Abstract
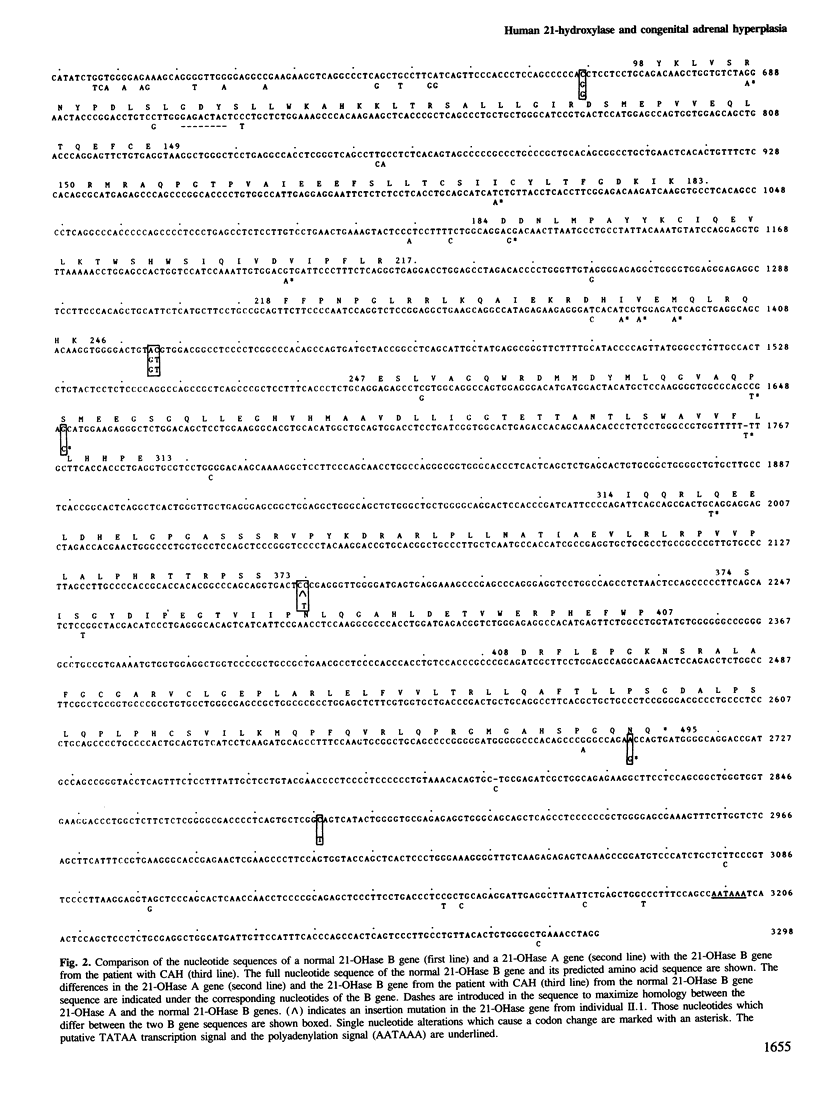
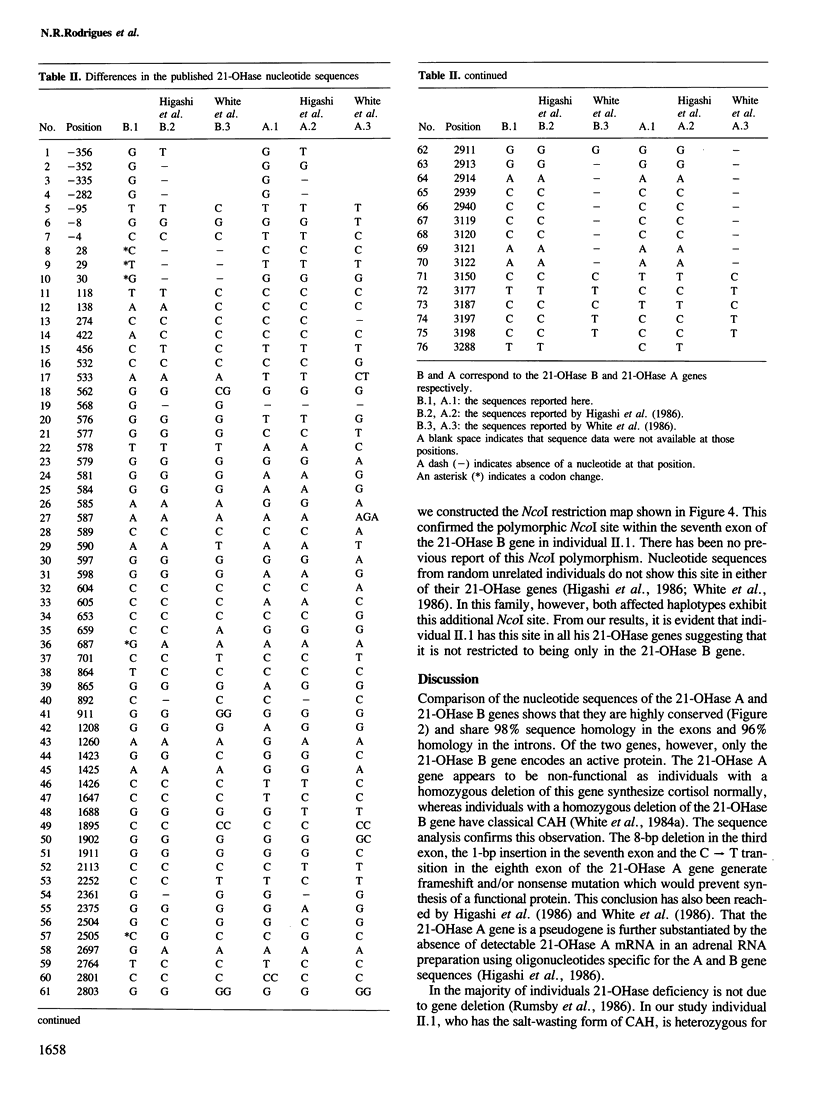
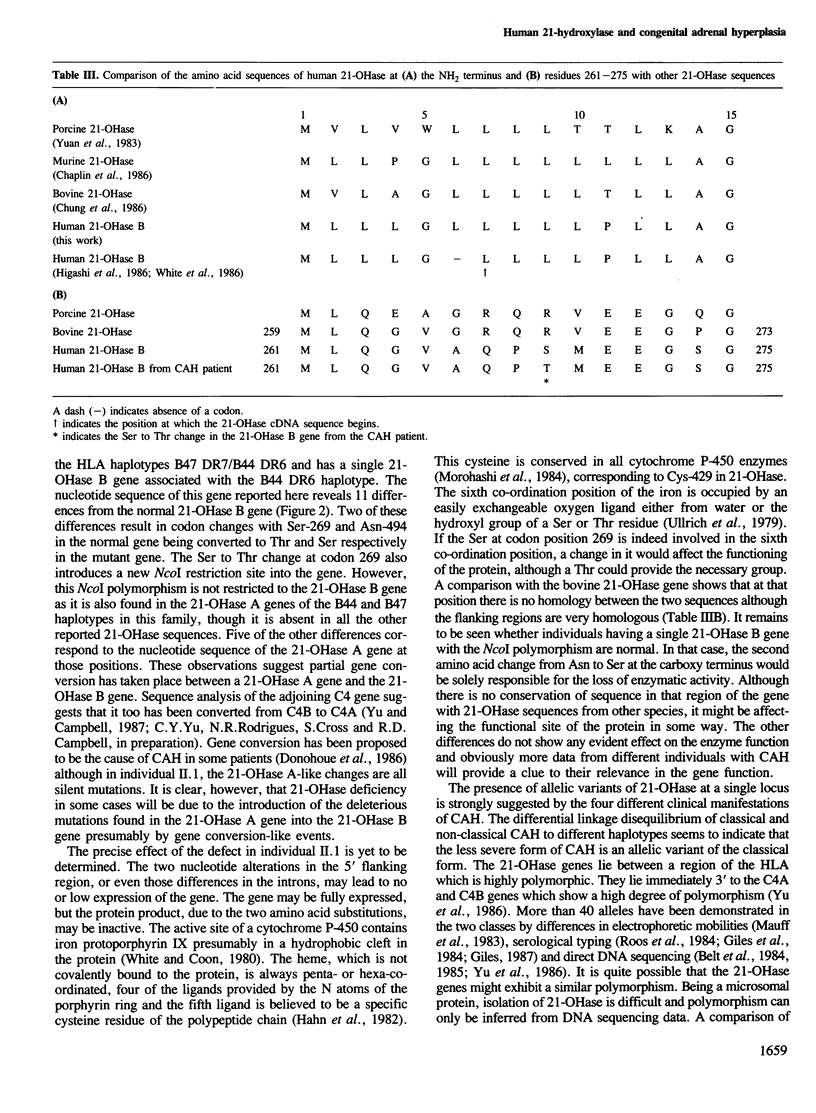
21-Hydroxylase deficiency which causes congenital adrenal hyperplasia is one of the most common defects of adrenal steroidogenesis. There are two 21-hydroxylase genes in man, A and B, and these have been mapped to the HLA class III region. Only the 21-hydroxylase B gene is thought to be active. To understand the molecular basis of congenital adrenal hyperplasia in a patient with the salt-wasting form of the disease, we cloned and characterized his single 21-hydroxylase B gene. The nucleotide sequence of this gene and a 21-hydroxylase B gene from a normal individual have been determined. Comparison of the two sequences has revealed 11 nucleotide alterations, of which two are in the 5' flanking region, four are in introns, one is in the 3' untranslated region and four are in exons. Two of the differences in exons cause codon changes, with Ser-269 and Asn-494 in the normal 21-hydroxylase B gene being converted to Thr and Ser, respectively. These amino acid substitutions may give an insight into those residues necessary for 21-hydroxylase enzymatic activity. We have also confirmed that the 21-hydroxylase A gene is a pseudogene due to three deleterious mutations in the exons. In addition, comparison of the 21-hydroxylase B gene sequence with other published sequences indicates that this microsomal cytochrome P-450 may be polymorphic.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anson D. S., Choo K. H., Rees D. J., Giannelli F., Gould K., Huddleston J. A., Brownlee G. G. The gene structure of human anti-haemophilic factor IX. EMBO J. 1984 May;3(5):1053–1060. doi: 10.1002/j.1460-2075.1984.tb01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. I., Karam J. H., Rutter W. J. Polymorphic DNA region adjacent to the 5' end of the human insulin gene. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5759–5763. doi: 10.1073/pnas.78.9.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belt K. T., Carroll M. C., Porter R. R. The structural basis of the multiple forms of human complement component C4. Cell. 1984 Apr;36(4):907–914. doi: 10.1016/0092-8674(84)90040-0. [DOI] [PubMed] [Google Scholar]
- Belt K. T., Yu C. Y., Carroll M. C., Porter R. R. Polymorphism of human complement component C4. Immunogenetics. 1985;21(2):173–180. doi: 10.1007/BF00364869. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPER D. Y., LEVIN S., NARASIMHULU S., ROSENTHAL O. PHOTOCHEMICAL ACTION SPECTRUM OF THE TERMINAL OXIDASE OF MIXED FUNCTION OXIDASE SYSTEMS. Science. 1965 Jan 22;147(3656):400–402. doi: 10.1126/science.147.3656.400. [DOI] [PubMed] [Google Scholar]
- Carroll M. C., Belt T., Palsdottir A., Porter R. R. Structure and organization of the C4 genes. Philos Trans R Soc Lond B Biol Sci. 1984 Sep 6;306(1129):379–388. doi: 10.1098/rstb.1984.0098. [DOI] [PubMed] [Google Scholar]
- Carroll M. C., Campbell R. D., Bentley D. R., Porter R. R. A molecular map of the human major histocompatibility complex class III region linking complement genes C4, C2 and factor B. Nature. 1984 Jan 19;307(5948):237–241. doi: 10.1038/307237a0. [DOI] [PubMed] [Google Scholar]
- Carroll M. C., Campbell R. D., Porter R. R. Mapping of steroid 21-hydroxylase genes adjacent to complement component C4 genes in HLA, the major histocompatibility complex in man. Proc Natl Acad Sci U S A. 1985 Jan;82(2):521–525. doi: 10.1073/pnas.82.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll M. C., Palsdottir A., Belt K. T., Porter R. R. Deletion of complement C4 and steroid 21-hydroxylase genes in the HLA class III region. EMBO J. 1985 Oct;4(10):2547–2552. doi: 10.1002/j.1460-2075.1985.tb03969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin D. D., Galbraith L. J., Seidman J. G., White P. C., Parker K. L. Nucleotide sequence analysis of murine 21-hydroxylase genes: mutations affecting gene expression. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9601–9605. doi: 10.1073/pnas.83.24.9601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung B. C., Matteson K. J., Miller W. L. Structure of a bovine gene for P-450c21 (steroid 21-hydroxylase) defines a novel cytochrome P-450 gene family. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4243–4247. doi: 10.1073/pnas.83.12.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoue P. A., van Dop C., McLean R. H., White P. C., Jospe N., Migeon C. J. Gene conversion in salt-losing congenital adrenal hyperplasia with absent complement C4B protein. J Clin Endocrinol Metab. 1986 May;62(5):995–1002. doi: 10.1210/jcem-62-5-995. [DOI] [PubMed] [Google Scholar]
- Dupont B., Oberfield S. E., Smithwick E. M., Lee T. D., Levine L. S. Close genetic linkage between HLA and congenital adrenal hyperplasia (21-hydroxylase deficiency). Lancet. 1977 Dec 24;2(8052-8053):1309–1312. doi: 10.1016/s0140-6736(77)90362-2. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Finkelstein M., Shaefer J. M. Inborn errors of steroid biosynthesis. Physiol Rev. 1979 Apr;59(2):353–406. doi: 10.1152/physrev.1979.59.2.353. [DOI] [PubMed] [Google Scholar]
- Giles C. M., Batchelor J. R., Dodi I. A., Fielder A. H., Rittner C., Mauff G., Bender K., Levene C., Schreuder G. M., Wells L. J. C4 and HLA haplotypes associated with partial inhibition of anti-Rg and anti-Ch. J Immunogenet. 1984 Oct-Dec;11(5-6):305–317. doi: 10.1111/j.1744-313x.1984.tb00817.x. [DOI] [PubMed] [Google Scholar]
- Hahn J. E., Hodgson K. O., Andersson L. A., Dawson J. H. Endogenous cysteine ligation in ferric and ferrous cytochrome P-450. Direct evidence from x-ray absorption spectroscopy. J Biol Chem. 1982 Sep 25;257(18):10934–10941. [PubMed] [Google Scholar]
- Higashi Y., Yoshioka H., Yamane M., Gotoh O., Fujii-Kuriyama Y. Complete nucleotide sequence of two steroid 21-hydroxylase genes tandemly arranged in human chromosome: a pseudogene and a genuine gene. Proc Natl Acad Sci U S A. 1986 May;83(9):2841–2845. doi: 10.1073/pnas.83.9.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauff G., Alper C. A., Awdeh Z., Batchelor J. R., Bertrams J., Bruun-Petersen G., Dawkins R. L., Démant P., Edwards J., Grosse-Wilde H. Statement on the nomenclature of human C4 allotypes. Immunobiology. 1983 Mar;164(2):184–191. doi: 10.1016/s0171-2985(83)80009-6. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Morohashi K., Fujii-Kuriyama Y., Okada Y., Sogawa K., Hirose T., Inayama S., Omura T. Molecular cloning and nucleotide sequence of cDNA for mRNA of mitochondrial cytochrome P-450(SCC) of bovine adrenal cortex. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4647–4651. doi: 10.1073/pnas.81.15.4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New M. I., Levine L. S. Recent advances in 21-hydroxylase deficiency. Annu Rev Med. 1984;35:649–663. doi: 10.1146/annurev.me.35.020184.003245. [DOI] [PubMed] [Google Scholar]
- Nussinov R. Some guidelines for identification of recognition sequences: regulatory sequences frequently contain (T)GTG/CAC(A), TGA/TCA and (T)CTC/GAG(A). Biochim Biophys Acta. 1986 Mar 26;866(2-3):93–108. doi: 10.1016/0167-4781(86)90106-5. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roos M. H., Giles C. M., Demant P., Mollenhauer E., Rittner C. Rodgers (Rg) and Chido (Ch) determinants on human C4: characterization of two C4 B5 subtypes, one of which contains Rg and Ch determinants. J Immunol. 1984 Nov;133(5):2634–2640. [PubMed] [Google Scholar]
- Ruf H. H., Wende P., Ullrich V. Models for ferric cytochrome P450. Characterization of hemin mercaptide complexes by electronic and ESR spectra. J Inorg Biochem. 1979 Nov;11(3):189–204. doi: 10.1016/s0162-0134(00)80017-3. [DOI] [PubMed] [Google Scholar]
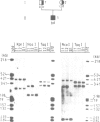
- Rumsby G., Carroll M. C., Porter R. R., Grant D. B., Hjelm M. Deletion of the steroid 21-hydroxylase and complement C4 genes in congenital adrenal hyperplasia. J Med Genet. 1986 Jun;23(3):204–209. doi: 10.1136/jmg.23.3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Speiser P. W., Dupont B., Rubinstein P., Piazza A., Kastelan A., New M. I. High frequency of nonclassical steroid 21-hydroxylase deficiency. Am J Hum Genet. 1985 Jul;37(4):650–667. [PMC free article] [PubMed] [Google Scholar]
- Ullrich V., Sakurai H., Ruf H. H. Model systems for the coordination chemistry of cytochrome P-450. Acta Biol Med Ger. 1979;38(2-3):287–297. [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P. C., Grossberger D., Onufer B. J., Chaplin D. D., New M. I., Dupont B., Strominger J. L. Two genes encoding steroid 21-hydroxylase are located near the genes encoding the fourth component of complement in man. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1089–1093. doi: 10.1073/pnas.82.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P. C., New M. I., Dupont B. Cloning and expression of cDNA encoding a bovine adrenal cytochrome P-450 specific for steroid 21-hydroxylation. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1986–1990. doi: 10.1073/pnas.81.7.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P. C., New M. I., Dupont B. HLA-linked congenital adrenal hyperplasia results from a defective gene encoding a cytochrome P-450 specific for steroid 21-hydroxylation. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7505–7509. doi: 10.1073/pnas.81.23.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P. C., New M. I., Dupont B. Structure of human steroid 21-hydroxylase genes. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5111–5115. doi: 10.1073/pnas.83.14.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. E., Coon M. J. Oxygen activation by cytochrome P-450. Annu Rev Biochem. 1980;49:315–356. doi: 10.1146/annurev.bi.49.070180.001531. [DOI] [PubMed] [Google Scholar]
- Yoshioka H., Morohashi K., Sogawa K., Yamane M., Kominami S., Takemori S., Okada Y., Omura T., Fujii-Kuriyama Y. Structural analysis of cloned cDNA for mRNA of microsomal cytochrome P-450(C21) which catalyzes steroid 21-hydroxylation in bovine adrenal cortex. J Biol Chem. 1986 Mar 25;261(9):4106–4109. [PubMed] [Google Scholar]
- Yu C. Y., Belt K. T., Giles C. M., Campbell R. D., Porter R. R. Structural basis of the polymorphism of human complement components C4A and C4B: gene size, reactivity and antigenicity. EMBO J. 1986 Nov;5(11):2873–2881. doi: 10.1002/j.1460-2075.1986.tb04582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P. M., Nakajin S., Haniu M., Shinoda M., Hall P. F., Shively J. E. Steroid 21-hydroxylase (cytochrome P-450) from porcine adrenocortical microsomes: microsequence analysis of cysteine-containing peptides. Biochemistry. 1983 Jan 4;22(1):143–149. doi: 10.1021/bi00270a021. [DOI] [PubMed] [Google Scholar]