Abstract
Background
The prognostic significance of ABO blood type for lymphoma is largely unknown. We evaluated the prognostic role of ABO blood type in patients with extranodal natural killer (NK)/T-cell lymphoma (ENKTL).
Methods
We retrospectively analyzed clinical data of 697 patients with newly diagnosed ENKTL from three cancer centers. The prognostic value of ABO blood type was evaluated using Kaplan–Meier curves and Cox proportional hazard models. The prognostic values of the International Prognostic Index (IPI) and the Korean Prognostic Index (KPI) were also evaluated.
Results
Compared with patients with blood type O, those with blood type non-O tended to display elevated baseline serum C-reactive protein levels (P = 0.038), lower rate of complete remission (P = 0.005), shorter progression-free survival (PFS, P < 0.001), and shorter overall survival (OS, P = 0.001). Patients with blood type O/AB had longer PFS (P < 0.001) and OS (P = 0.001) compared with those with blood type A/B. Multivariate analysis demonstrated that age >60 years (P < 0.001), mass ≥5 cm (P = 0.001), stage III/IV (P < 0.001), elevated serum lactate dehydrogenase (LDH) levels (P = 0.001), and blood type non-O were independent adverse predictors of OS (P = 0.001). ABO blood type was found to be superior to both the IPI in discriminating patients with different outcomes in the IPI low-risk group and the KPI in distinguishing between the intermediate-to-low- and high-to-intermediate-risk groups.
Conclusions
ABO blood type was an independent predictor of clinical outcome for patients with ENKTL.
Keywords: ABO blood type, Extranodal natural killer/T-cell lymphoma, Prognosis, The International Prognostic Index, The Korean Prognostic Index
Background
Extranodal natural killer (NK)/T-cell lymphoma (ENKTL), nasal type, is a distinct subtype of non-Hodgkin’s lymphoma (NHL) with unique clinicopathologic characteristics and geographic distribution [1]. ENKTL is relatively prevalent in Asia (e.g., China, Japan, and Korea) and Central/South America (e.g., Mexico and Peru) [2]. ENKTL accounts for 11% of all NHL cases in Chinese populations [3] and accounts for 5%–10% of all NHL cases in the populations of Asia and Central/South America [2, 4]. ENKTL is rarely diagnosed in European and North American populations (e.g., only accounts for 0.2% of NHL cases in the US populations) [5].
The treatment outcomes of ENKTL are generally poor in the CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) era with 5-year overall survival (OS) rates less than 50% [2, 6–9]. However, the application of l-asparaginase-containing chemotherapy significantly improves the outcomes of ENKTL patients with 5-year OS rate ranging from 50% to 86.9% [6, 10–13]. To date, optimal treatment strategies and prognosis for patients with ENKTL have not been fully defined. Although the prognostic value of the International Prognostic Index (IPI) has been well validated in many subtypes of NHL [14–16], its prognostic value remains controversial in ENKTL [4, 17]. Recently, the prognostic significance of the Korean Prognostic Index (KPI) in ENKTL has been confirmed by several studies, and this model may be further improved by including other laboratory parameters (e.g., C-reactive protein [CRP], albumin levels, and absolute lymphocyte count) [4, 11, 18, 19]. A better understanding of an ideal biomarker that is readily available, inexpensive, and reproducible for ENKTL could improve the prognosis and treatment strategies.
The ABO blood type system was a widely used blood test in clinical practice. Several studies have found that certain ABO blood types are associated with a high risk of certain cancers [20, 21]. Recently, the association between ABO blood type and the survival outcome of cancer patients has also drawn much attention, which has been demonstrated in various solid tumors, including pancreatic cancer [22], colon cancer [23], lung cancer [24], and esophageal cancer [25]. However, to the best of our knowledge, the prognostic value of ABO blood type in lymphoma has never been investigated. We therefore performed this triple-center study to evaluate the prognostic significance of ABO blood type in patients with ENKTL.
Patients and methods
Ethics statement
Written informed consent for the patients’ blood samples and other medical information to be stored in our hospitals’ databases and to be used in research were obtained from all patients. This study was approved by the Institutional Review Board of the National Cancer Institute and the ethics committees of Sun Yat-sen University Cancer Center, Hunan Cancer Hospital, and The Second Xiangya Hospital of Central South University. The study was performed in accordance with the Declaration of Helsinki and the institutional guidelines of the local ethics committee.
Patient selection
We screened consecutive patients with newly diagnosed ENKTL, nasal type, at Sun Yat-sen University Cancer Center, Hunan Cancer Hospital, and The Second Xiangya Hospital of Central South University between January 1998 and June 2015. All of the patients included in this study met the following criteria: (a) pathologically confirmed diagnosis of ENKTL, nasal type, by expert pathologists, according to the World Health Organization (WHO) classification [1], (b) no previous malignancy or any second primary tumor, (c) no previous anti-cancer treatment, (d) available data on ABO blood type, and (e) adequate clinical, laboratory, and follow-up data. Patients with blastic NK-cell lymphoma/leukemia, aggressive NK-cell lymphoma/leukemia, or peripheral T-cell lymphoma, unspecified, were excluded.
All pathologic specimens were reviewed and reclassified by central review, according to the WHO criteria for pathologic diagnosis. Antibodies (Dako, Glostrup, Denmark) to the following antigens were used for immunophenotyping: CD3, CD56, T-cell intracellular antigen (TIA-1), Gram-B, CD45RO, CD20, CD79a, CD30, Ki67, and anaplastic large-cell lymphoma kinase. In situ hybridization was used to detect Epstein–Barr virus (EBV)-encoded RNA.
Before treatment, the following baseline clinical data were collected: patient demographics, physical examinations, Eastern Cooperative Oncology Group performance status (ECOG PS), primary tumor site, B symptoms, treatment modalities and response, ABO blood type, serum lactate dehydrogenase (LDH) level, baseline serum CRP levels, serum EBV-DNA copy number, Ann Arbor stage, and computed tomography (CT) or magnetic resonance (MR) images of the nasopharynx, neck, chest, abdomen, and pelvis or positron emission tomography/computed tomography (PET/CT) images of the entire body. All patients were staged using the Ann Arbor staging system. The IPI (involving age, ECOG PS, stage, LDH level, extranodal sites) and KPI (involving stage, LDH level, B symptoms, regional lymphoma nodes) for nasal NK/T-cell lymphoma were used to perform survival analysis [18, 26].
In addition to the ENKTL patient group, we also selected a hospital-based control group of age- and sex-matched inpatients (case–control ratio = 1) who were diagnosed with nonmalignant disease based on surgical or other clinical management from the above three centers. The number of patients in the control group in each hospital was the same as the corresponding number of patients with ENKTL. If more than one control were matched with a case, one was picked randomly by computer. The data and distribution of ABO antigens in the control group were collected and compared with those of the ENKTL patient group.
Response criteria and statistical analysis
The response to treatment was assessed according to the International Working Group Recommendations for Response Criteria for non-Hodgkin lymphoma [27]. Progression-free survival (PFS) was defined as the interval between the date of diagnosis and the date of first relapse, progression, death from any cause, or the last date at which the patients were censored. Overall survival (OS) was defined as the duration between the date of diagnosis and either the time of death from any cause or the last date at which patients were censored. The relationships between ABO blood type and clinical and laboratory variables were assessed using Pearson’s Chi square test or Fisher’s test for categorical variables. The log-rank test and Kaplan–Meier method were applied for univariate survival analysis. Variables significant at P < 0.05 in univariate analysis were included in multivariate analysis. Multivariate analysis was performed using the Cox proportional hazards model. A two-tailed P < 0.05 was considered statistically significant. The statistical software package SPSS 16.0 (SPSS Inc., Chicago, IL, USA) was used for statistical calculations.
Results
Patient characteristics
In total, 697 ENKTL patients (492 males and 205 females), with a median age of 43 years (range 10–82 years), met the inclusion criteria. The median ages for blood type O, A, B, and AB groups were 44 years (range 10–80 years), 43 years (range 14–82 years), 42 years (range 16–76 years), and 49 years (range 21–76 years), respectively. The clinical characteristics of the 697 patients are listed in Table 1.
Table 1.
Basic characteristics of patients with extranodal natural killer (NK)/T-cell lymphoma (ENKTL) in distinct ABO blood type groups
| Characteristic | Total (cases) | Blood four-type group [cases (%)] | P | Blood two-type group [cases (%)] | P | ||||
|---|---|---|---|---|---|---|---|---|---|
| O | A | B | AB | O | Non-O | ||||
| Total | 697 | 255 | 195 | 188 | 59 | 255 | 442 | ||
| Age (years) | 0.313 | 0.722 | |||||||
| ≤60 | 597 | 220 (86.3) | 162 (83.1) | 167 (88.8) | 48 (81.4) | 220 (86.3) | 377 (85.3) | ||
| >60 | 100 | 35 (13.7) | 33 (16.9) | 21 (11.2) | 11 (18.6) | 35 (13.7) | 65 (14.7) | ||
| Gender | 0.854 | 0.730 | |||||||
| Male | 492 | 178 (69.8) | 137 (70.3) | 137 (72.9) | 40 (67.8) | 178 (69.8) | 314 (71.0) | ||
| Female | 205 | 77 (30.2) | 58 (29.7) | 51 (27.1) | 19 (32.2) | 77 (30.2) | 128 (29.0) | ||
| ECOG PS | 0.960 | 0.911 | |||||||
| 0–1 | 680 | 249 (97.6) | 190 (97.4) | 184 (97.9) | 57 (96.6) | 249 (97.6) | 431 (97.5) | ||
| ≥2 | 17 | 6 (2.4) | 5 (2.6) | 4 (2.1) | 2 (3.4) | 6 (2.4) | 11 (2.5) | ||
| B symptoms | 0.295 | 0.133 | |||||||
| Yes | 324 | 109 (42.7) | 101 (51.8) | 86 (45.7) | 28 (47.5) | 109 (42.7) | 215 (48.6) | ||
| No | 373 | 146 (57.3) | 94 (48.2) | 102 (54.3) | 31 (52.5) | 146 (57.3) | 227 (51.4) | ||
| LDH (U/L) | 0.596 | 0.602 | |||||||
| >245 | 194 | 68 (26.7) | 53 (27.2) | 59 (31.4) | 14 (23.7) | 68 (26.7) | 126 (28.5) | ||
| ≤245 | 503 | 187 (73.3) | 142 (72.8) | 129 (68.6) | 45 (76.3) | 187 (73.3) | 316 (71.5) | ||
| Tumor size (cm) | 0.399 | 0.953 | |||||||
| ≥5 | 65 | 24 (9.4) | 21 (10.8) | 18 (9.6) | 2 (3.4) | 24 (9.4) | 41 (9.3) | ||
| <5 | 632 | 231 (90.6) | 174 (89.2) | 170 (90.4) | 57 (96.6) | 231 (90.6) | 401 (90.7) | ||
| Extranodal sites ≥2 | 0.685 | 0.966 | |||||||
| Yes | 77 | 28 (11.0) | 22 (11.3) | 18 (9.6) | 9 (15.3) | 28 (11.0) | 49 (11.1) | ||
| No | 620 | 227 (89.0) | 173 (88.7) | 170 (90.4) | 50 (84.7) | 227 (89.0) | 393 (88.9) | ||
| Regional LN involvement | 0.605 | 0.239 | |||||||
| Yes | 171 | 69 (27.1) | 42 (21.5) | 46 (24.5) | 14 (23.7) | 69 (27.1) | 102 (23.1) | ||
| No | 526 | 186 (72.9) | 153 (78.5) | 142 (75.5) | 45 (76.3) | 186 (72.9) | 340 (76.9) | ||
| EBV-DNA (copies/mL)a | 0.435 | 0.829 | |||||||
| <1530 | 85 | 31 (50.8) | 20 (46.5) | 22 (44.9) | 12 (66.7) | 31 (50.8) | 54 (49.1) | ||
| ≥1530 | 86 | 30 (49.2) | 23 (53.5) | 27 (55.1) | 6 (33.3) | 30 (49.2) | 56 (50.9) | ||
| CRP (mg/L)b | 0.197 | 0.038 | |||||||
| ≤10 | 128 | 55 (61.1) | 26 (50.0) | 36 (46.3) | 11 (42.1) | 55 (61.1) | 73 (47.1) | ||
| >10 | 100 | 35 (38.9) | 26 (50.0) | 31 (53.7) | 8 (57.9) | 35 (38.9) | 65 (52.9) | ||
| Ann Arbor stage | 0.749 | 0.894 | |||||||
| I/II | 619 | 227 (89.0) | 173 (88.7) | 169 (89.9) | 50 (84.7) | 227 (89.0) | 392 (88.7) | ||
| III/IV | 78 | 28 (11.0) | 22 (11.3) | 19 (10.1) | 9 (15.3) | 28 (11.0) | 50 (11.3) | ||
| IPI score | 0.763 | 0.421 | |||||||
| 0–1 | 597 | 222 (87.1) | 164 (84.1) | 162 (86.2) | 49 (83.1) | 222 (87.1) | 375 (84.8) | ||
| 2–5 | 100 | 33 (12.9) | 31 (15.9) | 26 (13.8) | 10 (16.9) | 33 (12.9) | 67 (15.2) | ||
| KPI score | 0.974 | 0.984 | |||||||
| 0–1 | 478 | 175 (68.6) | 133 (68.2) | 128 (68.1) | 42 (71.2) | 175 (68.6) | 303 (68.6) | ||
| 2–4 | 219 | 80 (31.4) | 62 (31.8) | 60 (31.9) | 17 (28.8) | 80 (31.4) | 139 (31.4) | ||
ECOG PS Eastern Cooperative Oncology Group performance status, LDH lactate dehydrogenase, LN lymph node, EBV Epstein–Barr virus, CRP C-reactive protein, IPI International Prognostic Index, KPI Korean Prognostic Index
aData of EBV-DNA copy number were available for 171 patients, and the median value was 1530 copies/mL
bData of serum CRP levels were available for 228 patients, and the CRP level >10 mg/L was used as the cutoff value
Most of the patients (680, 97.6%) displayed a favorable performance status (ECOG PS 0–1). Three hundred and twenty-four patients (46.5%) presented with B symptoms. Elevated LDH levels were observed in 194 (27.8%) patients. Sixty-five patients (9.3%) had a mass ≥5 cm, and only 15 (2.2%) displayed bone marrow involvement. One hundred and seventy-one patients (24.5%) displayed regional lymph node involvement, and 77 (11.0%) displayed at least two extranodal involvement sites. Most of the patients (619, 88.8%) had localized disease (stage I/II). According to the IPI, 597 cases (85.7%) were classified as low-risk disease (IPI = 0–1), and 100 (14.3%) were categorized as high-risk disease (IPI = 2–5). The number of patients with KPI = 0–1 was higher than those with KPI = 2–4 [478 (68.6%) vs. 219 (31.4%)]. The baseline CRP levels were available in 228 patients (range 0.16–154.92 mg/L; median value 7.00 mg/L), and the baseline plasma EBV-DNA data were available in 171 patients (range 0–48,500,000 copies/mL; median value 1530 copies/mL).
The distribution of ABO blood types in the ENKTL group was blood type O in 255 (36.6%) patients, blood type A in 195 (28.0%) patients, blood type B in 188 (27.0%) patients, and blood type AB in 59 (8.5%) patients. According to the previous matching method, 697 patients with nonmalignant disease were randomly selected as the control group. The distribution of ABO blood types in the control group was blood type O in 279 (40.0%) patients, blood type A in 175 (25.1%) patients, blood type B in 162 (23.2%) patients, and blood type AB in 81 (11.6%) patients. There was no significant difference in blood type distribution between the ENKTL and control groups (P = 0.056). ABO blood type was not associated with patient age, gender, ECOG PS, B symptoms, LDH levels, tumor size, number of extranodal sites, regional lymph node involvement, baseline EBV-DNA copies, Ann Arbor stage, IPI score, or KPI score (all P > 0.1, Table 1). However, we found that patients with blood type non-O had a higher percentage of elevated CRP serum level compared with those with blood type O (P = 0.038, Table 1).
Treatment modalities and response
The primary treatment modalities were as follows: 436 (62.6%) patients received chemotherapy combined with radiotherapy, 171 (24.5%) received chemotherapy alone, 72 (10.3%) received radiotherapy alone, and 18 (2.6%) received only best supportive care. The treatment details and responses are listed in Table 2. No significant difference was found in treatment modalities when dividing patient into either four blood type groups (O vs. A vs. B vs. AB, P = 0.701) or two groups (O vs. non-O, P = 0.690). After the initial treatment, 509 (75.0%) of the 679 treated patients displayed a complete response (CR) or CR unconfirmed (CRu). The rate of CR to initial treatment was significantly higher in the blood type O group than in the blood type non-O group (79.2% vs. 69.5%, P = 0.005).
Table 2.
Primary treatment modalities and responses in patients with ENKTL
| Characteristic | Total (cases) | Blood four-type group [cases (%)] | P | Blood two-type group [cases (%)] | P | ||||
|---|---|---|---|---|---|---|---|---|---|
| O | A | B | AB | O | Non-O | ||||
| Treatment modality | 0.701 | 0.690 | |||||||
| CT combined RT | 436 | 164 (64.3) | 118 (60.5) | 120 (63.8) | 34 (57.6) | 164 (64.3) | 272 (61.5) | ||
| CT alone | 171 | 57 (22.4) | 49 (25.1) | 47 (25.0) | 18 (30.5) | 57 (22.4) | 114 (25.8) | ||
| RT alone | 72 | 26 (10.2) | 23 (11.8) | 16 (8.5) | 7 (11.9) | 26 (10.2) | 46 (10.4) | ||
| Best supportive care | 18 | 8 (3.1) | 5 (2.6) | 5 (2.7) | 0 (0) | 8 (3.1) | 10 (2.3) | ||
| CT regimen | 0.675 | 0.534 | |||||||
| CHOP or CHOP-like | 218 | 73 (28.6) | 68 (34.9) | 61 (32.4) | 16 (27.1) | 73 (28.6) | 145 (32.8) | ||
| EPOCH | 105 | 43 (16.9) | 24 (12.3) | 32 (17.0) | 6 (10.2) | 43 (16.9) | 62 (14.0) | ||
| ATT | 43 | 15 (5.9) | 13 (6.7) | 12 (6.4) | 3 (5.1) | 15 (5.9) | 28 (6.3) | ||
| GEMOX + L-asp | 232 | 85 (33.3) | 61 (31.3) | 60 (31.9) | 26 (44.1) | 85 (33.3) | 147 (33.3) | ||
| SMILE | 9 | 5 (2.0) | 1 (0.4) | 2 (1.1) | 1 (0.4) | 5 (2.0) | 4 (0.9) | ||
| Complete response | 509 | 202 (79.2) | 133 (68.2) | 131 (69.7) | 43 (72.9) | 0.040 | 202 (79.2) | 307 (69.5) | 0.005 |
CT chemotherapy, RT radiotherapy, CHOP cyclophosphamide + doxorubicin + vincristine + prednisone, EPOCH etoposide + doxorubicin + vincristine + cyclophosphamide + prednisone, ATT alternating triple therapy (CHOP-B, cyclophosphamide + doxorubicin + vincristine + bleomycin + prednisone, IMVP-16 ifosfamide + methotrexate + etoposide; DHAP, dexamethasone + cisplatin + cytarabine), GEMOX + L-asp gemcitabine + oxaliplatin + l-asparaginase, SMILE dexamethasone + methotrexate + ifosfamide + l-asparaginase + etoposide
The effect of ABO blood type on survival of patients with ENKTL
There were 302 deaths (43.3%) during a median follow-up of 41 months (range 1–214 months). The deaths were due to tumor progression (n = 289), treatment-related toxicities (n = 3), cardiovascular diseases (n = 2), and unknown causes (n = 8). The estimated 5-year PFS and OS rates for all 697 patients were 47.3% and 53.6%. The 5-year PFS rates for blood type A, B, AB, and O groups were 43.1%, 39.7%, 53.3%, and 55.0%, respectively (P = 0.002, Fig. 1a). The 5-year OS rates were 49.6%, 45.5%, 58.0%, and 62.0%, respectively (P = 0.007, Fig. 1b). Because the ENKTL patients with blood type O had longer PFS and OS compared with those with blood type A, B, or AB, we therefore divided these patients into blood type O and non-O (A, B, and AB) groups. The patients with blood type O had significantly higher 5-year PFS rate (55.0% vs. 42.9%, P < 0.001, Fig. 1c) and 5-year OS rate (62.0% vs. 48.9%, P = 0.001, Fig. 1d) compared with those with blood type non-O. Since patients with blood type AB and O have similar PFS and OS, we next examined the effect of ABO blood type on survival by comparing type O/AB versus type A/B. We found that patients with blood type O/AB had significantly higher 5-year PFS rate (54.6% vs. 41.5%, P < 0.001, Fig. 1e) and 5-year OS rate (61.3% vs. 47.6%, P = 0.001, Fig. 1f) compared with those with blood type A/B.
Fig. 1.

Survival curves of patients with extranodal natural killer (NK)/T-cell lymphoma (ENKTL) according to ABO blood type. a Progression-free survival (PFS) curves of patients according to blood types A, B, AB, and O. b Overall survival (OS) curves of patients according to blood types A, B, AB, and O. c PFS curves of patients according to blood types O and non-O (A, B, and AB). d OS curves of patients according to blood types O and non-O. e PFS curves of patients according to blood types O/AB versus type A/B. f OS curves of patients according to blood types O/AB versus type A/B
Blood type non-O was significantly associated with shorter OS in patients with Ann Arbor stage I/II disease (P = 0.002), but not in advanced cases (P = 0.151). For patients receiving chemotherapy plus radiotherapy, blood type non-O was also significantly associated with shorter OS (P = 0.003), whereas among patients receiving chemotherapy or radiotherapy alone, blood type non-O did not significantly affect the survival (P = 0.158 and P = 0.352, respectively). Blood type non-O was also associated with a worse outcome among patients receiving anthracyclines-containing chemotherapy (P = 0.010) or l-asparaginase-containing chemotherapy (P = 0.011). Table 3 displays the detailed data of prognostic significance of ABO blood type (O vs. non-O) in different subgroups.
Table 3.
The prognostic significance of ABO blood type in different subgroups of ENKTL patients
| Subgroup | 5-year OS rate (%) | P | |
|---|---|---|---|
| Blood type O | Blood type non-O | ||
| Ann Arbor stage | |||
| I/II | 66.1 | 53.2 | 0.002 |
| III/IV | 30.6 | 12.1 | 0.151 |
| Treatment modality | |||
| CT combined RT | 74.5 | 57.2 | 0.003 |
| CT alone | 37.7 | 30.2 | 0.158 |
| RT alone | 56.7 | 55.5 | 0.352 |
| Chemotherapy regimen | |||
| Anthracyclines-containing | 59.4 | 42.8 | 0.010 |
| l-Asparaginase-containing | 80.6 | 62.3 | 0.011 |
OS overall survival, CT chemotherapy, RT radiotherapy
Univariate and multivariate Cox regression analysis
Table 4 displays the results of the univariate and multivariate analysis of potential predictors of PFS and OS. Multivariate analysis using the forward conditional Cox region model identified tumor size ≥5 cm (risk ratio [RR] = 1.555, 95% confidence interval [CI] 1.130–2.139, P = 0.007), regional lymph node involvement (RR = 1.323, 95% CI 1.041–1.680, P = 0.022), blood type non-O (RR = 1.539, 95% CI 1.266–1.932, P < 0.001), and an IPI score ≥2 (RR = 2.285, 95% CI 1.751–2.983, P < 0.001) as adverse factors for PFS. In the multivariate analysis for OS, age >60 years (RR = 1.904, 95% CI 1.434–2.527, P < 0.001), tumor size ≥5 cm (RR = 1.720, 95% CI 1.233–2.400, P = 0.001), stage III/IV (RR = 2.114, 95% CI 1.554–2.875, P < 0.001), elevated LDH levels (RR = 1.514, 95% CI 1.183–1.936, P = 0.001), and blood type non-O (RR = 1.491, 95% CI 1.166–1.906, P = 0.001) were significant independent predictors of OS.
Table 4.
Univariate and multivariate analysis of prognostic factors for PFS and OS in patients with ENKTL
| Variable | PFS | OS | ||||
|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||
| P | RR (95% CI) | P | P | RR (95% CI) | P | |
| Age (>60 years vs. ≤60 years) | 0.002 | 1.140 (0.886–1.467) | 0.308 | <0.001 | 1.904 (1.434–2.527) | <0.001 |
| B symptoms (yes vs. no) | 0.052 | – | – | 0.019 | 1.285 (0.981–1.685) | 0.069 |
| Tumor size ≥5 cm (yes vs. no) | <0.001 | 1.555 (1.130–2.139) | 0.007 | <0.001 | 1.720 (1.233–2.400) | 0.001 |
| Extranodal sites ≥2 (yes vs. no) | <0.001 | 0.974 (0.577–1.642) | 0.920 | <0.001 | 0.857 (0.491–1.496) | 0.586 |
| Regional LN involvement (yes vs. no) | <0.001 | 1.323 (1.041–1.680) | 0.022 | 0.030 | 1.230 (0.898–1.686) | 0.197 |
| Stage III/IV (yes vs. no) | <0.001 | 1.142 (0.650–2.009) | 0.644 | <0.001 | 2.114 (1.554–2.875) | <0.001 |
| LDH >245 U/L (yes vs. no) | <0.001 | 1.293 (0.937–1.783) | 0.118 | <0.001 | 1.514 (1.183–1.936) | 0.001 |
| Blood group non-O (yes vs. no) | <0.001 | 1.539 (1.266–1.932) | <0.001 | 0.001 | 1.491 (1.166–1.906) | 0.001 |
| IPI score ≥2 (yes vs. no) | <0.001 | 2.285 (1.751–2.983) | <0.001 | <0.001 | 1.021 (0.570–1.828) | 0.945 |
| KPI score ≥2 (yes vs. no) | <0.001 | 0.970 (0.642–1.466) | 0.886 | <0.001 | 0.846 (0.539–1.327) | 0.467 |
PFS progression-free survival, OS overall survival, RR relative risk, CI confidence interval, LN lymph node, LDH lactate dehydrogenase, IPI International Prognostic Index, KPI Korean Prognostic Index, – not assessed
The comparison of prognostic value between ABO blood type and IPI and KPI models
Using the IPI predictive model, we identified 3 categories of patients with different survival outcomes: 598 (85.8%) patients in the low-risk (IPI = 0–1) group, 87 (12.5%) in the intermediate-risk (IPI = 2–3) group, and 12 (1.7%) in the high-risk (IPI = 4–5) group. The 5-year OS rate was 58.6% for the low-risk group, 25.3% for the intermediate-risk group, and 22.2% for the high-risk group (P < 0.001, Fig. 2a). Significant differences in survival were also found between the low-risk and intermediate-risk groups (P < 0.001) and between the intermediate-risk and high-risk groups (P = 0.024). However, based on the IPI data, 85.8% of the patients were disproportionately grouped into the low-risk group, and the IPI score was unable to identify patients with different survival statuses within the low-risk group, whereas ABO blood type (O vs. non-O) efficiently categorized patients in the low-risk IPI group into two subgroups with different survival outcomes (P = 0.010, Fig. 2b).
Fig. 2.
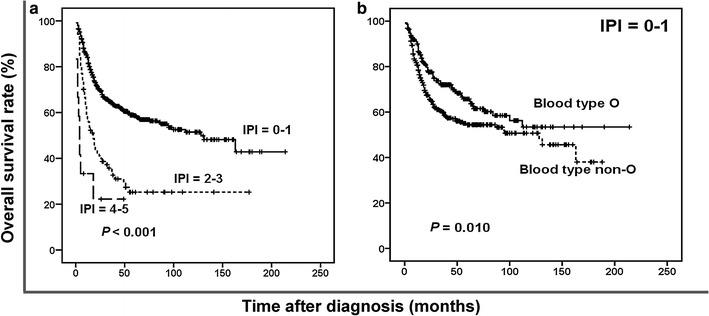
Survival curves of patients with ENKTL according to the International Prognostic Index (IPI) score. a OS curves of patients according to the IPI. b OS curves of patients with IPI score = 0–1 according to blood types O and non-O
The KPI model balanced the distribution of patients in different risk categories more efficiently than the IPI model (score 0:233 cases, 33.4%; score 1:245 cases, 35.2%; score 2:145 cases, 20.8%; and score 3–4:74 cases, 10.6%), and it was able to discriminate between patients with different survival outcomes. The 5-year OS rate was 63.8% for the KPI = 0 group, 54.2% for the KPI = 1 group, 50.0% for the KPI = 2 group, and 27.1% for the KPI = 3–4 group (P < 0.001). Moreover, the KPI model significantly distinguished between the low- and intermediate-to-low-risk groups (KPI = 0 vs. KPI = 1, P = 0.010, Fig. 3a), but not between the intermediate-to-low- and high-to-intermediate-risk groups (KPI = 1 vs. KPI = 2, P = 0.321, Fig. 3b). The KPI model also significantly distinguished between the high-to-intermediate- and high-risk groups (KPI = 2 vs. KPI = 3–4, P = 0.002, Fig. 3c). In contrast, ABO blood type (O vs. non-O) was efficient at discriminating patients with a KPI score = 0–1 (P = 0.020, Fig. 3d), 1–2 (P = 0.015, Fig. 3e), or 2–4 (P = 0.019, Fig. 3f).
Fig. 3.
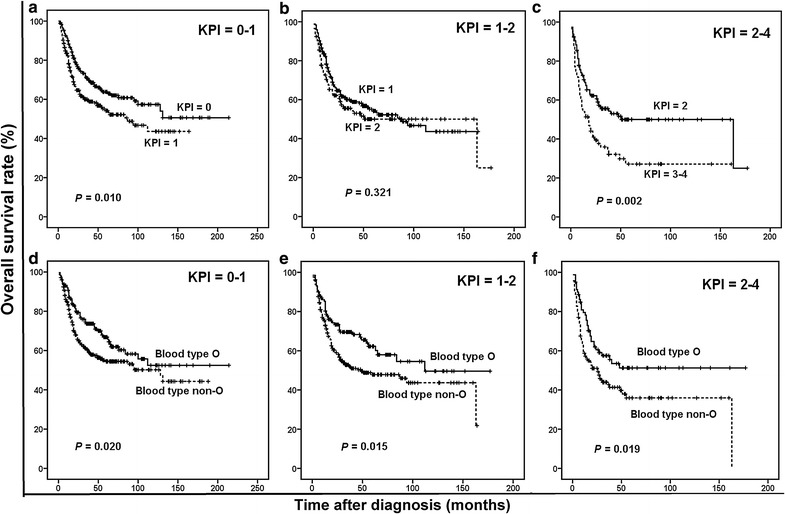
Survival curves of patients with ENKTL according to the Korean Prognostic Index (KPI) score. a OS curves of patients with KPI score = 0–1 according to the KPI model. b OS curves of patients with KPI score = 1–2 according to the KPI model. c OS curves of patients with KPI score = 2–4 according to the KPI model. d OS curves of patients with KPI score = 0–1 according to blood types O and non-O. e OS curves of patients with KPI score = 1–2 according to blood types O and non-O. f OS curves of patients with KPI score = 2–4 according to blood types O and non-O
Discussion
In the present triple-center study, we found that ABO blood type (O vs. non-O) is an independent prognostic factor of PFS and OS and a useful predictor of treatment response in patients with ENKTL. Moreover, the proportions of blood types O, A, B, and AB were similar to those reported previously for Chinese patients with solid tumors [23, 24]. However, because no studies published in English to date have investigated the prognostic role of ABO blood type on the outcome of lymphoma, we could not compare our results with published information. Despite this, the results of the current study are consistent with several previous studies in which blood type O was reported as a favorable prognostic factor for various types of solid tumors [22–25, 28, 29]. Rahbari et al. [29] investigated the influence of ABO blood type on 627 patients with pancreatic cancer and found blood type O as a favorable prognostic factor (hazard ratio [HR] = 0.78, P = 0.037). Similarly, Li et al. [24] evaluated the prognostic role of ABO blood type in 1601 patients with curatively resected non-small cell lung cancer (NSCLC), and they found that patients with blood type O or B had significantly prolonged OS and disease-free survival (DFS) compared with those with blood type A or AB. Moreover, in the study of Gershman et al. [28] of a large cohort of 2086 patients with bladder cancer, the results indicated that blood type non-O was associated with significantly shorter recurrence-free survival (RFS, P = 0.04) and cancer-specific survival (CSS, P = 0.02). In contrast, some studies did not find any effect of ABO blood type on survival outcome in some other cancers [30, 31]. Gates et al. [30] evaluated the impact of ABO blood type on the survival of 2036 invasive breast cancer patients and found no association. In addition, Lee et al. [31] found that ABO blood type was not associated with survival outcomes and was not a prognostic factor in patients who underwent surgery for renal carcinoma. Thus, further studies with larger sample sizes are warranted to confirm the prognostic role of ABO blood type in patients with ENKTL.
Moreover, in the present study, we found that the significant differences in PFS and OS holds true not only when patients were grouped into blood types O and non-O but also when patients were grouped into blood types O/AB and A/B. In addition, we observed longer survival in patients with blood type AB than in those with blood type A/B, although the difference was not significant (data not shown). It seems that blood type AB is a favorable factor for ENKTL. Our results are consistent with a previous study in which blood type AB was reported as a favorable prognostic factor for colon cancer [23]. In contrast, some studies found that blood type AB was an unfavorable prognostic factor for several other types of cancer [24, 25]. Because the number of patients with blood type AB is the lowest among all blood types, the influence of blood type AB on the prognostic value of ABO blood type in ENKTL may be rather small. More studies are needed to evaluate the prognostic role of blood type AB.
In our study, the prognostic significance of ABO blood type was observed not only in the entire cohort analysis but also in subgroup analysis. Blood type non-O was significantly associated with shorter OS as compared with blood type O in patients with Ann Arbor stage I/II disease, patients receiving chemotherapy plus radiotherapy, patients receiving anthracyclines-containing chemotherapy, or patients receiving l-asparaginase-containing chemotherapy. The prognostic value of the IPI score has been widely validated for diffuse large B-cell lymphoma (DLBCL) and many other subtypes of NHL. However, its prognostic role in ENKTL remains controversial, and the patients were allocated disproportionately by the IPI model [4, 17]. In the present study, although the IPI score was significantly predictive in the univariate analysis, it failed to identify patients with varying survival rates within the low-risk group, which accounted for a majority of the patients. When ABO blood type was added to the IPI model, the low-risk patients were separated into two groups with significantly different survival outcomes. The KPI model yielded a balanced distribution of patients with different levels of risk and separated them into four groups with different survival outcomes. However, the KPI model failed to significantly distinguish between the intermediate-to-low- and high-to-intermediate-risk groups. Conversely, ABO blood type was able to discriminate between the intermediate-to-low- and high-to-intermediate-risk groups, between the low- and intermediate-to-low-risk groups, and between the high-to-intermediate- and high-risk groups. Taken together, these results indicated that ABO blood type has a powerful prognostic value. Further studies are required to determine whether ABO blood type can improve the currently widely used KPI model in patients with ENKTL.
The mechanisms underlying the influence of ABO blood type on cancer development and progression are still largely unclear. However, several potential explanations have been proposed for the association of ABO blood type and cancer. Modified expression of blood group antigens on cancer cells has been hypothesized to influence the development and spread of cancer, by altering glycosyltransferase specificity or by altering cell motility, resistance to apoptosis, and immune escape [32]. Moreover, variations in the surface antigen expression on the surrounding epithelial and endothelial cells would affect the adhesion and signaling of cancer cells [33]. Other recent studies demonstrated that ABO blood type is associated with serum levels of soluble intercellular adhesion molecule-1 (sICAM-1), tumor necrosis factor-alpha (TNF-α), P-selectin, and soluble E-selectin, suggesting that blood group antigens may influence the chronic systemic inflammatory response, which is associated with the processes of angiogenesis, tumor growth, invasion, and migration [34, 35]. In addition, the present study found that the patients with blood type non-O had a higher percentage of elevated CRP serum level compared with those with blood type O. CRP is an acute-phase protein secreted by hepatocytes during the inflammatory response, and it is regulated by pro-inflammatory cytokines [36]. This finding suggests that ABO blood type may affect the prognosis of patients with ENKTL by changing the concentration of inflammatory cytokines. Moreover, multiple previous studies demonstrated that blood type non-O was independently associated with a risk of venous thromboembolism (VTE) [37, 38]. Although there was no data on the association between ABO blood type and VTE in patients with lymphoma, Mizrahi et al. [38] found that blood type non-O was an independent risk factor for VTE in children with acute lymphoblastic leukemia. In the current study, we indeed found that two patients, whose ABO blood types were A and B, died from acute pulmonary thromboembolism. Based on these data, we speculate that an increasing death rate caused by thromboembolism is another possible mechanism underlying the relationship between blood type non-O and poor prognosis.
We acknowledge some important limitations of our study. First and foremost, the study design was retrospective and non-randomized. Second, the relatively small sample size prevented us from statistically analyzing the survivor factors. Third, the therapeutic heterogeneity of the treatment strategy of patients may confound the results of our study.
Conclusions
In conclusion, our study showed an association between ABO blood type and survival in patients with lymphoma. We found that ABO blood type is an independent predictor of clinical outcome for ENKTL patients. Further studies with a large series and more uniform treatment are warranted to confirm the prognostic value of ABO blood type in patients with ENKTL.
Authors’ contributions
WQJ and PYY designed the research study. YJL, JWL, PYZ and TT collected the data. YJL and JWL analyzed the data. YJL wrote the paper. PYY, WQJ and XLL provided comments on the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This study were supported by the grants from the Hunan Provincial Science and Technology Department (No. 2016JJ3083) and the grants from the Heath and Family Planning Commission of Hunan Province (No. c2015-52).
Competing interests
The authors declare that they have no competing interests.
Abbreviations
- ENKTL
extranodal natural killer (NK)/T-cell lymphoma
- NHL
non-Hodgkin’s lymphoma
- PFS
progression-free survival
- OS
overall survival
- IPI
International Prognostic Index
- KPI
Korean Prognostic Index
- LDH
lactate dehydrogenase
- CRP
C-reactive protein
- RT
radiotherapy
- CR
complete response
- RR
relative risk
- CI
confidence interval
- TNF
tumor necrosis factor-alpha
- VTE
venous thromboembolism
- ALL
acute lymphoblastic leukemia
- PTE
pulmonary thromboembolism
Contributor Information
Ya-Jun Li, Email: liyajun9@yahoo.com.
Ping-Yong Yi, Phone: (86-731)89762281, Email: yipingy1964@163.com.
Ji-Wei Li, Email: lijiwei19909091@yahoo.com.
Xian-Ling Liu, Email: xianlingliuliuxl@126.com.
Tian Tang, Email: tiantang19841027@163.com.
Pei-Ying Zhang, Email: zhangpeiying88092@163.com.
Wen-Qi Jiang, Email: wqjiang12@yahoo.com.
References
- 1.Swerdlow S, Campo E, Harris N, Jaffe E, Pileri S, Stein H, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4. Lyon: International Agency for Research on Cancer; 2008. [Google Scholar]
- 2.Liang R. Advances in the management and monitoring of extranodal NK/T-cell lymphoma, nasal type. Br J Haematol. 2009;147(1):13–21. doi: 10.1111/j.1365-2141.2009.07802.x. [DOI] [PubMed] [Google Scholar]
- 3.Sun J, Yang Q, Lu Z, He M, Gao L, Zhu M, et al. Distribution of lymphoid neoplasms in China: analysis of 4,638 cases according to the World Health Organization classification. Am J Clin Pathol. 2012;138(3):429–434. doi: 10.1309/AJCP7YLTQPUSDQ5C. [DOI] [PubMed] [Google Scholar]
- 4.Au WY, Weisenburger DD, Intragumtornchai T, Nakamura S, Kim WS, Sng I, et al. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood. 2009;113(17):3931–3937. doi: 10.1182/blood-2008-10-185256. [DOI] [PubMed] [Google Scholar]
- 5.Chihara D, Ito H, Matsuda T, Shibata A, Katsumi A, Nakamura S, et al. Differences in incidence and trends of haematological malignancies in Japan and the United States. Br J Haematol. 2014;164(4):536–545. doi: 10.1111/bjh.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Y, Zhu Y, Cao JZ, Zhang YJ, Xu LM, Yuan ZY, et al. Risk-adapted therapy for early-stage extranodal nasal-type NK/T-cell lymphoma: analysis from a multicenter study. Blood. 2015;126(12):1424–1432. doi: 10.1182/blood-2015-04-639336. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki R, Suzumiya J, Yamaguchi M, Nakamura S, Kameoka J, Kojima H, et al. Prognostic factors for mature natural killer (NK) cell neoplasms: aggressive NK cell leukemia and extranodal NK cell lymphoma, nasal type. Ann Oncol. 2010;21(5):1032–1040. doi: 10.1093/annonc/mdp418. [DOI] [PubMed] [Google Scholar]
- 8.Guo HQ, Pu XX, Guo CC, Rao HL, Li HR, Lin TY. The role of Skp2 in extranodal NK/T-cell lymphoma. Chin J Cancer. 2010;29(5):567–571. doi: 10.5732/cjc.009.10645. [DOI] [PubMed] [Google Scholar]
- 9.Niu SQ, Yang Y, Li YY, Wen G, Wang L, Li ZM, et al. Primary site and regional lymph node involvement are independent prognostic factors for early-stage extranodal nasal-type natural killer/T cell lymphoma. Chin J Cancer. 2016;35:34. doi: 10.1186/s40880-016-0096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwong YL, Kim WS, Lim ST, Kim SJ, Tang T, Tse E, et al. SMILE for natural killer/T-cell lymphoma: analysis of safety and efficacy from the Asia Lymphoma Study Group. Blood. 2012;120(15):2973–2980. doi: 10.1182/blood-2012-05-431460. [DOI] [PubMed] [Google Scholar]
- 11.Li YJ, Jiang WQ, Huang JJ, Xia ZJ, Huang HQ, Li ZM. The Glasgow Prognostic Score (GPS) as a novel and significant predictor of extranodal natural killer/T-cell lymphoma, nasal type. Am J Hematol. 2013;88(5):394–399. doi: 10.1002/ajh.23422. [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Wang ZH, Chen XQ, Li YJ, Wang KF, Xia YF, et al. First-line combination of gemcitabine, oxaliplatin, and l-asparaginase (GELOX) followed by involved-field radiation therapy for patients with stage IE/IIE extranodal natural killer/T-cell lymphoma. Cancer. 2013;119(2):348–355. doi: 10.1002/cncr.27752. [DOI] [PubMed] [Google Scholar]
- 13.Yuan F, Wei X, Yin Q, Li Y, Mi R, Ai H, et al. A multi-center retrospective study of l-asparaginase-based regimens as first-line treatment in newly diagnosed extranodal NK/T-cell lymphoma. Zhonghua xue ye xue za zhi = Zhonghua xueyexue zazhi. 2014;35(7):614–618. doi: 10.3760/cma.j.issn.0253-2727.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 14.He XH, Li B, Yang S, Lu N, Zhang X, Zou SM, et al. R-CHOP regimen can significantly decrease the risk of disease relapse and progression in patients with non-germinal center B-cell subtype diffuse large B-cell lymphoma. Chin J Cancer. 2012;31(6):306–314. doi: 10.5732/cjc.011.10420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei WX, Huang JJ, Li WY, Zhang X, Xia Y, Jiang WQ, et al. Prognostic values of interim and post-therapy 18F-FDG PET/CT scanning in adult patients with Burkitt’s lymphoma. Chin J Cancer. 2015;34(12):608–613. doi: 10.1186/s40880-015-0057-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai QQ, Hu LY, Geng QR, Chen J, Lu ZH, Rao HL, et al. New risk factors and new tendency for central nervous system relapse in patients with diffuse large B-cell lymphoma: a retrospective study. Chin J Cancer. 2016;35(1):87. doi: 10.1186/s40880-016-0150-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chim CS, Ma SY, Au WY, Choy C, Lie AK, Liang R, et al. Primary nasal natural killer cell lymphoma: long-term treatment outcome and relationship with the International Prognostic Index. Blood. 2004;103(1):216–221. doi: 10.1182/blood-2003-05-1401. [DOI] [PubMed] [Google Scholar]
- 18.Lee J, Suh C, Park YH, Ko YH, Bang SM, Lee JH, et al. Extranodal natural killer T-cell lymphoma, nasal-type: a prognostic model from a retrospective multicenter study. J Clin Oncol. 2006;24(4):612–618. doi: 10.1200/JCO.2005.04.1384. [DOI] [PubMed] [Google Scholar]
- 19.Huang JJ, Jiang WQ, Lin TY, Huang Y, Xu RH, Huang HQ, et al. Absolute lymphocyte count is a novel prognostic indicator in extranodal natural killer/T-cell lymphoma, nasal type. Ann Oncol. 2011;22(1):149–155. doi: 10.1093/annonc/mdq314. [DOI] [PubMed] [Google Scholar]
- 20.Wolpin BM, Chan AT, Hartge P, Chanock SJ, Kraft P, Hunter DJ, et al. ABO blood group and the risk of pancreatic cancer. J Natl Cancer Inst. 2009;101(6):424–431. doi: 10.1093/jnci/djp020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iavarone M, Della Corte C, Pelucchi C, Marconi M, Trotti R, Triolo M, et al. Risk of hepatocellular carcinoma in relation to ABO blood type. Dig Liver Dis. 2016;48(1):94–96. doi: 10.1016/j.dld.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 22.Ben Q, Wang K, Yuan Y, Li Z. Pancreatic cancer incidence and outcome in relation to ABO blood groups among Han Chinese patients: a case–control study. Int J Cancer. 2011;128(5):1179–1186. doi: 10.1002/ijc.25426. [DOI] [PubMed] [Google Scholar]
- 23.Cao X, Wen ZS, Sun YJ, Li Y, Zhang L, Han YJ. Prognostic value of ABO blood group in patients with surgically resected colon cancer. Br J Cancer. 2014;111(1):174–180. doi: 10.1038/bjc.2014.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li N, Xu M, Li CF, Ou W, Wang BX, Zhang SL, et al. Prognostic role of the ABO blood types in Chinese patients with curatively resected non-small cell lung cancer: a retrospective analysis of 1601 cases at a single cancer center. Chin J Cancer. 2015;34(10):475–482. doi: 10.1186/s40880-015-0054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun P, Chen C, Zhang F, An X, Li XY, Li YH, et al. The ABO blood group predicts survival in esophageal squamous cell carcinoma in patients who ever smoked: a retrospective study from China. Tumour Biol. 2014;35(7):7201–7208. doi: 10.1007/s13277-014-1960-7. [DOI] [PubMed] [Google Scholar]
- 26.Shipp MA, Harrington DP, Anderson JR, Armitage JO, Bonadonna G, Brittinger G, Cabanillas F, Canellos GP, Coiffier B, Connors JM, Cowan RA. A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329(14):987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 27.Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17(4):1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 28.Gershman B, Moreira DM, Tollefson MK, Frank I, Cheville JC, Thapa P, et al. The association of ABO blood type with disease recurrence and mortality among patients with urothelial carcinoma of the bladder undergoing radical cystectomy. Urol Oncol. 2016;34(1):4.e1–4.e9. doi: 10.1016/j.urolonc.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 29.Rahbari NN, Bork U, Hinz U, Leo A, Kirchberg J, Koch M, et al. ABO blood group and prognosis in patients with pancreatic cancer. BMC Cancer. 2012;12:319. doi: 10.1186/1471-2407-12-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gates MA, Xu M, Chen WY, Kraft P, Hankinson SE, Wolpin BM. ABO blood group and breast cancer incidence and survival. Int J Cancer. 2012;130(9):2129–2137. doi: 10.1002/ijc.26220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee C, You D, Sohn M, Jeong IG, Song C, Kwon T, et al. Prognostic value of ABO blood group in patients with renal cell carcinoma: single-institution results from a large cohort. J Cancer Res Clin Oncol. 2015;141(8):1441–1447. doi: 10.1007/s00432-015-1908-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Pendu J, Marionneau S, Cailleau-Thomas A, Rocher J, Le Moullac-Vaidye B, Clement M. ABH and Lewis histo-blood group antigens in cancer. APMIS. 2001;109(1):9–31. doi: 10.1111/j.1600-0463.2001.tb00011.x. [DOI] [PubMed] [Google Scholar]
- 33.Meany DL, Chan DW. Aberrant glycosylation associated with enzymes as cancer biomarkers. Clin Proteom. 2011;8(1):7. doi: 10.1186/1559-0275-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barbalic M, Dupuis J, Dehghan A, Bis JC, Hoogeveen RC, Schnabel RB, et al. Large-scale genomic studies reveal central role of ABO in sP-selectin and sICAM-1 levels. Hum Mol Genet. 2010;19(9):1863–1872. doi: 10.1093/hmg/ddq061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marnell L, Mold C, Du Clos TW. C-reactive protein: ligands, receptors and role in inflammation. Clin Immunol. 2005;117(2):104–111. doi: 10.1016/j.clim.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 37.Ohira T, Cushman M, Tsai MY, Zhang Y, Heckbert SR, Zakai NA, et al. ABO blood group, other risk factors and incidence of venous thromboembolism: the Longitudinal Investigation of Thromboembolism Etiology (LITE) J Thromb Haemost. 2007;5(7):1455–1461. doi: 10.1111/j.1538-7836.2007.02579.x. [DOI] [PubMed] [Google Scholar]
- 38.Mizrahi T, Leclerc JM, David M, Ducruet T, Robitaille N. ABO group as a thrombotic risk factor in children with acute lymphoblastic leukemia: a retrospective study of 523 patients. J Pediatr Hematol Oncol. 2015;37(5):e328–e332. doi: 10.1097/MPH.0000000000000333. [DOI] [PubMed] [Google Scholar]


