following acoustic trauma. Hear. Res. 1990;50:254–257. doi: 10.1016/0378-5955(90)90049-u. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Wang J, Ding D. Auditory plasticity and hyperactivity following cochlear damage. Hear. Res. 2000;147:261–274. doi: 10.1016/s0378-5955(00)00136-2. [DOI] [PubMed] [Google Scholar]
- Sheldrake J, Diehl PU, Schaette R. Audiometric characteristics of hyperacusis patients. Front. Neurol. 2015;6:105. doi: 10.3389/fneur.2015.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard A, Hayes SH, Chen G-D, Ralli M, Salvi R. Review of salicylate-induced hearing loss, neurotoxicity, tinnitus and neuropathophysiology. Acta Otorhinolaryngol. Ital. 2014;34:79–93. [PMC free article] [PubMed] [Google Scholar]
- Sherlock LP, Formby C. Estimates of loudness, loudness discomfort, and the auditory dynamic range: normative estimates, comparison of procedures, and test-retest reliability. J. Am. Acad. Audiol. 2005;16:85–100. doi: 10.3766/jaaa.16.2.4. [DOI] [PubMed] [Google Scholar]
- Stebbins WC. Auditory reaction time and the derivation of equal loudness contours for the monkey. J. Exp. Anal. Behav. 1966;9:135–142. doi: 10.1901/jeab.1966.9-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins WC, Miller JM. Reaction time as a function of stimulus intensity for the monkey. J. Exp. Anal. Behav. 1964;7:309–312. doi: 10.1901/jeab.1964.7-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzberg D, Chrostowski M, Salvi RJ, Allman BL. Intracortical circuits amplify sound-evoked activity in primary auditory cortex following systemic injection of salicylate in the rat. J. Neurophysiol. 2012a;108:200–214. doi: 10.1152/jn.00946.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzberg D, Hayes SH, Kashanian N, Radziwon K, Salvi RJ, Allman BL. A novel behavioral assay for the assessment of acute tinnitus in rats optimized for simultaneous recording of oscillatory neural activity. J. Neurosci. Methods. 2013;219:224–232. doi: 10.1016/j.jneumeth.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzberg D, Salvi RJ, Allman BL. Salicylate toxicity model of tinnitus. Front. Syst. Neurosci. 2012b;6:28. doi: 10.3389/fnsys.2012.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Deng A, Jayaram A, Gibson B. Noise exposure enhances auditory cortex responses related to hyperacusis behavior. Brain Res. 2012;1485:108–116. doi: 10.1016/j.brainres.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Sun W, Fu Q, Zhang C, Manohar S, Kumaraguru A, Li J. Loudness perception affected by early age hearing loss. Hear. Res. 2014;313:18–25. doi: 10.1016/j.heares.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Sun W, Lu J, Stolzberg D, Gray L, Deng A, Lobarinas E, Salvi RJ. Salicylate increases the gain of the central auditory system. Neuroscience. 2009;159:325–334. doi: 10.1016/j.neuroscience.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syka J, Rybalko N, Popelář J. Enhancement of the auditory cortex evoked responses in awake Guinea pigs after noise exposure. Hear. Res. 1994;78:158–168. doi: 10.1016/0378-5955(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Turner JG, Parrish J. Gap detection methods for assessing salicylate-induced tinnitus and hyperacusis in rats. Am. J. Audiol. 2008;17:S185–S192. doi: 10.1044/1059-0889(2008/08-0006). [DOI] [PubMed] [Google Scholar]
- Tyler RS, Pienkowski M, Roncancio ER, Jun HJ, Brozoski T, Dauman N, Coelho CB, Andersson G, Keiner AJ, Cacace A, Martin N, Moore BCJ. A review of hyperacusis and future directions: Part I. Definitions and manifestations. Am. J. Audiol. 2014;23:402–419. doi: 10.1044/2014_AJA-14-0010. [DOI] [PubMed] [Google Scholar]
- Wagner E, Florentine M, Buus S, McCormack J. Spectral loudness summation and simple reaction time. J. Acoust. Soc. Am. 2004;116:1681–1686. doi: 10.1121/1.1780573. [DOI] [PubMed] [Google Scholar]
- Zeng F-G. An active loudness model suggesting tinnitus as increased central noise and hyperacusis as increased nonlinear gain. Hear. Res. 2013;295:172–179. doi: 10.1016/j.heares.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]