Abstract
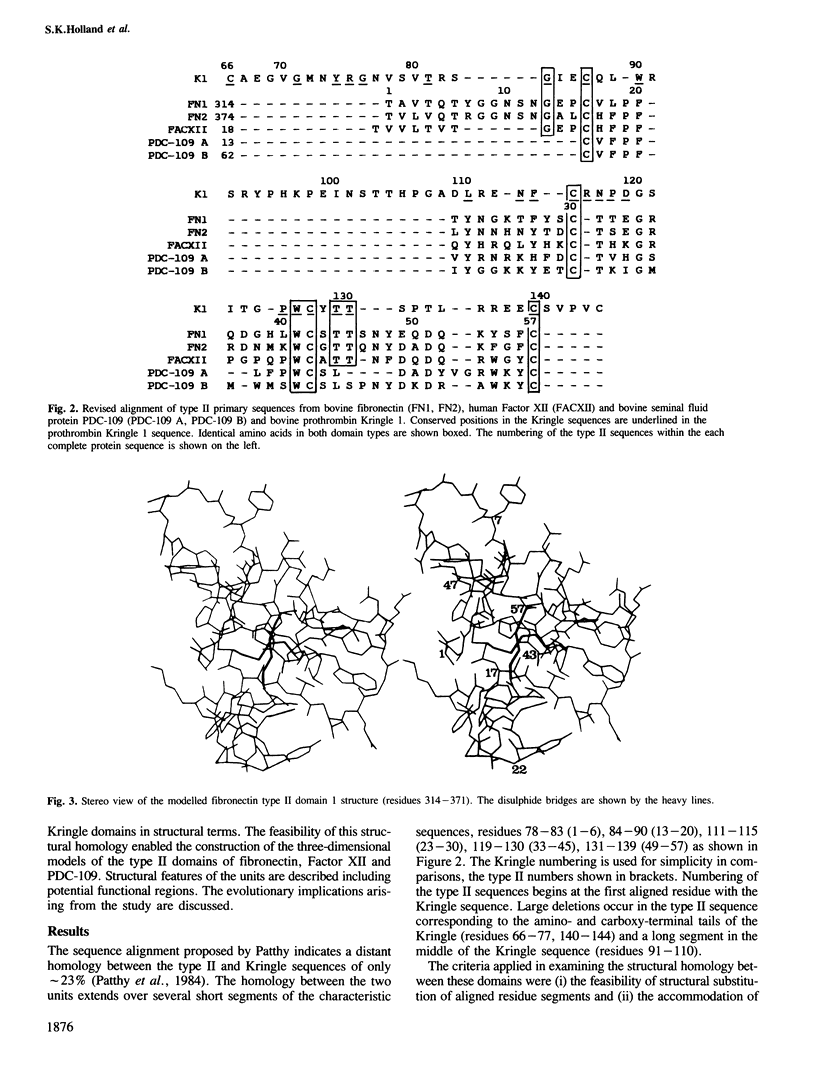
The proposed homology between the fibronectin type II domain and the Kringle domains of blood clotting and fibrinolytic proteins has been examined in three dimensions by substituting the type II sequence into the bovine prothrombin Kringle 1 tertiary structure, determined by X-ray crystallographical methods at 3.8 A. Structural substitution of aligned amino acids of the type II domains and the Kringle produces a compact chain fold and deletions and insertions in the type II sequence are accommodated within the modelled structure. This confirms the structural homology between the two domains and verifies the sequence alignment and common evolution of the type II and Kringle units. The two structures contain homologous hydrophobic cores, centered around the two disulphide bridges which link conserved beta-type strands. Gross differences between the two domains occur in exterior loops and potential functional sites in these regions of the type II structures as found in fibronectin, Factor XII and seminal fluid protein PDC-109 are proposed. We suggest that the domains evolved from a common ancestral protein comprising the hydrophobic core and disulphide arrangement which later diverged to bind different macromolecules through adaptation of the external loops.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- De Marco A., Laursen R. A., Llinas M. 1H-NMR spectroscopic manifestations of ligand binding to the kringle 4 domain of human plasminogen. Arch Biochem Biophys. 1986 Feb 1;244(2):727–741. doi: 10.1016/0003-9861(86)90642-9. [DOI] [PubMed] [Google Scholar]
- Esch F. S., Ling N. C., Böhlen P., Ying S. Y., Guillemin R. Primary structure of PDC-109, a major protein constituent of bovine seminal plasma. Biochem Biophys Res Commun. 1983 Jun 29;113(3):861–867. doi: 10.1016/0006-291x(83)91078-1. [DOI] [PubMed] [Google Scholar]
- Esmon C. T., Jackson C. M. The conversion of prothrombin to thrombin. IV. The function of the fragment 2 region during activation in the presence of factor V. J Biol Chem. 1974 Dec 25;249(24):7791–7797. [PubMed] [Google Scholar]
- Günzler W. A., Steffens G. J., Otting F., Kim S. M., Frankus E., Flohé L. The primary structure of high molecular mass urokinase from human urine. The complete amino acid sequence of the A chain. Hoppe Seylers Z Physiol Chem. 1982 Oct;363(10):1155–1165. doi: 10.1515/bchm2.1982.363.2.1155. [DOI] [PubMed] [Google Scholar]
- Jackson C. M., Nemerson Y. Blood coagulation. Annu Rev Biochem. 1980;49:765–811. doi: 10.1146/annurev.bi.49.070180.004001. [DOI] [PubMed] [Google Scholar]
- McMullen B. A., Fujikawa K. Amino acid sequence of the heavy chain of human alpha-factor XIIa (activated Hageman factor). J Biol Chem. 1985 May 10;260(9):5328–5341. [PubMed] [Google Scholar]
- Owens R. J., Baralle F. E. Mapping the collagen-binding site of human fibronectin by expression in Escherichia coli. EMBO J. 1986 Nov;5(11):2825–2830. doi: 10.1002/j.1460-2075.1986.tb04575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C. H., Tulinsky A. Three-dimensional structure of the kringle sequence: structure of prothrombin fragment 1. Biochemistry. 1986 Jul 15;25(14):3977–3982. doi: 10.1021/bi00362a001. [DOI] [PubMed] [Google Scholar]
- Patthy L. Evolution of the proteases of blood coagulation and fibrinolysis by assembly from modules. Cell. 1985 Jul;41(3):657–663. doi: 10.1016/s0092-8674(85)80046-5. [DOI] [PubMed] [Google Scholar]
- Patthy L., Trexler M., Váli Z., Bányai L., Váradi A. Kringles: modules specialized for protein binding. Homology of the gelatin-binding region of fibronectin with the kringle structures of proteases. FEBS Lett. 1984 Jun 4;171(1):131–136. doi: 10.1016/0014-5793(84)80473-1. [DOI] [PubMed] [Google Scholar]
- Pennica D., Holmes W. E., Kohr W. J., Harkins R. N., Vehar G. A., Ward C. A., Bennett W. F., Yelverton E., Seeburg P. H., Heyneker H. L. Cloning and expression of human tissue-type plasminogen activator cDNA in E. coli. Nature. 1983 Jan 20;301(5897):214–221. doi: 10.1038/301214a0. [DOI] [PubMed] [Google Scholar]
- Petersen T. E., Thøgersen H. C., Skorstengaard K., Vibe-Pedersen K., Sahl P., Sottrup-Jensen L., Magnusson S. Partial primary structure of bovine plasma fibronectin: three types of internal homology. Proc Natl Acad Sci U S A. 1983 Jan;80(1):137–141. doi: 10.1073/pnas.80.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorstengaard K., Thøgersen H. C., Vibe-Pedersen K., Petersen T. E., Magnusson S. Purification of twelve cyanogen bromide fragments from bovine plasma fibronectin and the amino acid sequence of eight of them. Overlap evidence aligning two plasmic fragments, internal homology in gelatin-binding region and phosphorylation site near C terminus. Eur J Biochem. 1982 Nov 15;128(2-3):605–623. doi: 10.1111/j.1432-1033.1982.tb07007.x. [DOI] [PubMed] [Google Scholar]
- Trexler M., Patthy L. Folding autonomy of the kringle 4 fragment of human plasminogen. Proc Natl Acad Sci U S A. 1983 May;80(9):2457–2461. doi: 10.1073/pnas.80.9.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
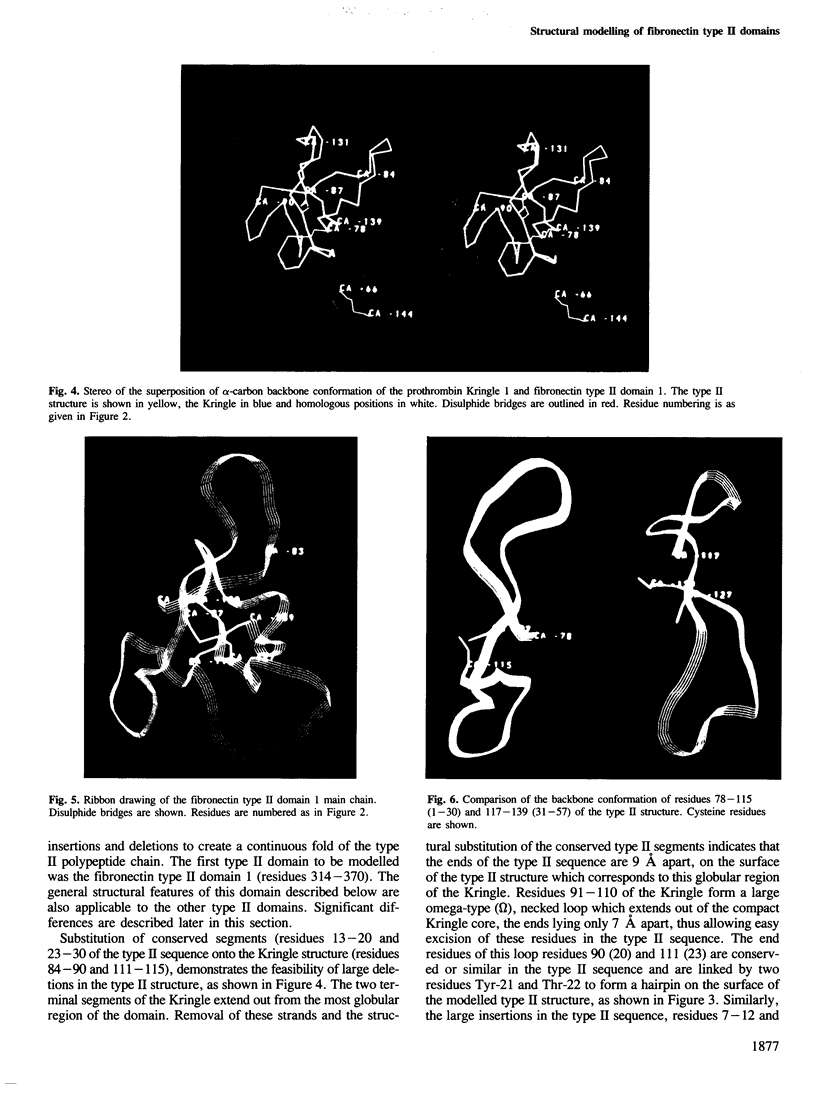
- Walz D. A., Hewett-Emmett D., Seegers W. H. Amino acid sequence of human prothrombin fragments 1 and 2. Proc Natl Acad Sci U S A. 1977 May;74(5):1969–1972. doi: 10.1073/pnas.74.5.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiman B., Lijnen H. R., Collen D. On the specific interaction between the lysine-binding sites in plasmin and complementary sites in alpha2-antiplasmin and in fibrinogen. Biochim Biophys Acta. 1979 Jul 25;579(1):142–154. doi: 10.1016/0005-2795(79)90094-1. [DOI] [PubMed] [Google Scholar]
- Yamada K. M. Cell surface interactions with extracellular materials. Annu Rev Biochem. 1983;52:761–799. doi: 10.1146/annurev.bi.52.070183.003553. [DOI] [PubMed] [Google Scholar]