Abstract
Purpose
Extracorporeal Membrane Oxygenation (ECMO) is a cardiopulmonary bypass device that is used to temporarily support the most critically ill of patients with respiratory and/or cardiac failure. Infection and its sequelae may be an indication for ECMO or infections may be acquired while on ECMO, and are associated with a mortality of greater than 50%.1 Effective therapy requires optimal dosing. However, optimal dosing can be different in patients on ECMO, because the ECMO circuit can alter drug pharmacokinetics. This review assessed the current literature for pharmacokinetic data and subsequent dosing recommendations for anti-infective drugs in patients on ECMO.
Methods
We searched the PubMed and EMBASE databases (1965 to February 2016) and included case reports, case series, or studies that provided pharmacokinetic data for anti-infective drugs including antibiotics, antifungals, and antivirals being used to treat patients of all age groups on ECMO. Pharmacokinetic parameters and dosing recommendations based on these data are presented.
Findings
The majority of data on this topic come from neonatal studies of antibiotics from the 1980s and 1990s. These studies generally demonstrate a larger volume of distribution (V) due to ECMO and therefore, higher doses are needed initially. More adult data is now emerging, but with a predominance of case reports and case series without comparison to critically ill controls. The available pharmacokinetic analyses do suggest that V and clearance (CL) are unchanged in the adult population and therefore dosing recommendations largely remain unchanged. There are a lack of data in children >1 year of age. The data support the importance of therapeutic drug monitoring (TDM) when available in this population of patients.
Implications
This review found reasonably robust dosing recommendations for some drugs and scant or no data for other important anti-infectives. In order to better determine optimal dosing on ECMO, a systematic approach is needed. Approaches that combine ex vivo ECMO experiments, animal studies, specialized pharmacokinetic modeling, and human clinical trials are being developed.
Keywords: ECMO, pharmacokinetics, anti-infectives, antibiotics, antifungals, antivirals
INTRODUCTION
Extracorporeal Membrane Oxygenation (ECMO) is a cardiopulmonary bypass device that supports patients with respiratory and/or cardiac failure. Mechanically, blood is drained from the venous system, pumped through an artificial lung membrane, and then returned to either the venous or arterial circulation. ECMO support comes in two forms: veno-venous (VV) and veno-arterial (VA). VV ECMO provides pulmonary support and VA ECMO provides both pulmonary and cardiac support. Regardless of whether the ECMO circuit is configured for VV or VA ECMO, the components are the same: tubing, pump, and oxygenator (Figure 1).
Figure 1.
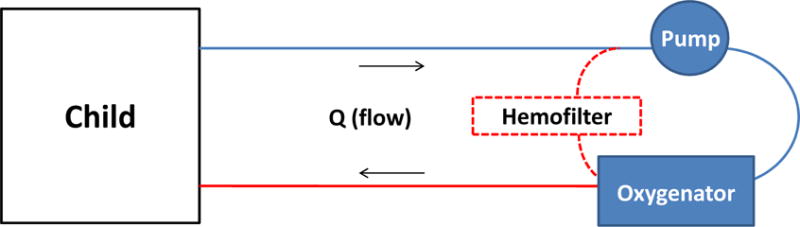
Basic ECMO diagram
While ECMO is life-saving in a very critically ill subset of patients, it also presents new challenges related to their management. One of these challenges is understanding and appropriately compensating for the effect of ECMO on drug pharmacokinetics (PK). This topic has been studied since the late 1980’s in neonates and isolated ex vivo ECMO circuits, and more recently in older children and adults. In general, ECMO has been shown to impact PK in three primary ways, as discussed below.
Direct extraction by the circuit
Drug extraction by the ECMO circuit is a well-recognized contributor to PK alterations across all patient populations that depends on both the circuit component materials and the drug physicochemical properties. The interaction between the drug and the ECMO circuit has been studied primarily in ex vivo experiments in which drug is administered to isolated ECMO circuits.2–11 Because there is no human attached to the circuit, any change in concentration over time is due to extraction by the circuit or drug degradation. In general, ex vivo studies have demonstrated increased extraction of highly lipophilic and protein bound drugs.4,9,10
However, the degree of extraction, even for the same drug, can be markedly affected by circuit materials. A study of fentanyl (LogP 4, protein binding 80%) in an ECMO circuit using a silicone membrane oxygenator showed >99% fentanyl loss in 180 minutes.11 When the same experiment was repeated in a circuit with a microporous, hollow-fiber polypropylene oxygenator, fentanyl loss was only 66% at 180 minutes.11 Another study compared the effect of six different coatings on drug extraction by the PVC tubing by administering fentanyl to circuits constructed with only a pump and tubing (no oxygenator).12,13 In the uncoated circuit, fentanyl loss at 120 minutes was 80%.12 In the coated tubing, fentanyl loss at 120 minutes ranged from 40–75%.13
Drug extraction by the circuit is likely due to non-specific adsorption.4,12–15 Adsorption is a function of 1) interactions between the drug and the material surface, 2) the maximal amount of binding per unit of surface area, and 3) the affinity of the drug for the surface. The process of adsorption is driven primarily by electrostatic and hydrophobic interactions. Interactions with polymers such as those used in ECMO circuit equipment tend to be due to hydrophobic interactions (i.e., hydrophobic drugs adhere to hydrophobic alkyl groups on polymers). Electrostatic interactions tend to dominate when surface coatings are applied to ECMO circuit components. Surface coatings are increasingly applied to ECMO circuit components to minimize the inflammatory response triggered when blood comes into contact with a foreign material,16–18 but coatings also can change the nature of interaction between the drug and material surface. When the drug and surface are oppositely charged, the degree of ionization driven by blood pH and drug pKa can influence the degree of adsorption. The extent and irreversibility of adsorption depend on the number of binding sites on the material surface and the affinity of the drug for the surface. In the ECMO system data are conflicting as to whether binding is saturable.3,11
Increased volume of distribution
ECMO support can increase volume of distribution (V) via multiple mechanisms: 1) drug extraction via direct interaction with the circuit as mentioned above; 2) hemodilution; and 3) physiologic changes related to ECMO support and critical illness. Hemodilution occurs due to the large volume of exogenous blood required to prime the circuit, frequent transfusions of blood products, and administration of crystalloid to maintain circuit flows. Hemodilution has the largest effect on drugs whose distribution is limited to the plasma compartment (i.e. low V drugs). Drugs that distribute widely to tissues (i.e. high V drugs) may be less impacted because drug extracted by the circuit may be replaced by drug stored in the tissue. The impact of hemodilution is likely inversely related to age. For a 3kg infant, the circuit prime volume (250–400mL) might exceed the infant’s native blood volume (~250mL), while in a 30kg child, the prime volume is ~20% of the child’s blood volume (~2.1L) and therefore represents ~40% of their total circulating blood volume while on ECMO (Figure 2). In addition, ongoing hemolysis and the need to maintain hemostasis results in frequent transfusions of blood products, sometimes totaling 6–8L over the course of an ECMO run.19
Figure 2.
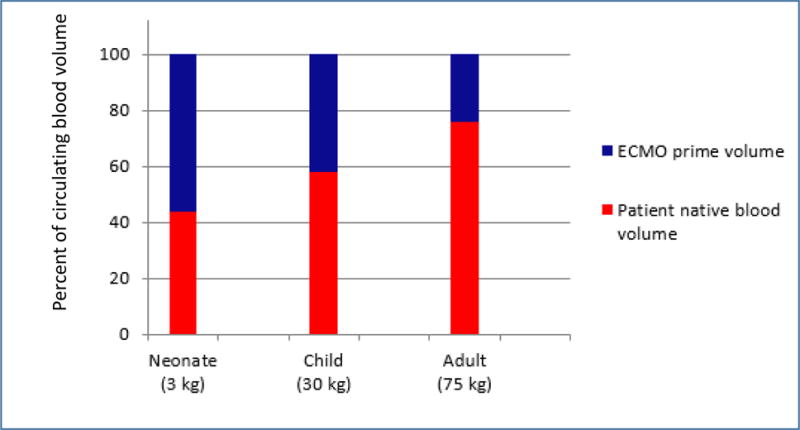
Age-dependent impact of ECMO prime volume on native blood volume for neonates, children, and adults on ECMO
The disease state can also impact V. Exposure to the ECMO circuit results in an inflammatory response.20–22 Inflammation often results in capillary leak and edema, which can increase V.20,23,24 In addition, patients on ECMO can have altered blood pH, which can affect a drug’s ionization and distribution into tissues. Finally, the renin-angiotensin system in the kidney can be upregulated, possibly related to non-pulsatile blood flow seen in VA EMCO.25 Upregulation of the renin-angiotensin system alters handling of fluids and can change the ratio of fluids in the body fluid compartments.
Our understanding of the impact of ECMO on V is limited by the fact that most studies were done in infants or an ex vivo system. Although ex vivo studies are useful in understanding how drugs interact with an ECMO circuit in isolation, direct translation of those results into humans is challenging. Translating results from infant studies is challenging because infants differ from older children and adults in important ways. In addition to the different ratio of exogenous to native blood volume described above, infants have a higher proportion of body water and lower protein binding, both of which can impact V.26–28 For these reasons, extrapolation of infant ECMO data to older children and adults must be done with caution.
Altered clearance
ECMO alters the PK of certain drugs by the effect it has on various organ systems. Renal dysfunction is common in patients on ECMO, occurring in >30% of ECMO patients.29 Reasons for the renal dysfunction are not entirely clear but appear to be multifactorial. Hypoxia and poor organ perfusion prior to ECMO support likely contributes. Non-pulsatile blood flow seen with VA ECMO is associated with decreased glomerular filtration rate (GFR).25 Of note, in VV ECMO where blood flow is pulsatile, the incidence of renal dysfunction (32%) is almost as high as that observed in VA ECMO (47%).29 Altered renal function can substantially increase exposure of renally-cleared drugs and places patients at risk for toxicity. The effect is complicated if hemofiltration or dialysis is combined with ECMO support. Some drugs are cleared by hemodialysis (compensating for decreased renal function), while others are not.
The impact of ECMO on metabolic capacity is not well described. It is postulated that decreased regional flow to the liver could result in decreased metabolism of hepatically-cleared drugs, especially those where extraction is blood-flow dependent.30 Further, we know that ECMO causes inflammation and that inflammation tends to decrease the expression and function of drug metabolizing enzymes.22,31–35 There are no data describing the impact of ECMO on drug transporters.
Patients on ECMO are critically ill and receive multiple drugs; therefore understanding the PK alterations that occur on ECMO is crucial. Anti-infective drugs are frequently used in patients on ECMO. Optimal dosing is important, because infections are common in patients on ECMO and result in substantial morbidity and mortality. According to the Extracorporeal Life Support Organization (ELSO), infection rates among all age groups on ECMO are as high as 15 per 1,000 ECMO days36. The mortality of patients on ECMO with reported infections ranges from 56–68%1. The objective of this paper is to review the current literature and provide dosing recommendations for antimicrobial drugs in patients on ECMO.
METHODS
We performed a literature review by searching PubMed (1965 to February 2016) and EMBASE (1965 to February 2016). Search terms included “pharmacokinetics OR dosing”, “Extracorporeal Membrane Oxygenation OR ECMO”, and “anti-infectives”. The search terms were developed in conjunction with a Duke University Medical Center librarian. Animal studies, ex vivo studies, and studies in languages other than English were excluded. Studies were included if they contained PK data of anti-infective drugs for study populations including neonates (0–28 days of age), infants (29 days-2 years of age), children (2-<12 years of age), adolescents (12-<18 years of age), and adults (≥ 18 years of age) on ECMO. References from searched articles were also considered and cited if they met the aforementioned criteria.
RESULTS
Dosing recommendations based on the available data are presented by age in Tables 1–3. In many cases, limited data existed for a given drug. In these instances we provide a dosing recommendation but note the limitations of those recommendations.
Table 1.
Anti-infective pharmacokinetics and dosing in neonates/infantsa on ECMO
| Drug | Volume of Distribution compared to non-ECMO patients | Clearance compared to non- ECMO patients | Standard Dosing in critically ill patientsb, c | Dosing Recommendations for patients on ECMOb |
|---|---|---|---|---|
| Antibiotics | ||||
| Cefotaxime | Increased | Unchanged | General: ≤7 days: 50 mg/kg q12h 7–28 days: 50 mg/kg q8h >28 days: 150 mg/kg/day divided q6-q8h |
Standard dosing |
| Gentamicin | Unchanged or increased | Decreased | < 3 months: 4–5 mg/kg q24h 3 months-2 years: 9.5 mg/kg q24hd |
<3 months: 4–5 mg/kg once then check 2 and 8–12 hour concentrations 3 months-2 years: 9.5 mg/kg once then check 2 and 8–12 hour concentrations |
| Meropenem | Not reported | Increased | General: 20 mg/kg q8h Meningitis: 40 mg/kg q8h |
40 mg/kg loading dose then 200 mg/kg/day continuous infusione or 33.3 mg/kg over 3 hours q4h |
| Vancomycin | Increased | Decreased | <7 days: 30 mg/kg/day divided q8–12h ≥7–28 days: 40–45 mg/kg/day divided q6–8h >28 days: 45–60 mg/kg/day divided q6–8h |
20 mg/kg once then check 2 and 8–12 hour concentrations |
| Anti-fungals | ||||
| Caspofungin | Increased | Increased | <3 months: 25 mg/m2 daily >3 months: 70 mg/m2 on day 1 then 50 mg/m2 daily Enzyme inducersf: 70 mg/m2 daily |
>78 mg/m2 dailyg |
| Fluconazole | Increased | Affected by renal function, not ECMO | Prophylaxis: ≤28 days: 3 mg/kg once daily or 6 mg/kg q48h >28 days: 6 mg/kg q24h Treatment: 25 mg/kg loading dose then 12 mg/kg q24h |
Prophylaxis: 12 mg/kg loading dose then 6 mg/kg q24hh Treatment: 35 mg/kg loading dose then 12 mg/kg q24hh |
| Micafungin | Increased | Decreased | Prophylaxis: ≥4months: 1–3 mg/kg q24h Treatment: <4 months: 7–10 mg/kg q24h ≥4 months: 3–7 mg/kg q24h |
Prophylaxis: 3 mg/kg q24hh Treatment: 5 mg/kg q24hh |
| Anti-virals | ||||
| Ganciclovir | Not reported | Not reported | Prophylaxis: 5 mg/kg q24h Treatment: 5 mg/kg q24h Congenital CMV: 6 mg/kg q12h |
6 mg/kg q12hi |
| Ribavirin | Decreased | Unchanged | Not established for patients <3 years of age | 5 mg/kg q6hj |
neonates are children <30 days of age, infants 1 month-2 years of age
all doses intravenous (IV)
Unless otherwise noted, all dosing recommendations for standard of care dosing were obtained from Lexicomp Online, Pediatric & Neonatal Lexi-Drugs, Hudson, Ohio: Lexi-Comp, Inc: April 2016
McDade EJ, Wagner JL, Moffett BS, Palazzi DL. Once-daily gentamicin dosing in pediatric patients without cystic fibrosis. Pharmacotherapy. 2010;30(3):248–253.
case report of 8 month old infant
enzyme inducing medication include rifampin, phenytoin, carbamazepine, dexamethasone, etc.
case report of 11 month old infant, dose still resulted in sub-therapeutic levels
doses for infants aged 0–2 years
case report of a neonate with congenital CMV
case report of a neonate with disseminated adenovirus
Table 3.
Anti-infective pharmacokinetics and dosing in adults on ECMO. Many of the recommendations are based on a single case study or case series and must be used acknowledging this limitation.
| Drug | Volume of Distribution compared to non-ECMO patients | Clearance compared to non-ECMO patients | Standard Dosing in critically ill patientsa, b | Dosing Recommendations for patients on ECMOa |
|---|---|---|---|---|
| Antibiotics | ||||
| Azithromycin | Decreased | Unchanged | 500 mg q24h or 500 mg × 1 then 250 mg q24h | Standard dosingc |
| Ethambutol | Not reported | Not reported | Weight 40–55 kg: 800 mg q24h Weight 56–75 kg: 1200 mg q24h Weight 76–90 kg: 1600 mg q24h |
50% increase over standard dosingd |
| Imipenem | Not reported | Not reported | 500–1000 mg q6–8h | Standard dosing unless treating resistant organisme |
| Levofloxacin | Not reported | Not reported | 500–1000 mg q24h | Standard dosingd |
| Linezolid | Unchanged | Not reported | 600 mg IV q12h | Standard dosing unless MRSA MIC is >1mg/Lf |
| Meropenem | Unchanged | Unchanged | General: 500 mg q6h or 1 g q8h Meningitis/Cystic Fibrosis: 2 g q8h |
1 g q8h for susceptible organisms (MIC≤2) 2 g q8h for resistant organisms (MIC >2 to 8) |
| Piperacillin/tazobactam | Unchanged | Unchanged | 3.375 g q6h- 4.5 g q6–8h | Standard dosing may be adequate for susceptible organisms |
| Pyrazinamide | Weight 40–55 kg: 1000 mg q24h Weight 56–75 kg: 1500 mg q24h Weight 76–90 kg: 2000 mg q24h |
50% increase over standard dosingd | ||
| Rifampin | Not reported | Not reported | Tuberculosis treatment: 10 mg/kg q24h | 20 mg/kg q24he |
| Tigecycline | Unchanged | Unchanged | 100 mg once then 50 mg q12h | Standard dosingg |
| Vancomycin | Unchanged | Unchanged | 15–20 mg/kg/dose q8–12h | Standard dosing |
| Anti-fungals | ||||
| Liposomal Amphotericin B | Not reported | Not reported | 3–5 mg/kg q24h | Standard dosingh |
| Caspofungin | Not reported | Not reported | 70 mg on day 1 then 50 mg q24h Enzyme inducersi: 70 mg q24h | Definitive dosing recommendations cannot be made |
| Voriconazole | Not reported | Not reported | 6 mg/kg q12h × 2 doses then 4 mg/kg q12h | Definitive dosing recommendations cannot be made |
| Anti-virals | ||||
| Oseltamivir | Unchanged | Unchanged | 75 mg or 150 mg enterally BID for severe illness | Standard dosing unless impaired renal function or enteral absorption |
| Ganciclovir | Not reported | Not reported | 6 mg/kg q12h | Standard dosingj |
all doses intravenous (IV) except oseltamivir (enteral)
Unless otherwise noted, all recommendations for standard of care dosing were obtained from Lexicomp Online, Lexi-Drugs, Hudson, Ohio: Lexi-Comp, Inc: April 2016
case series of three patients with ARDS
dose required to achieve target serum levels in a case report of a patient with miliary tuberculosis
case series of two lung transplant recipients with refractory ARDS
case series of three patients with MRSA
case report of a patient with vancomycin-resistant Staphylococcus epidermidis pneumonia
case report of a patient with Aspergillus fumigatus pneumonia
enzyme inducing medication include rifampin, phenytoin, carbamazepine, dexamethasone, etc.
case series of two patients with Candida albicans and Aspergillus
Antibiotics
Vancomycin
As mentioned previously, the majority of the PK data on anti-infectives used on ECMO come from neonatal studies, with vancomycin being the most commonly studied drug.37–40 Understanding of vancomycin PK on ECMO is of particular importance as Staphylococcus spp. are some of the most common organisms cultured from ECMO patients.36
Four PK studies including 49 neonates and infants showed an increased V and decreased CL compared to infants not on ECMO.37–40 To account for the increased V and decreased CL, the authors recommended increasing the dose and decreasing dosing frequency, respectively. The absolute dose recommendations from these studies, with the exception of Mulla et al.,39 are not relevant as these studies were performed in the 1990’s when dosing recommendations were different from those today. However, based on the PK changes, we recommend an initial 20 mg/kg dose in neonates and infants. Given the variability in circuit configurations we would also recommend therapeutic drug monitoring (TDM) at 2 and 8–12 hours after the end of the infusion. Per Infectious Diseases Society of America (IDSA) guidelines, the goal trough is 15–20 μg/mL and using first dose kinetics, V and CL can be estimated from these early levels and subsequent doses adjusted accordingly. Using early levels rather than waiting for a true trough level is important to avoid significant time spent below therapeutic targets.
The PK data for vancomycin in children over 2 years are limited to a single study by Mulla et al.39 Compared to critically ill children not on ECMO, children supported with ECMO demonstrated increased V and decreased CL. CL was found to be strongly associated with renal function so dosing recommendations were made based on serum creatinine values. For children on ECMO, we recommend a 20 mg/kg initial dose followed by TDM at 2 and 8–12 hours post-infusion.
Adult data have largely shown that ECMO does not significantly affect vancomycin V and CL.41–43 Both intermittent39,42,43 and continuous infusions41 were evaluated, and these studies provided a variety of dosing recommendations. Even though the PK differences between ECMO and non-ECMO patients were not statistically significant, there were clinically, and in some cases statistically, significant differences in dosing regimens between the two cohorts.42 Patients on ECMO required a higher dose to reach adequate trough concentrations after the first dose. Differences in ECMO’s impact on vancomycin PK in adults versus children are not clear, but may be due to age-related effects on V discussed above. Variability in CL likely reflects the small sample sizes and critically ill nature of the population. Based on the results from all of the adult studies, standard vancomycin dosing is likely adequate in adults on ECMO, but we would start with a loading dose of 30 mg/kg with TDM at 2 and 8–12 hours after the infusion to guide additional dosing. The necessity of a loading dose is highlighted by one particular study where vancomycin levels in 95% of patients on ECMO remained subtherapeutic for more than 3 days with conventional dosing.42
Gentamicin
Gentamicin PK is also well-described in infants on ECMO because the drug is commonly prescribed in this population and TDM is routine. However, results are variable. When compared to infants not on ECMO, one study reported that infants on ECMO had increased V and decreased CL,44 two others reported similar V with decreased CL,2,45 and a fourth reported no difference in V or CL.46 Decreased CL is not surprising given that gentamicin is cleared primarily by the kidneys and ECMO is associated with decreased renal function. Although V is higher in infants than children,47 gentamicin is still a low V drug; and we would expect that ECMO-related hemodilution would result in a larger V in infants on ECMO.
While the data do not present a solid consensus, the studies tended to recommend standard dosing with a longer dosing interval to account for decreased CL seen in infants on ECMO. Similar to vancomycin, the studies of gentamicin were performed in the late 1980s and early 1990s when standard dosing for critically ill neonates was 2.5 mg/kg every 12 hours. Current dosing recommendations are 4–5 mg/kg every 24 hours for infants <3 months and 9.5 mg/kg every 24 hours in infants from 3 months to 2 years of age.48 We recommend using these updated doses as an initial dose in infants on ECMO, followed by TDM at 2 and 8–12 hours after the infusion to guide further dosing. A decreased initial dose may be considered for neonates who are <3.5 kg. There are no studies describing gentamicin PK for children or adults on ECMO at this time.
Cefotaxime
The PK of cefotaxime and its active metabolite, desacetylcefotaxime, were recently evaluated in a group of 37 neonates and infants on ECMO.49 Increased V was noted but there was no significant alteration in CL compared to neonates not on ECMO. Despite the increased V, standard age-based dosing of 50 mg/kg every 12 hours for patients <7 days of age, 50 mg/kg every 8 hours daily for patients 7–28 days of age, and 37.5 mg/kg every 6 hours for patients >28 days of age50 was sufficient to maintain therapeutic drug concentrations (time above the minimum inhibitory concentration [t>MIC] of at least 50% of the dosing interval, assuming a MIC of 8 mg/L) in 36/37 patients. This led the authors to posit that non-ECMO patients may be receiving higher than necessary doses of the drug. Given these results we agree with the authors’ recommendations in neonates and infants. There are no studies describing cefotaxime PK in children or adults on ECMO.
Meropenem
Both intermittent dosing and continuous-infusion meropenem PK on ECMO have been studied.51–53 The pediatric data are limited to a case report describing an 8 month old infant on ECMO receiving continuous-infusion meropenem for a Pseudomonas pneumonia.51 This infant’s CL (4.5 ml/min/kg) was more than double that of two cohorts of critically ill term and pre-term infants <91 days and 2 months old, respectively, who were not on ECMO.54,55 Other PK data that include older infants is from cohorts with patients up to 13 years of age, making a direct comparison difficult. Two studies with median ages of approximately 3 years both showed greater CL (7.13 and 6.9 ml/min/kg) than the 8 month old on ECMO.56,57
A 40 mg/kg bolus followed by a 200 mg/kg/day continuous infusion achieved the target serum and lung drug concentrations (t>MIC for 40% of the dosing interval) given the MIC of the patient’s pathogen (0.25 μg/ml in blood and 0.5 μg/ml in BAL). It should be noted that the MICs observed for Pseudomonas in this patient are much lower than the MIC for susceptible Pseudomonas of 2 mg/L as determined by the European Committee of Antimicrobial Susceptibility Testing (EUCAST). Consequently it is difficult to extrapolate this dosing regimen to other patients. As meropenem is stable at room temperature for less than 4 hours, the logistics of a continuous infusion would be challenging and require frequent introduction of new batches of drug. A simpler solution may be an extended infusion regimen of 33.3 mg/kg given over 3 hours, every 4 hours.
Adult data showed no alteration in meropenem PK for patients on ECMO.52,53 One study in 6 adults on ECMO with normal renal function showed that standard dosing of 1000 mg every 8 hours58 achieved t>MIC for 100% of the dosing interval assuming a MIC of 2 mg/L.53 However, for more resistant organisms (e.g., Pseudomonas aeruginosa, MIC 8 mg/L) dosing simulations suggested 2000 mg every 8 hours are necessary to maintain concentrations above 8 mg/L for 100% of the doing interval. A retrospective study of 26 adults on ECMO and 41 matched controls evaluated a different target (t>MIC for 40% of the dosing interval assuming a MIC of 8 mg/L) and found that with standard dosing, ~10% of patients were subtherapeutic. We agree with the more conservative approach by Shekar et al.53 and recommend 1000 mg every 8 hours for susceptible organisms and 2000 mg every 8 hours for organisms with a MIC of 8 mg/L. Meropenem is renally cleared and dosing needs to be adjusted for patients with either decreased or augmented renal clearance.
Imipenem
Two patients on ECMO after lung transplantation were treated with imipenem, 1 gram every 6 hours, for acute respiratory distress syndrome (ARDS).59 Patient 1 had Enterobacter cloacae with an MIC of 0.125 mg/L and patient 2 had Klebsiella pneumoniae with an MIC of 0.25 mg/L. While trough concentrations between the two patients were quite discordant, both resulted in a t>MIC of 100% for each patient’s isolated organism. Data have shown that if treating a more resistant organism such as Pseudomonas aeruginosa (MIC >2 mg/L), better outcomes are achieved in critically ill patients by maintaining drug concentration of 4 times the MIC for 100% of the treatment period.60 If this is the case then the authors state that a higher dose should be considered.
Piperacillin/Tazobactam
In the previously mentioned study by Donadello et al, piperacillin/tazobactam PK were also studied.52 While PK parameters were similar between ECMO and non-ECMO patients, only 40% of adults achieved the target exposure for treatment of Pseudomonas aeruginosa when receiving a dose of 4 g every 6 hours. The target PD parameters were a concentration 4–8 times the clinical MIC breakpoint of 2 mg/L with 50% of time spent above the MIC. Based on these results, the authors concluded that meropenem is a better drug in adults on ECMO.
Linezolid
In the first report of linezolid PK in patients on ECMO, three adults received a standard dosing regimen of 600 mg every 12 hours.61 Despite inter-patient variability, drug exposure was adequate (i.e., AUC/MIC ratio >80) to treat methicillin-resistant Staphylococcus aureus with an MIC <1 mg/L. In more resistant organisms with MIC of 2 mg/L, satisfactory PK parameters were achieved in two out of three of the patients and with an MIC of 4 mg/L, they were adequate in only one patient. This suggests that increased dosing should be considered for isolates with MICs >1 mg/L.
Azithromycin
Three adults with ARDS requiring ECMO were treated with standard doses of azithromycin (500 mg daily58) and ECMO had no appreciable effect on plasma concentrations.62 Maximum and minimum concentrations and AUC were similar when compared to hospitalized non-ECMO patients. Compared to healthy volunteers, CL was similar but V was lower. A decreased V on ECMO is unusual and while the authors acknowledged this, they stated that the small sample size made it difficult to determine the cause. They did note that all patients were obese and receiving vasopressor therapy during sample collection, both of which could impact PK.
Tigecycline
A case report of an adult on ECMO treated with tigecycline suggested that ECMO has no effect on the drug’s PK.63 Plasma concentrations were not significantly different from those measured in critically ill patients not on ECMO.
Rifampin, pyrazinamide, ethambutol
Anti-tuberculosis agents have been described in a case report of an adult with tuberculosis on ECMO.64 TDM was used and to achieve therapeutic concentrations, this patient required daily rifampicin 1200mg, pyrazinamide 1500mg, ethambutol 1200mg, streptomycin 750mg, and levofloxacin 750mg. This patient required nearly 23 mg/kg/day of rifampin as compared to the standard of 10 mg/kg/day58 and 50% more pyrazinamide and ethambutol than recommended for her weight (52kg). Levofloxacin concentrations were therapeutic using standard dosing.
Another case report described the PK of rifampin and ethambutol in a patient on ECMO and renal replacement therapy (RRT).65 Because both of these drugs are cleared by RRT, it is difficult to assess the impact of ECMO.
Given the higher doses required in the first patient we recommend starting with a higher dose and using TDM to adjust dosing.
Antifungals
Although less studied than antibiotics, there are emerging PK data on antifungal drugs used on ECMO. Candida species represent the most commonly cultured organisms in infants, children, and adults on ECMO and are the second most common in neonates.36 Candida infections cause substantial morbidity and mortality66 and are difficult to eradicate due to the organism’s ability to adhere to indwelling catheters. For this reason, routine management for candidiasis consists not only of antifungal agents but also removal of catheters.67 Catheter removal for patients on ECMO is often impossible, because the ECMO cannulas connect the patient to the ECMO circuit. Therefore, therapy on ECMO relies on either prevention of invasive candidiasis or optimal therapeutic dosing in patients with infection.
Fluconazole
A PK trial of fluconazole in ten infants on ECMO performed by our group demonstrated a significantly higher V but a similar CL compared to critically ill infants not on ECMO.68 This was followed by a population PK analysis that included 21 infants and children on ECMO and 19 critically ill infants not on ECMO.69 The final population PK model showed that children on ECMO had ~40% higher V than children not on ECMO but similar CL. Based on the final population PK model, simulations were done to evaluate multiple dosing regimens for children on ECMO. In infants on ECMO who received 12mg/kg daily, none reached the therapeutic target of area under the curve for a 24 hour dosing interval (AUC24 >400 mg*h/liter) in the first 24 hours and it took 10 days for 90% of these simulated infants to reach this target. A 25 mg/kg loading dose, which is adequate to achieve therapeutic targets in infants not on ECMO, followed by 12 mg/kg daily was similarly inadequate. However, with a 35 mg/kg load followed by 12 mg/kg daily, fluconazole exposure was therapeutic in 88% of children in the first 24 hours. We recommend this regimen with maintenance dosing adjusted according to the product label for renal dysfunction.
For prophylaxis, children receiving the standard dosing of 6 mg/kg daily did not achieve therapeutic exposure (AUC24 >200 mg*h/liter) until day 7 of therapy. With a loading dose of 12 mg/kg followed by daily dosing of 6 mg/kg, 69% of children reached the therapeutic target by day 2 and 90% by day 5. We recommend a prophylactic dosing regimen of a 12 mg/kg loading dose followed by 6 mg/kg daily.
There were not enough children or adults in the study to adequately describe the PK and determine dosing in older populations. However, given the safety of fluconazole and devastating consequences of a fungal infection on ECMO, a 35 mg/kg load with standard maintenance therapy could also be considered for younger children as well. Providers should adhere to the manufacturer’s recommendations for dose adjustment in patients with renal insufficiency.
Micafungin
Our group has also studied micafungin in infants on ECMO.70 Micafungin’s activity against a broad spectrum of Candida species as well as its ability to treat biofilms makes it an appealing antifungal choice in this population. However, high protein binding (>99%) raised the concern that micafungin might be extracted by the ECMO circuit.10 Twelve infants received either prophylactic (4 mg/kg/day) or treatment doses (8 mg/kg/day) of micafungin depending on whether or not they had known fungal disease. Increased V was observed in infants on ECMO compared to historical controls not on ECMO. CL in infants on ECMO was on the upper end of the range reported in historical controls not on ECMO. In order to match exposures observed in adults not on ECMO, we recommend dosing of 2.5 and 5 mg/kg/day for prophylaxis and treatment of invasive candidiasis in infants, respectively. Median (range) exposures (AUC0–24) were 74 mg*h/L (53, 106) and 213 mg*h/L for 4 mg/kg and 8 mg/kg, respectively. However, even higher doses may be appropriate for two reasons: 1) the target exposure used in this study was derived from a phase III efficacy study in which >85% of adults had their central catheter removed during candidemia treatment, a procedure that is not possible on ECMO,71 and 2) micafungin is well tolerated up to 15 mg/kg in neonates (median [range] AUC0–24 437.5 μg*h/mL.72 It should be noted that these recommendations exclude treatment of premature neonates at risk for Candida meningoencephalitis as the higher doses (e.g. 10 mg/kg) usually recommended for this condition were not studied. Micafungin has not been studied in children or adults on ECMO.
Caspofungin
The PK of caspofungin on ECMO has been evaluated in three case reports, involving one infant and two adults, with varying results.73–75 The PK in an 11 month old infant demonstrated a lower AUC and increased CL when compared to previously described caspofungin PK in infants not on ECMO.73 This patient received substantially higher doses (78 mg/m2 daily) compared to standard dosing of 50–70 mg/m2 daily.50 Even with the higher dosing, the AUC was sub-therapeutic at 69 mg*h/L and the patient died before further dose adjustments were made. Caspofungin has low lipophilicity but is highly protein bound so sequestration by the circuit is a possible explanation for these findings.10 Additionally, increased V due to hemodilution or disease state may have decreased exposure. No specific dosing recommendations were made.
In an adult on ECMO who received both caspofungin and voriconazole, normal caspofungin concentrations were achieved with standard dosing of 70 mg daily.58,75 However, in a second adult treated with the same antifungal combination, standard dosing of caspofungin resulted in undetectable concentrations.74 Given the incongruence of these case reports and the overall very small sample size, it is difficult to make specific dosing recommendations for this drug.
Voriconazole
A child treated with voriconazole required a dose of 14 mg/kg every 12 hours to achieve target plasma concentrations of >1 mg/L.76 This is compared to the recommended standard dosing of a 10–12 mg/kg every 12 hours for two doses followed by 8–9 mg/kg every 12 hours.50 The authors felt that it was unclear if ECMO contributed to the patient’s higher dosing requirement but hypothesized that an increased V may have played a role. They endorse a dose of at least 10 mg/kg twice daily in children with the use of TDM in severely ill patients.
Varying PK effects have been seen in two adults on ECMO treated with voriconazole.74,75 Spriet et al administered higher than standard doses (400 mg vs 280 mg twice daily58) of voriconazole to account for possible circuit extraction due to the drug’s lipophilicity.75 Concentrations were initially similar to those prior to initiation of ECMO in the same patient, but then began to increase on the same dose, eventually reaching toxic concentrations. The authors felt that this was due to saturation of binding sites in the ECMO circuit over time and that because of this. An alternative explanation is that higher exposure was due to decreased CL combined with voriconazole’s non-linear kinetics.
In the aforementioned case report of caspofungin and voriconazole by Ruiz et al, the adult patient also received standard doses of voriconazole.74 As with caspofungin, serum concentrations were not achieved, leading the authors to recommend against using these two agents in patients on ECMO. Voriconazole, however, has the advantage of readily available TDM. If voriconazole is needed, close TDM is recommended.
Amphotericin B deoxycholate and Liposomal Amphotericin B
Amphotericin B deoxycholate was used treat a 15 year old male with Acute Respiratory Distress Syndrome (ARDS) secondary to pulmonary blastomycosis.77 He received standard doses of amphotericin B deoxycholate of 1 mg/kg every 24 hours58 and drug concentrations were maintained within a therapeutic range. The authors found no increase in V in this adult-sized adolescent, but did not comment on CL. Additionally, there were multiple exchanges of the ECMO membrane oxygenator as well as an entire circuit change, without any apparent effect on amphotericin concentrations.
In the previously mentioned case report by Ruiz et al, liposomal amphotericin B at standard doses of 3 mg/kg every 24 hours58 was used to treat an adult patient.74 This drug was initiated after they were unable to reach therapeutic concentrations of caspofungin and voriconazole. Although they did not report PK parameters, they did report that plasma concentrations of liposomal amphotericin B at this dose were therapeutic. Based on these limited data, we recommend standard dosing of these drugs for adult-sized adolescents and adults on ECMO.
Antivirals
Oseltamivir
While antiviral drugs have been the least studied class of anti-infective drugs on ECMO, their importance became clear during the H1N1 influenza pandemic of 2009 and 2010 when ECMO was required to support the sickest patients. Oseltamivir is unique among the drugs discussed thus far in that it exists only as an enteral formulation.
In three children on ECMO (6–15 years of age), the standard weight based dose was doubled in an effort to account for the potential PK alterations seen with other drugs on ECMO.78 However, in two of the three patients, nearly two-fold higher plasma concentrations were achieved, suggesting that this preemptive increase was unnecessary. The third patient had significant gastrointestinal co-morbidities and therapeutic concentrations were not achieved. This led the authors to posit that ECMO does not affect oseltamivir PK but that dosing adjustment should be considered in patients with altered gastric motility or other issues affecting enteral absorption.
Adult data are similar in demonstrating a lack of effect of ECMO itself on oseltamivir’s PK.79–81 Fourteen patients received standard oseltamivir dosing of 75 mg twice daily.58,81 Mean systemic exposures at this dose were well above the MIC for H1N1 and comparable to those seen in non-ECMO patients and healthy volunteers. However, there was significant variability of PK parameters between patients in this study, which the authors attributed to differences in enteral absorption and renal function.
Data from seven patients on ECMO, some of whom required RRT, also showed that ECMO does not have a significant effect on oseltamivir, but that renal function does affect PK.80 Patients on ECMO with preserved renal function had concentrations similar to healthy and non-critically ill controls. Those patients on ECMO and RRT, however, had significant accumulation of the drug suggesting that in this cohort, oseltamivir was not cleared by dialysis.
Overall, the adult data of this enteral medication support the use of standard dosing regimens for patients on ECMO with preserved renal function and normal gastric motility.
Peramivir
Peramivir has been used to treat oseltamivir-resistant H1N1 influenza. A case report of a 10 year old immunosuppressed kidney transplant recipient on ECMO and RRT describes use of the drug through an Emergency Investigational New Drug Application.82 The patient was initially given 2.2 mg/kg every 24 hours but required a dose increase to 5.4 mg/kg every 24 hours to achieve target trough concentrations >1500 ng/mL. Standard dosing for a child of this age with normal renal function is 10 mg/kg q24h.50 There are dosing recommendations for children with renal impairment but not those on RRT. Definitive dosing recommendations cannot be made, but based on this case, initial dosing should be adjusted for renal function rather than ECMO itself.
Ribavirin
Ribavirin was used to treat a neonate on ECMO with disseminated adenovirus.83 The patient received 20 mg/kg/day and plasma concentrations were comparable to those previously reported after single 6–10 mg/kg doses in children. This patient was also undergoing hemofiltration and the percentage eliminated via hemofiltration was similar to those seen with normal renal function. Although this is only one patient, a dose of 20 mg/kg/day may be considered in neonates on ECMO with normal renal function or those requiring dialysis.
Ganciclovir
There is a single case report of a neonate with congenital Cytomegalovirus (CMV) pneumonia on ECMO, treated with ganciclovir.84 This infant received 5 mg/kg every 12 hours, which is less than the currently recommended doses of 6 mg/kg every 12 hours but higher than the standard dose at the time (4 mg/kg every 12 hours). With the 5 mg/kg dose, peak plasma concentrations were considered therapeutic as they were consistently above the MIC of most clinical isolates of human CMV (2.75 μg/ml) at the time this paper was published. Today’s higher standard dosing is reflective of improved viral response to the higher dose85 so we would recommend using at least 6 mg/kg every 12 hours in this patient population for treatment of CMV. Given the known toxicity of the drug, careful laboratory monitoring is recommended.
DISCUSSION
This review evaluated the currently available literature on the PK of anti-infective drugs on ECMO and found reasonably robust dosing recommendations for some drugs and scant or no data for other important anti-infectives. The dosing recommendations about which we have the most confidence are those generated using dedicated PK trials (e.g., vancomycin, fluconazole). However, even with these trials, there are important limitations. First, the effect of ECMO on exposure is drug specific, necessitating a trial for each drug of interest.10 Second, the impact of ECMO can vary by age of the patient.69 Most ECMO PK trials were done in infants; and it is unclear whether the results can be extrapolated to older children and adults. Third, ECMO technology changes over time, and with it, the impact on drug disposition.11 Given the differences reported in drug extraction between older components (e.g., silicone membrane oxygenators) and newer technology (e.g., polymethylepentane hollow-fiber oxygenators), it is unknown whether dosing recommendations derived from trials using the older technology can be extrapolated to patients supported with modern ECMO circuits.
For these reasons it is difficult to draw broad conclusions about dosing on ECMO. Most of the infant trials on ECMO showed increased V and decreased CL while many of the adult studies showed no difference in PK parameters between ECMO and non-ECMO patients. Although it is reasonable to suggest that infants are more susceptible to hemodilution and increased V, there are many other factors that could contribute to the differences observed between infants and adults. In addition to the different ratio of exogenous to native blood volume described above, infants have a higher proportion of body water and lower protein binding, both of which can impact V.26–28 Further, many of the infant trials were performed in the 1990’s with circuit components that are no longer used today.
In order to better determine optimal dosing on ECMO, a systematic approach is needed. The ECMO PK Project is coordinated by The Critical Care Research Group at The Prince Charles Hospital in Brisbane, Australia.86 This approach combines 1) ex vivo studies in isolated ECMO circuits to determine drug interaction with the circuit, 2) ECMO animal experiments to elucidate mechanisms of PK changes, and 3) human PK trials in adults to evaluate mechanisms and develop population PK models to provide dosing guidelines in patients on ECMO. This approach should provide robust dosing recommendations for commonly used drugs and elucidate the mechanisms responsible for PK changes on ECMO. This approach has some limitations. As ECMO technology evolves, it is impossible to know if recommendations determined using current equipment will be able to be extrapolated as new equipment is introduced. While ECMO ex vivo experiments quantify drug extraction by the ECMO circuit, direct translation of these results into dosing recommendations is challenging. Additionally, care must be taken when extrapolating population PK model results to populations that were not used in the model-building process. Because of the age-related differences in PK, dedicated trials will likely need to be performed in all age groups.
Our group is using a complementary approach that translates ECMO ex vivo results to bedside dosing recommendations using Physiologically Based Pharmacokinetic (PBPK) modeling. PBPK offers an alternative modeling platform with the flexibility, efficiency, and the ability to account for the complex physiology of critically ill patients. PBPK models expand on traditional compartmental models by incorporating the key physiological, biochemical, and physicochemical determinants of drug disposition. The models are parameterized using a physiologic structure (e.g., liver compartment, kidney compartment, etc.) with mathematical equations that describe drug disposition as the drugs transit the compartments. These models can account for the effect that physiologic changes have on organ function (e.g., decreased renal perfusion) and provide a mechanistic understanding of drug disposition. By including information about organ ontogeny, the PBPK models can predict dosing in populations across the pediatric and adult age spectrum, including populations that were not studied in the trials. In patients on ECMO, an ECMO compartment can be added to the PBPK model to account for the effect of ECMO on drug disposition. Drug interaction (e.g. adsorption) within the ECMO compartment is informed by ex vivo ECMO experiments. While the model predictions will still need to be validated with PK data from children on ECMO, the number of children required will be less and the trial design more efficient. Further, if ECMO technology changes, the ex vivo experiment can be repeated with the new circuit to understand whether the drug interaction is different. Based on the interaction with the new circuit, parameterization of the ECMO compartment can be updated in the PBPK model and new predictions of drug disposition can be generated.
CONCLUSIONS
This review describes PK data and dosing recommendations for anti-infective drugs in patients on ECMO. We found that while the neonatal data are the most robust, they are the oldest, which raises concerns for its validity today as ECMO technology has continued to evolve over time. In addition, optimal therapeutic targets, and therefore standard dosing recommendations, have been updated. The most well defined PK effect of ECMO seems to be an increased in V in neonates. This effect is lost in adults, evidenced by very few recommendations for dose adjustments in this population. Data for pediatric patients who fit neither the neonatal or adult categories are lacking and require further investigation. There is also a need for expansion of the drugs studied and an improvement in methodology to assist in making more definitive dosing recommendations. There are multiple projects currently in process to fill this void.
Table 2.
Anti-infective pharmacokinetics and dosing in childrena on ECMO
| Drug | Volume of Distribution compared to non-ECMO patients | Clearance compared to non-ECMO patients | Standard Dosing in critically ill patientsb, c | Dosing Recommendations for patients on ECMOb |
|---|---|---|---|---|
| Antibiotics | ||||
| Vancomycin | Increased | Decreased | 60 mg/kg/dose divided q6–8h | 20 mg/kg once then check 2 and 8–12 hour concentrations |
| Anti-fungals | ||||
| Amphotericin B (conventional) | Unchanged | Unchanged | 1 mg/kg q24h | Standard dosingd |
| Voriconazole | Not reported | Decreased | 10–12 mg/kg q12h × 2 doses then 8- 9 mg/kg q12h | 14 mg/kg q12he |
| Anti-virals | ||||
| Oseltamivir | Unchanged | Unchanged | <15 kg: 30 mg BID 15–23 kg: 45 mg BID 23–40 kg: 60 mg BID >40 kg: 75 mg BID |
Standard dosing with caution in patients with decreased gastric mobility or other gastrointestinal co-morbidities |
| Peramivir | Not reported | Not reported | 180 days-5 years: 12 mg/kg q24h >5 years: 10 mg/kg q24h |
5.4 mg/kg q24hf |
children >2–18 years of age
all doses intravenous (IV) except oseltamivir (enteral)
Unless otherwise noted, all recommendations for standard of care dosing were obtained from Lexicomp Online, Pediatric & Neonatal Lexi-Drugs, Hudson, Ohio: Lexi-Comp, Inc: April 2016
case report of an adult-sized 15 year old with pulmonary blastomycosis
case report of a 5 year old with Aspergillus fumigatas
case report of a 10 year old renal transplant recipient with H1N1; pt required CRRT for renal failure
References
- 1.Extracorporeal Life Support Organization Registry Report: International Summary. January 2016.
- 2.Bhatt-Mehta V, Johnson CE, Schumacher RE. Gentamicin pharmacokinetics in term neonates receiving extracorporeal membrane oxygenation. Pharmacotherapy. 1992;12(1):28–32. [PubMed] [Google Scholar]
- 3.Dagan O, Klein J, Gruenwald C, Bohn D, Barker G, Koren G. Preliminary studies of the effects of extracorporeal membrane oxygenator on the disposition of common pediatric drugs. Ther Drug Monit. 1993;15(4):263–266. doi: 10.1097/00007691-199308000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Harthan AA, Buckley KW, Heger ML, Fortuna RS, Mays K. Medication adsorption into contemporary extracorporeal membrane oxygenator circuits. J Pediatr Pharmacol Ther. 2014;19(4):288–295. doi: 10.5863/1551-6776-19.4.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koren G, Crean P, Klein J, Goresky G, Villamater J, MacLeod SM. Sequestration of fentanyl by the cardiopulmonary bypass (CPBP) Eur J Clin Pharmacol. 1984;27(1):51–56. doi: 10.1007/BF00553154. [DOI] [PubMed] [Google Scholar]
- 6.Lemaitre F, Hasni N, Leprince P, et al. Propofol, midazolam, vancomycin and cyclosporine therapeutic drug monitoring in extracorporeal membrane oxygenation circuits primed with whole human blood. Crit Care. 2015;19:40. doi: 10.1186/s13054-015-0772-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta NM, Halwick DR, Dodson BL, Thompson JE, Arnold JH. Potential drug sequestration during extracorporeal membrane oxygenation: results from an ex vivo experiment. Intensive Care Med. 2007;33(6):1018–1024. doi: 10.1007/s00134-007-0606-2. [DOI] [PubMed] [Google Scholar]
- 8.Mulla H, Lawson G, von Anrep C, et al. In vitro evaluation of sedative drug losses during extracorporeal membrane oxygenation. Perfusion. 2000;15(1):21–26. doi: 10.1177/026765910001500104. [DOI] [PubMed] [Google Scholar]
- 9.Shekar K, Roberts JA, McDonald CI, et al. Sequestration of drugs in the circuit may lead to therapeutic failure during extracorporeal membrane oxygenation. Crit Care. 2012;16(5):R194. doi: 10.1186/cc11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shekar K, Roberts JA, McDonald CI, et al. Protein-bound drugs are prone to sequestration in the extracorporeal membrane oxygenation circuit: results from an ex vivo study. Crit Care. 2015;19:164. doi: 10.1186/s13054-015-0891-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wildschut ED, Ahsman MJ, Allegaert K, Mathot RA, Tibboel D. Determinants of drug absorption in different ECMO circuits. Intensive Care Med. 2010;36(12):2109–2116. doi: 10.1007/s00134-010-2041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Preston TJ, Hodge AB, Riley JB, Leib-Sargel C, Nicol KK. In vitro drug adsorption and plasma free hemoglobin levels associated with hollow fiber oxygenators in the extracorporeal life support (ECLS) circuit. J Extra Corpor Technol. 2007;39(4):234–237. [PMC free article] [PubMed] [Google Scholar]
- 13.Preston TJ, Ratliff TM, Gomez D, et al. Modified surface coatings and their effect on drug adsorption within the extracorporeal life support circuit. J Extra Corpor Technol. 2010;42(3):199–202. [PMC free article] [PubMed] [Google Scholar]
- 14.Palmgren JJ, Monkkonen J, Korjamo T, Hassinen A, Auriola S. Drug adsorption to plastic containers and retention of drugs in cultured cells under in vitro conditions. Eur J Pharm Biopharm. 2006;64(3):369–378. doi: 10.1016/j.ejpb.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Unger JK, Kuehlein G, Schroers A, Gerlach JC, Rossaint R. Adsorption of xenobiotics to plastic tubing incorporated into dynamic in vitro systems used in pharmacological research–limits and progress. Biomaterials. 2001;22(14):2031–2037. doi: 10.1016/s0142-9612(00)00389-6. [DOI] [PubMed] [Google Scholar]
- 16.De Somer F, Francois K, van Oeveren W, et al. Phosphorylcholine coating of extracorporeal circuits provides natural protection against blood activation by the material surface. Eur J Cardiothorac Surg. 2000;18(5):602–606. doi: 10.1016/s1010-7940(00)00508-x. [DOI] [PubMed] [Google Scholar]
- 17.Palatianos GM, Foroulis CN, Vassili MI, et al. A prospective, double-blind study on the efficacy of the bioline surface-heparinized extracorporeal perfusion circuit. Ann Thorac Surg. 2003;76(1):129–135. doi: 10.1016/s0003-4975(03)00338-2. [DOI] [PubMed] [Google Scholar]
- 18.Tayama E, Hayashida N, Akasu K, et al. Biocompatibility of heparin-coated extracorporeal bypass circuits: new heparin bonded bioline system. Artif Organs. 2000;24(8):618–623. doi: 10.1046/j.1525-1594.2000.06615.x. [DOI] [PubMed] [Google Scholar]
- 19.Buck ML. Pharmacokinetic changes during extracorporeal membrane oxygenation: implications for drug therapy of neonates. Clin Pharmacokinet. 2003;42(5):403–417. doi: 10.2165/00003088-200342050-00001. [DOI] [PubMed] [Google Scholar]
- 20.Butler J, Pathi VL, Paton RD, et al. Acute-phase responses to cardiopulmonary bypass in children weighing less than 10 kilograms. Ann Thorac Surg. 1996;62(2):538–542. [PubMed] [Google Scholar]
- 21.Kozik DJ, Tweddell JS. Characterizing the inflammatory response to cardiopulmonary bypass in children. Ann Thorac Surg. 2006;81(6):S2347–2354. doi: 10.1016/j.athoracsur.2006.02.073. [DOI] [PubMed] [Google Scholar]
- 22.McIlwain RB, Timpa JG, Kurundkar AR, et al. Plasma concentrations of inflammatory cytokines rise rapidly during ECMO-related SIRS due to the release of preformed stores in the intestine. Lab Invest. 2010;90(1):128–139. doi: 10.1038/labinvest.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seghaye MC, Grabitz RG, Duchateau J, et al. Inflammatory reaction and capillary leak syndrome related to cardiopulmonary bypass in neonates undergoing cardiac operations. J Thorac Cardiovasc Surg. 1996;112(3):687–697. doi: 10.1016/s0022-5223(96)70053-3. [DOI] [PubMed] [Google Scholar]
- 24.Anderson HL, 3rd, Coran AG, Drongowski RA, Ha HJ, Bartlett RH. Extracellular fluid and total body water changes in neonates undergoing extracorporeal membrane oxygenation. J Pediatr Surg. 1992;27(8):1003–1007. doi: 10.1016/0022-3468(92)90547-k. discussion 1007–1008. [DOI] [PubMed] [Google Scholar]
- 25.Many M, Soroff HS, Birtwell WC, Giron F, Wise H, Deterling RA., Jr The physiologic role of pulsatile and nonpulsatile blood flow. II. Effects on renal function. Arch Surg. 1967;95(5):762–767. doi: 10.1001/archsurg.1967.01330170070009. [DOI] [PubMed] [Google Scholar]
- 26.Ehrnebo M, Agurell S, Jalling B, Boreus LO. Age differences in drug binding by plasma proteins: studies on human foetuses, neonates and adults. Eur J Clin Pharmacol. 1971;3(4):189–193. doi: 10.1007/BF00565004. [DOI] [PubMed] [Google Scholar]
- 27.Friis-Hansen B. Water distribution in the foetus and newborn infant. Acta Paediatr Scand Suppl. 1983;305:7–11. doi: 10.1111/j.1651-2227.1983.tb09852.x. [DOI] [PubMed] [Google Scholar]
- 28.McNamara PJ, Alcorn J. Protein binding predictions in infants. AAPS PharmSci. 2002;4(1):E4. doi: 10.1208/ps040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Extracorporeal Life Support Organization. ECLS Registry Report: International Summary. 2016 https://www.elso.org/Registry/Statistics/InternationalSummary.aspx.
- 30.Mulla H, Lawson G, Firmin R, Upton DR. Drug Disposition During Extracorporeal Membrane Oxygenation (ECMO) Pediatric and Perinatal Drug Therapy. 2001;4(3):109–120. [Google Scholar]
- 31.Morgan ET. Regulation of cytochromes P450 during inflammation and infection. Drug Metab Rev. 1997;29(4):1129–1188. doi: 10.3109/03602539709002246. [DOI] [PubMed] [Google Scholar]
- 32.Abdel-Razzak Z, Loyer P, Fautrel A, et al. Cytokines down-regulate expression of major cytochrome P-450 enzymes in adult human hepatocytes in primary culture. Mol Pharmacol. 1993;44(4):707–715. [PubMed] [Google Scholar]
- 33.Rivory LP, Slaviero KA, Clarke SJ. Hepatic cytochrome P450 3A drug metabolism is reduced in cancer patients who have an acute-phase response. Br J Cancer. 2002;87(3):277–280. doi: 10.1038/sj.bjc.6600448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siewert E, Bort R, Kluge R, Heinrich PC, Castell J, Jover R. Hepatic cytochrome P450 down-regulation during aseptic inflammation in the mouse is interleukin 6 dependent. Hepatology. 2000;32(1):49–55. doi: 10.1053/jhep.2000.8532. [DOI] [PubMed] [Google Scholar]
- 35.Richardson TA, Sherman M, Kalman D, Morgan ET. Expression of UDP-glucuronosyltransferase isoform mRNAs during inflammation and infection in mouse liver and kidney. Drug Metab Dispos. 2006;34(3):351–353. doi: 10.1124/dmd.105.007435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bizzarro MJ, Conrad SA, Kaufman DA, Rycus P, Extracorporeal Life Support Organization Task Force on Infections EMO Infections acquired during extracorporeal membrane oxygenation in neonates, children, and adults. Pediatr Crit Care Med. 2011;12(3):277–281. doi: 10.1097/PCC.0b013e3181e28894. [DOI] [PubMed] [Google Scholar]
- 37.Amaker RD, DiPiro JT, Bhatia J. Pharmacokinetics of vancomycin in critically ill infants undergoing extracorporeal membrane oxygenation. Antimicrob Agents Chemother. 1996;40(5):1139–1142. doi: 10.1128/aac.40.5.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buck ML. Vancomycin pharmacokinetics in neonates receiving extracorporeal membrane oxygenation. Pharmacotherapy. 1998;18(5):1082–1086. [PubMed] [Google Scholar]
- 39.Mulla H, Pooboni S. Population pharmacokinetics of vancomycin in patients receiving extracorporeal membrane oxygenation. Br J Clin Pharmacol. 2005;60(3):265–275. doi: 10.1111/j.1365-2125.2005.02432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoie EB, Swigart SA, Leuschen MP, et al. Vancomycin pharmacokinetics in infants undergoing extracorporeal membrane oxygenation. Clin Pharm. 1990;9(9):711–715. [PubMed] [Google Scholar]
- 41.Donadello K, Roberts JA, Cristallini S, et al. Vancomycin population pharmacokinetics during extracorporeal membrane oxygenation therapy: a matched cohort study. Crit Care. 2014;18(6):632. doi: 10.1186/s13054-014-0632-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park SJ, Yang JH, Park HJ, et al. Trough Concentrations of Vancomycin in Patients Undergoing Extracorporeal Membrane Oxygenation. PLoS One. 2015;10(11):e0141016. doi: 10.1371/journal.pone.0141016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu CC, Shen LJ, Hsu LF, Ko WJ, Wu FL. Pharmacokinetics of vancomycin in adults receiving extracorporeal membrane oxygenation. J Formos Med Assoc. 2015 doi: 10.1016/j.jfma.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 44.Cohen P, Collart L, Prober CG, Fischer AF, Blaschke TF. Gentamicin pharmacokinetics in neonates undergoing extracorporal membrane oxygenation. Pediatr Infect Dis J. 1990;9(8):562–566. doi: 10.1097/00006454-199008000-00007. [DOI] [PubMed] [Google Scholar]
- 45.Southgate WM, DiPiro JT, Robertson AF. Pharmacokinetics of gentamicin in neonates on extracorporeal membrane oxygenation. Antimicrob Agents Chemother. 1989;33(6):817–819. doi: 10.1128/aac.33.6.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Munzenberger PJ, Massoud N. Pharmacokinetics of gentamicin in neonatal patients supported with extracorporeal membrane oxygenation. ASAIO Trans. 1991;37(1):16–18. doi: 10.1097/00002480-199101000-00006. [DOI] [PubMed] [Google Scholar]
- 47.Kelman AW, Thomson AH, Whiting B, et al. Estimation of gentamicin clearance and volume of distribution in neonates and young children. Br J Clin Pharmacol. 1984;18(5):685–692. doi: 10.1111/j.1365-2125.1984.tb02530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McDade EJ, Wagner JL, Moffett BS, Palazzi DL. Once-daily gentamicin dosing in pediatric patients without cystic fibrosis. Pharmacotherapy. 2010;30(3):248–253. doi: 10.1592/phco.30.3.248. [DOI] [PubMed] [Google Scholar]
- 49.Ahsman MJ, Wildschut ED, Tibboel D, Mathot RA. Pharmacokinetics of cefotaxime and desacetylcefotaxime in infants during extracorporeal membrane oxygenation. Antimicrob Agents Chemother. 2010;54(5):1734–1741. doi: 10.1128/AAC.01696-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lexicomp Online PNL-D. Hudson, Ohio: Lexi-Comp, Inc; Apr, 2016. [Google Scholar]
- 51.Cies JJ, Moore WS, 2nd, Dickerman MJ, et al. Pharmacokinetics of continuous-infusion meropenem in a pediatric patient receiving extracorporeal life support. Pharmacotherapy. 2014;34(10):e175–179. doi: 10.1002/phar.1476. [DOI] [PubMed] [Google Scholar]
- 52.Donadello K, Antonucci E, Cristallini S, et al. beta-Lactam pharmacokinetics during extracorporeal membrane oxygenation therapy: A case-control study. Int J Antimicrob Agents. 2015;45(3):278–282. doi: 10.1016/j.ijantimicag.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 53.Shekar K, Fraser JF, Taccone FS, et al. The combined effects of extracorporeal membrane oxygenation and renal replacement therapy on meropenem pharmacokinetics: a matched cohort study. Crit Care. 2014;18(6):565. doi: 10.1186/s13054-014-0565-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bradley JS, Sauberan JB, Ambrose PG, Bhavnani SM, Rasmussen MR, Capparelli EV. Meropenem pharmacokinetics, pharmacodynamics, and Monte Carlo simulation in the neonate. Pediatr Infect Dis J. 2008;27(9):794–799. doi: 10.1097/INF.0b013e318170f8d2. [DOI] [PubMed] [Google Scholar]
- 55.Smith PB, Cohen-Wolkowiez M, Castro LM, et al. Population pharmacokinetics of meropenem in plasma and cerebrospinal fluid of infants with suspected or complicated intra-abdominal infections. Pediatr Infect Dis J. 2011;30(10):844–849. doi: 10.1097/INF.0b013e31822e8b0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Du X, Li C, Kuti JL, Nightingale CH, Nicolau DP. Population pharmacokinetics and pharmacodynamics of meropenem in pediatric patients. J Clin Pharmacol. 2006;46(1):69–75. doi: 10.1177/0091270005283283. [DOI] [PubMed] [Google Scholar]
- 57.Ohata Y, Tomita Y, Nakayama M, Kozuki T, Sunakawa K, Tanigawara Y. Optimal dosage regimen of meropenem for pediatric patients based on pharmacokinetic/pharmacodynamic considerations. Drug Metab Pharmacokinet. 2011;26(5):523–531. doi: 10.2133/dmpk.dmpk-11-rg-027. [DOI] [PubMed] [Google Scholar]
- 58.Lexicomp Online L-D. Hudson, Ohio: Lexi-Comp, Inc; Apr, 2016. [Google Scholar]
- 59.Welsch C, Augustin P, Allyn J, Massias L, Montravers P, Allou N. Alveolar and serum concentrations of imipenem in two lung transplant recipients supported with extracorporeal membrane oxygenation. Transpl Infect Dis. 2015;17(1):103–105. doi: 10.1111/tid.12327. [DOI] [PubMed] [Google Scholar]
- 60.McKinnon PS, Paladino JA, Schentag JJ. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents. 2008;31(4):345–351. doi: 10.1016/j.ijantimicag.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 61.De Rosa FG, Corcione S, Baietto L, et al. Pharmacokinetics of linezolid during extracorporeal membrane oxygenation. Int J Antimicrob Agents. 2013;41(6):590–591. doi: 10.1016/j.ijantimicag.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 62.Turner RB, Rouse S, Elbarbry F, Wanek S, Grover V, Chang E. Azithromycin Pharmacokinetics in Adults With Acute Respiratory Distress Syndrome Undergoing Treatment With Extracorporeal-Membrane Oxygenation. Ann Pharmacother. 2015 doi: 10.1177/1060028015612105. [DOI] [PubMed] [Google Scholar]
- 63.Veinstein A, Debouverie O, Gregoire N, et al. Lack of effect of extracorporeal membrane oxygenation on tigecycline pharmacokinetics. J Antimicrob Chemother. 2012;67(4):1047–1048. doi: 10.1093/jac/dkr550. [DOI] [PubMed] [Google Scholar]
- 64.Kim HS, Lee ES, Cho YJ. Insufficient serum levels of antituberculosis agents during venovenous extracorporeal membrane oxygenation therapy for acute respiratory distress syndrome in a patient with miliary tuberculosis. ASAIO J. 2014;60(4):484–486. doi: 10.1097/MAT.0000000000000096. [DOI] [PubMed] [Google Scholar]
- 65.Strunk AK, Ciesek S, Schmidt JJ, et al. Single- and multiple-dose pharmacokinetics of ethambutol and rifampicin in a tuberculosis patient with acute respiratory distress syndrome undergoing extended daily dialysis and ECMO treatment. Int J Infect Dis. 2015 doi: 10.1016/j.ijid.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 66.Gardner AH, Prodhan P, Stovall SH, et al. Fungal infections and antifungal prophylaxis in pediatric cardiac extracorporeal life support. J Thorac Cardiovasc Surg. 2011 doi: 10.1016/j.jtcvs.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 67.Eppes SC, Troutman JL, Gutman LT. Outcome of treatment of candidemia in children whose central catheters were removed or retained. Pediatr Infect Dis J. 1989;8(2):99–104. [PubMed] [Google Scholar]
- 68.Watt KM, Benjamin DK, Jr, Cheifetz IM, et al. Pharmacokinetics and safety of fluconazole in young infants supported with extracorporeal membrane oxygenation. Pediatr Infect Dis J. 2012;31(10):1042–1047. doi: 10.1097/INF.0b013e31825d3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Watt KM, Gonzalez D, Benjamin DK, Jr, et al. Fluconazole population pharmacokinetics and dosing for prevention and treatment of invasive Candidiasis in children supported with extracorporeal membrane oxygenation. Antimicrob Agents Chemother. 2015;59(7):3935–3943. doi: 10.1128/AAC.00102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Autmizguine J, Hornik CP, Benjamin DK, Jr, et al. Pharmacokinetics and Safety of Micafungin in Infants Supported with Extracorporeal Membrane Oxygenation. Pediatr Infect Dis J. 2016 doi: 10.1097/INF.0000000000001268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kuse ER, Chetchotisakd P, da Cunha CA, et al. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet. 2007;369(9572):1519–1527. doi: 10.1016/S0140-6736(07)60605-9. [DOI] [PubMed] [Google Scholar]
- 72.Smith PB, Walsh TJ, Hope W, et al. Pharmacokinetics of an elevated dosage of micafungin in premature neonates. Pediatr Infect Dis J. 2009;28(5):412–415. doi: 10.1097/INF.0b013e3181910e2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koch BC, Wildschut ED, Goede AL, Hoog M, Bruggemann RJ. Insufficient serum caspofungin levels in a paediatric patient on ECMO. Med Mycol Case Rep. 2012;2:23–24. doi: 10.1016/j.mmcr.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ruiz S, Papy E, Da Silva D, et al. Potential voriconazole and caspofungin sequestration during extracorporeal membrane oxygenation. Intensive Care Med. 2009;35(1):183–184. doi: 10.1007/s00134-008-1269-3. [DOI] [PubMed] [Google Scholar]
- 75.Spriet I, Annaert P, Meersseman P, et al. Pharmacokinetics of caspofungin and voriconazole in critically ill patients during extracorporeal membrane oxygenation. J Antimicrob Chemother. 2009;63(4):767–770. doi: 10.1093/jac/dkp026. [DOI] [PubMed] [Google Scholar]
- 76.Bruggemann RJ, Antonius T, Heijst A, Hoogerbrugge PM, Burger DM, Warris A. Therapeutic drug monitoring of voriconazole in a child with invasive aspergillosis requiring extracorporeal membrane oxygenation. Ther Drug Monit. 2008;30(6):643–646. doi: 10.1097/FTD.0b013e3181898b0c. [DOI] [PubMed] [Google Scholar]
- 77.Hertzog JHBE, Sale M, Hauser GJ, Dalton HJ. Amphotericin B Pharmacokinetics During Extracorporeal Membrane Oxygenation: A Case Report. The Journal of Extra-Corporeal Technology. 1996;28(2):94–98. [Google Scholar]
- 78.Wildschut ED, de Hoog M, Ahsman MJ, Tibboel D, Osterhaus AD, Fraaij PL. Plasma concentrations of oseltamivir and oseltamivir carboxylate in critically ill children on extracorporeal membrane oxygenation support. PLoS One. 2010;5(6):e10938. doi: 10.1371/journal.pone.0010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eyler RF, Heung M, Pleva M, et al. Pharmacokinetics of oseltamivir and oseltamivir carboxylate in critically ill patients receiving continuous venovenous hemodialysis and/or extracorporeal membrane oxygenation. Pharmacotherapy. 2012;32(12):1061–1069. doi: 10.1002/phar.1151. [DOI] [PubMed] [Google Scholar]
- 80.Lemaitre F, Luyt CE, Roullet-Renoleau F, et al. Impact of extracorporeal membrane oxygenation and continuous venovenous hemodiafiltration on the pharmacokinetics of oseltamivir carboxylate in critically ill patients with pandemic (H1N1) influenza. Ther Drug Monit. 2012;34(2):171–175. doi: 10.1097/FTD.0b013e318248672c. [DOI] [PubMed] [Google Scholar]
- 81.Mulla H, Peek GJ, Harvey C, Westrope C, Kidy Z, Ramaiah R. Oseltamivir pharmacokinetics in critically ill adults receiving extracorporeal membrane oxygenation support. Anaesth Intensive Care. 2013;41(1):66–73. doi: 10.1177/0310057X1304100112. [DOI] [PubMed] [Google Scholar]
- 82.Shetty AK, Ross GA, Pranikoff T, et al. Oseltamivir-resistant 2009 H1N1 influenza pneumonia during therapy in a renal transplant recipient. Pediatr Transplant. 2012;16(5):E153–157. doi: 10.1111/j.1399-3046.2011.01489.x. [DOI] [PubMed] [Google Scholar]
- 83.Aebi C, Headrick CL, McCracken GH, Lindsay CA. Intravenous ribavirin therapy in a neonate with disseminated adenovirus infection undergoing extracorporeal membrane oxygenation: pharmacokinetics and clearance by hemofiltration. J Pediatr. 1997;130(4):612–615. doi: 10.1016/s0022-3476(97)70246-4. [DOI] [PubMed] [Google Scholar]
- 84.Hocker JR, Cook LN, Adams G, Rabalais GP. Ganciclovir therapy of congenital cytomegalovirus pneumonia. Pediatr Infect Dis J. 1990;9(10):743–745. doi: 10.1097/00006454-199010000-00013. [DOI] [PubMed] [Google Scholar]
- 85.Whitley RJ, Cloud G, Gruber W, et al. Ganciclovir treatment of symptomatic congenital cytomegalovirus infection: results of a phase II study. National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. J Infect Dis. 1997;175(5):1080–1086. doi: 10.1086/516445. [DOI] [PubMed] [Google Scholar]
- 86.Shekar K, Roberts JA, Smith MT, Fung YL, Fraser JF. The ECMO PK Project: an incremental research approach to advance understanding of the pharmacokinetic alterations and improve patient outcomes during extracorporeal membrane oxygenation. BMC Anesthesiol. 2013;13:7. doi: 10.1186/1471-2253-13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


