Abstract
Importance
Gene transfer has rarely been tested in randomized clinical trials.
Objective
To evaluate the safety and efficacy of intracoronary delivery of adenovirus 5 encoding adenylyl cyclase 6 (Ad5.hAC6) in heart failure.
Design, Setting, and Participants
A randomized, double-blind, placebo-controlled, phase 2 clinical trial was conducted in US medical centers (randomization occurred from July 19, 2010, to October 30, 2014). Participants 18 to 80 years with symptomatic heart failure (ischemic and nonischemic) and an ejection fraction (EF) of 40% or less were screened; 86 individuals were enrolled, and 56 were randomized. Data analysis was of the intention-to-treat population. Participants underwent exercise testing and measurement of left ventricular EF (echocardiography) and then cardiac catheterization, where left ventricular pressure development (+dP/dt)and decline (−dP/dt) were recorded. Participants were randomized (3:1 ratio) to receive 1 of 5 doses of intracoronary Ad5.hAC6 or placebo. Participants underwent a second catheterization 4 weeks later for measurement of dP/dt. Exercise testing and EF were assessed 4 and 12 weeks after randomization.
Interventions
Intracoronary administration of Ad5.hAC6 (3.2 × 109 to 1012 virus particles) or placebo.
Main Outcomes and Measures
Primary end points included exercise duration and EF before and 4 and 12 weeks after randomization and peak rates of +dP/dt and −dP/dt before and 4 weeks after randomization. Fourteen placebo participants were compared (intention to treat) with 24 Ad5.hAC6 participants receiving the highest 2 doses (D4 + 5).
Results
Fifty-six individuals were randomized and monitored for up to 1 year. Forty-two participants (75%) received Ad5.hAC6 (mean [SE] age, 63 [1] years; EF, 30% [1%]), and 14 individuals (25%) received placebo (age, 62 [1] years; EF, 30% [2%]). Exercise duration showed no significant group differences (4 weeks, P = .27; 12 weeks, P = .47, respectively). The D4 + 5 participants had increased EF at 4 weeks (+6.0 [1.7] EF units; n = 21; P < .004), but not 12 weeks (+3.0 [2.4] EF units; n = 21; P= .16). Placebo participants showed no increase in EF at 4 weeks or 12 weeks. Exercise duration showed no between-group differences (4-week change from baseline: placebo, 27 [36] seconds; D4 + 5, 44 [25] seconds; P = .27; 12-week change from baseline: placebo, 44 [28] seconds; D4 + 5, 58 [29 seconds, P = .47). AC6 gene transfer increased basal left ventricular peak −dP/dt (4-week change from baseline: placebo, +93 [51] mm Hg/s; D4 + 5, -39 [33] mm Hg/s; placebo [n = 21]; P < .03); AC6 did not increase arrhythmias. The admission rate for patients with heart failure was 9.5% (4 of 42) in the AC6 group and 28.6% (4 of 14) in the placebo group (relative risk, 0.33 [95% CI, 0.08-1.36]; P = .10).
Conclusions and Relevance
AC6 gene transfer safely increased LV function beyond standard heart failure therapy, attainable with one-time administration. Larger trials are warranted.
Trial Registration
clinicaltrials.gov Identifier: NCT00787059
Heart failure affects more than 28 million patients worldwide and is the only cardiovascular disease that is increasing in prevalence.1 Despite improvement in drug and device therapy for heart failure, hospitalization rates and mortality have changed little in the past decade2; new therapies are needed.
Adenylyl cyclase type 6 (AC6), a dominant AC type in heart muscle cells, is a 130-kD membrane protein that catalyzes the conversion of adenosine triphosphate to cyclic adenosine monophosphate (cAMP), a second messenger that is an important determinant of heart function.3 Preclinical studies4-9 have shown benefits of increased cardiac AC6 content on cardiac myocytes and the heart. The amount and function of AC6 are reduced in failing hearts.10-12
Pronounced increases in AC6 content do not alter basal intracellular cAMP production in rat cardiac myocytes or in mouse hearts.4,5 Recent studies13-15 indicate that AC6 has prominent effects on calcium handling and consequently influences heart function independently of cAMP. For example, AC6 gene transfer increases sarco/endoplasmic reticulumcalcium adenosine triphosphatase 2a (SERCA2a) calcium uptake,13 reduces phospholamban expression14 and increases its phosphorylation, and increases protein kinase B (Akt) activation through inhibition of a protein kinase B–specific phosphatase.15 These unique features make AC6 gene transfer fundamentally different from other agents that increase intracellular cAMP (dobutamine and milrinone), but increase mortality in heart failure.16 Increased expression of AC6 restores left ventricular (LV) function and prolongs life in a genetic murine model of cardiomyopathy.6,7 Expression of AC6 does not lead to unrelenting stimulation of the heart,5,6,8,9 and it normalizes prolonged action potential duration and reduces ventricular arrhythmias in cardiomyopathy.17
The extensive preclinical and clinical safety profile of intracoronary delivery of adenovirus vectors,8,9,18-20 the efficacy of this approach in preclinical models of heart failure, and the need for new therapies for heart failure compelled us to conduct the present clinical trial. The aim of the trial was to determine safety (heart failure hospitalizations and mortality) and heart function 4 and 12 weeks after intracoronary delivery of an E1/E3-deleted human adenovirus 5 encoding human AC6 (Ad5.hAC6) in participants with stable but symptomatic heart failure and reduced ejection fraction (EF). Our hypothesis was that a dose of intracoronary Ad5.hAC6 could be found that would safely increase the function of the failing hearts of participants with symptomatic heart failure and reduced EF.
Methods
Design
This randomized, double-blind, placebo-controlled, phase 2 study was conducted at 7 US medical centers (1 Veterans Affairs medical center, 5 academic centers, and 1 community hospital). Randomization occurred between July 19, 2010, and October 30, 2014. The objective of the trial was to evaluate the safety of one-time intracoronary injection of Ad5.hAC6 in participants with heart failure and to identify effective doses (if any) for subsequent trials. The ethics of the trial were considered and approved by the National Institutes of Health Recombinant DNA Advisory Committee and by the institutional review boards of the 7 centers (the trial protocol is available in Supplement 1). The participants provided written informed consent before the study procedures. No financial compensation was provided, although travel expenses were reimbursed. The National Institutes of Health Data and Safety Monitoring Board monitored the trial.
Adenovirus 5 encoding adenylyl cyclase consists of a human replication-deficient, serotype 5, E1/E3-deleted adeno-virus encoding human AC6, driven by a cytomegalovirus promoter. The infectivity of Ad5.hAC6 lots used for the clinical trial was 22 virus particles per fluorescent focus units. The core research pharmacist at Veterans Affairs San Diego Healthcare System distributed the vials of the study product (Ad5.hAC6 or placebo) to the participating sites in Kapac bags on dry ice with temperature monitoring.
Participants
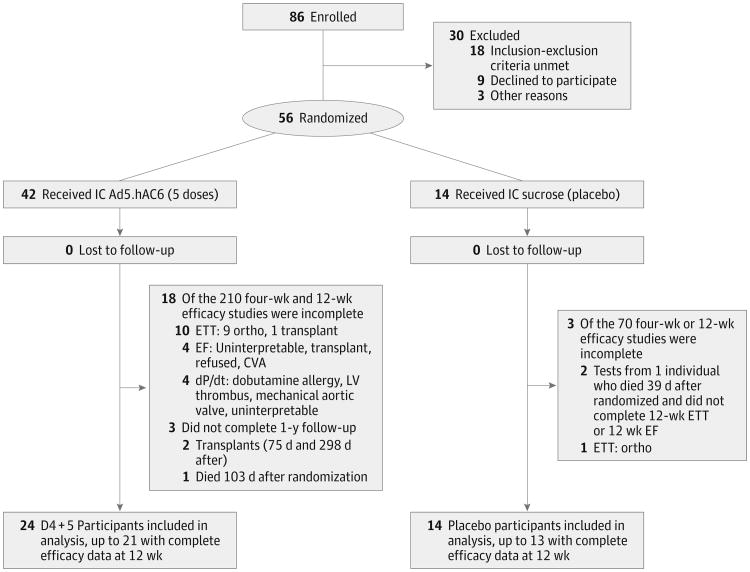
Male or nonpregnant female participants aged 18 to 80 years receiving optimal medical and device therapy as defined by the American Heart Association/American College of Cardiology guidelines21 with an LVEF of 40% or less and a history of stable symptomatic heart failure for more than 3 months were recruited. Participants were required to have an implanted cardiac defibrillator (ICD) and at least 1 major coronary artery or graft with less than 50% proximal obstruction. Figure 1 is a flow diagram of enrollment, randomization, follow-up, and analysis. Details regarding enrollment are included in the eMethods in Supplement 2.
Figure 1. Flow Diagram of Enrollment, Randomization, Follow-up, and Analysis.
CVA indicates cerebrovascular accident; D4 + 5, doses 4 and 5; dP/dt, rate of left ventricular (LV) pressure development and decay; EF, ejection fraction; ETT, exercise treadmill test; and IC, intracoronary.
Procedures
Vials were labeled with the dose to enable randomization within each dose and included a unique number that provided a means to later identify whether the vial contained Ad5.hAC6 or placebo. Details regarding randomization are reported in the eMethods in Supplement 2.
Participants received a single administration of intra-coronary Ad5.hAC6 at 1 of 5 ascending doses (D) (3.2 × 109 to 1012 virus particles) or intracoronary phosphate-buffered 3% sucrose (placebo) along with intracoronary nitroprusside (50 μg/min) in both groups, using a 3:1 randomization ratio. The 5 dose groups included: D1: 3.2 × 109 virus particles (6 active, 2 placebo participants); D2: 3.2 × 1010 virus particles (6 active, 2 placebo participants); D3: 1010 virus particles (6 active, 2 placebo participants); D4: 3.2 × 1011 virus particles (12 active, 4 placebo participants); and D5: 1012 virus particles (12 active, 4 placebo participants). This resulted in randomization of 14 individuals who received placebo and 42 who received 1 of 5 doses of Ad5.hAC6 (eTable 1 in Supplement 2). Nitroprusside was used to increase gene transfer efficiency. Intracoronary nitroprusside increases cardiac gene transfer efficiency 4-fold, presumably by increasing transvascular movement of adenovirus into the cardiac interstitium.18 The study product was distributed into patent coronary arteries or grafts in proportion to their perfused territory. All participants receiving the lowest dose were randomized before dose advancement (eTable 1 in Supplement 2). Safety was assessed during sequential clinic visits and testing (eTable 2 in Supplement 2). The National Institutes of Health Data and Safety Monitoring Board reviewed data after the first 3 participants and the last participant in each dose group before approving dose advancement.
Before delivery of the study product in the same single procedure, right and left heart catheterization was performed, and pressures, cardiac output, blood pressure, and LV end-diastolic pressure were recorded. Measurement of LV pressure development (+dP/dt) and decline (−dP/dt)was then conducted as described below. The study product (Ad5.hAC6 or placebo) was administered via an infusion catheter placed over a 0.014-inch guide wire into the proximal segment of each coronary artery or graft simultaneously with intracoronary nitro-prusside (eMethods in Supplement 2). Right heart catheterization and measurement of LV dP/dt were repeated 4 weeks after study product delivery.
Outcomes
Key efficacy end points included (1) LVEF (echocardiography, before and during dobutamine infusion), (2) LV peak +dP/dt and peak −dP/dt before and during dobutamine infusion, and (3) exercise capacity assessed by exercise treadmill testing. Secondary end points included symptoms, ICD events, and hemodynamic values obtained at catheterization.
Evaluation
Conventional 2-dimension echocardiography was performed before and 4 weeks and 12 weeks after randomization. Images were acquired before and during graded intravenous infusions of dobutamine (5, 10, and 20 μg/kg/min; 5 minutes each). Perflutren (Definity, Lantheus Medical Imaging; or Optison, GE Healthcare) was used to optimize endocardial border identification. Echocardiography data analysis, performed by a single experienced reader (N.D.D.), was conducted at a core laboratory (Veterans Affairs San Diego Medical Center) to increase the consistency of the analysis. Image acquisition and analysis were conducted blinded to group identity.
Left ventricular +dP/dt and −dP/dt were assessed using a 5F pressure catheter (Millar) before study product administration and 4 weeks after randomization. Data were acquired before and during graded intravenous infusions of dobutamine (5, 10, and 20 μg/kg/min; 5 minutes each) (eMethods in Supplement 2).
A modification of the modified Naughton protocol22 was used in treadmill testing, selected so participants could endure approximately 5 minutes of exercise. Participants completed the Kansas City Cardiomyopathy Questionnaire23 before and 4 weeks and 12 weeks after randomization to report their symptoms.
Information on right heart catheterization is presented in the eMethods and eResults in Supplement 2. No group differences were seen in hemodynamic measurements.
Implanted cardiac defibrillators, which were required for enrollment, enabled interrogation of devices before randomization and sequentially afterward. Implanted cardiac defibrillator discharges, antitachycardia pacing episodes, and episodes of nonsustained ventricular tachycardia were evaluated.
Quantitative polymerase chain reaction assays were performed on serum samples to detect Ad5.hAC6 presence 1 hour, 1 week, and 2 weeks after vector delivery. Detailed methods and results are in eMethods and eResults in Supplement 2. Of 14 participants who had detectable Ad5.hAC6 DNA in serum 1 hour after the completion of vector delivery, 4 (3 in D3, 1 in D5) had detectable Ad5.hAC6 DNA inserum 1 week but not 2 weeks after delivery.
We initially had excluded individuals with anti-Ad5 titers greater than 1:256, which resulted in unacceptably high screen failure. We eliminated this exclusion after D1. Previous studies19,20,24 had shown that Ad5 titers did not predictably influence the results (eMethods and eResults in Supplement 2).
Statistical Analysis
The protocol for this randomized, double-blind, placebo-controlled, phase 2 trial specified pairwise comparisons of the highest dose group or the highest 2 dose groups vs placebo (eTable 1 in Supplement 2). Sample sizes were calculated based on a mean estimated within-group SD of the key efficacy measurements (EF, dP/dt, and exercise duration) of 25%, and a detectability threshold of 25% in any 1 of the 3 end points. A comparison between 24 Ad5.hAC6 participants and 14 control participants, assuming a β error of 0.2 (80% power) and a 2-sided α error of .05 (without adjustment for multiple end points because of the hypothesis-generating nature of this study), would be suitable to detect a difference of 1.08 SD units between 2 study groups. Within-group differences in the 3 key end points (before and after study product administration) were tested using 2-sided t tests and a 5% significance level. Data are presented as mean (SE).
The intention-to-treat analyses for primary and secondary end points included paired t tests for within-group changes (2-sided). The Fisher exact test was used to determine between-group differences in the numbers of participants with heart failure admissions, transplantation, and mortality. The heart failure admission rate was assessed using a large-sample z test for Poisson rates. The effect of the cause of heart failure on EF response was evaluated by 2-way, repeated-measures analysis of variance. The association between Ad5.hAC6 dose and change in EF from baseline was examined using a log-linear regression analysis. To examine the effect of prerandomization anti-Ad5 antibody titer, a covariate adjustment approach was used. SAS, version 9.3 (SAS Institute Inc) was used for all analyses. Data analysis of the intention-to-treat population was conducted from May 31, 2015, to August 20, 2015.
Results
Figure 1 is a flow diagram of the study. In the key efficacy comparison groups (placebo, 14 participants; D4 + 5, 24), complete data were available in 91% (51 of 56) of the participants for EF, 93% (52 of 56) for LV dP/dt, and 79% (44 of 56) for exercise treadmill testing. Exercise treadmill testing was not required for randomization, which enabled enrollment of individuals with orthopedic limitations. Enrollment ended when 56 individuals had been randomized. No participants were lost to 1-year follow-up. Data for key efficacy measures in all dose groups and at all time points are reported in eTables 3-5 in Supplement 2.
Table 1 contains baseline characteristics of the 56 randomized participants. Mean (SE) EF was low (31% [1%]), and a balanced proportion of participants with New York Heart Association class 2 (25 [45%]) and 3 (29 [51%]) symptoms were randomized. An ischemic etiology for heart failure was present in 27 (48%) of the participants. There was uniformity between placebo and D4 + 5, the key comparison groups. Use of heart failure medication was similar and consistent throughout the trial.
Table 1. Baseline Characteristics.
| Characteristic | No. (%) | |||
|---|---|---|---|---|
| All (N = 56) | AC6 (n = 42) | Placebo (n = 14) | AC6 D4 + 5 (n = 24) | |
| Age, mean (SE), y | 63 (1) | 63 (1) | 62 (1) | 66 (2) |
| Male sex, % | 51 (91) | 39 (93) | 12 (86) | 23 (96) |
| Weight, mean (SE), kg | 95 (3) | 95 (4) | 97 (7) | 94 (4) |
| White race | 47 (84) | 35 (83) | 12 (86) | 20 (83) |
| EF, mean (SE), % | 31 (1) | 30 (1) | 30 (2) | 30 (2) |
| NYHA class 2/3/4 | 25 (45)/29 (51)/2 (4) | 19 (44)/21 (51)/2 (5) | 7 (50)/7 (50)/0 | 10 (43)/13 (52)/1 (4) |
| BNP, mean (SE), ng/mL | 464 (118) | 513 (154) | 318 (89) | 661 (260) |
| HF cause | ||||
| Ischemic | 27 (48) | 20 (48) | 7 (50) | 12 (50) |
| Nonischemica | 29 (52) | 22 (52) | 7 (50) | 12 (50) |
| Comorbidities | ||||
| Hypertension | 40 (71) | 30 (71) | 10 (71) | 15 (63) |
| Type 2 DM | 26 (46) | 18 (43) | 8 (57) | 9 (38) |
| CVA | 11 (20) | 10 (24) | 1 (7) | 6 (25) |
| PVD | 7 (13) | 6 (14) | 1 (7) | 1 (4) |
| Medication | ||||
| β-Blocker | 53 (95) | 40 (95) | 13 (93) | 23 (96) |
| ACEI or ARB | 49 (88) | 34 (81) | 14 (100) | 19 (79) |
| Aldosterone inhibitor | 21 (38) | 13 (31) | 7 (50) | 8 (33) |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; AC6, adenylyl cyclase 6; Ad5.hAC6, adenovirus 5 encoding AC6; ARB, angiotensin receptor blocker; BNP, brain natriuretic peptide; CVA, cerebrovascular accident; DM, diabetes mellitus; EF, ejection fraction; HF, heart failure; NYHA, New York Heart Association; PVD, peripheral vascular disease.
The sources of nonischemic HF included idiopathic, 19 (66%); viral myocarditis, 5 (17%); hypertension, 4 (14%); and alcohol, 1 (3%).
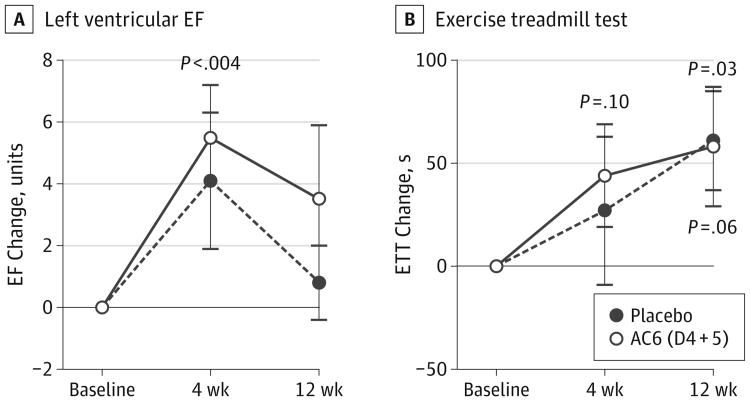
Figure 2A displays the effects of intracoronary administration of Ad5.hAC6 on basal LVEF. AC6 gene transfer in the highest 2 dose groups (D4 + 5) increased EF 4 weeks after intracoronary delivery (before, 29.7% [2.1%]; 4 weeks, 36.3% [2.1%]; (n = 21); mean increase, 6.0 [1.7] EF units; P < .004), but not at 12 weeks (before, 29.7% [2.1%]; 12 weeks, 34.2% [2.4%]; n = 21; mean increase, 3.0 [2.4] EF units; P = .16). In contrast, participants who had received placebo showed no significant increase in EF at 4 weeks (before, 29.6% [2.4%]; 4 weeks, 33.7% [3.0%]; n = 14; mean increase, 4.1 [2.2] EF units; P = .08) or at 12 weeks (before, 29.6% [2.4%]; 12 weeks, 31.6% [2.0%]; n = 13; mean increase, 0.8 [1.2] EF units; P = .31) (Figure 2A). No between-group difference was seen in mean values (D4 + 5 vs placebo: 4 weeks, P = .59; 12 weeks, P = .42).
Figure 2. Effects of Intracoronary Administration of Adenovirus 5 Encoding Adenylyl Cyclase 6 on Left Ventricular (LV) Ejection Fraction (EF) and Exercise Duration.
A, Placebo participants (baseline, n = 14; 4 and 12 weeks, n = 13) did not show significantly increased EF at 4 weeks (mean [SE] +4.1 [2.2] EF units; P = .08) or at 12 weeks (+0.8 [1.2] EF units; P = .48). Dose 4 and 5 (D4 + 5) participants (baseline, n = 23; 4 and 12 weeks, n = 21) showed increased EF at 4 weeks (+5.5 [1.7] EF units; P < .004) but less increase at 12 weeks (+3.5 [2.3] EF units; P = .16). Between-group findings for D4 + 5 and placebo were not significant (4 weeks, P = .59; 12 weeks, P = .42). B, Placebo participants (baseline, n = 14; 4 weeks, n = 13; 12 weeks, n = 12) did not have increased exercise treadmill test (ETT) duration at 4 weeks, but did at 12 weeks (P = .03). D4 + 5 participants (baseline, n = 19; 4 and 12 weeks, n = 17) showed no significant increases in ETT duration at 4 weeks (P = .10) or 12 weeks (P = .06). Error bars denote SE. Between-group findings for D4 + 5 and placebo were not significant (4 weeks, P = .70; 12 weeks, P = .94). AC6 indicates adenylyl cyclase 6.
To ascertain whether clinical features might influence EF response, we examined D4 + 5 participants with ischemic heart failure (n = 11), nonischemic heart failure (n = 10), and participants who received placebo (n = 13) (eFigure in Supplement 2). Although all 3 groups showed an EF increase at 4 weeks (ischemic, 4.4 [2.1] EF units; nonischemic, 6.8 [2.7] EF units; and placebo, 3.9 [2.2] EF units), only nonischemic participants showed a significant increase in EF at 12 weeks (ischemic, EF fell by 0.8 [3.0] EF units; nonischemic, EF increased by 8.2 [3.3] EF units; and placebo, EF increased by just 1.2 [1.1] EF units). Repeated-measures analysis of variance confirmed a benefit of AC6 on EF over time in nonischemic D4 + 5 participants compared with (1) participants with ischemic etiology (n = 11; P = .02), (2) placebo participants (n = 13; P = .02), and (3) nonischemic placebo participants (n = 6; P < .02) (eFigure in Supplement 2). These exploratory comparisons will require confirmation in subsequent trials.
In addition, the increase in EF at 4 weeks in AC6 participants was dose related (P < .04) (eTable 6 in Supplement 2). At 12 weeks, doses 1 to 5 failed to show a dose-response effect due to a surprising effect in dose 1 (4.7 [1.6]-EF unit increase; n = 6), the consequence of 2 participants showing 8- and 11-EF unit increases vs baseline. Both participants were Ad5 antibody-free and both had nonischemic etiology heart failure. However, doses 2 to 5 showed a dose-response effect at 12 weeks (P < .01) (eTable 6 in Supplement 2).
Twelve weeks after randomization, mean end-systolic volumes were 35 (9.8) mL lower in nonischemic D4 + 5 participants (P < .08 vs placebo) (eTable 7 in Supplement 2). Dobutamine stimulation was not associated with within-group or between-group differences in EF or LV volumes.
Treadmill time was not increased in D4 + 5 participants at 4 weeks (before, 429 [42] seconds; 4 weeks, 487 [49] seconds; n = 17; P = .10) or in placebo participants (before, 416 [56] seconds; 4 weeks, 446 [70] seconds; n = 13; P = .46) (Figure 2B). Treadmill time increased in both groups to similar degrees 12 weeks after randomization, reaching within-group statistical significance in placebo participants (before, 416 [56] seconds; 12 weeks, 501 [65] seconds; n = 12; P = .03) but not in D4 + 5 participants (before, 429 [42] seconds; 12 weeks, 501 [45] seconds; n = 17; P = .06). There was no between-group difference in treadmill time (D4 + 5 vs placebo: 4 weeks, P = .70; 12 weeks, P = .94).
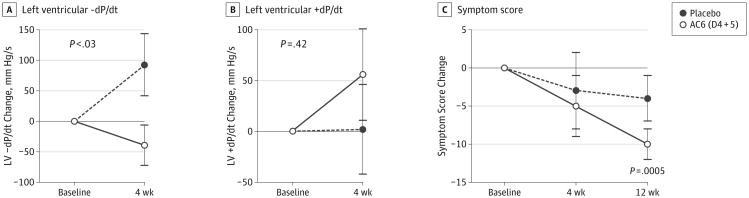
There was a between-group difference in change in basal LV peak −dP/dt 4 weeks after randomization (placebo, a +93 [51]-mm Hg/s change, n = 14; D4 + 5, a -39 [33]-mm Hg/s change, n = 21; P < .03) (Figure 3A). Change in basal LV peak +dP/dt showed no difference (placebo, a 2 [44]-mm Hg/s increase; n = 14; D4 + 5, a 56 [45]-mm Hg/s increase, n = 21; P = .42) (Figure 3B). Dobutamine stimulation was not associated with differences in peak −dP/dt or in peak+dP/dt; D4 + 5 participants showed significant reduction in symptoms 12 weeks after randomization (before, 28.8 [2.4]; 12 weeks, 20.5 [3.0]; n = 22; P = .0005) (Figure 3C). Placebo participants showed no reduction in symptoms at 12 weeks (before, 30.8 [5.1]; 12 weeks, 23.0 [4.4]; n = 13; P = .16). There was no decline in symptoms in either group at 4 weeks and no between-group differences after study product administration (D4 + 5 vs placebo: 4 weeks, P = .85; 12 weeks, P = .24). In the placebo group, median New York Heart Association classification fell by 0.5 units (from 2.5 to 2.0) at both 4 weeks (P = .07) and 12 weeks (P < .02); in the D4 + 5 group the decrease was 1.0 unit (from 3.0 to 2.0) at both 4 weeks (P < .11) and 12 weeks (P < .01).
Figure 3. Left Ventricular (LV) Pressure Development (+dP/dt), LV Decline (−dP/dt), and Symptom Scores.
A, LV peak −dP/dt decreased (LV pressure decline more rapid) in D4 + 5 participants (n = 22) compared with placebo participants (n = 14) 4 weeks after treatment. This between-group difference was statistically significant (P < .03). B, There was no between-group difference in LV peak +dP/dt between 14 placebo and 21 D4 + 5 participants (P = .42). C, D4 + 5 participants (baseline, n = 22; 4 weeks, n = 23; 12 weeks, n = 22) had reduced symptoms 12 weeks after randomization (P = .0005); no significant changes were noted in the placebo group (baseline, n = 13; 4 weeks, n = 14; 12 weeks, n = 13) (P = .16). Error bars denote SE. There was no between-group difference in symptom scores between D4 + 5 and placebo at 4 weeks (P = .85) and 12 weeks (P = .24). AC6 indicates adenylyl cyclase 6.
Right atrial, pulmonary artery wedge, and LV end-diastolic pressures were elevated to similar degrees in the placebo and D4 + 5 participants, and there were no within-group differences in the 4 weeks vs baseline study (eTable 8 in Supplement 2). Cardiac indices were low but showed no group difference or within-group benefits of AC6 gene transfer.
No significant adverse events occurred during product administration. Transient troponin I elevations occurred the morning after study product administration leading to 1-day hospitalization in 3 participants, 1 of whom received placebo. These adverse events were not associated with electrocardio-graphic changes, chest pain, or elevation of creatine kinase MB. The troponin I levels were increased compared with baseline levels on days 2 and 4 in both the placebo and D4 + 5 participants, with no group differences (day 2, P = .80; day 4, P = .50). No increases in creatine kinase MB in D4 + 5 or placebo participants were seen at any point (eTable 9 in Supplement 2). Similarly, there was no evidence for hepatotoxicity in patients receiving D4 + 5 (eTable 10 in Supplement 2).
Serious adverse events during a mean follow-up of 361 days (range, 305-365) are reported in Table 2. The percentage of participants with 1 or more serious adverse events was similar between groups.
Table 2. Serious Adverse Events.
| Characteristic | No. (%) | RR (95% CI) | P Value | |
|---|---|---|---|---|
| Placebo (n = 14) | Ad5.hAC6 (n = 42) | |||
| ≥1 SAEa | 5 (36) | 18 (43) | 1.20 (0.59-3.75) | .76b |
| HF admissions | 4 (28.6) | 4 (9.5) | 0.33 (0.08-1.36) | .10c |
| Death | 1 (7) | 1 (2) | 0.33 (0.01-11.10) | .40b |
Abbreviations: Ad5.hAC6, adenovirus 5 encoding adenylyl cyclase 6; HF, heart failure; RR, relative risk; SAE, serious adverse event.
A minority of SAEs were procedure or vector related: 1 (2%) each: groin hematoma, fever, cerebrovascular accident; and 3 (5%) troponin I level elevations at initial catheterization: placebo, 1 (7%); and Ad5.hAC6, 2 (5%).
Analysis conducted using Fisher exact test, as described in Methods section.
Analysis conducted using Z-test for Poisson rates, as described in Methods section.
Heart failure admission rate was 9.5% (4 of 42) among the AC6 participants and 28.6% (4 of 14) placebo participants (relative risk [RR], 0.33 [95% CI, 0.08-1.36]; P = .10). There were 2 deaths, 1 in each group: (AC6, 2%; placebo, 7%; RR, 0.33 [95% CI, 0.01-11.10]; P = .40). One death in a placebo participant was associated with progressive heart failure; the other, which occurred 3 months after administration of Ad5.hAC6 (D5), was in a participant with ischemic etiology heart failure who experienced myocardial infarction and cardiac arrest. There were 2 transplantations, both in AC6 participants (P > .99 vs placebo). The overall rates of ICD events and episodes of nonsustained ventricular tachycardia showed no group differences (eTable 11 in Supplement 2).
Anti-Ad5 titers ranged from less than 1:18 (undetectable) to greater than 1:4608, and mean (SE) values were 509 (155) (n = 51). There was no association between prerandomization anti-Ad5 titer and any efficacy end point. The correlation between antibody titer and EF change inD4 + 5 participants was not significant: P = .78 (at 4 weeks) and P = .22 (at 12 weeks).
Discussion
The most important findings of this initial clinical study are that intracoronary delivery of Ad5.hAC6 in patients with heart failure appears to be safe and that AC6 gene transfer provides a dose-related beneficial effect on cardiac function. We identified doses that merit additional study in subsequent larger trials.
Participants with nonischemic heart failure who received D4 + 5 had larger and persistent increases in EF vs D4 + 5 participants with ischemic etiology heart failure (P = .02), vs all placebo (P = .02), and vs placebo with ischemic heart failure (P < .02) (eFigure in Supplement 2). These data suggest that targeting Ad5.hAC6 to participants with nonischemic etiology heart failure would have the most benefit.
The 8.2 (3.3)–EF unit increase at 12 weeks in D4 + 5 participants with nonischemic heart failure (eFigure in Supplement 2), the dose-response relationship of Ad5.hAC6, and the absolute and relative increases in EF among D5 participants (4 weeks: 7.7 [2.7] EF units, a 26.5% relative increase in basal EF; 12 weeks: 4.2 [3.3] EF units, a 14.4% relative increase in basal EF) (eTable 6 in Supplement 2) indicate an important biological effect of AC6 gene transfer on LV function. The LV end-systolic volume index was 35 mL lower 12 weeks after randomization in D4 + 5 participants with nonischemic heart failure (P < .08 vs placebo) (eTable 7 in Supplement 2). Why would individuals with nonischemic heart failure have a superior response? Perhaps reduced viable myocardium in those with ischemic heart failure impedes the response to AC6 gene transfer. Alternatively, because ischemia affects endothelial function, its presence may impede transvascular movement of the vector into the cardiac interstitium and thereby may curtail gene transfer. The dose-response data (eTable 6 in Supplement 2) also indicate that the effect on LV function had not reached a plateau; higher doses may have provided increased response.
Left ventricular peak -dP/dt, a key end point of the study, was increased by AC6 gene transfer (P < .03) (Figure 3A). The peak rate of LV pressure decline is linked to LV relaxation and is abnormal in heart failure with reduced EF. This effect on LV relaxation also suggests that AC6 gene transfer may be effective to treat heart failure with preserved EF, a prevalent entity for which no therapy has been found to reduce mortality.25 One of the key mechanisms of AC6's action is enhanced calcium handling,13-15 which can benefit both diastolic and systolic function. The AC6-related increase in EF, seen particularly in nonischemic heart failure (eFigure in Supplement 2) indicates improved systolic function.
Preclinical data consistently showed an AC6-related increase in basal and stimulated rates of LV pressure development and decay,5,6,8,9 which served as a rationale for evaluating LV dP/dt in the present study. Although we found significant between-group differences in basal LV peak −dP/dt (P < .03) (Figure 3A), no between-group differences in basal LV +dP/dt were seen (Figure 3B). The dP/dt data were collected 4 weeks after randomization. Many participants showed maximal EF improvement at 12 weeks rather than 4 weeks (eFigure in Supplement 2), which may have reduced the likelihood of seeing an increase in LV +dP/dtat 4 weeks. The absence of do-but amine-stimulated group differences in peak +dP/dt and peak −dP/dt may reflect increased variability in these data.
A placebo effect on treadmill time, as we saw in the present study, is seen commonly in cardiovascular gene transfer trials.18,19 Previous studies have failed to show a benefit on exercise duration in heart failure participants after treatment with ACE inhibitors26 or long-acting β-blockers27 despite their benefits on mortality and heart failure hospitalization rates.
The absence of treatment differences in hemodynamic parameters (eTable 8 in Supplement 2) likely reflects the stability of participants in the trial. Symptoms of heart failure decreased in both groups (Figure 3C). However, the reduction in symptoms at 12 weeks was significant only in D4 + 5 participants (P = .0005), although no between-group difference (D4 + 5 vs placebo) was seen.
Similar increases in troponin I were seen in both groups the day after study product delivery (placebo, 6.4-fold increase; D4 + 5, 6.7-fold increase) (eTable 9 in Supplement 2). By day 4, levels had decreased (placebo, a 3.7-fold increase; D4 + 5: a 5.6-fold increase); by week 2, values were back to baseline. Electrocardiographic abnormalities or a clinical picture suggesting acute coronary syndrome were absent. The disappearance of elevation and the absence of elevation in aspartate aminotransferase or alanine aminotransferase (eTable 10 in Supplement 2) make clinically important adeno-virus-associated inflammation unlikely.
Although the 1-year mortality rate was decreased in participants who received AC6 gene transfer (AC6, 2%; placebo, 7%; RR, 0.33 [95% CI, 0.01-11.10]; P = .40), the numbers of events (1 per group) were too low to draw a conclusion. The heart failure admission rate (Table 2) was 9.5% in the 42 AC6 participants but 28.6% in the placebo group (RR, 0.33 [95% CI, 0.08-1.36]; P = .10).
Implanted cardiac defibrillators were a requirement for enrollment because of the possibility that AC6 could increase cAMP and thereby provoke cardiac arrhythmias, as was seen in a milrinone trial.16 However, AC6 gene transfer was not associated with increased rates of ICD events, which were uncommon in both groups. Furthermore, there was no increase in episodes of nonsustained ventricular tachycardia (eTable 11 in Supplement 2).
To date, adenovirus has been the most commonly used vector in clinical gene transfer,28 but a bias against its use persists. We initially selected adenovirus rather than adeno-associated virus, because AC6's large size in combination with the cytomegalovirus promoter made its expression in adeno-associated virus difficult. Two potential limitations of recombinant adenovirus vectors are inflammation and transient expression, which often are linked. However, cardiac and hepatic inflammation were not seen in the present study, as reflected by normal troponin I and liver enzyme levels in the weeks following vector delivery. These findings are consistent with those in 450 participants who received intracoronary Ad5.hFGF4 in the Angiogenic Gene Therapy (AGENT) trials,19,20 albeit at lower maximal doses (the AGENT maximal dose was 3.3 × 1010 virus particles vs 1012 virus particles in the present trial).
Adenovirus, like adeno-associated virus, directs extra-chromosomal transgene expression, so only one daughter cell continues to express transgene after cell division. However, our target cell, the cardiac myocyte, does not divide or does so rarely; therefore, transgene expression is not diluted in the heart. As a consequence of targeting nondividing cells and the absence of inflammation, AC6 function persisted at similar increased levels 2 to 18 weeks after intracoronary delivery in pigs.8 Intracoronary Ad5.hAC6 provides LV AC6transgene expression in pigs that persists with no reduction between day 28 and day 70 (IND 13751 for the present study).
Virus vectors previously have been used in cardiovascular diseases, including adenovirus encoding human fibroblast growth factor 4 in participants with angina (AGENT3),20 which failed to show a treatment effect in the overall patient population in a phase 2b/3 trial, and adeno-associated virus type 1 encoding human SERCA2a (AAV1.SERCA2a) in participants with heart failure and reduced EF.29 The phase 2b clinical trial of AAV1.SERCa2a failed to meet any primary or secondary end points.30
The current trial has some limitations. This was necessarily a small trial of a new gene therapy, and it was consequently not sufficiently powered to draw definitive conclusions regarding heart failure hospitalization rate or mortality. Although we monitored participants for 1 year for mortality and heart failure hospitalization rates–crucial elements in such a trial–additional efficacy end points (EF, dP/dt, and exercise treadmill testing duration) were examined only 4 and 12 weeks after randomization. The primary goals were to determine the safety of intracoronary administration of Ad5.hAC6 and to identify doses to test in subsequent larger clinical trials. Intracoronary Ad5.hAC6 appears to be safe even at doses of 1012 virus particles. Further testing will be required to confirm the safety and efficacy of IC Ad5.hAC6.
Conclusions
Intracoronary delivery of Ad5.hAC6 appears to be safe in patients with heart failure and reduced EF. Heart failure admission rate was 9.5% in participants who received AC6 and 28.6% in those who received placebo (P = .10). The rates of serious adverse events were similar in both groups, and ICD events were not increased. Despite limited sample size, 2 end points showed significant between-group differences: (1) AC6 gene transfer increased LV peak −dP/dt (P < .03); and (2) AC6 gene transfer increased EF in participants with nonischemic heart failure (P = .02). AC6 gene transfer safely increased LV function beyond optimal heart failure therapy through a single administration. Larger trials are warranted to assess the safety and efficacy of AC6 gene transfer for patients with heart failure.
Supplementary Material
eTable 1. Dose Groups
eTable 2. Clinical Tests and Frequency
eTable 3. Ejection Fraction
eTable 4. Left Ventricular Peak dP/dt
eTable 5. Exercise Treadmill Test Duration
eTable 6. Ad5.hAC6 Dose-Response Relationships
eTable 7. LV Volume
eTable 8. Hemodynamic Measurements
eTable 9. Assessment of Cardiac Injury
eTable 10. Assessment of Hepatotoxicity
eTable 11. Frequency of Non-Sustained VT and ICD Therapy Events
eFigure. Cause of Heart Failure and Ejection Fraction Response. Cause of heart failure determines ejection fraction (EF) response in Dose 4 + 5 subjects. Shown is EF change at 4 and 12 weeks comparing D4 + 5 subjects with ischemic (n=11) vs non-ischemic heart failure (n=10) vs placebo subjects (n=13). All 3 groups showed an EF increase at 4 weeks, but only subjects with non-ischemic heart failure showed an effect at 12 weeks, a mean +8.2±3.3 EF unit increase vs a mean +0.8±1.2 EF unit increase in the 13 placebo subjects (p=0.024). Participants with non-ischemic heart failure also fared better than those with ischemic heart failure (p=0.02) and better than placebo subjects with ischemic heart failure (P<0.02; n=6; not shown). P values from analysis of variance; symbols denote mean ±SE.
Key Points.
Question
Can intracoronary delivery of adenovirus 5 encoding adenylyl cyclase 6 (Ad5.hAC6) safely increase function of the failing heart?
Findings
In this randomized, double-blind, placebo-controlled clinical trial that included 56 participants with symptomatic heart failure and reduced left ventricular ejection fraction, intracoronary delivery of Ad5.hAC6 was not associated with increased adverse events and significantly increased left ventricular function.
Meaning
Larger trials are warranted to assess the safety and efficacy of AC6 gene transfer for patients with heart failure.
Acknowledgments
Funding/Support: This work was funded by National Institutes of Health (NIH) grants P01HL66941, National Heart, Lung, and Blood Institute (NHLBI) Gene Therapy Resource Program grant HHSN2682012000021, and Renova Therapeutics, Inc, via an NIH Public Private Partnership.
Role of the Funder/Sponsor: The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Supplemental content at jamacardiology.com
Author Contributions: Dr Hammond had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Hammond, Adler, Bhargava, Maisel, Narayan, Gao.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Hammond, Ross, Bhargava, Maisel, Narayan.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Hammond, Bhargava, Maisel, Dalton, Lee.
Obtained funding: Hammond.
Administrative, technical, or material support: Hammond, Penny, Traverse, Watkins, Yancy, Murray, Ross, Bhargava, Barnard, Lai, Dalton, Narayan, Blanchard, Gao.
Study supervision: Hammond, Penny, Patel, Ross, Narayan, Blanchard.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Hammond reported being a founder, board member, and unpaid consultant for Renova Therapeutics, a start-up private biotechnology company. Renova had no role in study design, data collection, data analysis, data interpretation, or writing of the report. Dr Lee reported receiving financial support from Renova Therapeutics. Dr Narayan reported equity interest in Topera, a paid consultancy with Abbott, and receiving honoraria from Medtronic, Boston Scientific, St Jude Medical Inc, and Biotronik. No other disclosures were reported.
Additional Contributions: The Belfer Gene Therapy Core Facility at Weill-Cornell manufactured Ad5.hAC6. The following groups and individuals helped in the conduct of this trial: NHLBI Gene Therapy Group: Sonia I. Skarlatos, PhD (deceased); Cheryl L McDonald, MD, PhD; Nora Rivera; MEd, Ray Ebert, PhD; Pankaj Qasba; PhD, Rita Sarkar, PhD; NHLBI Gene Therapy Resource Center Clinical Coordinating Center: Sue Sepelak MS, MSM; Meg Diggins; Eric Daniels; Jenee' Bevett; NHLBI Gene and Cell Therapy Data and Safety Monitoring Board, US Food and Drug Administration Center for Biologics Evaluation and Research Office of Cellular, Tissue, and Gene Therapies Staff: Stephen Funk and Rosa Cesarini (core research pharmacists); Eileen Mary D'Souza (administrative assistance); and Tamsin Lisa Kelly, JD, MD (reviewed the manuscript). We thank the participants who enrolled in the trial and their families.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall MJ, Levant S, DeFrances CJ. Hospitalization for Congestive Heart Failure: United States, 2000-2010. Hyattsville, MD: Centers for Disease Control and Prevention, National Center for Health Statistics; Oct, 2012. National Center for Health Statistics, Data Brief, No. 108. [Google Scholar]
- 3.Sunahara RK, Dessauer CW, Gilman AG. Complexity and diversity of mammalian adenylyl cyclases. Annu Rev Pharmacol Toxicol. 1996;36:461–480. doi: 10.1146/annurev.pa.36.040196.002333. [DOI] [PubMed] [Google Scholar]
- 4.Gao M, Ping P, Post S, Insel PA, Tang R, Hammond HK. Increased expression of adenylylcyclase type VI proportionately increases β-adrenergic receptor–stimulated production of cAMP in neonatal rat cardiac myocytes. Proc Natl Acad Sci U S A. 1998;95(3):1038–1043. doi: 10.1073/pnas.95.3.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao MH, Lai NC, Roth DM, et al. Adenylylcyclase increases responsiveness to catecholamine stimulation in transgenic mice. Circulation. 1999;99(12):1618–1622. doi: 10.1161/01.cir.99.12.1618. [DOI] [PubMed] [Google Scholar]
- 6.Roth DM, Gao MH, Lai NC, et al. Cardiac-directed adenylyl cyclase expression improves heart function in murine cardiomyopathy. Circulation. 1999;99(24):3099–3102. doi: 10.1161/01.cir.99.24.3099. [DOI] [PubMed] [Google Scholar]
- 7.Roth DM, Bayat H, Drumm JD, et al. Adenylyl cyclase increases survival in cardiomyopathy. Circulation. 2002;105(16):1989–1994. doi: 10.1161/01.cir.0000014968.54967.d3. [DOI] [PubMed] [Google Scholar]
- 8.Lai NC, Roth DM, Gao MH, et al. Intracoronary delivery of adenovirus encoding adenylyl cyclase VI increases left ventricular function and cAMP-generating capacity. Circulation. 2000;102(19):2396–2401. doi: 10.1161/01.cir.102.19.2396. [DOI] [PubMed] [Google Scholar]
- 9.Lai NC, Roth DM, Gao MH, et al. Intracoronary adenovirus encoding adenylyl cyclase VI increases left ventricular function in heart failure. Circulation. 2004;110(3):330–336. doi: 10.1161/01.CIR.0000136033.21777.4D. [DOI] [PubMed] [Google Scholar]
- 10.Bristow MR, Ginsburg R, Minobe W, et al. Decreased catecholamine sensitivity and β-adrenergic–receptor density in failing human hearts. N Engl J Med. 1982;307(4):205–211. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- 11.Ping P, Anzai T, Gao M, Hammond HK. Adenylyl cyclase and G protein receptor kinase expression during development of heart failure. Am J Physiol. 1997;273(2, pt 2):H707–H717. doi: 10.1152/ajpheart.1997.273.2.H707. [DOI] [PubMed] [Google Scholar]
- 12.Ishikawa Y, Sorota S, Kiuchi K, et al. Downregulation of adenylylcyclase types V and VI mRNA levels in pacing-induced heart failure in dogs. J Clin Invest. 1994;93(5):2224–2229. doi: 10.1172/JCI117219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang T, Gao MH, Roth DM, Guo T, Hammond HK. Adenylyl cyclase type VI corrects cardiac sarcoplasmic reticulum calcium uptake defects in cardiomyopathy. Am J Physiol Heart Circ Physiol. 2004;287(5):H1906–H1912. doi: 10.1152/ajpheart.00356.2004. [DOI] [PubMed] [Google Scholar]
- 14.Gao MH, Tang T, Guo T, Sun SQ, Feramisco JR, Hammond HK. Adenylyl cyclase type VI gene transfer reduces phospholamban expression in cardiac myocytes via activating transcription factor 3. J Biol Chem. 2004;279(37):38797–38802. doi: 10.1074/jbc.M405701200. [DOI] [PubMed] [Google Scholar]
- 15.Gao MH, Miyanohara A, Feramisco JR, Tang T. Activation of pH-domain leucine-rich protein phosphatase 2 (PHLPP2) by agonist stimulation in cardiac myocytes expressing adenylyl cyclase type 6. Biochem Biophys Res Commun. 2009;384(2):193–198. doi: 10.1016/j.bbrc.2009.04.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DiBianco R, Shabetai R, Kostuk W, Moran J, Schlant RC, Wright R. A comparison of oral milrinone, digoxin, and their combination in the treatment of patients with chronic heart failure. N Engl J Med. 1989;320(11):677–683. doi: 10.1056/NEJM198903163201101. [DOI] [PubMed] [Google Scholar]
- 17.Timofeyev V, He Y, Tuteja D, et al. Cardiac-directed expression of adenylyl cyclase reverses electrical remodeling in cardiomyopathy. J Mol Cell Cardiol. 2006;41(1):170–181. doi: 10.1016/j.yjmcc.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Roth DM, Lai NC, Gao MH, et al. Nitroprusside increases gene transfer associated with intracoronary delivery of adenovirus. Hum Gene Ther. 2004;15(10):989–994. doi: 10.1089/hum.2004.15.989. [DOI] [PubMed] [Google Scholar]
- 19.Grines CL, Watkins MW, Helmer G, et al. Angiogenic Gene Therapy (AGENT) trial in patients with stable angina pectoris. Circulation. 2002;105(11):1291–1297. doi: 10.1161/hc1102.105595. [DOI] [PubMed] [Google Scholar]
- 20.Henry TD, Grines CL, Watkins MW, et al. Effects of Ad5FGF-4 in patients with angina: an analysis of pooled data from the AGENT-3 and AGENT-4 trials. J Am Coll Cardiol. 2007;50(11):1038–1046. doi: 10.1016/j.jacc.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Jessup M, Abraham WT, Casey DE, et al. 2009 Focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119(14):1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 22.Page E, Cohen-Solal A, Jondeau G, et al. Comparison of treadmill and bicycle exercise in patients with chronic heart failure. Chest. 1994;106(4):1002–1006. doi: 10.1378/chest.106.4.1002. [DOI] [PubMed] [Google Scholar]
- 23.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35(5):1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 24.Roth DA, McKirnan MD, Canestrelli I, et al. Intracoronary delivery of an adenovirus encoding fibroblast growth factor-4 in myocardial ischemia: effect of serum antibodies and previous exposure to adenovirus. Hum Gene Ther. 2006;17(2):230–238. doi: 10.1089/hum.2006.17.230. [DOI] [PubMed] [Google Scholar]
- 25.Cheng RK, Cox M, Neely ML, et al. Outcomes in patients with heart failure with preserved, borderline, and reduced ejection fraction in the Medicare population. Am Heart J. 2014;168(5):721–730. doi: 10.1016/j.ahj.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Gundersen T, Swedberg K, Amtorp O, Remes J, Nilsson B Ramipril Study Group. Absence of effect on exercise capacity of 12-weeks treatment with ramipril in patients with moderate congestive heart failure. Eur Heart J. 1994;15(12):1659–1665. doi: 10.1093/oxfordjournals.eurheartj.a060449. [DOI] [PubMed] [Google Scholar]
- 27.Gullestad L, Manhenke C, Aarsland T, et al. Effect of metoprolol CR/XL on exercise tolerance in chronic heart failure—a substudy to the MERIT-HF trial. Eur J Heart Fail. 2001;3(4):463–468. doi: 10.1016/s1388-9842(01)00146-5. [DOI] [PubMed] [Google Scholar]
- 28.Gene Therapy Clinical Trials Worldwide. 2015 Update. [Accessed October 1, 2015];J Gene Med. http://www.wiley.co.uk/genmed/clinical. Published 2015.
- 29.Greenberg B, Yaroshinsky A, Zsebo KM, et al. Design of a phase 2b trial of intracoronary administration of AAV1/SERCA2a in patients with advanced heart failure: the CUPID 2 trial (Calcium Up-Regulation by Percutaneous Administration of Gene Therapy in Cardiac Disease Phase 2b) JACC Heart Fail. 2014;2(1):84–92. [Google Scholar]
- 30.Greenberg B, Butler J, Felker GM, et al. Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2): a randomised, multinational, double-blind, placebo-controlled, phase 2b trial. Lancet. doi: 10.1016/S0140-6736(16)00082-9. published online January 21, 2016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Dose Groups
eTable 2. Clinical Tests and Frequency
eTable 3. Ejection Fraction
eTable 4. Left Ventricular Peak dP/dt
eTable 5. Exercise Treadmill Test Duration
eTable 6. Ad5.hAC6 Dose-Response Relationships
eTable 7. LV Volume
eTable 8. Hemodynamic Measurements
eTable 9. Assessment of Cardiac Injury
eTable 10. Assessment of Hepatotoxicity
eTable 11. Frequency of Non-Sustained VT and ICD Therapy Events
eFigure. Cause of Heart Failure and Ejection Fraction Response. Cause of heart failure determines ejection fraction (EF) response in Dose 4 + 5 subjects. Shown is EF change at 4 and 12 weeks comparing D4 + 5 subjects with ischemic (n=11) vs non-ischemic heart failure (n=10) vs placebo subjects (n=13). All 3 groups showed an EF increase at 4 weeks, but only subjects with non-ischemic heart failure showed an effect at 12 weeks, a mean +8.2±3.3 EF unit increase vs a mean +0.8±1.2 EF unit increase in the 13 placebo subjects (p=0.024). Participants with non-ischemic heart failure also fared better than those with ischemic heart failure (p=0.02) and better than placebo subjects with ischemic heart failure (P<0.02; n=6; not shown). P values from analysis of variance; symbols denote mean ±SE.