Abstract
Skin tumours are among the most frequent tumour types of mankind. In the case of large tumours, field cancerization, or satellitosis scalping surgery is a possible option. The procedure can also be used in a palliative setting with tumour debulking. Less common indications are multiple benign tumours of the scalp and chronic inflammatory scalp dermatoses not responding to medical treatment. We present a case series and discuss surgical modalities beyond curative surgery of primary skin cancer.
Keywords: Skin tumours, scalping surgery, scalp, mesh graft transplantation, dermal template
Introduction
The most common skin tumours are non-melanoma skin cancers (NMSC). A very frequent localisation is the scalp. In the case of large tumours, a scalping surgery may become necessary.
We define scalping surgery as full-thickness soft tissue removal of at least one-third of the capillitium resulting in large defects. Scalping surgery may also include removal of periosteum or outer table of the scalp. Large defects with full thickness loss of soft tissue down to the bone require complex reconstructive options. In many cases, complete defect closure can be obtained by local flaps, tissue expander or free vascularized flaps [1, 2].
Comorbidities among elderly patients often limit anaesthetic tolerance and the use of distant flaps. In such cases, a defect closure is performed by mesh graft transplantation. The graft take can be improved by a combination of a dermal matrix template. Such a technique allows also mesh graft transplantation on bony underground [3-7].
Since permanent alopecia is a consequence of scalping surgery, the procedure needs to be discussed with every single patient in detail. Nevertheless, scalping surgery has its place in primary tumour surgery, in cases of field cancerization of the scalp with multiple NMSC, palliative oncology, and in rare benign conditions. We will discuss the possible indications, outcome and limitations of such an aggressive surgical approach aside from curative surgery of primary skin tumours.
Field cancerization
A 76-year-old female patient presented in 2011 with nevoid amelanotic melanoma and satellitosis of the scalp, which was BRAF-wild type. She was treated by Mohs surgery, and the defect was closed by a rotational flap. Since resection of satellitosis was incomplete, a second surgery was planned. But on return, she already presented multiple new non-pigmented cutaneous scalp metastases. We started with intralesional interleukin-2 (IL-2) treatment in 2011 and performed five courses until 2014. Due to the new formation of scalp satellitosis, palliative laser therapy – initially by erbium-YAG, later by pulsed 980nm diode laser – was performed as demanded. The treatment was better tolerated than IL-2 but no longer remission period could be achieved. More than 100 metastases have been observed (Fig. 1).
Figure 1.

Satellitosis of multiple non-pigmented melanoma metastases
Repeated imaging by X-ray, magnetic resonance imaging (MRI) and positron emission computerised tomography (PET) was negative for any metastatic spread distant from the scalp. Serum level of S100 remained low. Eventually, she was treated by scalping surgery followed by meshed skin graft transplantation. During follow-up of months, no relapse occurred (Fig. 2).
Figure 2.

After scalping surgery and meshed skin graft transplantation. The marked area was removed – it was a milia-like formation, no metastasis
A 40-year-old male patients with cranial dysplasia, thoracic deformities, scoliosis, and a mandibular cyst was referred to us because of multiple basal cell carcinomas (BCCs) of the head measuring up to 4.5 cm in diameter. Some of the tumours were ulcerated (Fig. 3).
Figure 3.

Gorlin-Goltz syndrome with multiple basal cell carcinomas, some ulcerated
Genetic analysis detected a heterozygous mutation c.290delA in exon 2 of the PTCH1 gene. Our findings confirmed the diagnosis of Gorlin-Goltz syndrome. The patient had been offered serial surgery, medical treatment with hedgehog inhibitor vismodegib, and scalping surgery. He decided to have scalping surgery which was performed under general anaesthesia (Fig. 4).
Figure 4.

After scalping surgery
The full-thickness defect was closed in the second step by meshed skin graft transplantation (Fig. 5). During follow-up of 10 months, there was no relapse.
Figure 5.

Five days after meshed skin graft transplantation
Debulking surgery
We performed debulking surgery in an 80-year-old male patient with a recurrent amelanotic lentigo melanoma of the scalp (Fig. 6).
Figure 6.

Relapsing Amelanotic lentigo maligna melanoma with penetration of the outer table of the scull
The tabula externa was already infiltrated by this tumour. We removed the periosteal layer and used a diamond drill for perforating the outer cortical layer of the scull to improve blood supply (Fig. 7).
Figure 7.
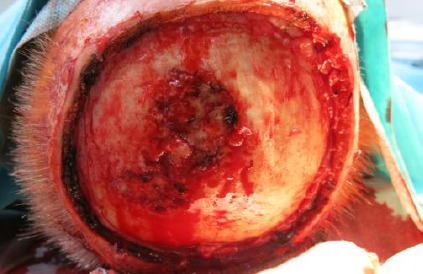
Operation situs
Sandwich transplantation with the elastin-collagen dermal template and meshed skin graft in the same session provided a stable defect closure until neurosurgical complete tumour resection, and skull repair was possible (Fig. 8).
Figure 8.

Stable meshed skin graft ten days after surgery
A 67-year-old male patient presented with a giant trichilemmal carcinoma of the scalp (20 x 12 x 2.4 cm), Broder grade 3 (Fig. 9).
Figure 9.

Giant trichilemmal carcinoma of the scalp
By cranial computerised tomography and gadolinium-enhanced vascular magnetic resonance imaging a parietal parasagittal cranial tumour invasion was detected (Fig. 10).
Figure 10.

MRI demonstrating penetration of the scull and impression of the sagittal sinus
There was a continuous growth to the meninges on the left side with infiltration and partial closure of the medial part of the superior sagittal sinus. Together with the neurosurgeon, we decided to perform debulking surgery as a palliative treatment (Fig. 11).
Figure 11.

Operation situs
Overall survival from the first diagnosis was three years. He did not die from cancer but traffic accident one week before planned neurosurgery of the intracranial tumour residues.
Chronic inflammatory scalp dermatoses
30-year-old male patient with severe dissecting cellulitis of the scalp, who was treated in a neoadjuvant setting with anti-tumor necrosis factor-alpha treatment compound infliximab, had been subjected to scalping surgery for treatment-resistant residues (Figs. 12 & 13).
Figure 12.

Dissecting folliculitis with severe inflammation and oozing lesions
Figure 13.

Two years after scalping surgery with stable meshed skin transplant take. No relapse of dissecting folliculitis.
He is in complete remission now for more than five years without any medical treatment.
Discussion
Scalping surgery is a niche technique with several possible indications. The advantage of the technique is its radical removal of multiple lesions, the complete change of the local immunity and the easiness of follow-up. The major drawback is the permanent hair loss in the treated area. This is an important issue to be discussed in detail with every patient before surgery.
Field cancerization is mostly used to describe the development of multiple actinic keratoses on the bald scalp. The (bald) scalp is an immunocompromised district according to the concept of Rucco [8]. In the case of only multiple superficial actinic keratoses, not responding to topical treatment, photodynamic therapy is a useful alternative [9].
We treated two patients with a related condition: A female patient with multiple cutaneous metastases of an amelanotic nevoid melanoma arising in a circumscribed area of her scalp without any metastasis to more distant sites and a male patient with Gorlin-Goltz syndrome (OMIM 109400) and multiple BCCs of the head [10].
Nevoid melanomas are melanomas that clinically and histologically resemble a nevus (only at low power magnification under the microscope), and can be divided into small cell and spitzoid types [11, 12]. The patient we present developed satellitosis without metastatic spread to more distant sites. We observed that microscopic satellitosis does not necessarily translate into the lymphovascular infiltration of the tumour [13]. In one study, the 5-year overall and disease-free survivals in patients with microscopic satellitosis were 34% and 18%, respectively, demonstrating an unfortunate prognosis [14].
Our patient had multiple treatments for numerous non-pigmented cutaneous metastases, such as 2.94 µm erbium-YAG and 980 nm pulsed dye laser, low-dose interferon alfa, and intralesional interleukin 2 [15-17]. Objective complete remissions have been achieved by intralesional interleukin-2 in up to 69% [18]. None of these treatments yielded a longer remission in the present patient. Therefore, we discussed the opportunity of scalping surgery for the area of local cutaneous metastases. The patients agreed, and so far, we did not observe any recurrence.
In Gorlin-Goltz syndrome, surgical excision allows removal BCCs with an overall chance of a cure between 94% to 98%. Surgical excision provides the advantage of three-dimensional margin control [19]. Recently, hedgehog inhibitor vismodegib was investigated for Gorlin-Goltz patients with unresectable or metastatic BCCs. In a pooled analysis of two vismodegib trials by Chang et al. (2016), 46 patients with Gorlin-Goltz syndrome were included. The investigator-rated best overall response reached 31 to 81 % in patients with unresectable BCC (n = 33) and 50 % in patients with metastatic BCC (n = 6) [20]. On the other hand, there is increasing evidence for either primary or secondary tumour resistance to hedgehog inhibitors, the transformation of BCC to squamous cell carcinoma (SCC) and increased risk of development of cutaneous SCC after vismodegib therapy [21-23]. We observed no recurrence of BCCs after scalping surgery in the treated area. Before, newly developed BCCs were noted almost every month.
In old patients, where other medical, surgical or radiological treatments and their combination is not tolerable anymore, a debulking surgery is a palliative approach to avoid tumour bleeding, secondary infection, diminish malodor and improve the quality of life of patients. Debulking surgery per se does not improve overall survival. However, in the case of possible combination with another non-surgical therapy, a positive effect on survival may be obtained.
Liao et al. (2017) treated a 92-year-old male patients with a higher multinodular mass on the scalp by this technique. The diagnosis was a cutaneous B-cell lymphoma of the parietooccipital region. Relapse-free survival was six months, and overall survival was two years [24].
We performed debulking surgery in an 82-year-old male patient with scull-penetrating amelanotic LMM and a 67-year-old male patient with scull-penetrating trichilemmal carcinoma [25]. Overall survival of the last patient was three years. The former patient was subjected to neurosurgical treatment.
Sometimes, scalping surgery is an option in patients with multiple benign scalp tumours. Sebastian and his group treated a 63-year-old man by en-bloc resection of multiple cylindromas of the scalp. After excision and granulation-stimulating local therapy, the wound was covered with meshed skin grafts from the thighs, but widespread tension blisters with ulcerations were recalcitrant to topical treatment. The defects were eventually closed with EpiDex, a tissue-engineered epidermal equivalent derived from outer root sheath keratinocytes [26].
Dissecting cellulitis of the scalp is a chronic inflammatory, oozing disorder with similarities to hidradenitis suppurative/ acne inverse. It can be recalcitrant to medical treatment. In such cases, scalping surgery with subsequent mesh graft transplantation is an option to improve patient’s quality of life [27].
In the case of exposed Calvary after scalping surgery, we employed two surgical techniques – either alone or in combination. Milling the exposed outer table of the scalp bone with a rose head burr driven by a pneumatic power drill exposes diploic veins in the cancellous bone. This increases survival of free transplants [28]. The use of a dermal template has been shown to increase skin graft survival and improve the mechanical quality of the transplant after healing [3-7]. We employed the sandwich technique with a reconstituted elastin-collagen matrix covered by meshed skin graft in a single operation [6].
Despite all advantages in medical treatments and laser technology, scalping surgery is an option in the management of skin cancer patients and severe chronic inflammatory disorders.
Footnotes
Funding: This research did not receive any financial support.
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Desai SC, Sand JP, Sharon JD, Branham G, Nussenbaum B. Scalp reconstruction:an algorithmic approach and systematic review. JAMA Facial Plast Surg. 2015;17:56–66. doi: 10.1001/jamafacial.2014.889. https://doi.org/10.1001/jamafacial.2014.889 PMid:25375669. [DOI] [PubMed] [Google Scholar]
- 2.Wollina U, Bayyoud Y, Krönert C, Nowak A. Giant epithelial malignancies (Basal cell carcinoma, squamous cell carcinoma):a series of 20 tumours from a single centre. J Cutan Aesthet Surg. 2012;5:12–19. doi: 10.4103/0974-2077.94328. https://doi.org/10.4103/0974-2077.94328 PMid:22557850 PMCid:PMC3339122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson MB, Wong AK. Integra-based reconstruction of large scalp wounds:A case report and systematic review of the literature. Plast Reconstr Surg Glob Open. 2016;4:e1074. doi: 10.1097/GOX.0000000000001074. https://doi.org/10.1097/GOX.0000000000001074 PMid:27826471 PMCid:PMC5096526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richardson MA, Lange JP, Jordan JR. Reconstruction of full-thickness scalp defects using a dermal regeneration template. JAMA Facial Plast Surg. 2016;18:62–67. doi: 10.1001/jamafacial.2015.1731. https://doi.org/10.1001/jamafacial.2015.1731 PMid:26606002. [DOI] [PubMed] [Google Scholar]
- 5.Gironi LC, Boggio P, Colombo E. Reconstruction of scalp defects with exposed bone after surgical treatment of basal cell carcinoma:the use of a bilayer matrix wound dressing. Dermatol Ther. 2015;28:114–117. doi: 10.1111/dth.12193. https://doi.org/10.1111/dth.12193 PMid:25546347. [DOI] [PubMed] [Google Scholar]
- 6.Wollina U. One-stage reconstruction of soft tissue defects with the sandwich technique:Collagen-elastin dermal template and skin graft. J Cutan Aesthet Surg. 2011;4:176–182. doi: 10.4103/0974-2077.91248. https://doi.org/10.4103/0974-2077.91248 PMid:22279382 PMCid:PMC3263127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faulhaber J, Felcht M, Teerling G, Klemke CD, Wagner C, Goerdt S, Koenen W. Long-term results after reconstruction of full thickness scalp defects with a dermal regeneration template. J Eur Acad Dermatol Venereol. 2010;24:572–527. doi: 10.1111/j.1468-3083.2009.03473.x. https://doi.org/10.1111/j.1468-3083.2009.03473.x PMid:19888947. [DOI] [PubMed] [Google Scholar]
- 8.Ruocco V, Ruocco E, Piccolo V, Brunetti G, Guerrera LP, Wolf R. The immunocompromised district in dermatology:A unifying pathogenic view of the regional immune dysregulation. Clin Dermatol. 2014;32:569–576. doi: 10.1016/j.clindermatol.2014.04.004. https://doi.org/10.1016/j.clindermatol.2014.04.004 PMid:25160098. [DOI] [PubMed] [Google Scholar]
- 9.Griffin LL, Lear JT. Photodynamic therapy and non-melanoma skin cancer. Cancers (Basel) 2016;8:E98. doi: 10.3390/cancers8100098. https://doi.org/10.3390/cancers8100098 PMid:27782094 PMCid:PMC5082388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards SL, Blessing K. Problematic pigmented lesions:approach to diagnosis. J Clin Pathol. 2000;53:409–418. doi: 10.1136/jcp.53.6.409. https://doi.org/10.1136/jcp.53.6.409 PMCid:PMC1731214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scolyer RA, Long GV, Thompson JF. Evolving concepts in melanoma classification and their relevance to multidisciplinary melanoma patient care. Mol Oncol. 2011;5:124–136. doi: 10.1016/j.molonc.2011.03.002. https://doi.org/10.1016/j.molonc.2011.03.002 PMid:21482206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimsey TF, Cohen T, Patel A, Busam KJ, Brady MS. Microscopic satellitosis in patients with primary cutaneous melanoma:implications for nodal basin staging. Ann Surg Oncol. 2009;16:1176–1183. doi: 10.1245/s10434-009-0350-7. https://doi.org/10.1245/s10434-009-0350-7 PMid:19224283. [DOI] [PubMed] [Google Scholar]
- 13.Kaley J, Patterson JW, Chokoeva AA, Lotti T, Wollina U, Tchernev G. Are microscopic satellites in melanoma indicative of lymphovascular invasion?A preliminary case study. J Biol Regul Homeost Agents. 2015;29(1 Suppl):65–68. PMid:26016970. [PubMed] [Google Scholar]
- 14.Austin E, Mamalis A, Ho D, Jagdeo J. Laser and light-based therapy for cutaneous and soft-tissue metastases of malignant melanoma:a systematic review. Arch Dermatol Res. 2017 doi: 10.1007/s00403-017-1720-9. https://doi.org/10.1007/s00403-017-1720-9 PMid:28314913. [DOI] [PubMed] [Google Scholar]
- 15.Boyd KU, Wehrli BM, Temple CL. Intra-lesional interleukin-2 for the treatment of in-transit melanoma. J Surg Oncol. 2011;104:711–717. doi: 10.1002/jso.21968. https://doi.org/10.1002/jso.21968 PMid:21744347. [DOI] [PubMed] [Google Scholar]
- 16.Bottoni U, Clerico R, Paolino G, Corsetti P, Ambrifi M, Brachini A, Richetta A, Nisticò S, Pranteda G, Calvieri S. Melanoma and IFN alpha:potential adjuvant therapy. J Biol Regul Homeost Agents. 2014;28:271–279. PMid:25001659. [PubMed] [Google Scholar]
- 17.Nouri N, Garbe C. Intralesional immunotherapy as a strategy to treat melanoma. Expert Opin Biol Ther. 2016;16:619–926. doi: 10.1517/14712598.2016.1157161. https://doi.org/10.1517/14712598.2016.1157161 PMid:2689⇐. [DOI] [PubMed] [Google Scholar]
- 18.Gorlin RJ, Goltz RW. Multiple nevoid basal-cell epithelioma, jaw cysts and bifid rib:a syndrome. N Engl J Med. 1960;262:908–912. doi: 10.1056/NEJM196005052621803. https://doi.org/10.1056/NEJM196005052621803 PMid:13851319. [DOI] [PubMed] [Google Scholar]
- 19.Lo Muzio L. Nevoid basal cell carcinoma syndrome (Gorlin syndrome) Orphanet J Rare Dis. 2008;3:32. doi: 10.1186/1750-1172-3-32. https://doi.org/10.1186/1750-1172-3-32 PMid:19032739 PMCid:PMC2607262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang AL, Arron ST, Migden MR, Solomon JA, Yoo S, Day BM, McKenna EF, Sekulic A. Safety and efficacy of vismodegib in patients with basal cell carcinoma nevus syndrome:pooled analysis of two trials. Orphanet J Rare Dis. 2016;11:120. doi: 10.1186/s13023-016-0506-z. https://doi.org/10.1186/s13023-016-0506-z PMid:27581207 PMCid:PMC5007799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansel G, Tchernev G, Chokoeva AA, Lotti T, Schönlebe J, Wollina U. Failure of vismodegib in advanced basal cell carcinoma. J Biol Regul Homeost Agents. 2015;29(1 Suppl):11–13. PMid:26016959. [PubMed] [Google Scholar]
- 22.Feigenbaum L, Scott BL, Moye MS, Nijhawan RI. Development of basal cell carcinoma with squamous differentiation during vismodegib treatment. Dermatol Surg. 2016 doi: 10.1097/DSS.0000000000001012. [DOI] [PubMed] [Google Scholar]
- 23.Mohan SV, Chang J, Li S, Henry AS, Wood DJ, Chang AL. Increased risk of cutaneous squamous cell carcinoma after vismodegib therapy for basal cell carcinoma. JAMA Dermatol. 2016;152:527–532. doi: 10.1001/jamadermatol.2015.4330. https://doi.org/10.1001/jamadermatol.2015.4330 PMid:26914338. [DOI] [PubMed] [Google Scholar]
- 24.Liao C, Yang M, Liu P, Zhang W. A 92-year-old man with primary cutaneous diffuse large B-cell non-Hodgkin’s lymphoma manifesting as a giant scalp mass:A case report. Medicine (Baltimore) 2017;96:e6270. doi: 10.1097/MD.0000000000006270. https://doi.org/10.1097/MD.0000000000006270 PMid:28272240 PMCid:PMC5348188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wollina U, Bayyoud Y, Kittner T, Dürig E. Giant tricholemmal squamous cell carcinoma with cranial infiltration. J Clin Aesthet Dermatol. 2011;4:34–37. PMid:21532876 PMCid:PMC3084608. [PMC free article] [PubMed] [Google Scholar]
- 26.Tausche AK, Richter-Huhn G, Sebastian G. Treatment of recalcitrant wounds with autologous epidermal equivalents. After excision of multiple cylindromas of the scalp. Hautarzt. 2004;55:296–300. doi: 10.1007/s00105-003-0637-8. https://doi.org/10.1007/s00105-003-0637-8 PMid:15029438. [DOI] [PubMed] [Google Scholar]
- 27.Wollina U, Gemmeke A, Koch A. Dissecting cellulitis of the scalp responding to intravenous tumor necrosis factor-alpha antagonist. J Clin Aesthet Dermatol. 2012;5:36–39. PMid:22708007 PMCid:PMC3366445. [PMC free article] [PubMed] [Google Scholar]
- 28.Mühlstädt M, Thomé C, Kunte C. Rapid wound healing of scalp wounds devoid of periosteum with milling of the outer table and split-thickness skin grafting. Br J Dermatol. 2012;167:343–347. doi: 10.1111/j.1365-2133.2012.10999.x. https://doi.org/10.1111/j.1365-2133.2012.10999.x PMid:22512740. [DOI] [PubMed] [Google Scholar]


