Abstract
Meta-C‒H arylation of free phenylacetic acid has been realized using 2-carbomethoxynorbornene (NBE-CO2Me) as a transient mediator. Both the modified norbornene and the mono-protected 3-amino-2-hydroxypyridine type ligand are crucial for this auxiliary-free meta-C‒H arylation reaction. A series of phenylacetic acids including mandelic acid and phenylglycine react smoothly with various aryl iodides to provide the meta-arylated products in high yields.
Keywords: 3-amino-2-hydroxypyridine ligand, meta-C–H arylation, phenylacetic acid
Graphical Abstract

Despite the rapid development in transition metal-catalyzed C‒H activation, meta-C‒H functionalization remains limited in scope and efficiency.1–3 Although sterics or the electronics of the arene substrates can lead to meta-selective C–H functionalizations,3 the successful control of meta-selectivity with mono-substituted arenes are rare.3j In addition, these approaches are not feasible when polar functional groups are remote to the arenes. The use of a U-shaped template with a tethered nitrile or pyridine directing group demonstrated the feasibility of directing meta-selective metal insertion into remote C‒H bonds regardless of the substitution patterns.1 More recently, our group2a and others2b have combined directed ortho-C‒H activation with Catellani’s norbornene process4 to achieve meta-C‒H functionalization.2 Using this strategy, the design of novel ligands2a,e and transient mediators2c has enabled a variety of new meta-C‒H transformations.2f–g However, the use of weakly coordinating native functional groups as the directing group to achieve this meta-C–H functionalization has not been realized to date, despite the plethora of work concerning carboxylic acid directed ortho-C‒H functionalizations (Scheme 1a).5–6 Herein we report an auxiliary-free meta-C‒H arylation of phenylacetic acids, using 2-carbomethoxynorbornene (NBE-CO2Me) as a transient mediator and mono-protected 3-amino-2-hydroxypyridine as the ligand. (Scheme 1c).
Scheme 1.
Meta-C‒H arylation of phenylacetic acid.
Although carboxylic acid directed ortho-C‒H functionalizations have been developed,6 we anticipate a number of challenges when using such weakly coordinating directing group for meta-C‒H functionalizations: 1) The lack of tunability of an external directing group to accommodate the multiple steps of the catalytic cycle is a significant challenge associated with the use of native carboxylic acid; 2) Due to the weak coordination of the carboxylic acid, the Catellani reaction of aryl iodides could outcompete the ortho-C‒H palladation of phenyl acetic acid to give homo-coupling products; 3) Following the ortho-C‒H palladation, the undesired ortho-C‒H arylation is known to take place.6f
Despite the aforementioned challenges, the previously observed ligand effect in this catalytic cycle offers a promising handle to overcome these hurdles. For instance, in the meta-arylation of phenylacetamide, the quinoline ligand was crucial to achieve the high reactivity2a,c (Scheme 1b). We therefore focused on searching for a suitable ligand which can promote both the carboxylic acid-directed ortho-C‒H activation and the subsequent norbornene-mediated palladation of the meta-C‒H bonds. Based on our previous findings that mono-N-protected amino acid (MPAA) ligands can significantly accelerate the ortho-C‒H olefination of phenylacetic acid,6d,f we initiated our study by stirring the mixture of 2-methyl phenylacetic acid (0.1 mmol), p-iodotoluene (2.5 equiv), K2HPO4 (2 equiv), Ag2CO3 (0.75 equiv), Pd(OAc)2 (10 mol%), NBE-CO2Me (1.5 equiv) in hexafluoroisopropanol (HFIP) at 100 °C in the presence of MPAA ligands (L1 and L2, Table 1). Unfortunately, no desired products were observed when employing MPAA ligands, indicating that this scaffold does not sufficiently orchestrate the fundamental steps following the carbopalladation of the norbornene mediator. Although quinoline and pyridine ligands have been found to be crucial for norbornene-mediated meta-C‒H arylation and alkylation of phenylacetamide,2a,c no desired products were obtained with phenylacetic acid substrates when ligands L3–5 were used. Pyridine and quinoline ligands are likely to inhibit the ortho-C–H palladation of the phenyl acetic acid.
Table 1.
Reaction conditions: 2-methyl phenylacetic acid 1a (0.1 mmol), p-iodotoluene (2.5 equiv), Pd(OAc)2 (10 mol %), K2HPO4 (2.0 equiv), Ag2CO3 (0.75 equiv), NBE-CO2Me (1.5 equiv), Ligand (20 mol %), HFIP (1.0 mL), 100 °C, 24 h.
The yield was determined by 1H NMR using CH2Br2 as the internal standard.
N.P.= no desired products.
Isolated yield.
Next, we turned to mono-protected 3-amino-2-hydroxypyridine ligands which were developed for the norbornene-mediated meta-C‒H functionalization of aniline and phenol derived substrates using a non-native directing group.2e–f To our delight, 3-acetylamino-2-hydroxypyridine L7 orchestrated the fundamental steps of this catalytic cycle and enabled the formation of meta-arylated product in 45% yield without detectable formation of the ortho-arylated side product. Removal of the 3-acetylamino group caused a decrease in reactivity (L6, 23% yield), which indicated the acetyl-protected amino group could enhance the efficiency of the ligand. Further examination of a series of substituted mono-protected 3-amino-2-hydroxypyridine ligands was carried out. A methyl group at the 6-position of the pyridine ring resulted in complete loss of reactivity (L8), presumably due to the imposed steric hindrance. While the methyl group at either 4- or 5-position gave lower yields (L9–10), the electron-withdrawing trifluoromethyl group at 5-position increased the yield to 78% (L11). With L11 identified as promising ligand scaffold, a series of protecting group (PG) on the amino group (L11–18) were evaluated. A benzoyl protecting group decreases the effectiveness of the ligand (L12). The strongly electron-withdrawing trifluoroacetyl decreased the yield from 78% to 54% (L13), while a slightly electron-withdrawing PG was effective (L14). Notably, 1-admantanecarbonyl group (L18) afforded the desired products in 85% NMR yield (83% isolated yield). We currently hypothesize that mono-protected 3-amino-2-hydroxypyridine ligands are uniquely effective for this transformation because they not only promote the initial carboxylic acid-directed C–H activation and subsequent olefin insertion (much like MPAA ligands), but also promote the remaining steps of the catalytic cycle (unlike MPAA ligands). Control experiments showed that the pyridone-type ligand L18 can indeed accelerate the carboxylic acid-directed ortho-C‒H olefination reaction.7 The loading of Pd(OAc)2 can be reduced to 5 mol% with only a slight decrease in yield (70%). The use of catalytic amount of NBE-CO2Me is also feasible, albeit leading to lower yield (50 mol% NBE-CO2Me, 64% yield). In the absence of NBE-CO2Me, only ortho-arylated product was obtained.6f The simple norbornene are completely inactive under these conditions.
With the optimal conditions in hand, we examined the reactivity of variety of substituted aryl iodide coupling partners using 2-methyl phenylacetic acid as the model substrate. Both electron-rich and electron-deficient aryl iodides were well-tolerated, affording meta-arylated products in good to excellent yields (Table 2). Ortho-substituents including methyl, methoxyl, fluoro, and trifluoromethyl groups are compatible with this protocol (3a–3d). It is worth-noting that these non-coordinating ortho-substitutents were previously found to adversely affect meta-C‒H arylation reactions.2e Surprisingly, methyl 2-iodobenzoate, a superior coupling partner in previous meta-C‒H arylation reactions, gave the desired product in low yield (45%) under our standard conditions. The use of 1.5 equivalents of Ag2CO3 improved the yield to 70% (3e). Para-substituted aryl iodides gave slightly lower yields (3l–3r), especially for electron-deficient aryl iodides (3o–3r). 3,5-Disubstituted aryl iodides afforded excellent yields, regardless of their electronic properties (3s, 3t). Although this protocol tolerates various functional groups including nitro, trifluoromethyl, fluoro, chloro, bromo and phosphate (3f, 3m–3p, 3r), coordinating heteroaryl iodides are not reactive under current conditions.
Table 2.
Reaction conditions: 2-methyl phenylacetic acid 1a (0.1 mmol), Aryl iodide (2.5 equiv), Pd(OAc)2 (10 mol %), K2HPO4 (2.0 equiv), Ag2CO3 (0.75 equiv), NBE-CO2Me (1.5 equiv), L18 (20 mol %), HFIP (1.0 mL), 100 °C, 24 h.
Isolated yield.
Ag2CO3 (1.5 equiv) was used.
Isolated as the methyl ester.
The substrate scope of phenylacetic acids was also examined using 1-iodo-2-nitrobenzene as the coupling partner (Table 3). Substrates containing either electron-withdrawing or electron-donating substituents on the phenyl ring were arylated at the meta-position with high efficiency (5b–5n). Simple phenylacetic acid afforded a mixture of mono- and di-arylated products in 85% combined yield with a mono:di ratio of 1.2:1.0 (5a). Both ortho- and meta-substituted substrates gave the mono-arylated products in high yields (5b–5i). Bromo- and chloro- subsituents remained intact under the reaction conditions (5g, 5h), allowing orthogonality with traditional Pd-catalyzed cross coupling reactions. The mono-selectivity of para-substituted substrates varied. While 4-fluorophenylacetic acid gave a mixture of mono- and di-arylated products (49% 5j-mono, 35% 5j–di), excellent mono-selectivity was observed with 4-methoxyphenylacetic acid giving mono-arylated product in 60% yield (5k). The steric hindrance of the para-methoxyl group being adjacent to the meta-position is sufficient to prevent the di-arylation.2e Disubstituted phenylacetic acids were compatible substrates, affording moderate to excellent yields (5l–5n). 1-Naphthleneacetic acid gave the desired product in 82% yield (5o). While α-alkyl substituted phenylacetic acids resulted in poor yields, the rigid bicyclic substrate 1,2,3,4-tetrahydronaphthalene-1-carboxylic acid worked well (5p). Notably, derivatives of mandelic acid and α-phenylglycine were compatible with this protocol (5q, 5r).
Table 3.
Reaction conditions: phenylacetic acid (0.1 mmol), 2-nitro-iodobenzene (2.5 equiv), Pd(OAc)2 (10 mol %), K2HPO4 (2.0 equiv), Ag2CO3 (0.75 equiv), NBE-CO2Me (1.5 equiv), L18 (20 mol %), HFIP (1.0 mL), 100 °C, 24 h.
Isolated yield.
2f (3.0 equiv) was used.
Isolated as the methyl ester.
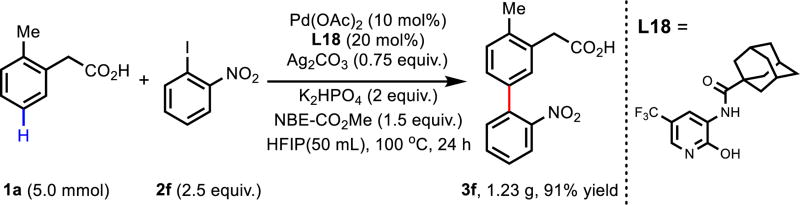
A gram-sale reaction was carried out to demonstrate the robustness of this reaction. Employing 2-methyl phenylacetic acid as substrate, the meta-arylated product was obtained in 91% isolated yield under the standard conditions (Scheme 2).
Scheme 2.
Gram-scale reaction.
In summary, meta-C‒H arylation of free phenylacetic acids was achieved by employing 2-carbomethoxynorbornene as the transient mediator and mono-protected-3-amino-2-hydroxypyridine as the ligand. Both the pyridone ligand and the modified norbornene mediator are crucial for realizing this auxiliary-free meta-C‒H arylation.
Supplementary Material
Acknowledgments
We gratefully acknowledge The Scripps Research Institute and the NIH (NIGMS, 2R01 GM102265) for financial support.
Footnotes
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201xxxxxx.
References
- 1.For selected examples of directed remote meta-C–H functionalization, see: Leow D, Li G, Mei T-S, Yu J-Q. Nature. 2012;486:518. doi: 10.1038/nature11158.Tang R, Li G, Yu J-Q. Nature. 2014;507:215. doi: 10.1038/nature12963.Kuninobu Y, Ida H, Nishi M, Kanai M. Nat. Chem. 2015;7:712. doi: 10.1038/nchem.2322.Davis HJ, Mihai MT, Phipps RJ. J. Am. Chem. Soc. 2016;138:12759. doi: 10.1021/jacs.6b08164.Zhang Z, Tanaka K, Yu J-Q. Nature. 2017;543:538. doi: 10.1038/nature21418.
- 2.For selected examples using Pd/norbornene relay process to achieve meta-C–H arylation, see: Wang X-C, Gong W, Fang L-Z, Zhu R-Y, Li S, Engle KM, Yu J-Q. Nature. 2015;519:334. doi: 10.1038/nature14214.Dong Z, Wang J, Dong G. J. Am. Chem. Soc. 2015;137:5887. doi: 10.1021/jacs.5b02809.Shen P-X, Wang X-C, Wang P, Zhu R-Y, Yu J-Q. J. Am. Chem. Soc. 2015;137:11574. doi: 10.1021/jacs.5b08914.Han J, Zhang L, Zhu Y, Zheng Y, Chen X, Huang Z-B, Shi D-Q, Zhao Y. Chem. Comm. 2016;52:6903. doi: 10.1039/c6cc02384c.Wang P, Farmer ME, Huo X, Jain P, Shen P-X, Ishoey M, Bradner JE, Wisniewski SR, Eastgate ME, Yu J-Q. J. Am. Chem. Soc. 2016;138:9269. doi: 10.1021/jacs.6b04966.Wang P, Li G-C, Jain P, Farmer ME, He J, Shen P-X, Yu J-Q. J. Am. Chem. Soc. 2016;138:14092. doi: 10.1021/jacs.6b08942.
- 3.For other examples of meta-C–H functionalizations: Saidi O, Marafie J, Ledger AEW, Liu PM, Mahon MF, Kociok-Köhn G, Whittlesey MK, Frost CG. J. Am. Chem. Soc. 2011;133:19298. doi: 10.1021/ja208286b.Hofmann N, Ackermann L. J. Am. Chem. Soc. 2013;135:5877. doi: 10.1021/ja401466y.Teskey CJ, Lui AYW, Greaney MF. Angew. Chem. Int. Ed. 2015;54:11677. doi: 10.1002/anie.201504390.Fan Z, Ni J, Zhang A. J. Am. Chem. Soc. 2016;138:8470. doi: 10.1021/jacs.6b03402.Phipps RJ, Gaunt MJ. Science. 2009;323:1593. doi: 10.1126/science.1169975.Duong HA, Gilligan RE, Cooke ML, Phipps RJ, Gaunt MJ. Angew. Chem. Int. Ed. 2011;50:463. doi: 10.1002/anie.201004704.Luo J, Preciado S, Larrosa I. J. Am. Chem. Soc. 2013;136:4109. doi: 10.1021/ja500457s.Ishiyama T, Takagi J, Ishida K, Miyaura N, Anastasi NR, Hartwig JF. J. Am. Chem. Soc. 2002;124:390. doi: 10.1021/ja0173019.Cho J-Y, Tse MK, Holmes D, Maleczka RE, Jr, Smith MR., III Science. 2002;295:305. doi: 10.1126/science.1067074.Zhang Y-H, Shi B-F, Yu J-Q. J. Am. Chem. Soc. 2009;131:5072. doi: 10.1021/ja900327e.
- 4.For reviews on norbornene mediated ortho-C–H functionalizations, see: Catellani M. Top. Organomet. Chem. 2005;14:21.Martins A, Mariampillai B, Lautens M. Top. Curr. Chem. 2010;292:1. doi: 10.1007/128_2009_13.Ye J, Lautens M. Nat. Chem. 2015;7:863. doi: 10.1038/nchem.2372.Della Ca’ N, Fontana M, Motti E, Catellani M. Acc. Chem. Res. 2016;49:1389. doi: 10.1021/acs.accounts.6b00165.For other system using norbornene as a transient mediator for indole C–H functionalizations: Jiao L, Bach T. J. Am. Chem. Soc. 2011;133:12990. doi: 10.1021/ja2055066.Jiao L, Herdtweck E, Bach T. J. Am. Chem. Soc. 2012;134:14563. doi: 10.1021/ja3058138.
- 5.Engle KM, Mei T-S, Wasa M, Yu J-Q. Acc. Chem. Res. 2012;45:788. doi: 10.1021/ar200185g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.a) Giri R, Maugel NL, Li J-J, Wang D-H, Breazzano SP, Saunders LB, Yu J-Q. J. Am. Chem. Soc. 2007;129:3510. doi: 10.1021/ja0701614. [DOI] [PubMed] [Google Scholar]; b) Chiong HA, Pham Q-N, Daugulis O. J. Am. Chem. Soc. 2007;129:9879. doi: 10.1021/ja071845e. [DOI] [PubMed] [Google Scholar]; c) Wang D-H, Mei T-S, Yu J-Q. J. Am. Chem. Soc. 2008;130:17676. doi: 10.1021/ja806681z. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Wang D-H, Engle KM, Shi B-F, Yu J-Q. Science. 2010;327:315. doi: 10.1126/science.1182512. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Satoh T, Miura M. Synthesis. 2010;20:3395. [Google Scholar]; f) Dastbaravardeh N, Toba T, Farmer ME, Yu J-Q. J. Am. Chem. Soc. 2015;137:9877. doi: 10.1021/jacs.5b04324. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Mei R, Zhu C, Ackermann L. Chem. Commun. 2016;52:13171. doi: 10.1039/c6cc07773k. [DOI] [PubMed] [Google Scholar]
-
7.The ortho-C‒H olefination of 2-methyl phenylacetic acid was carried out in the presence of L18, 70% combined yield of the ortho-olefinated product and cyclized product was obtained under the conditions similar to those used in the meta-functionalization reaction. Without ligand L18, only 30% olefination product was formed.

Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.