Abstract
Trabecular meshwork (TM) cells are the governing regulators of the TM structure. When the functionality of these cells is impaired, the structure of the TM is perturbed, which often results in increased ocular hypertension. High intraocular pressure is the most significant risk factor for steroid-induced glaucoma. Dexamethasone (Dex) induced phenotype of TM cells is widely utilized as a model system to gain insight into mechanisms underlying damaged TM in glaucoma. In this study, to assess the possible role of the abnormal Wnt signaling in steroid-induced glaucoma, we analyzed the effects of small-molecule Wnt signaling modulators on Dex-induced expression extracellular matrix proteins of primary human TM cells. While Dex-treated TM cells exhibited increased collagen and fibronectin expression, we found that Wnt signaling inhibitor 3235-0367 suppressed these Dex-induced effects. We therefore propose that Wnt signaling plays an important role in Dex-mediated impairment of TM cell functions. Moreover, the use of small molecule Wnt signaling inhibitors to treat TM cells may provide us an opportunity of restoring TM tissue in steroid-induced glaucoma.
Keywords: trabecular meshwork cell, Wnt, dexamethasone, glaucoma
1. Introduction
Glaucoma is a leading cause of blindness in the world and one of the causative factors is increased intraocular pressure (IOP). Increased IOP is a risk factor commonly found in patients with glaucoma caused by prolonged steroid treatment [1, 2]. The flow of aqueous humor through Schlemm’s canal is regulated by the trabecular meshwork (TM). When the TM becomes occluded, the flow of aqueous humor is hampered resulting in increased IOP. Ocular hypertension from the pressure can potentially damage the optic nerve, resulting in steroid-induced glaucoma [3].
TM cells are the predominant regulators of the structural integrity of the TM. The glaucomatous phenotypes of TM cells consistent with decreased outflow facility include: increased deposition of extracellular matrix (ECM) including collagen [4–6] and fibronectin [4–8], and increased cell stiffness [9]. However, the signaling mechanisms underlying this TM cell dysfunction are largely unknown. It has been established that dexamethasone (Dex) induces aberrant levels of myocilin [10], fibronectin [8, 11], and collagen in TM cells [11]. Indeed, Dex-induced glaucomatous phenotypes of TM cells have been widely used as a model to study TM cell dysfunction in glaucoma [12–14].
Abnormal Wnt signaling has been implicated in glaucoma. For example, the expression of sFRP1, a Wnt signaling antagonist, is up-regulated in glaucomatous TM cells [15], and the correlation of sFRP1 upregulation and increased IOP in both organ culture models and mice has been demonstrated [15]. The presence of active Wnt signaling has also been reported on a cellular level in vitro within TM cells [16, 17]. This active Wnt signaling was found to be involved in TM cell mediated ECM expression [18] and TM cell stiffening [19]. Overall, Wnt signaling appears to be a key player in TM regulation, with strong evidence suggesting that abnormal Wnt signaling may promote IOP [15]. Therefore, we decided to investigate whether a small molecule Wnt signaling activator, BML-284 [20], or a small molecule Wnt signaling inhibitor, 3235-0367 [21], can affect the Dex-induced glaucomatous phenotype of TM cells. We found that the inhibition of Wnt signaling abrogated Dex-mediated myocilin expression and ECM expression of primary human TM cells. This data reveals that Wnt signaling may exhibit pleiotropic roles in glaucomatous TM. In addition to deciphering the mechanism of Wnt signaling in TM cell dysfunction, small molecule Wnt signaling inhibitors may have potential therapeutic applications within steroid induced glaucoma.
2. Material and Methods
2.1. Primary human TM cells
Three independent human primary TM cell cultures were established from three donors at ScienCell Research Laboratories, Inc. (Carlsbad, CA). The first TM cell culture (HTM1; ScienCell catalog number 6590, lot number 4973) was obtained from a 25-year-old Caucasian male donor. The second TM cell culture (HTM2; ScienCell catalog number 6590, lot number 5975) was obtained from a 24-week-female-fetus. The third TM cell culture (HTM3; ScienCell catalog number 6590, lot number 5987) was from a 22-week-female-fetus. All experiments involving human tissue/cells were performed in compliance with the tenets of the Declaration of Helsinki. Internal review board approval was obtained for the procurement and use of human eye tissue for the study. Briefly, the eyes were rinsed with serum-free Dulbecco’s minimum essential medium (DMEM) three times. Under a dissecting microscope, a small incision was made 1.5 mm posterior to the limbus. Using vannas straight capsulotomy scissors, the incision was extended 360 degrees to remove the anterior portion of the eye. The iris and ciliary body were carefully removed using precision watchmaker’s, extra-fine point forceps, taking care to avoid damage to the angle area. Using a surgical blade, a thin layer (1.5 mm × 0.5 mm) of light gray TM tissue was cut from the angle area. The dissected TM tissues were further cut into smaller pieces, rinsed with serum-free DMEM, resuspended in fetal bovine serum (FBS), and plated as explants in a T-75 flask. The flask was left in the hood for TM tissues attachment, after which TM cell medium (ScienCell, Carlsbad, CA) was added. Cells were cultured at 37 °C, until day 7, when a monolayer of cells around the tissue blocks were harvested and frozen in cell freezing medium (ScienCell, Carlsbad, CA) as passage zero. The cells were maintained in DMEM low glucose (Thermo Fisher, Waltham, MA) with 10 % FBS (VWR, Radnor, PA), 1 % amino acids (Thermo Fisher, Waltham, MA) and 1 % TM cell growth supplement (ScienCell, Carlsbad, CA). Cells from passage 3–5 were used for all assays.
2.2. Compound preparation and treatment
Dex (Sigma-Aldrich, St. Louis, MO), Wnt signaling inhibitor 3235-0367 (ChemDiv, San Diego, CA) and Wnt signaling activator BML-824 (Santa Cruz Biotechnology, Dallas, TX) were dissolved in 100 % DMSO (Sigma-Aldrich, St. Louis, MO). Dex and small molecule Wnt signaling modulators were applied with the working concentration of 100 nM, 1 μM, and 0.1 μM respectively (individual final DMSO content of 0.001 %, therefore the vehicle control used was 0.002 % given that Dex was added together with the compound in some conditions). These concentrations were chosen after a viability assessment was carried out for cells treated with concentrations ranging from 0.1 μM– 50 μM. Compounds were added every 24-hours, and the cells were treated for seven days before harvesting for PCR analysis.
2.3. Quantitative PCR
Total mRNA was isolated from cells using the Qiagen RNeasy mini kit (Qiagen, Valencia, CA) as per manufacturer’s protocol. The Concentration of RNA was analyzed using a NanoDrop (Thermo Fisher, Waltham, MA). The Quantitect (Qiagen, Germantown, MD) reverse transcriptase process was utilized as per manufacturer’s protocol to obtain cDNA. Quantitative PCR was carried out using the Realplex2 (Eppendorf, Hauppauge, NY) with TaqMan primers for human GAPDH (Hs02758991_g1), human AXIN2 (AXIN2, Hs00610344_m1), human myocilin (MYOC, Hs00165345_m1), human collagen I (COL1A1, Hs00164004_m1), human collagen IV (COL4A1, Hs00266237_m1) and human fibronectin (FN1, Hs01549976_m1). Gene expression was normalized against the expression of GAPDH. We followed the manufacturer’s protocol for the qPCR reaction and utilized the delta-delta method of analysis. Each experimental condition was repeated at least three times.
2.4. Immunofluorescence
Cells grown to 90 % confluency on CC2 glass chamber slides (Thermo Fisher, Waltham, MA), were treated with Dex (100 nM), Dex with compound or DMSO vehicle for 7 days. Cells were washed once with PBS (Thermo Fisher, Waltham, MA) and fixed in 4 % paraformaldehyde (Sigma-Aldrich, St. Louis, MO) for 30 minutes at room temperature. Samples were washed 3 times for 5 minutes each, with PBS. Samples were blocked with PBS containing 5 % goat serum and 0.25 % Triton-X-100. The following primary antibodies used: rabbit anti human myocilin (Santa Cruz, Dallas, TX), rabbit anti human collagen I (34710, Abcam, Cambridge, MA), rabbit anti human collagen IV (6586, Abcam, Cambridge, MA), rabbit anti human fibronectin (2413, Abcam, Cambridge, MA) and phalloidin-TRITC (Sigma-Aldrich, St. Louis, MO) was added and incubated overnight at 4 °C in PBS containing 1 % goat serum. Samples were washed once with PBS. Secondary antibodies including: goat anti rabbit Alexafluor 488 (A-11034, Thermo Fisher, Waltham, MA) or goat anti rabbit Alexafluor 594 (A-11037, Thermo Fisher, Waltham, MA), were added to the membrane and incubated for one hour at room temperature. The samples were washed 3 times for 5 minutes per wash, with PBS and then counterstained and mounted with DAPI (Vector Labs, Burlingame, CA). Samples were visualized using Keyence BZ-X700 fluorescence microscope (Keyence, Japan) and the acquisition settings were identical for all treatments. Protein expressions were quantified by from 488 or 594 fluorescence images by using the software package Image J. Specifically, after splitting the DAPI channel and the 488 channel (or the 594 channel), calculations of the area covered by the fluorescence from the 488 (or 594 channel) was carried out and normalized against the area detected in the DAPI channel. There were three random fields of views photographed for each quantification.
2.5. TM cell collagen secretion
Collagen secreted into the media by the TM cultures was analyzed using the Sircol Assay Kit (Biocolor, Westbury) according to manufacturer’s instructions First, the TM cells were seeded into a 6-well plate at 2.0×105 cells/well and incubated for 4-hours. Then, the media was replaced with conditioned media either containing Dex (100 nM), Dex with compounds, DMSO (vehicle), and incubated at 37°C for 48-hours. After incubation, 1 ml of media was collected from each tube and were mixed with 200μL of Collagen Isolation and Concentration Reagent from the Sircol Assay Kit and incubated overnight at 4 °C. The following day, the samples were centrifuged at 12,000 rpm for 10 minutes and the supernatants were discarded. Then, 1mL of Sircol dye reagent (Sircol Assay Kit) that binds to collagen was added to each tube and gently mixed by inverting. The tubes were incubated for 30 minutes at room temperature and were then centrifuged at 12,000 rpm for 10 minutes. After, 750 μL/tube of ice cold Acid-Salt Wash (from the Sircol Assay Kit) was gently layered over the collagen-dye pellet, mixed and centrifuged at 12,000 rpm for 10 minutes to remove any unbound dye. The supernatant was disregarded and 250 μL/tube of the alkali reagent was added to the collagen-bound dye. The pellet formed was vortexed until completely dissolved back into solution and 200 μL aliquots of each sample was loaded on a 96-well plate. Using the FilterMax F5 microplate reader (Molecular Devices, Sunnyvale, CA), absorbance was measured at 555nm.
2.6. Western Blot analysis
Cells were treated with Dex for five days then lysed in RIPA lysis buffer (25mM Tris, 150mM NaCl, 1% NP-40, 1% sodium deoxycholate, and 0.1% SDS. The lysis buffer was supplemented with protease and phosphatase inhibitor cocktails (Thermofisher Scientific). The BCA assay (Pierce, Fisher Scientific) was use to quantify protein. Proteins were separated by molecular weight using SDS polyacrylamide gel electrophoresis, transferred onto a PVDF membrane (Bio-Rad Laboratories, Inc. Hercules, CA), and probed against myocilin and β-Actin (loading control) (Santa Cruz Biotechnology, Santa Cruz, CA).
2.7. Statistical analysis
Prism software was utilized for statistic calculations where One-way ANOVA was used together with Dunnett’s correction to obtain the standard error of mean and significance (p<0.05 was considered significant).
3. Results
3.1. TM cell characterization
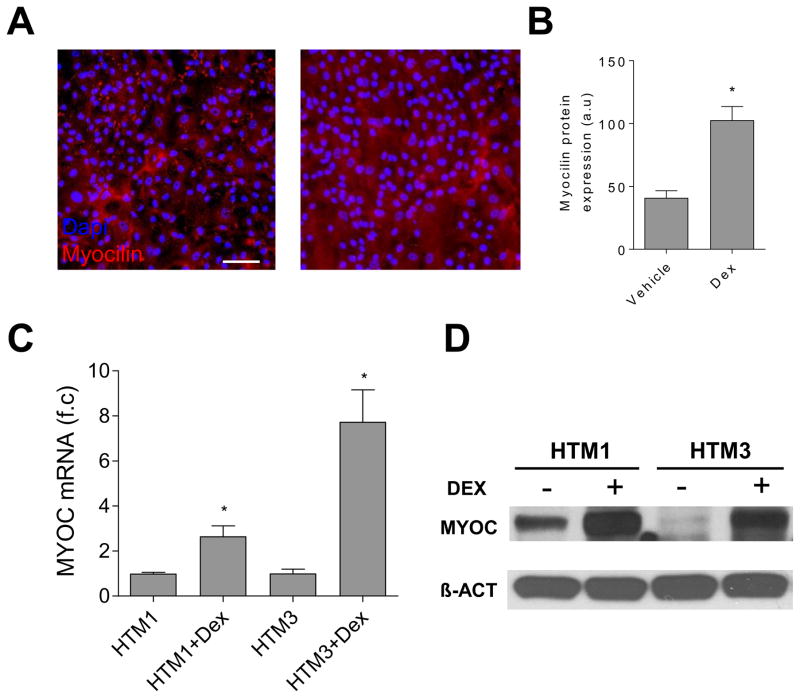
In the study, we used TM cells from a 25-year-old donor and two human fetal TM cells since TM cells from younger donors have increased and consistent doubling rates and greater passage capacity [22, 23]. The morphology of the cells we used in our study was similar to that reported by Rhee et al. [24], Morgan et al. [19], and Lin et al. [22], where they were small in size, slightly elongated, exhibited a moderate to low number of processes, and their cell bodies were both rounder and wider than those of scleral fibroblasts. The predominant marker of TM cells is increased myocilin expression following treatment with Dex [10, 25, 26]. We found that both the level of myocilin protein expression (Fig. 1A, 1B, and 1D) and myocilin mRNA (Fig. 1C) were increased in the TM cells following Dex treatment. The protein expression profile of these cells, including the increased myocilin expression levels after Dex treatment, is similar to that of reported TM cells [22].
Figure 1.
TM cell characterization. Representative immunofluorescence images (A) and the summary of the immunofluorescence study (B) showed that the myocilin level in the HTM2 cells was increased after Dex treatment (100 nM) for seven days. (C) The myocilin mRNA levels elevated in both the HTM1 cells and the HTM3 cells after Dex (100 nM) treatment for five days. Each experiment was carried out at two independent times; each measurement was done in triplicate. (D) The Western Blot studies showed that the myocilin protein levels increased in both HTM1 and HTM3 after Dex treatment for five days.
3.2. Dex induced myocilin expression in primary human TM cells is decreased with Wnt signaling inhibition
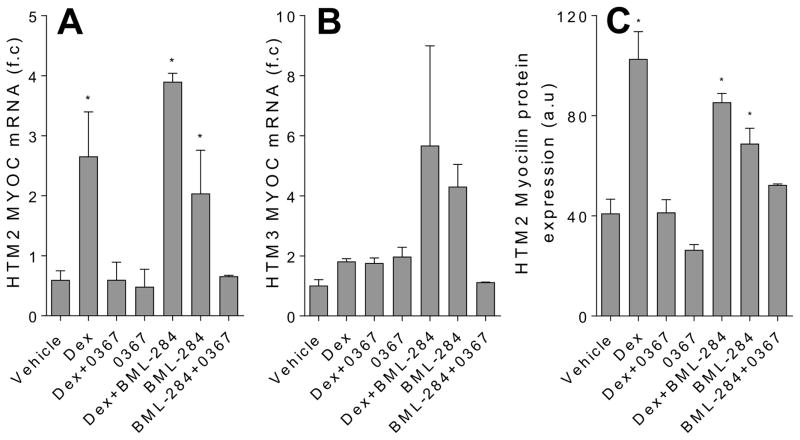
A known Wnt signaling antagonist, sFRP1, is upregulated in glaucomatous TM [15]. Given that Dex-mediated phenotype of TM cells is consistent with that of glaucomatous TM [8, 11, 17, 27–29], we speculated that activation of Wnt signaling would alleviate Dex-mediated TM cell activity. Hence, we examined whether Wnt signaling activator, BML-284 [20], would counteract the effects of Dex upon myocilin expression of the cells. We chose Wnt signaling activator BML-284 because this compound activates Wnt signaling without inhibiting GSK-3β [20]. As most of the known Wnt signaling activators active the Wnt signaling through inhibiting GSK-3β, a key element in the canonical Wnt signaling pathway, it has been suggested that the compound BML-284 may be able to activate both canonical and noncanonical Wnt signaling pathways [20]. We treated the Dex-induced TM cells with BML-284 and a Wnt inhibitor, 3235-0367, as a control, respectively. We chose compound 3235-0367 to inhibit Wnt signaling because this compound targets the interactions between Wnt protein and its cell-surface receptor, Frizzled [21]; therefore, it is considered as a “broad-spectrum” Wnt signaling inhibitor that can block both canonical and non-canonical Wnt signaling pathways. To our surprise, we found that BML-284 maintained the level of Dex-mediated myocilin expression and that it was inhibition of Wnt signaling with 3235-0367 that prevented Dex-induced myocilin expression (Fig 2). Furthermore, BML-284 alone increased myocilin expression of the TM cells, and this was not the case when the cell was treated with both BML-284 and 3235-0367. Collectively, these data suggest that Wnt signaling promotes myocilin expression in TM cells, and Dex induces myocilin expression in a Wnt signaling-dependent manner.
Figure 2.
Wnt signaling inhibition decreases Dex-mediated myocilin mRNA and protein expression in TM cells. Analysis of myocilin mRNA expression using qPCR with f.c stands for fold change in HTM2 (A) and HTM3 (B), as well as myocilin protein expression in HMT2 using immunofluorescence where a.u stands for arbitrary units (C). The cells were treated for 7 days with DMSO vehicle (0.002 %), Dex (100 nM), Dex in the presence of Wnt signaling activator BML-284 (0.1 μM), BML-284 alone (0.1 μM), Dex in the presence of Wnt signaling inhibitor 3235-0367 (1 μM), 3235-0367 alone (1 μM) or lastly BML-284 (0.1 μM) together with 3235-0367 (1 μM). The mRNA expression data were obtained from three independent experiments (* p<0.05 via One-way ANOVA with Dunnett’s correction) and the raw immunofluorescence images are shown in the Supplementary figure S1.
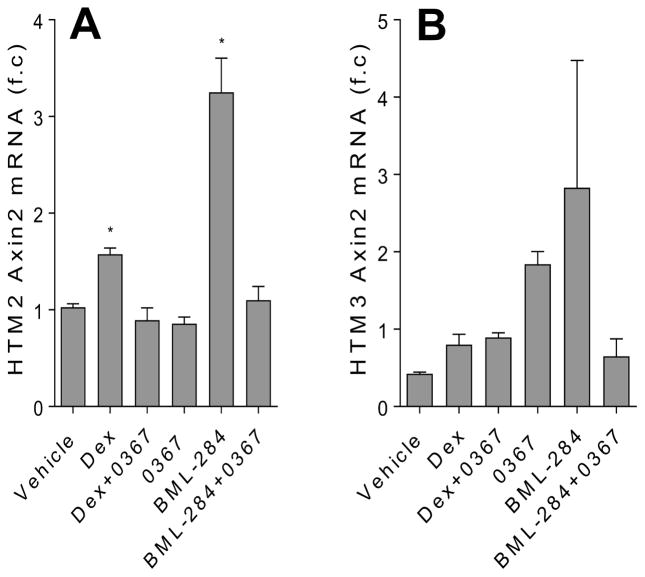
3.3. Dex mediated Axin2 expression is decreased with Wnt signaling inhibition
One of the known Wnt signaling target genes is Axin2 [30, 31]. Whereas most Wnt signaling target genes are tissue or developmental stage specific, the Axin2 gene is considered as a global transcriptional target [32]. For this reason, Axin2 is widely regarded as a general indicator of Wnt signaling pathway activity [33]. Therefore, to determine whether Dex directly activated Wnt signaling, we examined the expression of Axin2 in the Dex-treated cells. We found that Axin2 expression was increased in the TM cells treated with Dex (Fig. 3) and that when the TM cells received Dex together with 3235-0367, the Axin2 expression was not altered beyond control level. Additionally, BML-284 increased Axin2 expression in the TM cells; however, this expression was at control level when BML-284 was added to the cells together with 3235-0367. Overall, these findings suggest that Dex directly activates Wnt signaling and that small molecule modulators BML-284 and 3235-0367 can activate or inhibit Wnt signaling in the TM cells respectively.
Figure 3.
Dex-induced AXIN2 expression in TM cells is decreased with Wnt signaling inhibition. Analysis of AXIN2 mRNA expression was carried out using qPCR. The HTM2 (A) and HTM3 (B) cells were treated for 7 days with DMSO vehicle (0.002 %), Dex (100 nM), Dex in the presence of Wnt signaling inhibitor 3235-0367 (1 μM), 3235-0367 alone (1 μM), Wnt signaling activator BML-284 (0.1 μM) or BML-284 (0.1 μM) together with 3235-0367 (1 μM). The data were obtained from three independent experiments (* p<0.05 via One-way ANOVA with Dunnett’s correction).
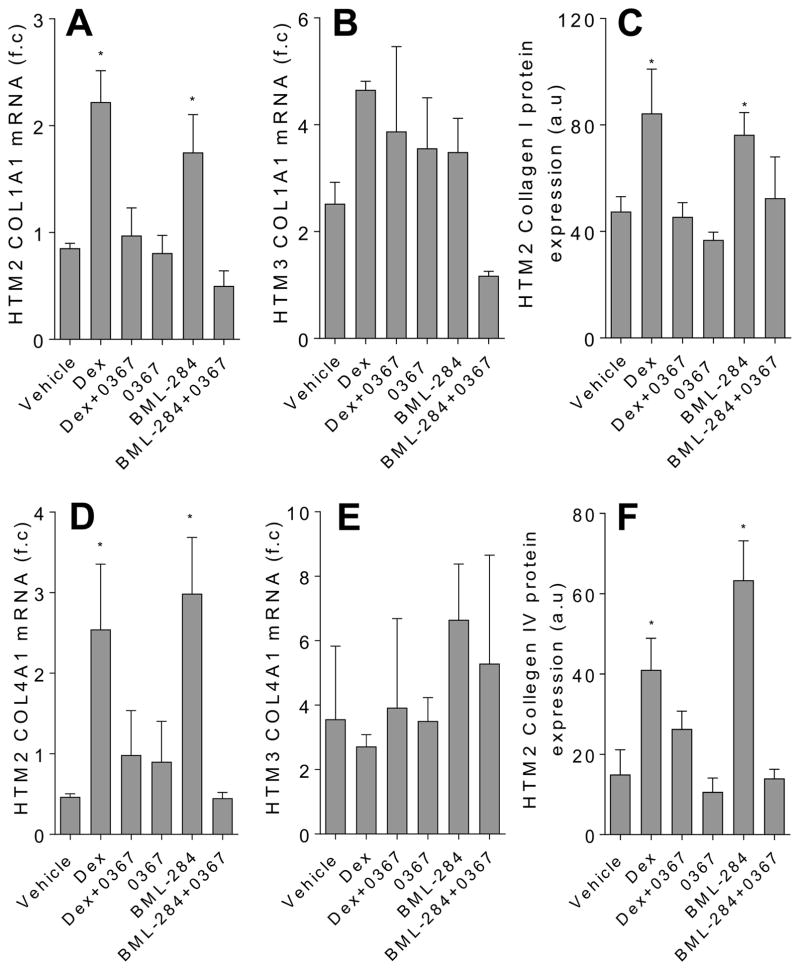
3.4. Extracellular matrix expression in primary human TM cells treated with Dex is decreased with Wnt signaling inhibition
Glaucomatous TM phenotype that is consistent with decreased outflow includes aberrant accumulations of ECM components such as collagen [4–6] and fibronectin [4–8, 11] and abnormal ECM structural remodeling [34–36]. Dex is known to increase expression of collagen [11] and fibronectin [8, 11] in TM cells and to change the ECM structural organization [34–36]. The mechanisms underlying how Dex orchestrates this profibrotic activity in TM cells is vastly unknown; however, it has been shown that expression of ECM proteins is regulated by Wnt signaling [18]. Therefore, using 3235-0367 and BML-284, we decided to examine whether Wnt signaling was involved in the Dex-mediated expression of ECM components in primary human TM cells. We first confirmed that Dex increased expression of collagen I (Fig. 4A–4C) as well as collagen IV (Fig. 4D–4F) mRNA and protein expression. Furthermore, as previously reported [8, 11], the expression of fibronectin mRNA and protein was increased upon Dex treatment (Fig. 5). We then found that Wnt signaling inhibitor 3523-0367 abolished this Dex-mediated increase of collagen and fibronectin expression. Intriguingly, Wnt signaling activator BML-284 alone also increased the expression of collagen I and collagen IV; however, their expression level was reverted back to normal when the Wnt signaling inhibitor 3235-0367 was added together with BML-284 into the TM cells. Overall, these data indicate that in the Dex-treated TM cells Wnt signaling plays a role in the aberrant collagen and fibronectin expression.
Figure 4.
Inhibition of Wnt signaling decreases the expression of collagen in the Dex-treated TM cells. The top panel shows the analysis of collagen I (COL1A1) mRNA in HTM2 (A) and HTM3 (B) cells and protein expression in the HTM2 cells (C) using qPCR and immunofluorescence respectively. The bottom panel shows the analysis of collagen IV (COL4A1) mRNA in the HTM2 (D) and HTM3 (E) cells and protein expression in the HTM2 cells (F). The cells were treated for 7 days with DMSO vehicle (0.002 %), Dex (100 nM), Dex in the presence of Wnt signaling inhibitor 3235-0367 (1 μM), 3235-0367 alone (1 μM), Wnt signaling activator BML-284 (0.1 μM) or BML-284 (0.1 μM) together with 3235-0367 (1 μM). Each experimental condition was repeated three times (* p<0.05 via One-way ANOVA with Dunnett’s correction). The raw immunofluorescent images are shown in the Supplementary figure S2.
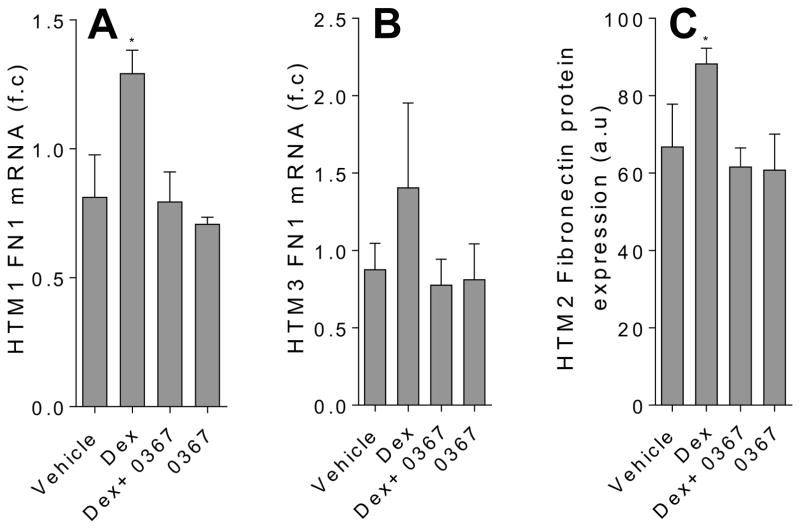
Figure 5.
Inhibition of Wnt signaling decreases the expression fibronectin the in Dex-treated TM cells. The analysis of fibronectin (FN1) mRNA in HTM2 (A) and HTM3 (B) cells and protein expression in HTM2 cells (C) were carried out using qPCR and immunofluorescence respectively. The cells were treated for 7 days with DMSO vehicle (0.002 %), Dex (100 nM), Dex in the presence of Wnt signaling inhibitor 3235-0367 (1 μM), and 3235-0367 alone (1 μM). Each experimental condition was repeated three times (* p<0.05 via One-way ANOVA with Dunnett’s correction). The raw immunofluorescent images are shown in the Supplementary figure S3.
Besides up-regulated collagen expression in the Dex-treated TM cells, we also found that within 48hours after Dex treatment, secretion of collagen from the Dex-treated TM cells was also up-regulated (Fig. 6). Moreover, consistent with our collagen expression study, Wnt signaling effectively abrogated the unregulated collagen secretion induced by the Dex-treatment. Also, when the TM cells were treated with BML-284, the Wnt signaling activator used in our study as a control, we found that collagen secretion was elevated (Fig. 6). These data further suggest that aberrant Wnt signaling activity in the TM cells may also increase collagen secretion.
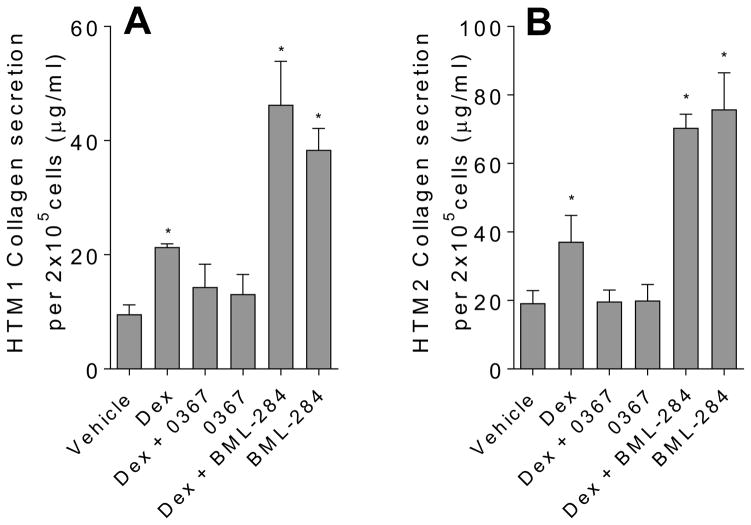
Figure 6.
Inhibition of Wnt signaling reduces collagen secretion from TM cells treated with Dex. The analysis of collagen secreted in the conditioned media were collected from HTM2 (A) and HTM3 (B) using the Sircol method of collagen detection. Cells were treated with the following conditions: ethanol (vehicle) (0.004%), Dex (100 nM), Dex (100 nM) in the presence of Wnt signaling inhibitor 3235-0367 (1 μM), 3235-0367 alone (1 μM), Dex (100 nM) in the presence of Wnt signaling activator BML-284 (0.1 μM), and activator BML-284 alone (0.1 μM). Each experiment condition was carried out in triplicates (*p<0.05 via One way ANOVA with Dunnett’s correction).
4. Discussion
TM cells are the governing regulators of the structural integrity of TM. When the functionality of these cells are impaired, the TM becomes occluded, and the flow of aqueous humor is hampered, ultimately resulting in ocular hypertension and steroid-induced glaucoma. The underlying mechanism of glaucomatous TM is largely unknown; however, Wnt signaling pathways have been implicated in glaucomatous TM [15, 16, 18, 19]. Using Dex-treated TM cells as a model of TM cell dysfunction in glaucoma [12], in this study, we demonstrated that a Wnt signaling inhibitor, 3235-0367, effectively abrogated Dex-induced phenotypes of primary human TM cells. Consistently, we also showed that applying a Wnt signaling activator, BML-284, to the TM cells induced the phenotypes similar to that of Dex-treated TM cells. Our data indicates that Dex-induced TM cell phenotypes are likely caused by aberrant activation of the Wnt signaling pathways.
Yuan et al. reported that activation of Wnt signaling with non-canonical Wnt5a induces cross linked actin networks (CLAN) formation [17], one of the glaucomatous phenotypes, and inhibition of Wnt signaling with Ror2 knockdown prevents Dex-mediated CLAN formation [17]. Mao et al. showed that addition of Wnt3A did not seem to have an effect on CLAN formation [16] suggesting that the non-canonical arm of Wnt signaling is important for CLAN formation. Furthermore, it was shown that Wnt signaling activation by way of myocilin overexpression and application of SB216763, a GSK-3β inhibitor that is able to activate the Wnt/β-catenin signaling pathway in cells [37], resulted in impaired actin expression of TM cells [38].
It is known that through the glucocorticoid response element pathway and/or the non-glucocorticoid response element pathway, glucocorticoids can alter expression of hundreds of genes in TM cells [13, 39]. Therefore, a combination of several genes is likely responsible for Wnt signaling activation in the Dex-treated TM cells. Dex also induces myocilin expression in the TM cells [40]. Moreover, it has been showed that myocilin is an effective modulator of the Wnt signaling pathways [41] and that overexpression of myocilin in the TM cells activates Wnt signaling [38]. However, because the Wnt signaling inhibitor, 3235-0367 could effectively abrogate the Dex-induced myocilin expression and the BML-284 increased myocilin expression, myocilin expression could be the consequence and not the cause of the initial Wnt signaling activation in the Dex-treated TM cells. Nevertheless, the expression of myocilin in the Dex-treated TM cells could further enhance the activated Wnt signaling.
Wnt signaling is not only a key player in regulating cell fate determination during embryonic development but also plays critical roles in maintaining tissue homeostasis [33, 42–44]. It has been shown that Wnt signaling is also important in maintaining TM tissue homeostasis and that suppressing Wnt signaling by constitutively overexpressing Wnt signaling inhibitors such as sFRP1 and Dkk1 in mice can cause damage to the TM tissues [15, 16]. Therefore, we speculate that Dex-induced Wnt signaling activation is a “protective” mechanism of the TM cells. However, to keep the homeostatic state of the TM tissues, the activated Wnt signaling has to be “reset” after an external insult. Therefore, prolonged Dex exposure could persistently activate Wnt signaling that then could lead to abnormal Wnt signaling activation and eventually cause glaucomatous phenotypes. It was shown that sFRP1, a negative Wnt signaling regulator, is upregulated in Dex-treated TM cells [9] and glaucomatous TM tissues [9, 15]. Plausibly, sFRP1 could be the negative feedback system generated by glaucomatous TM cells to suppress the abnormal Wnt signaling in the cells that is similar to that observed in cardiomyocyte differentiation [45]. Indeed, it was also shown that the expression level of DKK1 is also evaluated in the Dex-treated TM cells [9], and it is known that DKK1 is a target of the canonical Wnt signaling pathway [46].
Because our study showed that inhibiting the Wnt signaling pathway abrogated Dex-induced phenotypes of primary human TM cells, yet in the presence of Wnt signaling activator, BML-284, a phenotype similar to that induced by Dex in the TM cells, we propose that Wnt signaling may exhibit pleiotropic roles in Dex-induced glaucomatous TM. Like other glaucoma risk factors, prolonged steroid treatment may cause abnormal hyperactivity of Wnt signaling; and in turn, the abnormal Wnt signaling activation may promote TM cell malfunction and tissue damage that culminates in steroid glaucoma. Therefore, small molecule Wnt signaling inhibitors such as 3235-0367 may be helpful in developing a therapeutic target against the aberrant TM cellular activity in steroid glaucoma. Conversely, since Wnt signaling plays an important role in maintaining tissue homeostasis, we speculate that abnormally low Wnt signaling in the TM cells could also cause TM cell dysfunction that leads to glaucomatous phenotype [16]. In such case, a small molecule Wnt signaling activator, such as BML-284, can be used in the potential therapeutic development.
5. Conclusion
Our study shows that Dex-treated trabecular meshwork cells exhibit increased collagen and fibronectin expression. Wnt signaling inhibitor 3235-0367 can suppress these Dex-induced effects. Contrastingly, the Wnt signaling activator BML-284 can induce a phenotype similar to that of the Dex-treated TM cells. Taken together, we conclude that Wnt signaling plays an important role in the Dex-mediated impairment of TM cell functions and that the use of small molecule Wnt signaling inhibitors may provide us an opportunity of restoring TM cell and tissue in steroid-induced glaucoma.
Supplementary Material
Highlights.
Dex induced myocilin expression in primary human TM cells is decreased with Wnt signaling inhibition.
Dex mediated AXIN2 expression is decreased with Wnt signaling inhibition.
Extracellular matrix protein expression in primary human TM cells treated with Dex is decreased with Wnt signaling inhibition.
Small molecule Wnt signaling inhibition reduces Dex mediated collagen secretion by primary human TM cells.
Acknowledgments
The authors would like to thank Dr. Beatrice YJT Yue for her invaluable discussions regarding experimental set up, as well as editing of the paper. The authors would also like to thank the lab of Dr. Sophie X. Deng for the use of their fluorescence microscope and qPCR machine. We also acknowledge Alexandro Guerrero, Freddi Tran, Martin Ramirez and Tracy Yu for all their help within the lab. This work was supported in part by NIH grants GM100909 and by Research to Prevent Blindness.
Abbreviations
- TM
trabecular meshwork
- IOP
intraocular pressure
- Dex
dexamethasone
- ECM
extracellular matrix
- DMEM
Dulbecco’s minimum essential medium
- FBS
fetal bovine serum
- PBS
Phosphate Buffered Saline
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- MYOC
myocilin
- COL1A1
Collagen Type I
- COL4A1
collagen type IV
- FN1
fibronectin
- DMSO
Dimethyl sulfoxide
- DAPI
4′,6-diamidino-2-phenylindole
- ANOVA
analysis of variance
Footnotes
Conflicts of interest
The authors declare that they have no conflicts of interest with any of the content in this article except that J.S. is an employee of ScienCell Research Laboratories.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Armaly MF. Effect of Corticosteroids on Intraocular Pressure and Fluid Dynamics. Ii. The Effect of Dexamethasone in the Glaucomatous Eye. Arch Ophthalmol. 1963;70:492–499. doi: 10.1001/archopht.1963.00960050494011. [DOI] [PubMed] [Google Scholar]
- 2.Cubey RB. Glaucoma following the application of corticosteroid to the skin of the eyelids. Br J Dermatol. 1976;95:207–208. doi: 10.1111/j.1365-2133.1976.tb00830.x. [DOI] [PubMed] [Google Scholar]
- 3.Pleyer U, Ursell PG, Rama P. Intraocular Pressure Effects of Common Topical Steroids for Post-Cataract Inflammation: Are They All the Same? Ophthalmology and Therapy. 2013;2:55–72. doi: 10.1007/s40123-013-0020-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Junglas B, Yu AH, Welge-Lussen U, Tamm ER, Fuchshofer R. Connective tissue growth factor induces extracellular matrix deposition in human trabecular meshwork cells. Exp Eye Res. 2009;88:1065–1075. doi: 10.1016/j.exer.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Pattabiraman PP, Rao PV. Mechanistic basis of Rho GTPase-induced extracellular matrix synthesis in trabecular meshwork cells. Am J Physiol Cell Physiol. 2010;298:C749–763. doi: 10.1152/ajpcell.00317.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pattabiraman PP, Maddala R, Rao PV. Regulation of plasticity and fibrogenic activity of trabecular meshwork cells by Rho GTPase signaling. J Cell Physiol. 2014;229:927–942. doi: 10.1002/jcp.24524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medina-Ortiz WE, Belmares R, Neubauer S, Wordinger RJ, Clark AF. Cellular fibronectin expression in human trabecular meshwork and induction by transforming growth factor-beta2. Invest Ophthalmol Vis Sci. 2013;54:6779–6788. doi: 10.1167/iovs.13-12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steely HT, Browder SL, Julian MB, Miggans ST, Wilson KL, Clark AF. The effects of dexamethasone on fibronectin expression in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1992;33:2242–2250. [PubMed] [Google Scholar]
- 9.Raghunathan VK, Morgan JT, Park SA, Weber D, Phinney BS, Murphy CJ, Russell P. Dexamethasone Stiffens Trabecular Meshwork, Trabecular Meshwork Cells, and Matrix. Invest Ophthalmol Vis Sci. 2015;56:4447–4459. doi: 10.1167/iovs.15-16739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faralli JA, Clark RW, Filla MS, Peters DM. NFATc1 activity regulates the expression of myocilin induced by dexamethasone. Exp Eye Res. 2015;130:9–16. doi: 10.1016/j.exer.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou L, Li Y, Yue BY. Glucocorticoid effects on extracellular matrix proteins and integrins in bovine trabecular meshwork cells in relation to glaucoma. Int J Mol Med. 1998;1:339–346. [PubMed] [Google Scholar]
- 12.Gasiorowski JZ, Russell P. Biological properties of trabecular meshwork cells. Exp Eye Res. 2009;88:671–675. doi: 10.1016/j.exer.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wordinger RJ, Clark AF. Effects of glucocorticoids on the trabecular meshwork: towards a better understanding of glaucoma. Prog Retin Eye Res. 1999;18:629–667. doi: 10.1016/s1350-9462(98)00035-4. [DOI] [PubMed] [Google Scholar]
- 14.Stamer WD, Seftor RE, Williams SK, Samaha HA, Snyder RW. Isolation and culture of human trabecular meshwork cells by extracellular matrix digestion. Curr Eye Res. 1995;14:611–617. doi: 10.3109/02713689508998409. [DOI] [PubMed] [Google Scholar]
- 15.Wang WH, McNatt LG, Pang IH, Millar JC, Hellberg PE, Hellberg MH, Steely HT, Rubin JS, Fingert JH, Sheffield VC, Stone EM, Clark AF. Increased expression of the WNT antagonist sFRP-1 in glaucoma elevates intraocular pressure. J Clin Invest. 2008;118:1056–1064. doi: 10.1172/JCI33871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mao W, Millar JC, Wang WH, Silverman SM, Liu Y, Wordinger RJ, Rubin JS, Pang IH, Clark AF. Existence of the canonical Wnt signaling pathway in the human trabecular meshwork. Invest Ophthalmol Vis Sci. 2012;53:7043–7051. doi: 10.1167/iovs.12-9664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan Y, Call MK, Yuan Y, Zhang Y, Fischesser K, Liu CY, Kao WW. Dexamethasone induces cross-linked actin networks in trabecular meshwork cells through noncanonical wnt signaling. Invest Ophthalmol Vis Sci. 2013;54:6502–6509. doi: 10.1167/iovs.13-12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villarreal G, Jr, Chatterjee A, Oh SS, Oh DJ, Kang MH, Rhee DJ. Canonical wnt signaling regulates extracellular matrix expression in the trabecular meshwork. Invest Ophthalmol Vis Sci. 2014;55:7433–7440. doi: 10.1167/iovs.13-12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morgan JT, Raghunathan VK, Chang YR, Murphy CJ, Russell P. The intrinsic stiffness of human trabecular meshwork cells increases with senescence. Oncotarget. 2015;6:15362–15374. doi: 10.18632/oncotarget.3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Wu X, Mitchell B, Kintner C, Ding S, Schultz PG. A small-molecule agonist of the Wnt signaling pathway. Angew Chem Int Ed Engl. 2005;44:1987–1990. doi: 10.1002/anie.200462552. [DOI] [PubMed] [Google Scholar]
- 21.Lee HJ, Bao J, Miller A, Zhang C, Wu J, Baday YC, Guibao C, Li L, Wu D, Zheng JJ. Structure-based Discovery of Novel Small Molecule Wnt Signaling Inhibitors by Targeting the Cysteine-rich Domain of Frizzled. J Biol Chem. 2015;290:30596–30606. doi: 10.1074/jbc.M115.673202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin S, Lee OT, Minasi P, Wong J. Isolation, culture, and characterization of human fetal trabecular meshwork cells. Curr Eye Res. 2007;32:43–50. doi: 10.1080/02713680601107058. [DOI] [PubMed] [Google Scholar]
- 23.Stamer WD, Clark AF. The many faces of the trabecular meshwork cell. Exp Eye Res. 2016 doi: 10.1016/j.exer.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rhee DJ, Tamm ER, Russell P. Donor corneoscleral buttons: a new source of trabecular meshwork for research. Exp Eye Res. 2003;77:749–756. doi: 10.1016/j.exer.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Polansky JR, Fauss DJ, Zimmerman CC. Regulation of TIGR/MYOC gene expression in human trabecular meshwork cells. Eye (Lond) 2000;14(Pt 3B):503–514. doi: 10.1038/eye.2000.137. [DOI] [PubMed] [Google Scholar]
- 26.Stamer DW, Roberts BC, Epstein DL, Allingham RR. Isolation of primary open-angle glaucomatous trabecular meshwork cells from whole eye tissue. Curr Eye Res. 2000;20:347–350. [PubMed] [Google Scholar]
- 27.Wilson K, McCartney MD, Miggans ST, Clark AF. Dexamethasone induced ultrastructural changes in cultured human trabecular meshwork cells. Curr Eye Res. 1993;12:783–793. doi: 10.3109/02713689309020383. [DOI] [PubMed] [Google Scholar]
- 28.Clark AF, Wilson K, de Kater AW, Allingham RR, McCartney MD. Dexamethasone-induced ocular hypertension in perfusion-cultured human eyes. Invest Ophthalmol Vis Sci. 1995;36:478–489. [PubMed] [Google Scholar]
- 29.Clark AF, Brotchie D, Read AT, Hellberg P, English-Wright S, Pang IH, Ethier CR, Grierson I. Dexamethasone alters F-actin architecture and promotes cross-linked actin network formation in human trabecular meshwork tissue. Cell Motil Cytoskeleton. 2005;60:83–95. doi: 10.1002/cm.20049. [DOI] [PubMed] [Google Scholar]
- 30.Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan D, Wiesmann M, Rohan M, Chan V, Jefferson AB, Guo L, Sakamoto D, Caothien RH, Fuller JH, Reinhard C, Garcia PD, Randazzo FM, Escobedo J, Fantl WJ, Williams LT. Elevated expression of axin2 and hnkd mRNA provides evidence that Wnt/beta -catenin signaling is activated in human colon tumors. Proc Natl Acad Sci U S A. 2001;98:14973–14978. doi: 10.1073/pnas.261574498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, Behrens J. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 34.Keller KE, Aga M, Bradley JM, Kelley MJ, Acott TS. Extracellular matrix turnover and outflow resistance. Exp Eye Res. 2009;88:676–682. doi: 10.1016/j.exer.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tektas OY, Lutjen-Drecoll E. Structural changes of the trabecular meshwork in different kinds of glaucoma. Exp Eye Res. 2009;88:769–775. doi: 10.1016/j.exer.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 36.Vranka JA, Kelley MJ, Acott TS, Keller KE. Extracellular matrix in the trabecular meshwork: intraocular pressure regulation and dysregulation in glaucoma. Exp Eye Res. 2015;133:112–125. doi: 10.1016/j.exer.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coghlan MP, Culbert AA, Cross DA, Corcoran SL, Yates JW, Pearce NJ, Rausch OL, Murphy GJ, Carter PS, Roxbee Cox L, Mills D, Brown MJ, Haigh D, Ward RW, Smith DG, Murray KJ, Reith AD, Holder JC. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol. 2000;7:793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- 38.Shen X, Ying H, Yue BY. Wnt activation by wild type and mutant myocilin in cultured human trabecular meshwork cells. PLoS One. 2012;7:e44902. doi: 10.1371/journal.pone.0044902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clark AF, Wordinger RJ. The role of steroids in outflow resistance. Exp Eye Res. 2009;88:752–759. doi: 10.1016/j.exer.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen TD, Chen P, Huang WD, Chen H, Johnson D, Polansky JR. Gene structure and properties of TIGR, an olfactomedin-related glycoprotein cloned from glucocorticoid-induced trabecular meshwork cells. J Biol Chem. 1998;273:6341–6350. doi: 10.1074/jbc.273.11.6341. [DOI] [PubMed] [Google Scholar]
- 41.Kwon HS, Lee HS, Ji Y, Rubin JS, Tomarev SI. Myocilin is a modulator of Wnt signaling. Mol Cell Biol. 2009;29:2139–2154. doi: 10.1128/MCB.01274-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grigoryan T, Wend P, Klaus A, Birchmeier W. Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes Dev. 2008;22:2308–2341. doi: 10.1101/gad.1686208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- 45.Gibb N, Lavery DL, Hoppler S. sfrp1 promotes cardiomyocyte differentiation in Xenopus via negative-feedback regulation of Wnt signalling. Development. 2013;140:1537–1549. doi: 10.1242/dev.088047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niida A, Hiroko T, Kasai M, Furukawa Y, Nakamura Y, Suzuki Y, Sugano S, Akiyama T. DKK1, a negative regulator of Wnt signaling, is a target of the beta-catenin/TCF pathway. Oncogene. 2004;23:8520–8526. doi: 10.1038/sj.onc.1207892. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.