Abstract
BACKGROUND
Lentigo malignant melanoma is a melanoma subtype of chronic sun-damaged skin in elderly Caucasians. Amelanotic variants of lentigo malignant are extremely rare.
CASE PRESENTATION
This is a case report of an 80-year-old male patient who presented with a non-pigmented exophytic tumour of his bald head. After complete surgical excision under the suspicion of squamous cell carcinoma, three-dimensional histologic examination confirmed an amelanotic lentigo malignant melanoma with a tumour thickness of 1.76 mm, resected R0. Five years later he developed the first relapse, the other year a satellite metastasis was surgically removed. One year later, this patient had developed a large relapsing lentigo malignant melanoma with skull roof invasion. There was no evidence of distant metastatic spread. Amelanotic lentigo malignant melanoma is a very rare tumour.
CONCLUSIONS
Serial excision or slow Mohs and Mohs micrographic surgery are the treatments of choice especially in the head and neck area. These tumours may be locally very aggressive as it is shown by skull invasion in the present case.
Keywords: Melanoma, amelanotic lentigo malignant melanoma, relapses, surgery, skull invasion, follow-up
Introduction
Lentigo malignant melanoma (LMM) is a melanoma subtype that develops on chronic sun-damaged skin mostly in elderly patients (age >70 years). Its precursor, lentigo maligna or Hutchinson’s melanotic freckle is a melanoma in situ on average with a remarkable slow growth rate. In 2% to 20% of patients lentigo maligna will further progress to invasive melanoma. Lentigo maligna and LMM may have similar clinical presentation, what challenges their differential diagnosis [1].
LMM accounts for 4% to 15% of all melanomas. The propensity to lentigines is higher in LMM versus superficial spreading melanoma (SSM), while high nevus counts are a stronger indicator for SSM. In addition, SSM is stronger associated to non-melanoma skin cancer compared to SSM. Sunburns don’t seem to be not an independent risk factor for LMM [2]. A recent study from the Netherlands analysed age-standardized incidence rate for LMM between 1989 to 2013. The incidence rate increased from 0.24 to 1.19 [3].
On histopathologic examination LMM is characterised by the atypical junctional growth of melanocytic cells, pagetoid growth not only in interfollicular but follicular skin, cellular atypia, and increased proliferative activity. The affected skin shows marked epidermal atrophy, solar elastosis and dermal inflammatory infiltrate. LMM radial growth may regress, a feature that may notoriously lead to false negative surgical margins. Therefore, diagnosis of LMM is not an easy one especially in the interpretation of resection margins. To assist LMM diagnosis, various immunostains are used with monoclonal antibodies against S100, HMB-45 (against Pmel 17), soluble adenylyl cyclase (antibody R21), microphthalmia transcription factor or MART-1 (protein melan-A) [1, 4].
Dermoscopy may aid melanoma diagnosis, but studies so far have considered pigmented LMM only [5, 6]. Confocal microscopy could be a tool for the definition of tumour margins before surgery, but the technique is yet no standard procedure [7].
Case report
A 73-year-old male patient presented with a firm tumour on his bald head with a diameter of approximately 2 cm. The reason for the first presentation was frequent bleeding.
He had a medical history of prostate cancer (Gleason score 7) and subsequent radiotherapy, squamous cell cancer of the right hand, arterial hypertension, hyperlipidemia, chronic ischemic heart disease with four by-passes and pace maker implantation.
On examination, we observed a skin coloured exophytic nodule, covered by scales (Fig. 1a). Under the working diagnosis of a squamous cell carcinoma, the tumour was removed by standard excision followed by three-dimensional histology.
Figure 1.
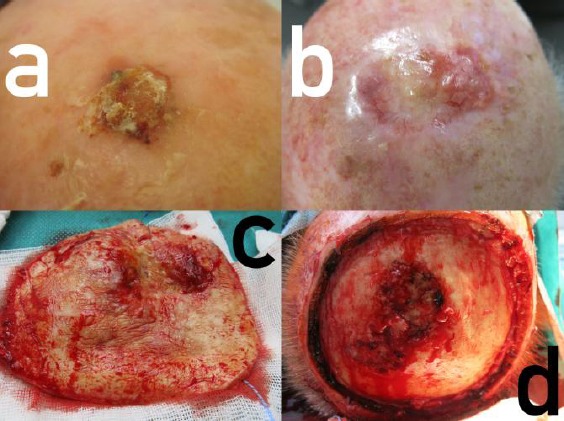
Amelanotic lentigo malignant melanoma of the bald head. (a) Initial presentation of the primary tumour resembling squamous cell carcinoma; (b) Relapse on the borders of the transplant, telangiectasias but again no pigmentation at all; (c) Surgical specimen; (d) Defect before closure with skull roof invasion located centrally
Histologic examination confirmed a lentigo malignant melanoma (LMM) with a tumour thickness of 1.76 mm and Clark level IV. The tumour cells were positive for S100 with coexpression of MART-1 and HMB45. They were negative for CD10 and CD68.
The surgical margins were free. The staging was performed by imaging techniques. No metastases were observed. This lead to the final diagnosis of LMM (pT2b N0 M0). Healing was unremarkable.
Five years later, he presented with an amelanotic relapse of his LMM, which was surgically removed by slow Mohs again. Tumour thickness was 3.00 mm (rpT3a). Tumour staging was unremarkable, no metastases.
One year later, he returned with another skin coloured nodule of the bald head which was removed by slow Mohs surgery. The defect was closed by sandwich transplantation using dermal equivalent (Matriderm) and meshed skin graft [8]. Histologic examination revealed a satellite metastasis of his LMM. Healing was uneventful. The tumour was now staged as rpT3a pN2c cM0, stage IIIb.
Almost exactly one year later, he presented with a firm tumour proliferation around the skin transplant with some telangiectasias but without pigmentation (Fig. 1b). A skin biopsy was taken. Histologically, there was a proliferation of atypical melanocytes singly and in nests along the basal layer of the epidermis and epitheloid nests in the adjacent dermis. The atypical cells were small with prominent nuclei. These findings confirmed LMM (Fig. 2). Staging demonstrated a tabula externa defect (Fig. 3). Surgery was planned a tumour debulking. The patient was treated surgically as a tumour by wide excision with 2 cm safety margins (Fig. 1c), after decortication the tabula externa was partially removed by a diamond drill. During surgery, it became obvious that the tumour had already infiltrated the skull roof in an area of about 4 x 2.5 cm (Fig. 1d). The defect was closed by sandwich transplantation (Fig. 4) and the patient was presented to the tumour board for either neurosurgery and radiotherapy. Since the tabula interna was still intact as demonstrated by cranial computerised tomography, he was referred to the neurosurgery and plastic surgery department for tumour removal and skull repair. Staging did not provide any hint for the further metastatic spread.
Figure 2.
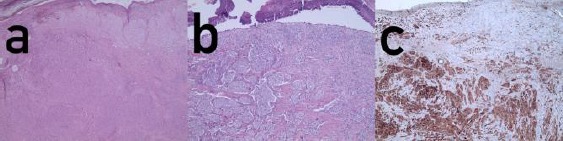
Histopathology of lentigo malignant melanoma. (a) Overview (hematoxylin-eosin x 2); (b) detail (hematoxylin-eosin x 4); (c) immunoperoxidase staining for S100 (x4)
Figure 3.
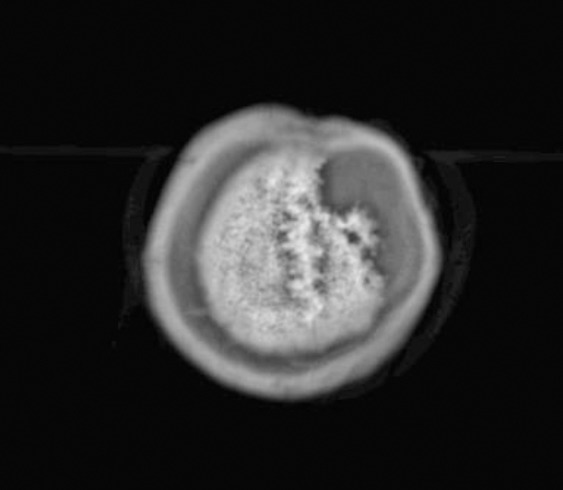
Skull defect in magnetic resonance imaging
Figure 4.
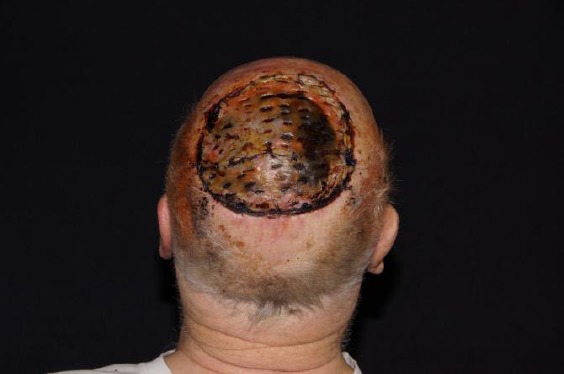
Four weeks after sandwich transplantation with a stable graft
Discussion
In our department, LMM is seen in 12% of female and 11% of male melanoma patients. Amelanotic melanoma comprises only two percent of all melanoma subtypes. Amelanotic LMM is an unusual rare tumour type with less than 25 cases reported worldwide (9-15). The clinical suspicion for amelanotic LMM is extremely low. Dermoscopy is not sensitive enough to detect this melanoma subtype [5, 6].
Major differential diagnoses are actinic keratosis and squamous cell carcinoma (SCC). Diagnosis can only be confirmed by histopathological examination.
Due to the growth characteristics of LMM with recurrent regression, the assessment of free surgical margins is not an easy task. The medical history of our patient demonstrated that even with slow Mohs, experienced dermatopathologist and dermasurgeon, recurrence is possible [5].
The present case is exceptional for several reasons: (i) it is an amelanotic LMM; (ii) recurrence was only locally; (iii) it invaded and destroyed the roof of the skull; and (iv) it remained locally over a period of 7 years. We could not find any other case with such a locally aggressive behaviour but without the further metastatic spread. The occurrence of a relapse five years after the first diagnosis is quite characteristic. Most LMM relapse between 3 to 5 years [16].
Treatment of LMM consists of surgery with wide excision, staged excision or Mohs surgery. Nevertheless, a higher recurrence rate has been reported for LMM, depending on histopathological methods for tumour margin examination, safety margins, and length of follow-up. With an average safety margin of 5 mm, only 56% of tumours could be cleared, while a safety margin of 15 mm achieved a 97% complete clearance rate (17Felton et al. 2016). Therefore, a 5-mm safety margin is inadequate for LMM. However, 15 mm is not always possible like in the face. A better choice is Mohs surgery or staged excision [17]. With staged excision, the recurrence rate within a mean of 11.5 years was 5.9 % [18]. By staged excision or slow Mohs technique, formalin-fixed tissue specimen are used that offer a better quality of tissue sections than frozen sections do. In a retrospective study comparing serial excision (slow Mohs) with Mohs micrographic technique on frozen sections, the recurrence rate was 7.3% and 33.0%, respectively [19]. On average, recurrence rates of 8% to 20% have been reported with standard surgery compared to 4% to 5 % by Mohs surgery [20].
Topical treatment with ingenol mebutate, imiquimod or cryotherapy is not recommended due to the ill-defined tumour borders and the microscopic growth pattern along the hair follicles deep downwards. Also, the progression of lentigo maligna into LMM has been reported after topical treatment [21]. Relapse rate after topical therapy or cryosurgery ranges from 20% to 100% after five years [20].
For patients who refuse surgery or could not be operated for medical reasons, a radiotherapy is a therapeutic option. An 88% complete clearance rate has been reported for lentigo maligna and early LMM using 100 to 160 Gy fractioned Grenz rays [22].
Debulking surgery is an established method to reduce tumour load in advanced melanoma [23]. In metastatic melanoma, debulking surgery may be necessary in emergency situations in case of heavily bleeding or obstructing tumour growth for instance [24].
Skull roof invasion by melanoma is very rare. It can occasionally be seen in patients with squamous cell carcinoma of the skin, basal cell carcinoma and rare neoplastic adnexal tumours of skin [25-30]. Research on PubMed with “lentigo maligna melanoma” and “skull roof invasion” provided not a single hit.
The management of skull invasion warrants interdisciplinary cooperation. Therefore, the patient had been presented to the interdisciplinary tumour board.
Footnotes
Funding: This research did not receive any financial support.
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Juhász ML, Marmur ES. Reviewing challenges in the diagnosis and treatment of lentigo maligna and lentigo-maligna melanoma. Rare Cancers Ther. 2015;3:133–145. doi: 10.1007/s40487-015-0012-9. https://doi.org/10.1007/s40487-015-0012-9 PMid:27182482 PMCid:PMC4837936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kvaskoff M, Siskind V, Green AC. Risk factors for lentigo maligna melanoma compared with superficial spreading melanoma:a case-control study in Australia. Arch Dermatol. 2012;148(2):164–170. doi: 10.1001/archdermatol.2011.291. https://doi.org/10.1001/archdermatol.2011.291 PMid:22004881. [DOI] [PubMed] [Google Scholar]
- 3.Greveling K, Wakkee M, Nijsten T, van den Bos RR, Hollestein LM. Epidemiology of lentigo maligna and lentigo maligna melanoma in the Netherlands 1989-2013. J Invest Dermatol. 2016;136(10):1955–1960. doi: 10.1016/j.jid.2016.06.014. https://doi.org/10.1016/j.jid.2016.06.014 PMid:27349862. [DOI] [PubMed] [Google Scholar]
- 4.Christensen KN, Hochwalt PC, Hocker TL, Roenigk RK, Brewer JD, Baum CL, Otley CC, Arpey CJ. Comparison of MITF and Melan-A immunohistochemistry during Mohs surgery for lentigo maligna-type melanoma in situ and lentigo maligna melanoma. Dermatol Surg. 2016;42(2):167–175. doi: 10.1097/DSS.0000000000000600. https://doi.org/10.1097/DSS.0000000000000600 PMid:26771682. [DOI] [PubMed] [Google Scholar]
- 5.Jaimes N, Marghoob AA, Rabinovitz H, Braun RP, Cameron A, Rosendahl C, Canning G, Keir J. Clinical and dermoscopic characteristics of melanomas on nonfacial chronically sun-damaged skin. J Am Acad Dermatol. 2015;72(6):1027–1035. doi: 10.1016/j.jaad.2015.02.1117. https://doi.org/10.1016/j.jaad.2015.02.1117 PMid:25824275. [DOI] [PubMed] [Google Scholar]
- 6.Bollea-Garlatti LA, Galimberti GN, Galimberti RL. Lentigo maligna:keys to dermoscopic diagnosis. Actas Dermosifiliogr. 2016;107(6):489–497. doi: 10.1016/j.ad.2016.01.001. https://doi.org/10.1016/j.ad.2016.01.001 PMid:26875792. [DOI] [PubMed] [Google Scholar]
- 7.Guitera P, Moloney FJ, Menzies SW, Stretch JR, Quinn MJ, Hong A, Fogarty G, Scolyer RA. Improving management and patient care in lentigo maligna by mapping with in vivo confocal microscopy. JAMA Dermatol. 2013;149(6):692–698. doi: 10.1001/jamadermatol.2013.2301. https://doi.org/10.1001/jamadermatol.2013.2301 PMid:23553208. [DOI] [PubMed] [Google Scholar]
- 8.Wollina U. One-stage reconstruction of soft tissue defects with the sandwich technique:Collagen-elastin dermal template and skin grafts. J Cutan Aesthet Surg. 2011;4(3):176–182. doi: 10.4103/0974-2077.91248. https://doi.org/10.4103/0974-2077.91248 PMid:22279382 PMCid:PMC3263127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaufmann R, Nikelski K, Weber L, Sterry W. Amelanotic lentigo maligna melanoma. J Am Acad Dermatol. 1995;32(2 Pt 2):339–342. doi: 10.1016/0190-9622(95)90399-2. https://doi.org/10.1016/0190-9622(95)90399-2. [DOI] [PubMed] [Google Scholar]
- 10.Cliff S, Otter M, Holden CA. Amelanotic lentigo maligna melanoma of the face:a case report and review of the literature. Clin Exp Dermatol. 1997;22(4):177–179. https://doi.org/10.1111/j.1365-2230.1997.tb01056.x PMid:9499607. [PubMed] [Google Scholar]
- 11.Allan SJ, Dicker AJ, Tidman MJ, McLaren KM, Hunter JA. Amelanotic lentigo maligna and amelanotic lentigo maligna melanoma:a report of three cases mimicking intraepidermal squamous carcinoma. J Eur Acad Dermatol Venereol. 1998;11(1):78–81. https://doi.org/10.1111/j.1468-3083.1998.tb00961.x PMid:9731974. [PubMed] [Google Scholar]
- 12.Conrad N, Jackson B, Goldberg L. Amelanotic lentigo maligna melanoma:a unique case presentation. Dermatol Surg. 1999;25(5):408–411. doi: 10.1046/j.1524-4725.1999.08271.x. https://doi.org/10.1046/j.1524-4725.1999.08271.x PMid:10469082. [DOI] [PubMed] [Google Scholar]
- 13.Rocamora V, Puig L, Romaní J, de Moragas JM. Amelanotic lentigo maligna melanoma:report of a case and review of the literature. Cutis. 1999;64(1):53–56. PMid:10431675. [PubMed] [Google Scholar]
- 14.Abdulla FR, Kerns MJ, Mutasim DF. Amelanotic lentigo maligna:a report of three cases and review of the literature. J Am Acad Dermatol. 2010;62(5):857–860. doi: 10.1016/j.jaad.2009.06.017. https://doi.org/10.1016/j.jaad.2009.06.017 PMid:19766347. [DOI] [PubMed] [Google Scholar]
- 15.Perera E, Mellick N, Teng P, Beardmore G. A clinically invisible melanoma. Australas J Dermatol. 2014;55(3):e58–e59. doi: 10.1111/ajd.12022. https://doi.org/10.1111/ajd.12022 PMid:23425084. [DOI] [PubMed] [Google Scholar]
- 16.Bub JL, Berg D, Slee A, Odland PB. Management of lentigo maligna and lentigo maligna melanoma with staged excision:a 5 year follow up. Arch Dermatol. 2004;140(5):552–558. doi: 10.1001/archderm.140.5.552. https://doi.org/10.1001/archderm.140.5.552 PMid:15148099. [DOI] [PubMed] [Google Scholar]
- 17.Felton S, Taylor RS, Srivastava D. Excision margins for melanoma in situ on the head and neck. Dermatol Surg. 2016;42(3):327–334. doi: 10.1097/DSS.0000000000000648. https://doi.org/10.1097/DSS.0000000000000648 PMid:26866286. [DOI] [PubMed] [Google Scholar]
- 18.Wilson JB, Walling HW, Scupham RK, Bean AK, Ceilley RI, Goetz KE. Staged excision for lentigo maligna and lentigo maligna melanoma:analysis of surgical margins and long-term recurrence in 68 cases from a single practice. J Clin Aesthet Dermatol. 2016;9(6):25–30. PMid:27386048 PMCid:PMC4928453. [PMC free article] [PubMed] [Google Scholar]
- 19.Walling HW, Scupham RK, Bean AK, Ceilley RI. Staged excision versus Mohs micrographic surgery for lentigo maligna and lentigo maligna melanoma. J Am Acad Dermatol. 2007;57(4):659–664. doi: 10.1016/j.jaad.2007.02.011. https://doi.org/10.1016/j.jaad.2007.02.011 PMid:17870430. [DOI] [PubMed] [Google Scholar]
- 20.McKenna JK, Florell SR, Goldman GD, Bowen GM. Lentigo maligna/lentigo maligna melanoma:current state of diagnosis and treatment. Dermatol Surg. 2006;32(4):493–504. doi: 10.1111/j.1524-4725.2006.32102.x. https://doi.org/10.1097/00042728-200604000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Tio D, van der Woude J, Prinsen CA, Jansma EP, Hoekzema R, van Montfrans C. A systematic review on the role of imiquimod in lentigo maligna and lentigo maligna melanoma:need for standardization of treatment schedule and outcome measures. J Eur Acad Dermatol Venereol. 2017;31(4):616–24. doi: 10.1111/jdv.14085. PMid:279√8. [DOI] [PubMed] [Google Scholar]
- 22.Hedblad MA, Mallbris L. Grenz ray treatment of lentigo maligna and early lentigo maligna melanoma. J Am Acad Dermatol. 2012;67(1):60–68. doi: 10.1016/j.jaad.2011.06.029. https://doi.org/10.1016/j.jaad.2011.06.029 PMid:22030019. [DOI] [PubMed] [Google Scholar]
- 23.Gajdos C, McCarter MD. Debulking surgery in advanced melanoma. Expert Rev Anticancer Ther. 2011;11(11):1703–1712. doi: 10.1586/era.11.98. https://doi.org/10.1586/era.11.98 PMid:22050019. [DOI] [PubMed] [Google Scholar]
- 24.Mantas D, Tsaparas P, Charalampoudis P, Gogas H, Kouraklis G. Emergency surgery for metastatic melanoma. Int J Surg Oncol. 2014;2014:987170. doi: 10.1155/2014/987170. https://doi.org/10.1155/2014/987170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guitera P, Moloney FJ, Menzies SW, Stretch JR, Quinn MJ, Hong A, Fogarty G, Scolyer RA. Improving management and patient care in lentigo maligna by mapping with in vivo confocal microscopy. JAMA Dermatol. 2013;149(6):692–698. doi: 10.1001/jamadermatol.2013.2301. https://doi.org/10.1001/jamadermatol.2013.2301 PMid:23553208. [DOI] [PubMed] [Google Scholar]
- 26.Wollina U, Tchernev G. Advanced basal cell carcinoma. Wien Med Wochenschr. 2013;163(15-16):347–353. doi: 10.1007/s10354-013-0193-5. https://doi.org/10.1007/s10354-013-0193-5 PMid:23589318. [DOI] [PubMed] [Google Scholar]
- 27.Wollina U, Bayyoud Y, Krönert C, Nowak A. Giant epithelial malignancies (Basal cell carcinoma, squamous cell carcinoma):a series of 20 tumors from a single center. J Cutan Aesthet Surg. 2012;5(1):12–19. doi: 10.4103/0974-2077.94328. https://doi.org/10.4103/0974-2077.94328 PMid:22557850 PMCid:PMC3339122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wollina U, Bayyoud Y, Kittner T, Dürig E. Giant tricholemmal squamous cell carcinoma with cranial infiltration. J Clin Aesthet Dermatol. 2011;4(4):34–37. PMid:21532876 PMCid:PMC3084608. [PMC free article] [PubMed] [Google Scholar]
- 29.Soma PF, Chibbaro S, Makiese O, Marsella M, Diemidio P, Fricia M, Passanisi M, Catania V, Siragò P, Ventura F. Aggressive scalp carcinoma with intracranial extension:a multidisciplinary experience of 25 patients with long-term follow-up. J Clin Neurosci. 2008;15(9):988–992. doi: 10.1016/j.jocn.2007.09.014. https://doi.org/10.1016/j.jocn.2007.09.014 PMid:18653348. [DOI] [PubMed] [Google Scholar]
- 30.Abo Sedira M, Amin AA, Rifaat MA, El-Sebai HI, El-Badawy MA, Aboul Kassem HA. Locally advanced tumors of the scalp:the Egyptian National Cancer Institute experience. J Egypt Natl Canc Inst. 2006;18(3):250–257. PMid:17671535. [PubMed] [Google Scholar]


