Abstract
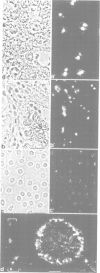
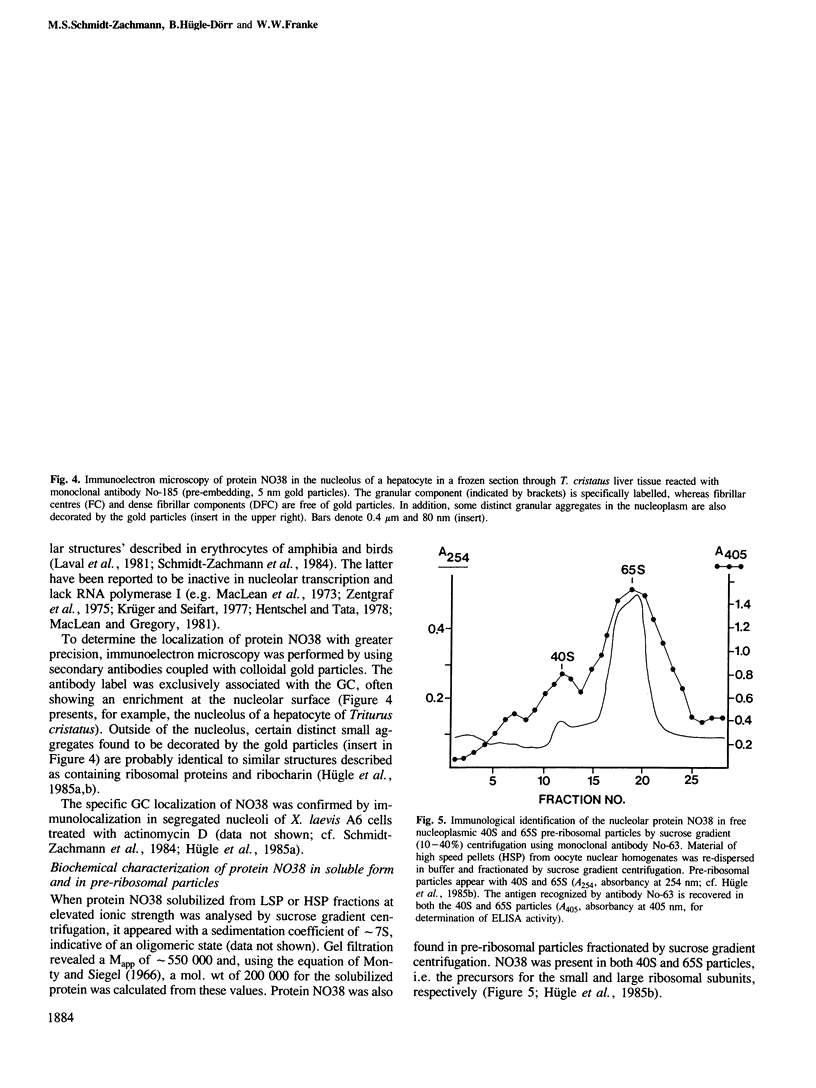
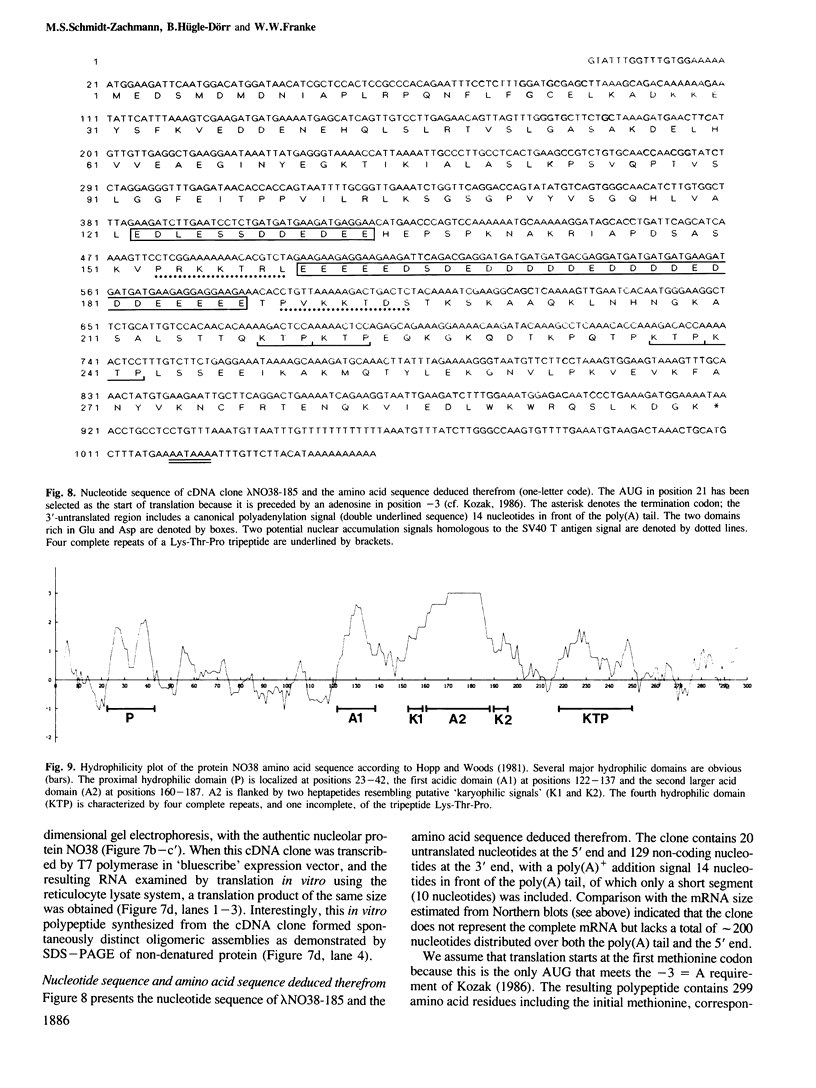
Using monoclonal antibodies we have localized a polypeptide, appearing on gel electrophoresis with a Mr of approximately 38,000 and a pI of approximately 5.6, to the granular component of the nucleoli of Xenopus laevis oocytes and a broad range of cells from various species. The protein (NO38) also occurs in certain distinct nucleoplasmic particles but is not detected in ribosomes and other cytoplasmic components. During mitosis NO38-containing material dissociates from the nucleolar organizer region and distributes over the chromosomal surfaces and the perichromosomal cytoplasm; in telophase it re-populates the forming nucleoli. With these antibodies we have isolated from a X. laevis ovary lambda gt11 expression library a cDNA clone encoding a polypeptide which, on one- and two-dimensional gel electrophoresis, co-migrates with authentic NO38. The amino acid sequence deduced from this clone defines a polypeptide of 299 amino acids of mol. wt 33,531 which is characterized by the presence of two domains exceptionally rich in aspartic and glutamic acid, one of them flanked by two putative karyophilic signal heptapeptides. Comparison with other protein sequences shows that NO38 is closely related to the histone-binding, karyophilic protein nucleoplasmin: the first 124 amino acids have 58 amino acid positions in common. Protein NO38 also shows striking homologies to the phosphopeptide region of rat nucleolar protein B23 and the carboxyterminal region of human B23. We propose that protein NO38, which forms distinct homo-oligomers of approximately 7S and Mr of approximately 230,000, is a member of a family of karyophilic proteins, the 'nucleoplasmin family'. It is characterized by its specific association with the nucleolus and might be involved in nuclear accumulation, nucleolar storage and pre-rRNA assembly of ribosomal proteins in a manner similar to that discussed for the role of nucleoplasmin in histone storage and chromatin assembly.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allis C. D., Ziegler Y. S., Gorovsky M. A., Olmsted J. B. A conserved histone variant enriched in nucleoli of mammalian cells. Cell. 1982 Nov;31(1):131–136. doi: 10.1016/0092-8674(82)90412-3. [DOI] [PubMed] [Google Scholar]
- Benavente R., Krohne G., Stick R., Franke W. W. Electron microscopic immunolocalization of a karyoskeletal protein of molecular weight 145 000 in nucleoli and perinucleolar bodies of Xenopus laevis. Exp Cell Res. 1984 Mar;151(1):224–235. doi: 10.1016/0014-4827(84)90370-7. [DOI] [PubMed] [Google Scholar]
- Benedum U. M., Baeuerle P. A., Konecki D. S., Frank R., Powell J., Mallet J., Huttner W. B. The primary structure of bovine chromogranin A: a representative of a class of acidic secretory proteins common to a variety of peptidergic cells. EMBO J. 1986 Jul;5(7):1495–1502. doi: 10.1002/j.1460-2075.1986.tb04388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M. Protein migration into nuclei. I. Frog oocyte nuclei in vivo accumulate microinjected histones, allow entry to small proteins, and exclude large proteins. J Cell Biol. 1975 Feb;64(2):421–430. doi: 10.1083/jcb.64.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugler B., Caizergues-Ferrer M., Bouche G., Bourbon H., Amalric F. Detection and localization of a class of proteins immunologically related to a 100-kDa nucleolar protein. Eur J Biochem. 1982 Nov 15;128(2-3):475–480. doi: 10.1111/j.1432-1033.1982.tb06989.x. [DOI] [PubMed] [Google Scholar]
- Chaly N., Bladon T., Setterfield G., Little J. E., Kaplan J. G., Brown D. L. Changes in distribution of nuclear matrix antigens during the mitotic cell cycle. J Cell Biol. 1984 Aug;99(2):661–671. doi: 10.1083/jcb.99.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P. K., Aldrich M., Cook R. G., Busch H. Amino acid sequence of protein B23 phosphorylation site. J Biol Chem. 1986 Feb 5;261(4):1868–1872. [PubMed] [Google Scholar]
- Chan P. K., Chan W. Y., Yung B. Y., Cook R. G., Aldrich M. B., Ku D., Goldknopf I. L., Busch H. Amino acid sequence of a specific antigenic peptide of protein B23. J Biol Chem. 1986 Oct 25;261(30):14335–14341. [PubMed] [Google Scholar]
- Colberg-Poley A. M., Voss S. D., Chowdhury K., Gruss P. Structural analysis of murine genes containing homoeo box sequences and their expression in embryonal carcinoma cells. 1985 Apr 25-May 1Nature. 314(6013):713–718. doi: 10.1038/314713a0. [DOI] [PubMed] [Google Scholar]
- Cotten M., Sealy L., Chalkley R. Massive phosphorylation distinguishes Xenopus laevis nucleoplasmin isolated from oocytes or unfertilized eggs. Biochemistry. 1986 Sep 9;25(18):5063–5069. doi: 10.1021/bi00366a014. [DOI] [PubMed] [Google Scholar]
- Dabauvalle M. C., Franke W. W. Determination of the intracellular state of soluble macromolecules by gel filtration in vivo in the cytoplasm of amphibian oocytes. J Cell Biol. 1986 Jun;102(6):2006–2014. doi: 10.1083/jcb.102.6.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabauvalle M. C., Franke W. W. Karyophilic proteins: polypeptides synthesized in vitro accumulate in the nucleus on microinjection into the cytoplasm of amphibian oocytes. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5302–5306. doi: 10.1073/pnas.79.17.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskal Y., Smetana K., Busch H. Evidence from studies on segregated nucleoli that nucleolar silver staining proteins C23 and B23 are in the fibrillar component. Exp Cell Res. 1980 Jun;127(2):285–291. doi: 10.1016/0014-4827(80)90434-6. [DOI] [PubMed] [Google Scholar]
- Davis L. I., Blobel G. Identification and characterization of a nuclear pore complex protein. Cell. 1986 Jun 6;45(5):699–709. doi: 10.1016/0092-8674(86)90784-1. [DOI] [PubMed] [Google Scholar]
- De Robertis E. M., Longthorne R. F., Gurdon J. B. Intracellular migration of nuclear proteins in Xenopus oocytes. Nature. 1978 Mar 16;272(5650):254–256. doi: 10.1038/272254a0. [DOI] [PubMed] [Google Scholar]
- De Robertis E. M. Nucleocytoplasmic segregation of proteins and RNAs. Cell. 1983 Apr;32(4):1021–1025. doi: 10.1016/0092-8674(83)90285-4. [DOI] [PubMed] [Google Scholar]
- DePinho R. A., Legouy E., Feldman L. B., Kohl N. E., Yancopoulos G. D., Alt F. W. Structure and expression of the murine N-myc gene. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1827–1831. doi: 10.1073/pnas.83.6.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derenzini M., Pession A., Betts-Eusebi C. M., Novello F. Relationship between the extended, non-nucleosomal intranucleolar chromatin in situ and ribosomal RNA synthesis. Exp Cell Res. 1983 Apr 15;145(1):127–143. doi: 10.1016/s0014-4827(83)80015-9. [DOI] [PubMed] [Google Scholar]
- Dingwall C., Dilworth S. M., Black S. J., Kearsey S. E., Cox L. S., Laskey R. A. Nucleoplasmin cDNA sequence reveals polyglutamic acid tracts and a cluster of sequences homologous to putative nuclear localization signals. EMBO J. 1987 Jan;6(1):69–74. doi: 10.1002/j.1460-2075.1987.tb04720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwall C., Sharnick S. V., Laskey R. A. A polypeptide domain that specifies migration of nucleoplasmin into the nucleus. Cell. 1982 Sep;30(2):449–458. doi: 10.1016/0092-8674(82)90242-2. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Honda B. M., Laskey R. A., Thomas J. O. Assembly of nucleosomes: the reaction involving X. laevis nucleoplasmin. Cell. 1980 Sep;21(2):373–383. doi: 10.1016/0092-8674(80)90474-2. [DOI] [PubMed] [Google Scholar]
- Escande M. L., Gas N., Stevens B. J. Immunolocalization of the 100 K nucleolar protein in CHO cells. Biol Cell. 1985;53(2):99–109. doi: 10.1111/j.1768-322x.1985.tb00359.x. [DOI] [PubMed] [Google Scholar]
- Fakan S., Bernhard W. Localisation of rapidly and slowly labelled nuclear RNA as visualized by high resolution autoradiography. Exp Cell Res. 1971 Jul;67(1):129–141. doi: 10.1016/0014-4827(71)90628-8. [DOI] [PubMed] [Google Scholar]
- Fakan S., Hernandez-Verdun D. The nucleolus and the nucleolar organizer regions. Biol Cell. 1986;56(3):189–205. doi: 10.1111/j.1768-322x.1986.tb00452.x. [DOI] [PubMed] [Google Scholar]
- Feldherr C. M., Kallenbach E., Schultz N. Movement of a karyophilic protein through the nuclear pores of oocytes. J Cell Biol. 1984 Dec;99(6):2216–2222. doi: 10.1083/jcb.99.6.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields A. P., Kaufmann S. H., Shaper J. H. Analysis of the internal nuclear matrix. Oligomers of a 38 kD nucleolar polypeptide stabilized by disulfide bonds. Exp Cell Res. 1986 May;164(1):139–153. doi: 10.1016/0014-4827(86)90461-1. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Kleinschmidt J. A., Spring H., Krohne G., Grund C., Trendelenburg M. F., Stoehr M., Scheer U. A nucleolar skeleton of protein filaments demonstrated in amplified nucleoli of Xenopus laevis. J Cell Biol. 1981 Aug;90(2):289–299. doi: 10.1083/jcb.90.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman J. W., Dowell B. L., Ochs R. L., Ross B. E., Busch H. Effect of differentiation on the expression of a nucleolar antigen with a molecular weight of 145,000 in HL-60 cells. Cancer Res. 1987 Jan 15;47(2):586–591. [PubMed] [Google Scholar]
- Gao B., Klein L. E., Britten R. J., Davidson E. H. Sequence of mRNA coding for bindin, a species-specific sea urchin sperm protein required for fertilization. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8634–8638. doi: 10.1073/pnas.83.22.8634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler N., Kaufmann E., Fischer S., Plessmann U., Weber K. Neurofilament architecture combines structural principles of intermediate filaments with carboxy-terminal extensions increasing in size between triplet proteins. EMBO J. 1983;2(8):1295–1302. doi: 10.1002/j.1460-2075.1983.tb01584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goessens G. Nucleolar structure. Int Rev Cytol. 1984;87:107–158. doi: 10.1016/s0074-7696(08)62441-9. [DOI] [PubMed] [Google Scholar]
- Goldfarb D. S., Gariépy J., Schoolnik G., Kornberg R. D. Synthetic peptides as nuclear localization signals. Nature. 1986 Aug 14;322(6080):641–644. doi: 10.1038/322641a0. [DOI] [PubMed] [Google Scholar]
- Goodall G. J., Dominguez F., Horecker B. L. Molecular cloning of cDNA for human prothymosin alpha. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8926–8928. doi: 10.1073/pnas.83.23.8926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon J. B. Nuclear transplantation and the control of gene activity in animal development. Proc R Soc Lond B Biol Sci. 1970 Dec 1;176(1044):303–314. doi: 10.1098/rspb.1970.0050. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Hereford L., Herskowitz I. Targeting of E. coli beta-galactosidase to the nucleus in yeast. Cell. 1984 Apr;36(4):1057–1065. doi: 10.1016/0092-8674(84)90055-2. [DOI] [PubMed] [Google Scholar]
- Hentschel C. C., Tata J. R. Template-engaged and free RNA polymerases during Xenopus erythroid cell maturation. Dev Biol. 1978 Aug;65(2):496–507. doi: 10.1016/0012-1606(78)90044-1. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hügle B., Hazan R., Scheer U., Franke W. W. Localization of ribosomal protein S1 in the granular component of the interphase nucleolus and its distribution during mitosis. J Cell Biol. 1985 Mar;100(3):873–886. doi: 10.1083/jcb.100.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hügle B., Scheer U., Franke W. W. Ribocharin: a nuclear Mr 40,000 protein specific to precursor particles of the large ribosomal subunit. Cell. 1985 Jun;41(2):615–627. doi: 10.1016/s0092-8674(85)80034-9. [DOI] [PubMed] [Google Scholar]
- Iacangelo A., Affolter H. U., Eiden L. E., Herbert E., Grimes M. Bovine chromogranin A sequence and distribution of its messenger RNA in endocrine tissues. Nature. 1986 Sep 4;323(6083):82–86. doi: 10.1038/323082a0. [DOI] [PubMed] [Google Scholar]
- Kalderon D., Roberts B. L., Richardson W. D., Smith A. E. A short amino acid sequence able to specify nuclear location. Cell. 1984 Dec;39(3 Pt 2):499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Kaufmann E., Geisler N., Weber K. SDS-PAGE strongly overestimates the molecular masses of the neurofilament proteins. FEBS Lett. 1984 May 7;170(1):81–84. doi: 10.1016/0014-5793(84)81373-3. [DOI] [PubMed] [Google Scholar]
- Kistler J., Duncombe Y., Laemmli U. K. Mapping nucleolar proteins with monoclonal antibodies. J Cell Biol. 1984 Dec;99(6):1981–1988. doi: 10.1083/jcb.99.6.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt J. A., Dingwall C., Maier G., Franke W. W. Molecular characterization of a karyophilic, histone-binding protein: cDNA cloning, amino acid sequence and expression of nuclear protein N1/N2 of Xenopus laevis. EMBO J. 1986 Dec 20;5(13):3547–3552. doi: 10.1002/j.1460-2075.1986.tb04681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt J. A., Fortkamp E., Krohne G., Zentgraf H., Franke W. W. Co-existence of two different types of soluble histone complexes in nuclei of Xenopus laevis oocytes. J Biol Chem. 1985 Jan 25;260(2):1166–1176. [PubMed] [Google Scholar]
- Kleinschmidt J. A., Franke W. W. Soluble acidic complexes containing histones H3 and H4 in nuclei of Xenopus laevis oocytes. Cell. 1982 Jul;29(3):799–809. doi: 10.1016/0092-8674(82)90442-1. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Krippl B., Ferguson B., Jones N., Rosenberg M., Westphal H. Mapping of functional domains in adenovirus E1A proteins. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7480–7484. doi: 10.1073/pnas.82.22.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krohne G., Benavente R. The nuclear lamins. A multigene family of proteins in evolution and differentiation. Exp Cell Res. 1986 Jan;162(1):1–10. doi: 10.1016/0014-4827(86)90421-0. [DOI] [PubMed] [Google Scholar]
- Krohne G., Franke W. W. A major soluble acidic protein located in nuclei of diverse vertebrate species. Exp Cell Res. 1980 Sep;129(1):167–189. doi: 10.1016/0014-4827(80)90341-9. [DOI] [PubMed] [Google Scholar]
- Krohne G., Franke W. W. Immunological identification and localization of the predominant nuclear protein of the amphibian oocyte nucleus. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1034–1038. doi: 10.1073/pnas.77.2.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krohne G., Stick R., Kleinschmidt J. A., Moll R., Franke W. W., Hausen P. Immunological localization of a major karyoskeletal protein in nucleoli of oocytes and somatic cells of Xenopus laevis. J Cell Biol. 1982 Sep;94(3):749–754. doi: 10.1083/jcb.94.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger C., Seifart K. H. RNA polymerases during differentiation of avian erythrocytes. Exp Cell Res. 1977 May;106(2):446–450. doi: 10.1016/0014-4827(77)90200-2. [DOI] [PubMed] [Google Scholar]
- Lanford R. E., Butel J. S. Construction and characterization of an SV40 mutant defective in nuclear transport of T antigen. Cell. 1984 Jul;37(3):801–813. doi: 10.1016/0092-8674(84)90415-x. [DOI] [PubMed] [Google Scholar]
- Lanford R. E., Kanda P., Kennedy R. C. Induction of nuclear transport with a synthetic peptide homologous to the SV40 T antigen transport signal. Cell. 1986 Aug 15;46(4):575–582. doi: 10.1016/0092-8674(86)90883-4. [DOI] [PubMed] [Google Scholar]
- Lapeyre B., Amalric F., Ghaffari S. H., Rao S. V., Dumbar T. S., Olson M. O. Protein and cDNA sequence of a glycine-rich, dimethylarginine-containing region located near the carboxyl-terminal end of nucleolin (C23 and 100 kDa). J Biol Chem. 1986 Jul 15;261(20):9167–9173. [PubMed] [Google Scholar]
- Laskey R. A., Honda B. M., Mills A. D., Finch J. T. Nucleosomes are assembled by an acidic protein which binds histones and transfers them to DNA. Nature. 1978 Oct 5;275(5679):416–420. doi: 10.1038/275416a0. [DOI] [PubMed] [Google Scholar]
- Laval M., Hernandez-Verdun D., Bouteille M. Remnant nucleolar structures and residual RNA synthesis in chick erythrocytes. Exp Cell Res. 1981 Mar;132(1):157–167. doi: 10.1016/0014-4827(81)90092-6. [DOI] [PubMed] [Google Scholar]
- Lewis S. A., Cowan N. J. Genetics, evolution, and expression of the 68,000-mol-wt neurofilament protein: isolation of a cloned cDNA probe. J Cell Biol. 1985 Mar;100(3):843–850. doi: 10.1083/jcb.100.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lischwe M. A., Ochs R. L., Reddy R., Cook R. G., Yeoman L. C., Tan E. M., Reichlin M., Busch H. Purification and partial characterization of a nucleolar scleroderma antigen (Mr = 34,000; pI, 8.5) rich in NG,NG-dimethylarginine. J Biol Chem. 1985 Nov 15;260(26):14304–14310. [PubMed] [Google Scholar]
- Maclean N., Hilder V. A., Baynes Y. A. RNA synthesis in Xenopus erythrocytes. Cell Differ. 1973 Dec;2(5):261–269. doi: 10.1016/0045-6039(73)90030-4. [DOI] [PubMed] [Google Scholar]
- Mamrack M. D., Olson M. O., Busch H. Amino acid sequence and sites of phosphorylation in a highly acidic region of nucleolar nonhistone protein C23. Biochemistry. 1979 Jul 24;18(15):3381–3386. doi: 10.1021/bi00582a026. [DOI] [PubMed] [Google Scholar]
- Mamrack M. D., Olson M. O., Busch H. Negatively charged phosphopeptides of nucleolar nonhistone proteins from Novikoff hepatoma ascites cells. Biochem Biophys Res Commun. 1977 May 9;76(1):150–157. doi: 10.1016/0006-291x(77)91680-1. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mills A. D., Laskey R. A., Black P., De Robertis E. M. An acidic protein which assembles nucleosomes in vitro is the most abundant protein in Xenopus oocyte nuclei. J Mol Biol. 1980 May 25;139(3):561–568. doi: 10.1016/0022-2836(80)90148-5. [DOI] [PubMed] [Google Scholar]
- Moran E., Mathews M. B. Multiple functional domains in the adenovirus E1A gene. Cell. 1987 Jan 30;48(2):177–178. doi: 10.1016/0092-8674(87)90418-1. [DOI] [PubMed] [Google Scholar]
- Moreau N., Angelier N., Bonnanfant-Jais M. L., Gounon P., Kubisz P. Association of nucleoplasmin with transcription products as revealed by immunolocalization in the amphibian oocyte. J Cell Biol. 1986 Sep;103(3):683–690. doi: 10.1083/jcb.103.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreland R. B., Nam H. G., Hereford L. M., Fried H. M. Identification of a nuclear localization signal of a yeast ribosomal protein. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6561–6565. doi: 10.1073/pnas.82.19.6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno Diaz de la Espina S., Franke W. W., Krohne G., Trendelenburg M. F., Grund C., Scheer U. Medusoid fibril bodies: a novel type of nuclear filament of diameter 8 to 12 nm with periodic ultrastructure demonstrated in oocytes of Xenopus laevis. Eur J Cell Biol. 1982 Jun;27(2):141–150. [PubMed] [Google Scholar]
- Moreno F. J., Hernandez-Verdun D., Masson C., Bouteille M. Silver staining of the nucleolar organizer regions (NORs) on Lowicryl and cryo-ultrathin sections. J Histochem Cytochem. 1985 May;33(5):389–399. doi: 10.1177/33.5.2580879. [DOI] [PubMed] [Google Scholar]
- Morris G. E., Nguyen thi Man, Head L. P. Monoclonal antibodies against a nucleolar protein from differentiating chick muscle cells. J Cell Sci. 1985 Jun;76:105–113. doi: 10.1242/jcs.76.1.105. [DOI] [PubMed] [Google Scholar]
- Ochs R. L., Lischwe M. A., Shen E., Carroll R. E., Busch H. Nucleologenesis: composition and fate of prenucleolar bodies. Chromosoma. 1985;92(5):330–336. doi: 10.1007/BF00327463. [DOI] [PubMed] [Google Scholar]
- Ochs R. L., Lischwe M. A., Spohn W. H., Busch H. Fibrillarin: a new protein of the nucleolus identified by autoimmune sera. Biol Cell. 1985;54(2):123–133. doi: 10.1111/j.1768-322x.1985.tb00387.x. [DOI] [PubMed] [Google Scholar]
- Ochs R., Lischwe M., O'Leary P., Busch H. Localization of nucleolar phosphoproteins B23 and C23 during mitosis. Exp Cell Res. 1983 Jun;146(1):139–149. doi: 10.1016/0014-4827(83)90332-4. [DOI] [PubMed] [Google Scholar]
- Paine P. L. Diffusive and nondiffusive proteins in vivo. J Cell Biol. 1984 Jul;99(1 Pt 2):188s–195s. doi: 10.1083/jcb.99.1.188s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierandrei-Amaldi P., Beccari E., Bozzoni I., Amaldi F. Ribosomal protein production in normal and anucleolate Xenopus embryos: regulation at the posttranscriptional and translational levels. Cell. 1985 Aug;42(1):317–323. doi: 10.1016/s0092-8674(85)80127-6. [DOI] [PubMed] [Google Scholar]
- Prestayko A. W., Klomp G. R., Schmoll D. J., Busch H. Comparison of proteins of ribosomal subunits and nucleolar preribosomal particles from Novikoff hepatoma ascites cells by two-dimensional polyacrylamide gel electrophoresis. Biochemistry. 1974 Apr 23;13(9):1945–1951. doi: 10.1021/bi00706a026. [DOI] [PubMed] [Google Scholar]
- Richardson W. D., Roberts B. L., Smith A. E. Nuclear location signals in polyoma virus large-T. Cell. 1986 Jan 17;44(1):77–85. doi: 10.1016/0092-8674(86)90486-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer U., Rose K. M. Localization of RNA polymerase I in interphase cells and mitotic chromosomes by light and electron microscopic immunocytochemistry. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1431–1435. doi: 10.1073/pnas.81.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Zachmann M. S., Hügle B., Scheer U., Franke W. W. Identification and localization of a novel nucleolar protein of high molecular weight by a monoclonal antibody. Exp Cell Res. 1984 Aug;153(2):327–346. doi: 10.1016/0014-4827(84)90604-9. [DOI] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Spector D. L., Ochs R. L., Busch H. Silver staining, immunofluorescence, and immunoelectron microscopic localization of nucleolar phosphoproteins B23 and C23. Chromosoma. 1984;90(2):139–148. doi: 10.1007/BF00292451. [DOI] [PubMed] [Google Scholar]
- Stanton L. W., Schwab M., Bishop J. M. Nucleotide sequence of the human N-myc gene. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1772–1776. doi: 10.1073/pnas.83.6.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Warner J. R. Distribution of newly formed ribosomal proteins in HeLa cell fractions. J Cell Biol. 1979 Mar;80(3):767–772. doi: 10.1083/jcb.80.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt R., Stanton L. W., Marcu K. B., Gallo R. C., Croce C. M., Rovera G. Nucleotide sequence of cloned cDNA of human c-myc oncogene. Nature. 1983 Jun 23;303(5919):725–728. doi: 10.1038/303725a0. [DOI] [PubMed] [Google Scholar]
- Williams M. A., Kleinschmidt J. A., Krohne G., Franke W. W. Argyrophilic nuclear and nucleolar proteins of Xenopus laevis oocytes identified by gel electrophoresis. Exp Cell Res. 1982 Feb;137(2):341–351. doi: 10.1016/0014-4827(82)90035-0. [DOI] [PubMed] [Google Scholar]
- Zentgraf H., Scheer U., Franke W. W. Characterization and localization of the RNA synthesized in mature avian erythrocytes. Exp Cell Res. 1975 Nov;96(1):81–95. doi: 10.1016/s0014-4827(75)80040-1. [DOI] [PubMed] [Google Scholar]