Abstract
Background:
Patients with severe cervical multilevel stenosis and an adequate lordotic curvature often undergo multilevel laminectomies with posterior instrumented fusions. Although the “gold standard” for the fusion mass remains iliac crest autograft, many require additional volume provided by bone graft expanders. Here, we studied the fusion rates for 32 patients undergoing multilevel cervical laminectomy and vertex/rod/eyelet/titanium cable fusions utilizing lamina/iliac autograft and the bone graft expander Nanoss (RTI Surgical, Alachua, FL, USA) with autogenous bone marrow aspirate (BMA).
Methods:
Thirty-two patients, averaging 63.0 years of age, presented with severe cervical myeloradiculopathy (average Nurick Grade 4.4). Magnetic resonance (MR) studies documented 2–3-level high intrinsic cord signals, whereas computed tomography (CT) scans confirmed marked stenosis and ossification of the posterior longitudinal ligament (OPLL)/ossification of the yellow ligament (OYL). Patients underwent multilevel lamnectomies/instrumented fusions, and were followed up for an average of 2.7 years.
Results:
Multilevel laminectomies (2.8 levels) and average 7.8-level vertex/rod/eyelet/cable fusions were performed utilizing lamina/iliac crest autograft and Nanoss/BMA. Fusion was confirmed on X-ray/CT studies an average of 4.7 months postoperatively in 31 of 32 patients (97%); there was just one pseudarthrosis requiring secondary surgery. The only other complication was a delayed transient C5 palsy that fully resolved in 6 postoperative months.
Conclusions:
Thirty-two severely myelopathic underwent 2.8-level cervical laminectomies/7.8 level fusions utilizing lamina/iliac autograft and Nanoss/BMA. Fusion was documented on both dynamic X-ray and CT studies in 31 of 32 (97%) patients an average of 4.7 months postoperatively. Nanoss/BMA appears to be a safe and effective bone graft expander that can be utilized for posterior cervical fusions.
Keywords: Bone graft expander, bone marrow aspirate (BMA), high fusion rates, Nanoss, posterior cervical fusions
INTRODUCTION
Following multilevel cervical laminectomies/posterior fusions, the volume of iliac crest autograft (e.g., the “gold standard”) may require supplementation with a bone graft expander. Although there are many types available here we prospectively performed 32 multilevel level cervical laminectomies/posterior instrumented fusions (vertex/rod/eyelet/titanium cable) utilizing lamina/iliac crest autograft supplemented with Nanoss (RTI Surgical Alachua, FL, USA) and autologous bone marrow aspirate (BMA). Over an average of 2.7 postoperative years, we utilized both dynamic X-ray and computed tomography (CT) studies to assess fusion rates.
MATERIALS AND METHODS
Prospectively, 32 patients averaging 63 years of age presented with severe cervical myeloradiculopathy (average Nurick Grade 4.4) [Table 1]. Marked focal stenosis was documented on both magnetic resonance (MR) and computed tomographic (CT) studies along with ossification of the posterior longitudinal ligament (OPLL) and/or ossification of the yellow ligament (OYL) [Figures 1–4]. Patients underwent multilevel laminectomies and posterior vertex rod/eyelet/titanium cable fusions [Table 2] [Figures 5 and 6]. The fusion mass consisted of lamina/iliac crest autograft and Nanoss with BMA. Fusion rates were analyzed over an average of 2.7 postoperative years [Figures 7 and 8].
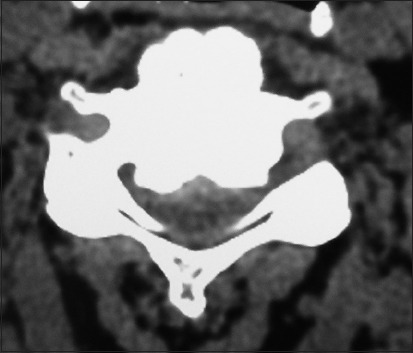
Table 1.
Clinical data for posterior cervical fusions using bone graft expander Nanoss with bone marrow aspirate to supplement fusion mass
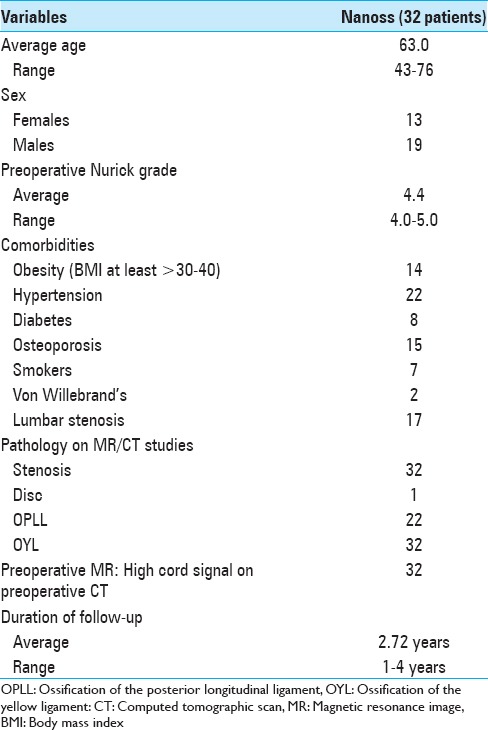
Figure 1.
This preoperative cervical T2-weighted sagittal midline MR documented posterolateral cord compression at the C3–C4 level and C6–C7 levels. Here, lamienctomies at C3 and C6/C7 resulted in sufficient posterolateral cord decompression with resection of ossification of the yellow ligament and decompression of dorsolateral shingling of the respective laminae. Here, laminectomy of C3, C6, and C7 with C2–T2 posterior fusion resulted in adequate cord decompression
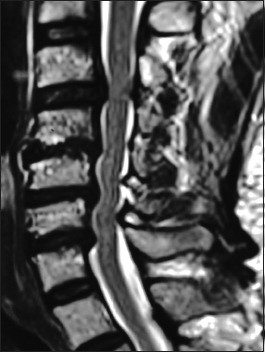
Figure 4.
On this preoperative axial noncontrast CT study obtained at the C5–C6 level, there is marked ventral ossification of the posterior longitudinal ligament accompanied by dorsolateral inward shingling of both the C5 and C6 laminae (note both laminae are seen on the same image posteriorly). The combined pathology reduced the AP diameter of the spinal canal to less than 6 mm
Table 2.
Surgical data for 32 cervical laminectomies/posterior fusions with Nanoss/bone marrow aspirate (BMA)
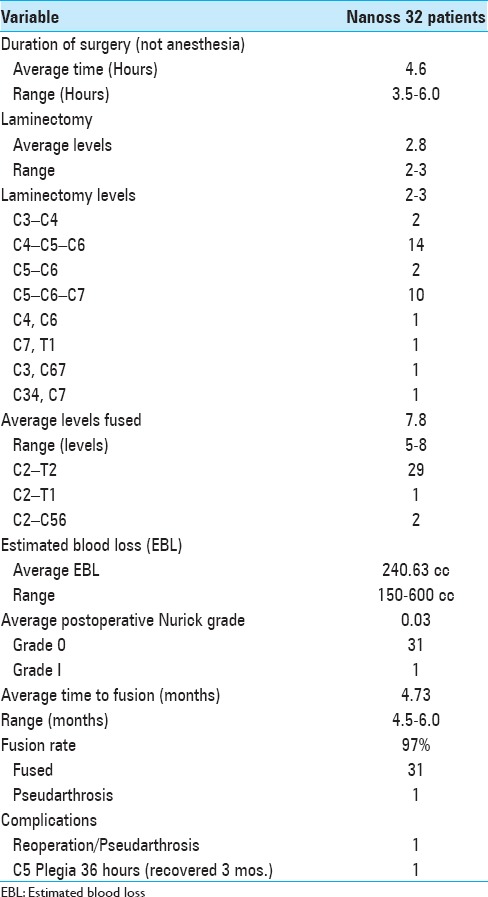
Figure 5.
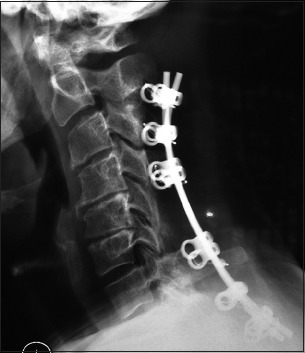
The postoperative plain X-ray documented the laminectomy defects at the C5, C6 levels, and the posterior vertex/rod/eyelet/titanium cable system applied to the spinous processes of C2, C3, C4, C7, T1, and T2. Note there was some pullout of C2 but the remaining cables remained in place until the patient fused without the need for further surgery
Figure 6.
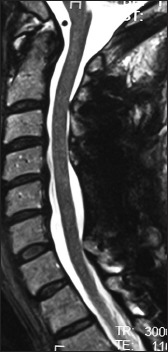
The 6-week postoperative T2 sagittal MR study documented excellent decompression of the spinal cord following laminectomy of C5, C6 with posterior vertex/rod/eyelet/titanium cable fusion C2T2. Note in this case the preoperative increased signal in the cord at the C5–C6 level fully resolved
Figure 7.
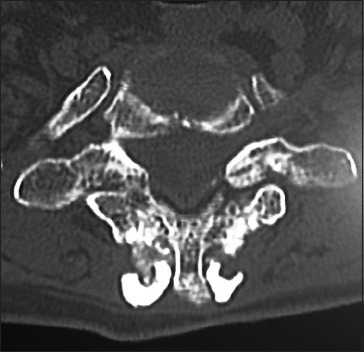
On the 6-month postoperative axial CT, fusion over the laminae/continuity of the bone fragments attributed to lamina/iliac crest autograft and Nanoss/BMA/ was noted
Figure 8.
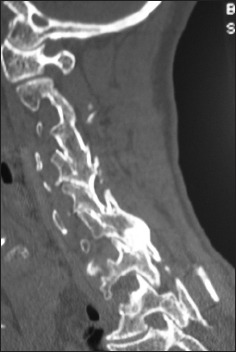
Parasagittal 6-month postoperative 2D-CT documented fusion across multiple lateral laminae and facet joints utilizing lamina/iliac crest autograft and Nanoss/BMA for posterolateral C2-T2 fusion.
Figure 2.
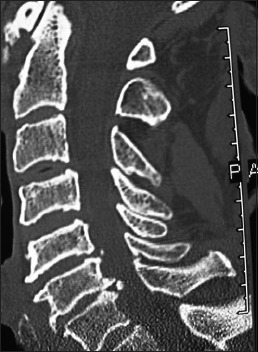
Preoperative 2D-sagittal CT documenting stenosis C45/56/67 associated with ossification of the posterior longitudinal ligament and dorsolateral shingling of the laminae. Here a laminectomy of C5, C6, C7 with posterior fusion C2–T2 resulted in adequate cord decompression
Figure 3.
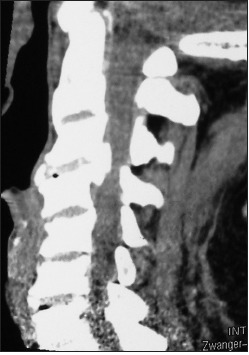
The preoperative soft tissue sagittal 2D-CT documented marked spinal stenosis at the C3–C4 level attributed to anterior ossification of the posterior longitudinal ligament (OPLL) and dorsolateral inward shingling/stenosis involving the C3 and C4 laminae. Here, a laminectomy of C3, C4 with posterior fusion C2–C5/C6 adequately decompressed the spinal cord
Fusion technique/mass: Vertex/rod/eyelet fusion, autograft, and Nanoss/BMA
The vertex/rod/eyelet/titanium cable system was applied to the intact spinous processes cephalad/caudad to the laminectomy levels [Figure 5]. Ten cc of BMA, collected during the iliac crest autograft harvesting, was then immediately applied to the 2.5 cm. × 10 cm Nanoss strips. The laminae and facet joints were then decorticated cephalad/caudad to the laminectomy levels; at the laminectomy levels, only the very lateral residual laminae/facets were decorticated. Lamina and iliac crest autograft (morcellized with a rongeur/but not turned into a paste with the bone mill) were applied over the decorticated surfaces, the largest volumes being placed where the laminae were intact, whereas only the smallest cancellous bone fragements were applied laterally at the laminectomy sites. Finally, Nanoss/BMA strips were placed dorsal to the autograft; ¼ inch strips over the intact laminar levels, but just 1/8th inch strips laterally at the laminectomy levels.
RESULTS
Cervical surgery required an average of 4.6 hours. Patients underwent average 2.8-level cervical laminectomies/7.8-level posterior fusions utilizing lamina/iliac crest autograft and Nanoss/BMA [Table 2] [Figures 5–8]. Most posterior fusions extended from C2–T2 (29 of 32 patients). Routine postoperative MR studies, obtained 0–6 weeks postoperatively, confirmed adequate cord decompression. Intrinsic cord on these MR studies resolved in half of the patients, whereas the other half exhibited residual myelomalacia. Dynamic X-ray/CT studies documented fusion in 31 of 32 patients (97%) an average of 4.7 months postoperatively. Only 1 patient developed a pseudarthrosis; this was attributed to severe osteoporosis and resultant kyphosis with wire pull-out requiring a secondary fusion. The only other complication was a delayed/transient bilateral C5 palsy. This occurred 36 hours postoperatively in a diabetic who underwent a C4, C5, C6 laminectomy with C2–T2 posterior fusion; his deficit spontaneously resolved within 6 postoperative months.
Avoidance of infections
No patient developed an infection. The avoidance of infections was largely attributed to the use of Hibiclens washes started 2 weeks preoperatively, the intraoperative use of antibiotic irrigation every 15 minutes, the use of postoperative prophylactic antibiotics, and utilizing a Silverlon dressing for up to 1 month postoperatively on the posterior cervical wound.
DISCUSSION
Iliac crest autograft (the “gold standard”) with Nanoss/BMA
Iliac crest autograft is still considered the “gold standard” for a fusion mass. Nevertheless, when the autograft fusion mass is insufficient, Nanoss provides a Food and Drug Administration (FDA) approved bone void filler/expander (e.g., approved for posterolateral spinal fusions) when combined with laminar/iliac autograft and BMA. Nanoss, composed of nanostructured hydroxyapatite (HA), is an engineered extracellular osteoconductive bioscaffold matrix that facilitates cell infiltration. In this study, we confirmed the efficacy of Nanoss/BMA in promoting a 97% posterior cervical fusion rate. In several prior studies, Nanoss was found to be comparable to Vitoss (Stryker, Kalamazoo, MI, USA).
Safety/efficacy of Vitoss, and Vitoss vs. Nanoss in Lumbar Spine Fusion
The safety and efficacy of Vitoss and Vitoss vs. Nanoss as bone graft expanders were documented in prior studies of the lumbar spine. In 2006, Epstein utilized Vitoss and lamina autograft (50:50 mix) to perform 40 laminectomies (average 3.7 levels), and 1 (27 patients) and 2 (13 patients) level posterolateral instrumented pedicle/screw fusions.[2] At 6 postoperative months, dynamic X-rays and CT studies confirmed fusion for 26 of 27 single-level fusions, and 11 of 13 two-level fusions; only 1 of the latter patients with symptomatic pseudarthrosis required secondary surgery. In a review of the literature in 2008, Epstein found similar fusion rates and outcomes for noninstrumented vs. instrumented lumbar fusions utilizing demineralized bone matrix (DBMs)/allografts, hydroxyapatite (HA), and Beta TriCalcium Phosphate (B-TCP: Vitoss).[3] In 2015, Epstein (2015) performed an initial comparison of the efficacy of Vitoss (213 patients) vs. Nanoss (45 patients) in promoting posterolateral lumbar noninstrumented fusions.[7] Patients underwent comparable multilevel lumbar laminectomies (average 4.6 vs. 4.5 levels, respectively), and noninstrumented fusions (average 1.3 vs. 1.2 levels, respectively). Both fusion groups utilizing Vitoss vs. Nanoss demonstrated nearly comparable; times to fusion (5.3 months vs. 4.8 months; notably somewhat shorter), fusion rates [210 (98.6%) vs. 45 (100%) patients], rates of pseudarthroses [3 (1.4%) vs. 0], incidence of postoperative seromas [2 (0.94%) vs. 0], and deep wound infections [2 (0.94%) vs. 0].
Safety/efficacy of Vitoss and Vitoss vs. Nanoss in Cervical Spine Fusion
The safety and efficacy of Vitoss and Vitoss vs. Nanoss as bone graft expanders were also previously documented in the cervical spine. In 2008, Epstein evaluated the fusion rates for 35 severely myelopathic adults (mean Nurick Grade 4.1) undergoing average 2-level laminectomies with average 7-level posterior cervical vertex/rod eyelet/braided titanium cable fusions utilizing lamina/iliac autograft and Vitoss/BMA.[4] For these adults averaging 65 years of age, 100% fusion was documented on both dynamic X-rays and CT studies an average of 5.2 months postoperatively. Complications included 2 transient root injuries (transient C5 palsies in diabetic patients that spontaneously resolved), 2 wound infections, 1 wound breakdown, no pseudarthroses, no reoperations, no cord injuries, and no mortalities. In 2011, Epstein next reviewed 53 severely myelopathic patients averaging 65.3 years of age undergoing cervical laminectomies (average 2.3 levels) and instrumented fusions (average 7.5 levels) again utilizing iliac crest autograft and B-TCP/Vitoss.[5] Fusion occurred on X-ray studies (100%) and 2D-CT studies (86.8% of patients) an average of 5.4 months postoperatively; 3 smokers demonstrated delayed fusions at 8 postoperative months. In 2015, comparing two successive cohorts, Epstein preliminarily confirmed the efficacy of Vitoss (72 patients) vs. Nanoss (20 patients) (RTI: Alachua, FL, USA) as bone graft expanders for posterior cervical fusions (vertex/rod/eyelet/braided titanium cables).[6] Fusion was respectively documented an average of 5.65 vs. 5.35 months postoperatively utilizing both dynamic X-ray and CT studies. There were 2 cases of pseudarthrosis among the 72 fused with Vitoss vs. none in the 20 patients receiving Nanoss/BMA. Notably, in the present study, 31 of 32 (97%) patients fused utilizing lamina/iliac crest autograft and Nanoss/BMA fused on postoperative dynamic-ray/CT studies obtained an average of 4.7 months postoperatively.
Other bone graft expanders
Bone morphogenetic protein, demineralized bone matrix, and ceramics
Other bone graft expanders/supplements, including bone morphogenetic protein [BMP: Infuse; Medtronic, Memphis, USA)], demineralized bone matrix (DBM), and ceramics, have promoted spinal fusions. BMP promoted fusions even without autograft; however, several authors were concerned about observed/anticipated complications (e.g. heterotopic ossification, osteolysis, postoperative seromas, increased infection, and increased cancer rates).[1,9,10] In 2013, Grabowski and Cornett acknowledged that, although iliac crest bone graft remained the “gold standard,” multiple different bone graft substitutes (BMP, DBM, other graft expanders/allograft) were increasingly in use.[9] They too noted the: “recent concern regarding their safety (BMP) has tempered enthusiasm regarding their use.” In 2015, Bauman et al. studied 101 posterolateral thoracolumbar instrumented fusions (PLF) addressing traumatic vertebral fractures.[1] The fusion rates for both groups were nearly comparable; 94% with DBM/PLF (16 patients) and 100% with autograft/PLF (46 patients). The complication rates were also similar for both groups; 1 deep infection in the DBM group and 2 superficial wound infections in the autograft bone group (ABG) group. In 2016, using Medline, Kadam et al. identified 181 clinical studies that utilized BMP (62 studies, 34.25%), ceramics (40 studies), and allografts (39 studies) as bone graft expanders spinal fusions.[10] Although clinical outcomes were comparable in all groups, the best fusion rates were obtained utilizing BMP, followed by allograft, and lastly DBM.
Actifuse (Baxter Corporation, Franklin Lakes, NJ, USA, Deerfield Il, USA)
Actifuse is another bone graft substitute/extender used in spine surgery. In Lerner and Liljenqvist study in 2013, Actifuse (Si-CaP) was combined with BMA to provide an adequate fusion mass for adolescent idiopathic scoliosis surgery (AIS).[11] They acknowledged local/iliac autograft was the “gold standard,” but cited the increased morbidity associated with donor site harvesting. Utilizing 20–40 ml of ACTIFUSE/BMA, they documented 100% fusion at 2 postoperative years based on X-ray studies alone (no CT studies); all patients fused without a loss of correction, implant failures, or adverse events. In a 2015 randomized controlled trial performed over a 2-year period, Licina et al. (Global Spine) found comparable high fusion rates following posterolateral instrumented lumbar fusions (PLF) utilizing either Actifuse (SiCaP: 9 of 9 patients) vs. BMP (rhBMP-2; 9 of 10 patients).[12] Note, however, this study involved an extremely small number of patients in each cohort. Later, in 2016, in a rabbit animal model, Fredericks et al. compared PLF (L5–L6 levels) rates using Signafuse® Bioactive Bone Graft Putty vs. Actifuse® ABX.[8] Postoperative assessment at 6–12 weeks included X-rays, biomechanical testing, and histology. Although the fusion rates were similar (50%) and MicroCT findings were comparable, histological fusion scores were higher for Signafuse.
CONCLUSION
Multiple bone graft expanders (e.g., BMP, DBM, Vitoss, Actifuse, and now Nanoss) have been trialed to supplement and/or occasionally supplant iliac crest bone graft. In this study, Nanoss/BMA combined with iliac/laminar autograft resulted in a 97% posterior cervical fusion rate in 31 of 32 patients, confirmed on both dynamic X-ray and CT studies an average of 4.7 months postoperatively. Notably, other than the one pseudarthrosis, there were no Nanoss-related complications. These preliminary data appear to confirm the safety/efficacy of Nanoss for posterolateral cervical spine fusions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
REFERENCES
- 1.Baumann F, Krutsch W, Pfeifer C, Neumann C, Nerlich M, Loibl M. Posterolateral fusion in acute traumatic thoracolumbar fractures: A comparison of demineralized bone matrix and autologous bone graft. Acta Chir Orthop Traumatol Cech. 2015;82:119–25. [PubMed] [Google Scholar]
- 2.Epstein NE. A preliminary study of the efficacy of Beta Tricalcium Phosphate as a bone expander for instrumented posterolateral lumbar fusions. J Spinal Disord Tech. 2006;19:424–9. doi: 10.1097/00024720-200608000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Epstein NE. Efficacy of different bone volume expanders for augmenting lumbar fusions. Surg Neurol. 2008;69:16–9. doi: 10.1016/j.surneu.2007.03.021. discussion 19. [DOI] [PubMed] [Google Scholar]
- 4.Epstein NE. An argument for traditional posterior cervical fusion techniques: Evidence from 35 cases. Surg Neurol. 2008;70:45–51. doi: 10.1016/j.surneu.2007.10.023. discussion 51-2. [DOI] [PubMed] [Google Scholar]
- 5.Epstein NE. Efficacy of posterior cervical fusions utilizing an artificial bone graft expander, beta tricalcium phosphate. Surg Neurol Int. 2011;2:15. doi: 10.4103/2152-7806.76458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Epstein NE. Preliminary documentation of the comparable efficacy of vitoss versus NanOss bioactive as bone graft expanders for posterior cervical fusion. Surg Neurol Int. 2015;6(Suppl 4):S164–71. doi: 10.4103/2152-7806.156559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Epstein NE. Preliminary study showing safety/efficacy of nanoss bioactive versus vitoss as bone graft expanders for lumbar noninstrumented fusions. Surg Neurol Int. 2015;6(Suppl 10):S318–22. doi: 10.4103/2152-7806.159380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fredericks D, Petersen EB, Watson N, Grosland N, Gibson-Corley K, Smucker J. Comparison of Two Synthetic Bone Graft Products in a Rabbit Posterolateral Fusion Model. Iowa Orthop J. 2016;36:167–73. [PMC free article] [PubMed] [Google Scholar]
- 9.Grabowski G, Cornett CA. Bone graft and bone graft substitutes in spine surgery: Current concepts and controversies. J Am Acad Orthop Surg. 2013;21:51–60. doi: 10.5435/JAAOS-21-01-51. [DOI] [PubMed] [Google Scholar]
- 10.Kadam A, Millhouse PW, Kepler CK, Radcliff KE, Fehlings MG, Janssen ME, et al. Bone substitutes and expanders in Spine Surgery: A review of their fusion efficacies. Int J Spine Surg. 2016;10:33. doi: 10.14444/3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lerner T, Liljenqvist U. Silicate-substituted calcium phosphate as a bone graft substitute in surgery for adolescent idiopathic scoliosis. Eur Spine J. 2013;22(Suppl 2):S185–94. doi: 10.1007/s00586-012-2485-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Licina P, Coughlan M, Johnston E, Pearcy M. Comparison of Silicate-Substituted Calcium Phosphate (Actifuse) with Recombinant Human Bone Morphogenetic Protein-2 (Infuse) in Posterolateral Instrumented Lumbar Fusion. Global Spine J. 2015;5:471–8. doi: 10.1055/s-0035-1566230. [DOI] [PMC free article] [PubMed] [Google Scholar]


