Abstract
Background
In HIV-uninfected populations, physical activity decreases mortality and inflammation. Inflammation is a potential cause for co-morbidities in HIV+ adults, the evidence examining the effect of physical activity on cardiometabolic health is limited. This analysis examines the relationship between physical activity, cardiometabolic health and inflammation.
Methods
We conducted a nested study within the SATURN-HIV trial in which 147 HIV+ adults were randomized to 10 mg daily rosuvastatin or placebo. Measures of physical activity, cardiometabolic health, inflammation, and vascular disease (carotid artery intima media thickness and Computed Tomography-acquired measures pericardial fat volume) were assessed at baseline and through 96 weeks . Spearman correlations and multivariable analyses were used to explore relationships between physical activity, cardiometabolic health and inflammation.
Results
Median age (Q1, Q3) was 46 (40.4, 52.7) years, 80% were male, 69% were African American and 46% on protease inhibitors. Baseline median physical activity was 44 minutes per week (0, 150), 24% of participants performed greater than 150 minutes per week. At baseline, physical activity correlated with several markers of cardiometabolic health and inflammation (all p≤0.05). Over all time points median physical activity was independently associated with carotid distensibility (β = 2.53, p = 0.008), pericardial fat volume (β = −6.13, p = 0.001) and IL 6 (β = −0.468, p < 0.001).
Conclusions
Physical activity is associated with vascular disease, endothelial function, and may be an adjuvant to decreasing co-morbidities in HIV+ adults. Further studies should examine long-term effects of physical activity on cardiometabolic health and inflammation in this population.
Keywords: Physical activity, HIV, cardiometabolic health, inflammation
Introduction
With improved anti-retroviral therapy, people living with HIV are aging and experiencing increased cardiometabolic comorbidities. Physical activity has gained renewed attention as a strategy to minimize the risk of developing these comorbidities in HIV+ adults. In HIV uninfected adults, regular physical activity lowers all-cause mortality by protecting against atherosclerosis and insulin resistance1,2. Physical activity increases energy expenditure3, decreases obesity and enhances cardiovascular health by improving lipid profiles and vascular function4. The long term benefits of physical activity by preventing diseases associated with chronic inflammation such as type 2 diabetes, atherosclerosis, and rheumatoid arthritis may also be related to an anti-inflammatory effect5,6. In healthy adults, increased physical activity is accompanied by reduced inflammatory markers independent of cardiovascular disease risk factors7,8. In populations characterized by low grade chronic inflammation, such as diabetes and rheumatoid arthritis, physical activity has been shown to decrease C reactive protein (CRP)7, Interleukin 10 (IL10)9, Tumor Necrosis Factor Alpha (TNF-α)10 and Intercellular Adhesion Molecule (ICAM) 11. The mechanism by which physical activity decreases inflammation is multifactorial and recent research has focused on two potential mechanistic pathways5. First, physical activity may increase the anti-inflammatory cytokine adiponectin and second it may decrease pro-inflammatory adipokines12,13.
Accordingly, recent small studies of HIV+ adults have found that supervised endurance training was shown to decrease CRP, Interleukin 6 (IL6), Interleukin 18 (IL18) and TNF-α14 and that supervised moderate intensity exercise for twelve weeks was associated with improvement in CRP, sCD14, d dimer, IL6 and IL 18 levels 15. No studies could be located that examined the effect of free-living exercise (i.e. physical activity conducted in and around their natural home setting) on inflammation in HIV+ adults. The effect of free-living exercise on inflammatory markers is a significant question in this population because HIV+ adults experience high levels of inflammation which may contribute to their increased cardiometabolic morbidity. If their baseline level of physical activity (that which occurs naturally, without supervision, in their home setting16) can decrease this inflammation, health care providers can provide counseling on how much and what type of physical activity can produce desirable outcomes as an adjunct to antiretroviral therapy.
Further, in HIV-uninfected populations, physical activity has also been consistently associated with improvement in markers of cardiovascular disease such as carotid intima media thickness (cIMT) 17, carotid distensibility 18 and flow mediated dilation (FMD) 19 which predict future cardiovascular events such as myocardial infarction and stroke20,21. In HIV+ adults, little is known about the effect of free-living physical activity on markers of cardiometabolic health and inflammation. Recent evidence demonstrated that physical activity is safe in healthy HIV+ adults at all ages and that it improves cardiopulmonary fitness, body composition and psychological status22,23, underscoring the timeliness of an investigation into its effects in a natural setting.
HIV+ adults experience impaired cardiometabolic pathways and persistent inflammation that are potential contributing factors to their increased chronic comorbidities. Beyond their effect of cholesterol lowering, statins, or 3 hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors can reduce inflammation and reactive oxygen species and can improve vascular function24,25. However, we lack evidence on the effect of both statins and physical activity on modulating inflammation and markers of cardiometabolic health. To help us understand the relationship between physical activity, cardiometabolic health, inflammation and statin use, here we present results of a secondary analysis on the relationship between free-living physical activity and markers of inflammation and cardiometabolic health in the Stopping Atherosclerosis and Treating Unhealthy Bone with Rosuvastatin in HIV (SATURN-HIV) trial.
Materials and Methods
Study Design
The data for our secondary analysis come from the SATURN-HIV study, a 96-week study designed to measure the effect of rosuvastatin on markers of cardiovascular risk, skeletal health, and immune activation in HIV disease26. The study was approved by the Institutional Review Board of University Hospitals Case Medical Center, Cleveland, Ohio. Written informed consent was provided by all participants. The study is registered on clinicaltrials.gov (NCT01218802). Participants were randomized 1:1 to rosuvastatin 10 mg daily vs. matching placebo. All participants were ≥18 years of age, with HIV-1 infection on stable ART for at least 3 months with cumulative ART duration of at least 6 months, HIV-1 RNA <1000 copies/mL, fasting LDL-cholesterol (LDL-C) ≤130 mg/dL and triglyceride ≤500 mg/dL. Additionally, participants were required to have evidence of either heightened T-cell activation, identified as the proportion of CD8+ T cells that expressed CD38+ and HLA-DR+ of ≥19% or levels of high-sensitivity C-reactive protein (hs-CRP) ≥2 mg/L. Participants were excluded if they had a history of coronary disease or diabetes, were pregnant or lactating, or had an active infectious or inflammatory condition.
Study evaluations
At entry, week 24, week 48 and 96, fasting (> 12 hours) blood draws were obtained for measurements of renal and lipid profiles, glucose and insulin levels. Additionally, blood was processed and plasma, serum and peripheral blood mononuclear cells were stored for measurement of markers of immune activation, systemic inflammation and coagulation as previously described26,27.
Soluble plasma biomarkers of monocyte activation (soluble CD14 and soluble CD 163), systemic inflammation (hs-CRP, IL-6), and coagulation (D-dimer) were measured as previously described26,27. Monocyte and T-cells were phenotyped by flow cytometry as previously described27.
Subclinical Vascular Disease
At entry, week 48 and week 96, mean-mean common carotid artery (CCA) intima media thickness (CCA-IMT) was measured by high resolution ultrasound as described previously28,29. Measurements were taken at three separate angles bilaterally and the average of the six measurements was used for analysis. To calculate carotid distensibility, the diameter of the distal 1cm of the right carotid artery was measured in systole (Ds) and diastole (Dd). Each diameter was averaged over three consecutive beats. Blood pressure was obtained at the time of carotid ultrasound in order to determine the pulse pressure (PP). Carotid distensibility was calculated using the same formula [(2*(Ds-Dd)/Dd)/PP] used in the Women’s Interagency Health Study (WIHS)30 and the Multicenter AIDS Cohort Study (MACS)31 and is reported in units of 10−6×N−1m2. Flow-mediated dilation (FMD) of the brachial artery and hyperemic velocity-time integral (VTI) were measured by brachial artery reactivity testing using a forearm occlusion method as previously described28. Pericardial fat volume and coronary artery calcium score (CAC) were quantified offline from gated, non-contrast coronary calcium scans performed on a 64-slice multi-detector CT scanner28.
Physical Activity
Free living physical activity was assessed using the NIAID Adult AIDS Clinical Trials Group Physical Activity Assessment32. This instrument asks participants to self-report the number of times they participated in one of 27 activities in the past two weeks and on average, the length of each activity bout. Examples of activities included walking for exercise, hiking, gardening, swimming, yoga. There is substantial evidence supporting the health benefits of exercise that is based on time spent in various intensity levels33, yet intensity of the activity bout was not assessed on this instrument. As our purpose was to assess the effect of free-living physical activity on inflammation and cardiometabolic health, we focused on intentional physical activity that was most likely to have at least a moderate level of intensity. We created three exercise variables to help us analyze this relationship. First, we created a moderate intensity variable by summing the amount of walking time and doing yoga. Second, a moderate to high intensity variable by summing the amount of time spent jogging/running, hiking, aerobics/dancing, calisthenics, biking, swimming and weight lifting, and third an overall exercise variable by summing the amount of time reported in each of these nine activities. After summing the exercise variables, we divided by two to equal the amount of exercise per week. We created a binary exercise variable, taking values yes or no, from the continuous overall exercise variable by classifying an individual to the yes category if performed American Heart Association recommended exercise of 2.5 hours per week,2 otherwise to no. We used this binary exercise variable in the regression analyses as a covariate of interest because it is more easily interpreted in relation to the outcome variables.
Statistical analysis
We performed descriptive analyses on all of the covariates (age, sex, race, nadir CD4, ART duration, etc) and outcomes of interests: markers of inflammation and cardiometabolic health. We checked validity of data distributions by running frequency analyses and graphical presentations. For example, we compared observations of a variable across various groups and time points using box plots. All baseline variables measured on a continuous scale were compared between groups using 2 samples Wilcoxon Rank Sum tests those measured as categorical variables were compared using Chi-Square tests. We used t-tests to assess the relationship between baseline physical activity and baseline markers of inflammation, immune activation, cardiometabolic risk.
Next we used a quantile regression approach to answer (1) what cardiometabolic and inflammatory markers over the 96 week period are associated with physical activity (2) is there an interaction between physical activity and statin use over the 96 week period for the same cardiometabolic and inflammatory markers. All cardiometabolic measures and inflammatory outcomes were determined based on existing literature 15,22,34,35. Separate models were constructed for each of the cardiometabolic and inflammatory outcome variables. Each model included physical activity and the following clinically relevant variables: age, sex, race, BMI, CD4 nadir and ART duration. To describe the additive effect of physical activity and statin use, we modeled these effects controlling for both statin use and added an interaction between physical activity and statin use.
For a tangible estimate of the regression coefficient we rescaled the covariate distribution by dividing a suitable value. For example, we rescaled age to decade by dividing 10 and some of the covariates are rescaled by dividing its standard deviation. We also studied the outcome variables distribution and found that many variables distributions are skewed. The quantile regression approach is a robust regression approach36. For correlated observations we have corrected the standard errors of the regression estimates using bootstrap procedure. We used 500 replicates in estimating the SE of the regression coefficient. All analyses were performed using statistical software STATA 11.0.
RESULTS
Baseline characteristics
All 147 SATURN-HIV participants were included in this analysis, of which 119 completed all measures. Demographic and baseline characteristics are displayed in Table 1 and were similar between groups (p>0.05) except for physical activity (p=0.003). Median age (Q1, Q3) was 46 (41, 53) years, 79% were male, 68% were African American. Median BMI was 27 (22, 30); systolic and diastolic blood pressures were 121 (110,136) and 79 (72, 85); HOMA-IR was 1.8(1, 4.45). Median current and nadir CD4+ T cell counts were 617 (398, 853) and 182 (84, 312). Median known duration of HIV was 11 years (6–19). All participants were on ART with 88% on tenofovir and 46% on protease inhibitors. Baseline median physical activity was 66 and 22.5 minutes per week for the statin and placebo group respectively (p=0.003).
Table 1.
Baseline Characteristics
| Rosuvastatin | Placebo | |
|---|---|---|
| N=72 | N=75 | |
| Age (years) | 45 (41,51) | 47 (39,53) |
| Male | 81% | 76% |
| African American | 69% | 67% |
| HIV duration (years) | 11 (6–17) | 12 (6–19) |
| Current CD4+ count (cells/mm3) | 608 (440–848) | 627 (398–853) |
| Nadir CD4+ count (cells/mm3) | 173 (84,312) | 190 (89,281) |
| Undetectable viral load (<50 copies/ml) | 56 (78%) | 58 (77%) |
| ART duration (years) | 5.2 (3.1,9.9) | 5.9(3.3,9.6) |
| Current Protease Inhibitor use | 50% | 48% |
| Current TDF use | 64 (89%) | 66 (88%) |
| Body Mass Index (kg/m2) | 27 (22,30) | 27 (23,30) |
| Systolic Blood Pressure (mm Hg) | 122 (112,136) | 120(110,132) |
| Diastolic Blood Pressure, mm Hg | 79 (73,85) | 80 (72,83) |
| HDL cholesterol (mg/dL) | 47 (38,58) | 46 (37,57) |
| LDL cholesterol (mg/dL) | 96 (76,107) | 97 (77,121) |
| Homeostatic Model Assessment of Insulin resistance (HOMA-IR) | 1.7 (1,2.81) | 1.95 (1.13,4.45) |
| Current Smoking | 43 (60) | 50 (67) |
| Carotid Distensibility (10-6*Newtons-1*m2) | 24.09 (19.17,31.83) | 23.48 (18.62,30.01) |
| Pericardial Fat Volume (cm3) | 69.5 (43.35,90.8) | 63.35 (50.2,91.8) |
| Coronary Artery Calcium score | 0 (0, 7.75) | 0 (0, 9) |
| Number of Subjects with Coronary Artery Calcium score >0 (%) | 24 (33%) | 30 (40%) |
| Hyperemic VTI (cm) | 0.80 (0.60,0.94) | 0.76 (0.67,0.94) |
| Flow Mediated Dilation | 3.94 (2.14, 6.24) | 4.02 (1.98, 0.75) |
| Mean-mean IMT (mm) | 0.66 (0.62, 0.77) | 0.67 (0.60, 0.75) |
| Interleukin-6 (pg/mL) | 2.89 (1.88,4.14) | 2.64 (1.96,5.27) |
| hsCRP (µg/mL) | 1.56 (0.77,4.86) | 2.02 (0.71,5.22) |
| Overall Physical Activity (minutes per week) | 66 (0,150) | 22.5 (0,150) |
| Number of Subjects Reporting Physical Activity >150 minutes per week | 17 (24%) | 18 (24%) |
Data presented as median (Q1,Q3) for continuous variables and by frequency (column percent) for nominal variables.
Baseline associations with physical activity
At baseline, in unadjusted analyses, moderate intensity physical activity was associated with lower HDL-C, IL-6, and hsCRP; moderate to high intensity physical activity was associated with lower BMI, HOMA-IR, leptin, pericardial fat and higher hyperemic VTI (better vascular dilation); and overall physical activity was associated with lower levels of leptin, IL-6, hsCRP and higher hyperemic VTI. We found no associations with TNF-α receptors I and II, sCD163, sCD14, CD14+ CD16+ monocytes, or CD14 dim CD16+ monocytes. Full results can be found in Table 2.
Table 2.
Univariable analysis estimate by physical activity intensity at baseline
| Moderate intensity | Moderate High Intensity | Overall Activity | ||||
|---|---|---|---|---|---|---|
| regression coefficient |
p-value | regression coefficient | p-value | regression coefficient |
p-value | |
| Metabolic Factors | ||||||
| Body Mass Index (kg/m2) | −0.039 | 0.64 | −0.17 | 0.04 | −0.14 | 0.09 |
| HDL (mg/dL) | −0.19 | 0.02 | −0.01 | 0.87 | −0.15 | 0.08 |
| LDL (mg/dL) | −0.05 | 0.56 | −0.09 | 0.27 | −0.04 | 0.62 |
| Triglycerides(mg/dL) | −0.01 | 0.93 | −0.06 | 0.48 | −0.04 | 0.65 |
| HOMA-IR | 0.04 | 0.64 | −0.18 | 0.03 | −0.11 | 0.21 |
| Leptin | −0.10 | 0.22 | −0.34 | <0.001 | −0.28 | 0.00 |
| Cardiovascular measures | ||||||
| Carotid Distensibility (10-6*Newtons-1*m2) | 0.10 | 0.22 | −0.00 | 0.99 | 0.08 | 0.32 |
| Pericardial Fat (cm3) | −0.06 | 0.44 | −0.16 | 0.05 | −0.12 | 0.14 |
| Hyperemic VTI (cm) | 0.11 | 0.18 | 0.18 | 0.03 | 0.19 | 0.02 |
| Flow Mediated Dilation | −0.04 | 0.70 | −0.02 | 0.76 | −0.06 | 0.47 |
| Mean-mean IMT (mm) | −0.00 | 0.95 | 0.02 | 0.81 | 0.02 | 0.83 |
| Inflammation and Immune activation | ||||||
| Interleukin 6 (pg/mL) | −0.19 | 0.02 | −0.14 | 0.09 | −0.23 | <0.001 |
| hsCRP (µg/mL) | −0.20 | 0.02 | −0.13 | 0.12 | −0.20 | 0.02 |
| sCD163 (ng/ml) | 0.05 | 0.53 | −0.00 | 0.98 | −0.03 | 0.75 |
| sCD14 (ng/ml) | −0.16 | 0.06 | 0.08 | 0.34 | −0.02 | 0.79 |
| CD14+ CD16+ monocytes (%) | −0.05 | 0.59 | −0.13 | 0.12 | −0.15 | 0.07 |
| CD14dimCD16+ monocytes (%) | −0.12 | 0.16 | 0.02 | 0.83 | −0.06 | 0.47 |
Only variables with p <0.1 included; variables tested but not included: Demographics and clinical parameters (waist-hip ratio, glucose, insulin); inflammation and immune activation (D-dimer, CD4+CD38+HLADR+ T-cells, CD8+CD38+HLADR+ T-cells, TNF-α receptors I and II); cardiovascular measures (Framingham score and CAC score)
Free-living physical activity
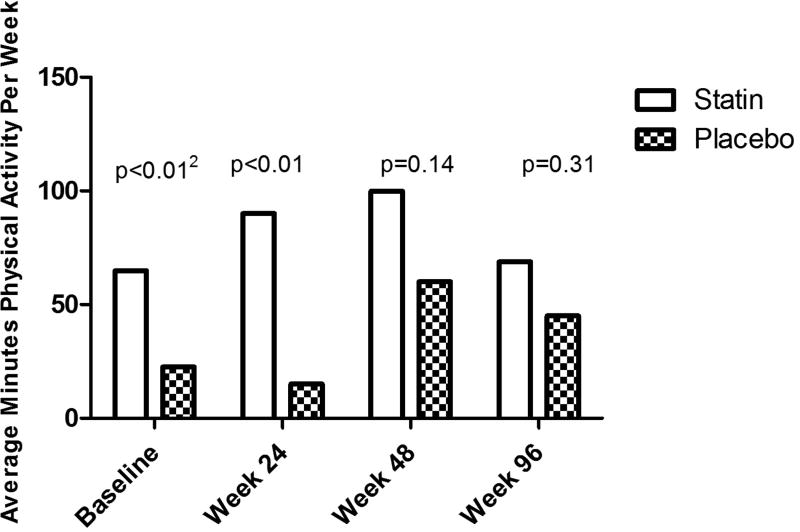
Figure 1 displays overall physical activity level by time and treatment group. Median (Q1,Q3) physical activity was different between the groups at baseline [66 min for the statin group (0,150) and 22.5 for the placebo group (0, 150); p=0.0003] and week 24 [90 min for the statin group (15, 225) and 15min for the placebo group (0, 105); p=0.002]. There were no differences between the groups at week 48 [180 min for the statin group (22.5, 300) and 60 min for the placebo group (0, 180); p=0.14] and week 96 [69 min for the statin group (0, 270) and 45 min for the placebo group (0, 180); p= 0.31).
Figure 1. Physical Activity between Treatment Groups.
1 Data are presented as median minutes of physical activity per week. 2 p-values indicate a difference in physical activity between statin and placebo group using a Wilxcoxon - Mann-Whitney Test, at each timepoint.
Over the 96 week study period, overall physical activity was associated with multiple measures of subclinical vascular disease (e.g. carotid distensiblity, pericardial fat volume) as well as with IL-6 when adjusting for age, sex, race, BMI, CD4+ T cell nadir and duration of antitretroviral therapy (Table 3). To further describe the additive effect of physical activity when using statins, we modelled these effects controlling for both statin use and an interaction between physical activity and statin use. We found that the relationship between free living physical activity and pericardial fat volume and IL-6 was enhanced when accounting for statin use (all p-values ≤ 0.05). In contrast, the relationship between physical activity and carotid distensibility was attenuated when accounting for statin use. Additionally, we found a significant interaction between free living physical activity, hsCRP, pericardial fat, carotid distensibility and statin use, signifying an additive effect of physical activity.
Table 3.
Multivariable analysis of relationship between physical activity and inflammatory and cardiovascular markers over all time points1
| Carotid IMT | Carotid Distensibility | Pericardial Fat Volume | IL6 | hsCRP | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β coefficient | p value | β coefficient | p value | β coefficient | p value | β coefficient | p value | β coefficient | p value | |
| Physical Activity (engaging in 2.5 hrs per week) | 0.005 | 0.22 | 2.533 | 0.008 | −6.13 | 0.001 | −0.468 | <0.001 | −0.170 | 0.273 |
| Age (per decade) | 0.065 | <0.001 | −5.327 | <0.001 | 8.161 | <0.001 | 0.285 | 0.010 | 0.012 | 0.932 |
| Sex | −0.018 | 0.09 | 6.172 | <0.001 | −25.72 | <0.001 | −0.460 | 0.050 | 0.838 | <0.001 |
| Race | 0.026 | <0.001 | −3.795 | 0.001 | −18.095 | <0.001 | 0.128 | 0.024 | −0.381 | <0.001 |
| BMI (kg/m2) | 0.001 | <0.001 | −0.259 | <0.001 | 3.982 | <0.001 | 0.106 | <0.001 | 0.187 | <0.001 |
| CD4 Nadir (cells/mm3) | 0.00 | 0.95 | −0.401 | 0.107 | −3.072 | <0.001 | −0.108 | 0.064 | −0.084 | 0.029 |
| ART duration (years) | 0.00 | 0.60 | −0.397 | 0.004 | 0.818 | 0.470 | −0.15 | 0.357 | −0.075 | 0.391 |
| STATIN2 | ||||||||||
| β coefficient | p value | β coefficient | p value | β coefficient | p value | β coefficient | p value | β coefficient | p value | |
| Physical Activity | −0.005 | 0.704 | 1.726 | 0.104 | −10.671 | <0.001 | −0.690 | 0.042 | −0.393 | 0.048 |
| Age (per decade) | 0.066 | <0.001 | −5.469 | <0.001 | 8.628 | 0.001 | 0.277 | 0.012 | 0.004 | 0.978 |
| Sex | −0.021 | 0.007 | 6.269 | <0.001 | −21.345 | <0.001 | −0.600 | 0.005 | 0.926 | <0.001 |
| Race | 0.032 | 0.005 | −3.123 | 0.007 | −19.236 | <0.001 | 0.136 | 0.254 | −0.400 | <0.001 |
| BMI (kg/m2) | 0.001 | <0.001 | −0.289 | <0.001 | 3.727 | <0.001 | 0.112 | <0.001 | 0.192 | <0.001 |
| CD4 Nadir (cells/mm3) | 0.002 | 0.421 | −0.723 | 0.090 | −3.769 | <0.001 | −0.097 | 0.003 | −0.060 | 0.175 |
| ART duration (years) | −0.001 | 0.812 | −0.483 | 0.077 | 0.981 | <0.001 | −0.089 | 0.521 | −0.094 | 0.239 |
| Statin use | −0.010 | 0.539 | 0.448 | 0.351 | −8.137 | 0.016 | −0.454 | 0.054 | −0.390 | 0.039 |
| Statin Physical Activity interaction | 0.027 | 0.424 | 1.072 | <0.001 | 12.928 | 0.001 | 0.483 | 0.412 | 0.431 | 0.041 |
The top panel regresses physical activity on select inflammatory and cardiovascular markers over time, controlling for age, sex, race, BMI, CD4 nadir and duration of ART use;
The bottom panel regresses physical activity on the same inflammatory and cardiovascular markers over time, controlling for age, sex, race, BMI, CD4 nadir, duration of ART use, statin use (group of randomization), and the interaction of statins and physical activity.
DISCUSSION
For the first time in HIV, we investigated the effects of free-living physical activity on markers of inflammation and cardiometabolic health, and we found that HIV+ adults who engage in at least 2.5 hours of moderate intensity exercise per week are likely to experience lower levels of inflammation and subclinical vascular disease.
Recent evidence suggested that some of the benefits of physical activity may be related to anti-inflammatory effects37,38. Prior studies of exercise in HIV + adults have focused on metabolic outcomes such as insulin resistance and dyslipidemia. Several randomized controlled trials assessed the effect of a supervised exercise regimen in HIV+ individuals. Six months of aerobic and resistance exercise in HIV + individuals improved self-efficacy, cognitive function and heart rate when compared to the control group39. A study on 20 sedentary HIV + adults with lipodystrophy demonstrated that strength and endurance training for 16 weeks reduced total and LDL cholesterol, free fatty acids, hsCRP, IL-6, IL-18 and TNF α and increased HDL cholesterol14. Recently, data from Longo et al demonstrated that 50 HIV + adults with a sedentary lifestyle who enrolled in moderate intensity (brisk walking) for 12 weeks had improved fitness and immune activation (sCD14, d dimer, IL6 and IL 18 levels)15. Our analysis adds to this growing body of evidence by showing that HIV+ adults on antiretrovirals who reported at least 2.5 hours of moderate intensity exercise per week, in their home setting, had lower levels of systemic inflammation as measured by IL6 and hsCRP. Taken together, these data suggest that free-living exercise may have an additive role in decreasing systemic inflammation in HIV+ adults. Previous findings have shown that physical activity reduces the proportion of inflammatory monocytes40. However, we did not find evidence of a relationship between inflammatory monocyte subsets/monocyte immune activation (CD14dimCD16+ monocytes, CD38+HLA-DR+ T cells, sCD14 and sCD163) and physical activity, suggesting that the anti-inflammatory effects of regular exercise in HIV+ adults may be mediated by a different mechanism such as reduction in fat mass.
Regular physical activity decreases the risk of metabolic and cardiovascular diseases in HIV-uninfected populations, but we were unable to find any prior data assessing the effect of free-living physical activity on markers of subclinical vascular disease in HIV. Previous studies in HIV-uninfected adults suggested that short term exercise training can improve endothelial function and vascular remodeling41. Additionally, enrollment in an exercise program improved FMD in young patients who were prehypertensive (aged 18–35) and overweight patients with coronary heart disease 42,43. Our data suggest that even in HIV+ adults selected for having heightened immune activation, physical activity of at least 2.5 hours per week is associated with improved carotid distensibility (i.e. reduced “stiffness”). This association persisted even after adjustment for demographics, HIV-related factors, and statin use. This suggests that physical activity in HIV+ adults is associated with improved vascular structure as well as function.
A novel finding is our observed relationship between physical activity and pericardial fat volume. Pericardial fat volume is increased in HIV infection compared to uninfected controls44,45 and is associated with systemic inflammation in patients on ART46. Because of close proximity and a lack of fascial separation, inflammation in this fat may contribute to coronary atherosclerosis and myocardial dysfunction47,48. We found that after controlling for known demographic and HIV characteristics, including total body adiposity (BMI) and statin use, HIV+ adults who engage in at least 2.5 hours of moderate intensity physical activity per week had on average a 10.7 cm3 reduction in pericardial fat, compared to those who did not meet the minimum exercise requirements. Furthermore, physical activity was associated with even larger reductions in pericardial fat among subjects who were taking a statin compared to those who were taking placebo. These data suggest that the pericardial fat depot is a surrogate marker of metabolically unfavorable fat distribution and may be particularly sensitive to interventions that aim to improve metabolic disease in patients with treated HIV infection.
Our study’ strengths include asking a clinically-relevant question pertaining to the effects of free-living exercise on inflammation and cardiometabolic health. Free-living exercise is a sustainable and acceptable intervention that is consistent with the health promotion initiatives of the Affordable Care Act. Our study is further strengthened by our detailed evaluations of inflammation, immune activation and cardiovascular disease risk. Additionally, its large sample size and prospective design provide the first evidence examining the long-term relationship of free-living physical activity, inflammation and cardiometabolic health in this population, significantly adding to the literature. As a secondary analysis, the main limitation in the analysis is that it included self- reported measure of physical activity. Though we used a common physical activity assessment for the population in clinical trials, it is a subjective self-reported measure. Questionnaires such as ours have several strengths including low burden to subjects, wide applicability, assessing different physical activity domains and intensities. However, they are limited by recall and social desirability, which can lead to over-reporting of activity49. Future, prospective studies primarily examining this relationship should consider an objective measure of exercise including activity monitors or heart rate monitors. In addition, the participants in this study had baseline heightened inflammation, normal LDL cholesterol level and were mostly black men, therefore our findings may not be applicable to other HIV infected populations.
In conclusion, we show that self-reported free-living physical activity in HIV + individuals is independently associated with markers of systemic inflammation, vascular disease and endothelial function and may be an adjuvant to improve the health of HIV + individuals. The effects of physical activity on cardiometabolic health may be mediated by reductions in inflammation and should be further investigated in HIV + individuals.
Acknowledgments
The authors would like to thank the patients who participated in this research.
Source of funding for study: The work was supported by the National Institutes of Health R01 NR012642 to GAM. Technical support was provided by the Center for AIDS Research, Case Western Reserve University (P30 AI36219).
Conflicts of Interest and other Sources of Funding:
GAM served as a consultant, speaker, and has received research funding from Bristol-Myers Squibb, ViiV, Gilead, Merck, and Pfizer. CTL is supported by the National Institutes of Health (K23 HL123341), a Wolf Family Foundation Scholars Grant, and Medtronic Philanthropy. He has received research grants from Bristol-Myers Squibb. ARW is supported by the American Heart Association (14CRP20380259).
Footnotes
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Study drugs and matching placebo were donated by Astra Zeneca. Preliminary results from this study were presented at CROI, 2015 in Seattle, WA, February 23–26, 2015. This trial is registered at clinicaltrials.gov (NCT01218802).
Author Contributions:
GM designed the study and obtained funding. AS provided statistical support. All authors contributed to data analysis and writing of the manuscript.
References
- 1.Pedersen BK. The anti-inflammatory effect of exercise: its role in diabetes and cardiovascular disease control. Essays Biochem. 2006;42:105–117. doi: 10.1042/bse0420105. [DOI] [PubMed] [Google Scholar]
- 2.Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular RiskA Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2013 doi: 10.1016/j.jacc.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Hollowell RP, Willis LH, Slentz CA, Topping JD, Bhakpar M, Kraus WE. Effects of exercise training amount on physical activity energy expenditure. Med Sci Sports Exerc. 2009 Aug;41(8):1640–1644. doi: 10.1249/MSS.0b013e31819c71a4. [DOI] [PubMed] [Google Scholar]
- 4.Kraus WE, Houmard JA, Duscha BD, et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002 Nov 7;347(19):1483–1492. doi: 10.1056/NEJMoa020194. [DOI] [PubMed] [Google Scholar]
- 5.Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011 Sep;11(9):607–615. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- 6.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007 May;132(6):2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 7.Geffken DF, Cushman M, Burke GL, Polak JF, Sakkinen PA, Tracy RP. Association between physical activity and markers of inflammation in a healthy elderly population. Am J Epidemiol. 2001 Feb 1;153(3):242–250. doi: 10.1093/aje/153.3.242. [DOI] [PubMed] [Google Scholar]
- 8.Abramson JL, Vaccarino V. Relationship between physical activity and inflammation among apparently healthy middle-aged and older US adults. Arch Intern Med. 2002 Jun 10;162(11):1286–1292. doi: 10.1001/archinte.162.11.1286. [DOI] [PubMed] [Google Scholar]
- 9.Kadoglou NP, Iliadis F, Angelopoulou N, et al. The anti-inflammatory effects of exercise training in patients with type 2 diabetes mellitus. Eur J Cardiovasc Prev Rehabil. 2007 Dec;14(6):837–843. doi: 10.1097/HJR.0b013e3282efaf50. [DOI] [PubMed] [Google Scholar]
- 10.Hopps E, Canino B, Caimi G. Effects of exercise on inflammation markers in type 2 diabetic subjects. Acta Diabetol. 2011 Sep;48(3):183–189. doi: 10.1007/s00592-011-0278-9. [DOI] [PubMed] [Google Scholar]
- 11.Zoppini G, Targher G, Zamboni C, et al. Effects of moderate-intensity exercise training on plasma biomarkers of inflammation and endothelial dysfunction in older patients with type 2 diabetes. Nutr Metab Cardiovasc Dis. 2006 Dec;16(8):543–549. doi: 10.1016/j.numecd.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Mujumdar PP, Duerksen-Hughes PJ, Firek AF, Hessinger DA. Long-term, progressive, aerobic training increases adiponectin in middle-aged, overweight, untrained males and females. Scand J Clin Lab Invest. 2011 Apr;71(2):101–107. doi: 10.3109/00365513.2011.554995. [DOI] [PubMed] [Google Scholar]
- 13.Lim S, Choi SH, Jeong IK, et al. Insulin-sensitizing effects of exercise on adiponectin and retinol-binding protein-4 concentrations in young and middle-aged women. J Clin Endocrinol Metab. 2008 Jun;93(6):2263–2268. doi: 10.1210/jc.2007-2028. [DOI] [PubMed] [Google Scholar]
- 14.Lindegaard B, Hansen T, Hvid T, et al. The effect of strength and endurance training on insulin sensitivity and fat distribution in human immunodeficiency virus-infected patients with lipodystrophy. J Clin Endocrinol Metab. 2008 Oct;93(10):3860–3869. doi: 10.1210/jc.2007-2733. [DOI] [PubMed] [Google Scholar]
- 15.Valeria Longo MB, Simona Bossolasco, Laura Galli, et al. Brisk Walking Improves Inflammatory Markers in cART-Treated Patients. Paper presented at: CROI2014; Boston. [Google Scholar]
- 16.McDermott MM, Liu K, Guralnik JM, et al. Home-based walking exercise intervention in peripheral artery disease: a randomized clinical trial. JAMA. 2013 Jul 3;310(1):57–65. doi: 10.1001/jama.2013.7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masley SC, Roetzheim R, Masley LV, McNamara T, Schocken DD. Emerging Risk Factors as Markers for Carotid Intima Media Thickness Scores. J Am Coll Nutr. 2015 Mar 9;:1–8. doi: 10.1080/07315724.2014.916238. [DOI] [PubMed] [Google Scholar]
- 18.Ashor AW, Lara J, Siervo M, Celis-Morales C, Mathers JC. Effects of exercise modalities on arterial stiffness and wave reflection: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2014;9(10):e110034. doi: 10.1371/journal.pone.0110034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seligman BG, Polanczyk CA, Santos AS, et al. Intensive practical lifestyle intervention improves endothelial function in metabolic syndrome independent of weight loss: a randomized controlled trial. Metabolism. 2011 Dec;60(12):1736–1740. doi: 10.1016/j.metabol.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Gokce N, Keaney JF, Jr, Hunter LM, et al. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003 May 21;41(10):1769–1775. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 21.Terai M, Ohishi M, Ito N, et al. Comparison of arterial functional evaluations as a predictor of cardiovascular events in hypertensive patients: the Non-Invasive Atherosclerotic Evaluation in Hypertension (NOAH) study. Hypertens Res. 2008 Jun;31(6):1135–1145. doi: 10.1291/hypres.31.1135. [DOI] [PubMed] [Google Scholar]
- 22.O'Brien K, Nixon S, Tynan AM, Glazier R. Aerobic exercise interventions for adults living with HIV/AIDS. Cochrane Database Syst Rev. 2010;(8):CD001796. doi: 10.1002/14651858.CD001796.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farinatti PT, Borges JP, Gomes RD, Lima D, Fleck SJ. Effects of a supervised exercise program on the physical fitness and immunological function of HIV-infected patients. J Sports Med Phys Fitness. 2010 Dec;50(4):511–518. [PubMed] [Google Scholar]
- 24.Tousoulis D, Oikonomou E, Siasos G, et al. Dose-dependent effects of short term atorvastatin treatment on arterial wall properties and on indices of left ventricular remodeling in ischemic heart failure. Atherosclerosis. 2013 Apr;227(2):367–372. doi: 10.1016/j.atherosclerosis.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 25.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008 Nov 20;359(21):2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 26.Eckard AR, Jiang Y, Debanne SM, Funderburg NT, McComsey GA. Effect of 24 weeks of statin therapy on systemic and vascular inflammation in HIV-infected subjects receiving antiretroviral therapy. J Infect Dis. 2014 Apr 15;209(8):1156–1164. doi: 10.1093/infdis/jiu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Funderburg NT, Jiang Y, Debanne SM, et al. Rosuvastatin treatment reduces markers of monocyte activation in HIV-infected subjects on antiretroviral therapy. Clin Infect Dis. 2014 Feb;58(4):588–595. doi: 10.1093/cid/cit748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Longenecker CT, Jiang Y, Orringer CE, et al. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS. 2014 Apr 24;28(7):969–977. doi: 10.1097/QAD.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Longenecker CT, Funderburg NT, Jiang Y, et al. Markers of inflammation and CD8 T-cell activation, but not monocyte activation, are associated with subclinical carotid artery disease in HIV-infected individuals. HIV Med. 2013 Jul;14(6):385–390. doi: 10.1111/hiv.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karim R, Mack WJ, Kono N, et al. T-cell activation, both pre- and post-HAART levels, correlates with carotid artery stiffness over 6.5 years among HIV-infected women in the WIHS. J Acquir Immune Defic Syndr. 2014 Nov 1;67(3):349–356. doi: 10.1097/QAI.0000000000000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seaberg EC, Benning L, Sharrett AR, et al. Association between human immunodeficiency virus infection and stiffness of the common carotid artery. Stroke. 2010 Oct;41(10):2163–2170. doi: 10.1161/STROKEAHA.110.583856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McComsey GA, Kendall MA, Tebas P, et al. Alendronate with calcium and vitamin D supplementation is safe and effective for the treatment of decreased bone mineral density in HIV. AIDS. 2007 Nov 30;21(18):2473–2482. doi: 10.1097/QAD.0b013e3282ef961d. [DOI] [PubMed] [Google Scholar]
- 33.Swain DP, Franklin BA. Comparison of cardioprotective benefits of vigorous versus moderate intensity aerobic exercise. Am J Cardiol. 2006 Jan 1;97(1):141–147. doi: 10.1016/j.amjcard.2005.07.130. [DOI] [PubMed] [Google Scholar]
- 34.Mesquita Soares TC, Galvao De Souza HA, De Medeiros Guerra LM, et al. Morphology and biochemical markers of people living with HIV/AIDS undergoing a resistance exercise program: clinical series. J Sports Med Phys Fitness. 2011 Sep;51(3):462–466. [PubMed] [Google Scholar]
- 35.Stanley TL, Grinspoon SK. Body composition and metabolic changes in HIV-infected patients. J Infect Dis. 2012 Jun;205(Suppl 3):S383–390. doi: 10.1093/infdis/jis205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koenker R, Hallock K. Quantile regression. Journal of Economic Perspectives. 2001;15:143–156. [Google Scholar]
- 37.Petelin A, Bizjak M, Cernelic-Bizjak M, Jurdana M, Jakus T, Jenko-Praznikar Z. Low-grade inflammation in overweight and obese adults is affected by weight loss program. J Endocrinol Invest. 2014 Jun 10; doi: 10.1007/s40618-014-0102-9. [DOI] [PubMed] [Google Scholar]
- 38.Lancaster GI, Febbraio MA. The immunomodulating role of exercise in metabolic disease. Trends Immunol. 2014 Jun;35(6):262–269. doi: 10.1016/j.it.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 39.Fillipas S, Oldmeadow LB, Bailey MJ, Cherry CL. A six-month, supervised, aerobic and resistance exercise program improves self-efficacy in people with human immunodeficiency virus: a randomised controlled trial. Aust J Physiother. 2006;52(3):185–190. doi: 10.1016/s0004-9514(06)70027-7. [DOI] [PubMed] [Google Scholar]
- 40.Timmerman KL, Flynn MG, Coen PM, Markofski MM, Pence BD. Exercise training-induced lowering of inflammatory (CD14+CD16+) monocytes: a role in the anti-inflammatory influence of exercise? J Leukoc Biol. 2008 Nov;84(5):1271–1278. doi: 10.1189/jlb.0408244. [DOI] [PubMed] [Google Scholar]
- 41.Feairheller DL, Diaz KM, Kashem MA, et al. Effects of moderate aerobic exercise training on vascular health and blood pressure in African Americans. J Clin Hypertens (Greenwich) 2014 Jul;16(7):504–510. doi: 10.1111/jch.12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beck DT, Casey DP, Martin JS, Emerson BD, Braith RW. Exercise training improves endothelial function in young prehypertensives. Exp Biol Med (Maywood) 2013 Apr;238(4):433–441. doi: 10.1177/1535370213477600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ades PA, Savage PD, Lischke S, et al. The effect of weight loss and exercise training on flow-mediated dilatation in coronary heart disease: a randomized trial. Chest. 2011 Dec;140(6):1420–1427. doi: 10.1378/chest.10-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guaraldi G, Scaglioni R, Zona S, et al. Epicardial adipose tissue is an independent marker of cardiovascular risk in HIV-infected patients. AIDS. 2011;25(9):1199–1205. doi: 10.1097/QAD.0b013e3283474b9f. [DOI] [PubMed] [Google Scholar]
- 45.Gabriella O, Giovanni G, Stefano Z, et al. Ectopic fat is linked to prior cardiovascular events in men with HIV. Journal of acquired immune deficiency syndromes. 2012 Apr 15;59(5):494–497. doi: 10.1097/QAI.0b013e31824c8397. [DOI] [PubMed] [Google Scholar]
- 46.Longenecker CT, Jiang Y, Yun CH, et al. Perivascular fat, inflammation, and cardiovascular risk in HIV-infected patients on antiretroviral therapy. Int J Cardiol. 2013 Oct 9;168(4):4039–4045. doi: 10.1016/j.ijcard.2013.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iacobellis G, Bianco AC. Epicardial adipose tissue: emerging physiological, pathophysiological and clinical features. Trends in endocrinology and metabolism: TEM. 2011 Nov;22(11):450–457. doi: 10.1016/j.tem.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bays HE. Adiposopathy is "sick fat" a cardiovascular disease? J Am Coll Cardiol. 2011 Jun 21;57(25):2461–2473. doi: 10.1016/j.jacc.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 49.Strath SJ, Kaminsky LA, Ainsworth BE, et al. Guide to the assessment of physical activity: Clinical and research applications: a scientific statement from the American Heart Association. Circulation. 2013 Nov 12;128(20):2259–2279. doi: 10.1161/01.cir.0000435708.67487.da. [DOI] [PubMed] [Google Scholar]