Abstract
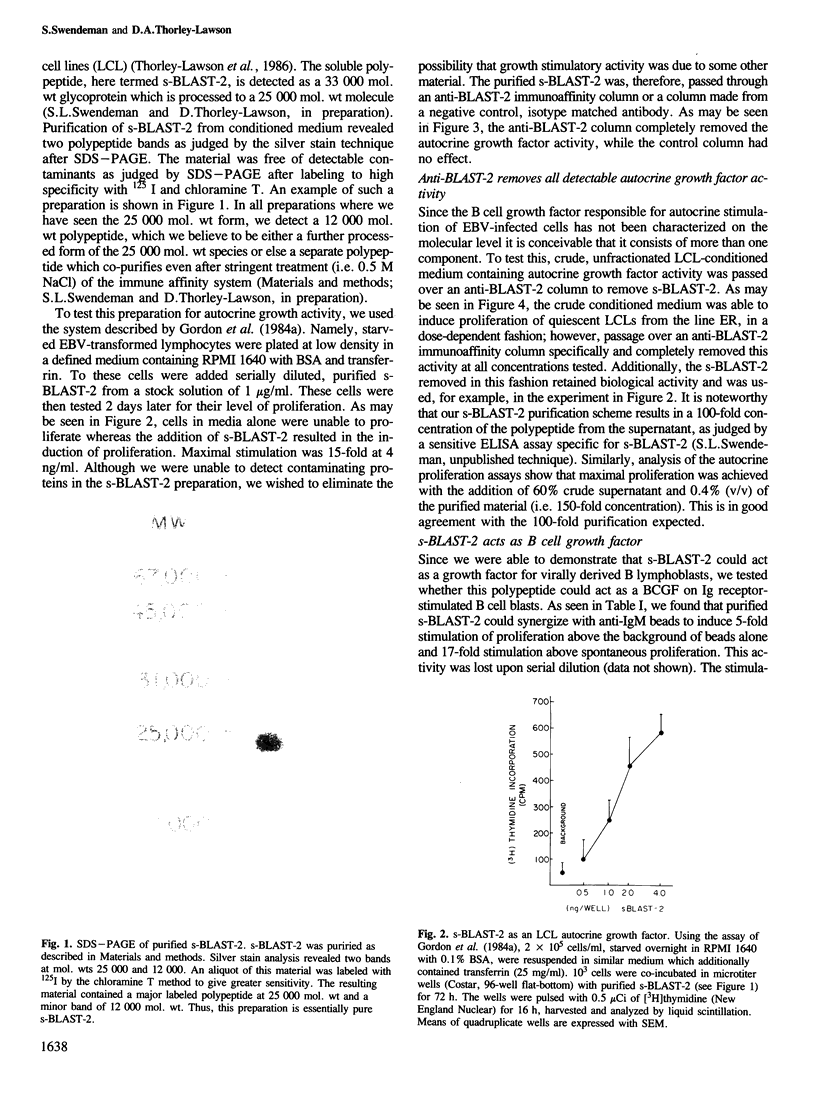
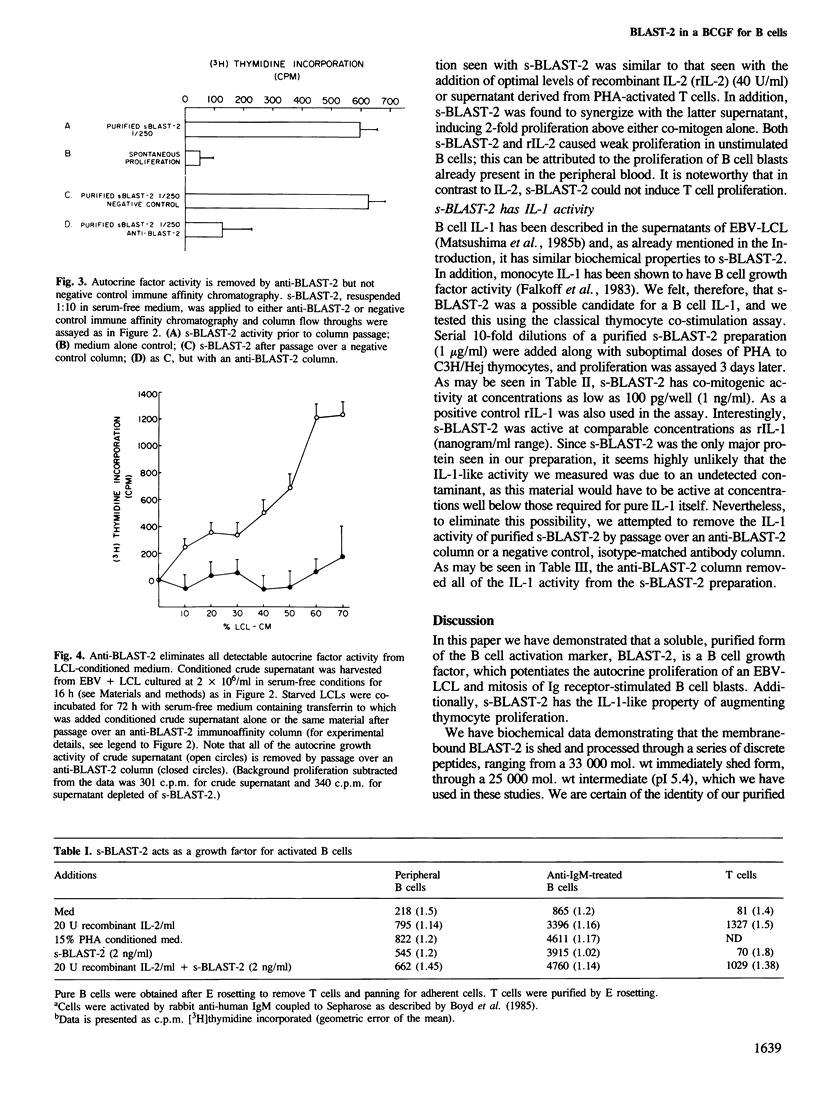
A shed form of the membrane-bound B cell activation marker, BLAST-2 (Epstein-Barr virus cell surface, CD 23) was immune-affinity purified from Epstein-Barr virus-transformed lymphoblast conditioned medium. SDS-PAGE analysis revealed a complex of two polypeptides, mol. wts 25,000 and 12,000, here termed s-BLAST-2. We show that this complex, when purified to homogeneity, can act as a growth factor for EBV-infected B lymphoblasts and normal receptor-stimulated B cell blasts. It has no effect on resting B or T cells. These data suggest that the BLAST-2 antigen has a role in autocrine B cell growth. Additionally, this complex is a co-mitogen for PHA-stimulated murine thymocytes, a property of interleukin-1.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrus J. L., Jr, Jurgensen C. H., Brown E. J., Fauci A. S. Purification to homogeneity of a high molecular weight human B cell growth factor; demonstration of specific binding to activated B cells; and development of a monoclonal antibody to the factor. J Exp Med. 1985 Oct 1;162(4):1319–1335. doi: 10.1084/jem.162.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazar B. A., Sutton L. M., Strome M. Self-stimulating growth factor production by B-cell lines derived from Burkitt's lymphomas and other lines transformed in vitro by Epstein-Barr virus. Cancer Res. 1983 Oct;43(10):4562–4568. [PubMed] [Google Scholar]
- Boyd A. W., Anderson K. C., Freedman A. S., Fisher D. C., Slaughenhoupt B., Schlossman S. F., Nadler L. M. Studies of in vitro activation and differentiation of human B lymphocytes. I. Phenotypic and functional characterization of the B cell population responding to anti-Ig antibody. J Immunol. 1985 Mar;134(3):1516–1523. [PubMed] [Google Scholar]
- Boyd A. W., Freedman A. S., Horowitz J. C., Anderson K. C., Fisher D. C., Rosen K. J., Schlossman S. F., Nadler L. M. Studies of the in vitro activation and differentiation of human B lymphocytes. II. Optimization of activation by anti-immunoglobulin antibody bound to beads: analysis of the role of autocrine effects on B-cell proliferation and of T-cell help in B-cell differentiation. Cell Immunol. 1986 Apr 15;99(1):228–240. doi: 10.1016/0008-8749(86)90231-5. [DOI] [PubMed] [Google Scholar]
- Chesnut R. W., Grey H. M. Studies on the capacity of B cells to serve as antigen-presenting cells. J Immunol. 1981 Mar;126(3):1075–1079. [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Falkoff R. J., Muraguchi A., Hong J. X., Butler J. L., Dinarello C. A., Fauci A. S. The effects of interleukin 1 on human B cell activation and proliferation. J Immunol. 1983 Aug;131(2):801–805. [PubMed] [Google Scholar]
- Gordon J., Aman P., Rosén A., Ernberg I., Ehlin-Henriksson B., Klein G. Capacity of B-lymphocytic lines of diverse tumor origin to produce and respond to B-cell growth factors: a progression model for B-cell lymphomagenesis. Int J Cancer. 1985 Feb 15;35(2):251–256. doi: 10.1002/ijc.2910350218. [DOI] [PubMed] [Google Scholar]
- Gordon J., Ley S. C., Melamed M. D., Aman P., Hughes-Jones N. C. Soluble factor requirements for the autostimulatory growth of B lymphoblasts immortalized by Epstein-Barr virus. J Exp Med. 1984 May 1;159(5):1554–1559. doi: 10.1084/jem.159.5.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J., Ley S. C., Melamed M. D., English L. S., Hughes-Jones N. C. Immortalized B lymphocytes produce B-cell growth factor. Nature. 1984 Jul 12;310(5973):145–147. doi: 10.1038/310145a0. [DOI] [PubMed] [Google Scholar]
- Gordon J., Rowe M., Walker L., Guy G. Ligation of the CD23,p45 (BLAST-2,EBVCS) antigen triggers the cell-cycle progression of activated B lymphocytes. Eur J Immunol. 1986 Sep;16(9):1075–1080. doi: 10.1002/eji.1830160908. [DOI] [PubMed] [Google Scholar]
- Jurgensen C. H., Ambrus J. L., Jr, Fauci A. S. Production of B cell growth factor by normal human B cells. J Immunol. 1986 Jun 15;136(12):4542–4547. [PubMed] [Google Scholar]
- Kehrl J. H., Muraguchi A., Butler J. L., Falkoff R. J., Fauci A. S. Human B cell activation, proliferation and differentiation. Immunol Rev. 1984 Apr;78:75–96. doi: 10.1111/j.1600-065x.1984.tb00477.x. [DOI] [PubMed] [Google Scholar]
- Klein G., Klein E. Evolution of tumours and the impact of molecular oncology. Nature. 1985 May 16;315(6016):190–195. doi: 10.1038/315190a0. [DOI] [PubMed] [Google Scholar]
- Kurt-Jones E. A., Kiely J. M., Unanue E. R. Conditions required for expression of membrane IL 1 on B cells. J Immunol. 1985 Sep;135(3):1548–1550. [PubMed] [Google Scholar]
- Lipsky P. E., Thompson P. A., Rosenwasser L. J., Dinarello C. A. The role of interleukin 1 in human B cell activation: inhibition of B cell proliferation and the generation of immunoglobulin-secreting cells by an antibody against human leukocytic pyrogen. J Immunol. 1983 Jun;130(6):2708–2714. [PubMed] [Google Scholar]
- Malynn B. A., Romeo D. T., Wortis H. H. Antigen-specific B cells efficiently present low doses of antigen for induction of T cell proliferation. J Immunol. 1985 Aug;135(2):980–988. [PubMed] [Google Scholar]
- Matsushima K., Kuang Y. D., Tosato G., Hopkins S. J., Oppenheim J. J. B-cell-derived interleukin 1 (IL-1)-like factor. I. Relationship of production of IL-1-like factor to accessory cell function of Epstein-Barr virus-transformed human B-lymphoblast lines. Cell Immunol. 1985 Sep;94(2):406–417. doi: 10.1016/0008-8749(85)90264-3. [DOI] [PubMed] [Google Scholar]
- Matsushima K., Procopio A., Abe H., Scala G., Ortaldo J. R., Oppenheim J. J. Production of interleukin 1 activity by normal human peripheral blood B lymphocytes. J Immunol. 1985 Aug;135(2):1132–1136. [PubMed] [Google Scholar]
- Matsushima K., Tosato G., Benjamin D., Oppenheim J. J. B-cell-derived interleukin-1 (IL-1)-like factor. II. Sources, effects, and biochemical properties. Cell Immunol. 1985 Sep;94(2):418–426. doi: 10.1016/0008-8749(85)90265-5. [DOI] [PubMed] [Google Scholar]
- Mehta S. R., Conrad D., Sandler R., Morgan J., Montagna R., Maizel A. L. Purification of human B cell growth factor. J Immunol. 1985 Nov;135(5):3298–3302. [PubMed] [Google Scholar]
- Melchers F., Andersson J. B cell activation: three steps and their variations. Cell. 1984 Jul;37(3):713–720. doi: 10.1016/0092-8674(84)90407-0. [DOI] [PubMed] [Google Scholar]
- Melchers F., Andersson J. Factors controlling the B-cell cycle. Annu Rev Immunol. 1986;4:13–36. doi: 10.1146/annurev.iy.04.040186.000305. [DOI] [PubMed] [Google Scholar]
- Monroe J. G., Cambier J. C. B cell activation. I. Anti-immunoglobulin-induced receptor cross-linking results in a decrease in the plasma membrane potential of murine B lymphocytes. J Exp Med. 1983 Jun 1;157(6):2073–2086. doi: 10.1084/jem.157.6.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraguchi A., Fauci A. S. Proliferative responses of normal human B lymphocytes. Development of an assay system for human B cell growth factor (BCGF). J Immunol. 1982 Sep;129(3):1104–1108. [PubMed] [Google Scholar]
- Rimsky L., Wakasugi H., Ferrara P., Robin P., Capdevielle J., Tursz T., Fradelizi D., Bertoglio J. Purification to homogeneity and NH2-terminal amino acid sequence of a novel interleukin 1 species derived from a human B cell line. J Immunol. 1986 May 1;136(9):3304–3310. [PubMed] [Google Scholar]
- Rubin L. A., Kurman C. C., Fritz M. E., Biddison W. E., Boutin B., Yarchoan R., Nelson D. L. Soluble interleukin 2 receptors are released from activated human lymphoid cells in vitro. J Immunol. 1985 Nov;135(5):3172–3177. [PubMed] [Google Scholar]
- Scala G., Kuang Y. D., Hall R. E., Muchmore A. V., Oppenheim J. J. Accessory cell function of human B cells. I. Production of both interleukin 1-like activity and an interleukin 1 inhibitory factor by an EBV-transformed human B cell line. J Exp Med. 1984 Jun 1;159(6):1637–1652. doi: 10.1084/jem.159.6.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Autocrine growth factors and cancer. 1985 Feb 28-Mar 6Nature. 313(6005):745–747. doi: 10.1038/313745a0. [DOI] [PubMed] [Google Scholar]
- Sugden B., Metzenberg S. Characterization of an antigen whose cell surface expression is induced by infection with Epstein-Barr virus. J Virol. 1983 Jun;46(3):800–807. doi: 10.1128/jvi.46.3.800-807.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Geilinger K. Monoclonal antibodies against the major glycoprotein (gp350/220) of Epstein-Barr virus neutralize infectivity. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5307–5311. doi: 10.1073/pnas.77.9.5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Mann K. P. Early events in Epstein-Barr virus infection provide a model for B cell activation. J Exp Med. 1985 Jul 1;162(1):45–59. doi: 10.1084/jem.162.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Nadler L. M., Bhan A. K., Schooley R. T. BLAST-2 [EBVCS], an early cell surface marker of human B cell activation, is superinduced by Epstein Barr virus. J Immunol. 1985 May;134(5):3007–3012. [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Schooley R. T., Bhan A. K., Nadler L. M. Epstein-Barr virus superinduces a new human B cell differentiation antigen (B-LAST 1) expressed on transformed lymphoblasts. Cell. 1982 Sep;30(2):415–425. doi: 10.1016/0092-8674(82)90239-2. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Swendeman S. L., Edson C. M. Biochemical analysis suggests distinct functional roles for the BLAST-1 and BLAST-2 antigens. J Immunol. 1986 Mar 1;136(5):1745–1751. [PubMed] [Google Scholar]
- Todaro G. J., De Larco J. E. Growth factors produced by sarcoma virus-transformed cells. Cancer Res. 1978 Nov;38(11 Pt 2):4147–4154. [PubMed] [Google Scholar]




