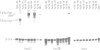Abstract
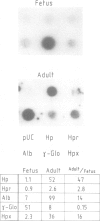
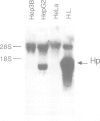
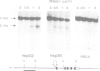
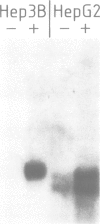
Haptoglobin is a plasma protein scarcely present in fetal but abundant in adult serum, where it is present at a concentration of approximately 150 mg/100 ml. In this paper we show by run-on experiments that the haptoglobin (Hp) gene is actively transcribed in adult but not in fetal liver nuclei. Studies with established cell lines indicate that the Hp gene is expressed in the hepatoma cells HepG2 but not in the hepatoma cell line Hep3B nor in HeLa cells. Plasmids carrying various segments of the 5' flanking region of the Hp gene fused to the chloramphenicol acetyl transferase (CAT) gene direct CAT transcription when introduced into HepG2 but are inactive in Hep3B and in HeLa cells, thus behaving like the resident chromosomal Hp gene. Deletion analysis defines a region, upstream to the transcription initiation site, essential for cell-specific expression. The Hp gene is induced in Hep3B cells by treatment with supernatant from LPS-stimulated monocytes (SMS), in a manner mimicking the acute phase reaction. We characterize the DNA segment necessary and sufficient for cell-specific expression of the Hp-CAT constructions in HepG2 and show that the same segment is also sufficient for acute phase induction in Hep3B.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altruda F., Poli V., Restagno G., Argos P., Cortese R., Silengo L. The primary structure of human hemopexin deduced from cDNA sequence: evidence for internal, repeating homology. Nucleic Acids Res. 1985 Jun 11;13(11):3841–3859. doi: 10.1093/nar/13.11.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann H., Hill R. E., Sauder D. N., Jahreis G. P. Regulation of major acute-phase plasma proteins by hepatocyte-stimulating factors of human squamous carcinoma cells. J Cell Biol. 1986 Feb;102(2):370–383. doi: 10.1083/jcb.102.2.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensi G., Raugei G., Klefenz H., Cortese R. Structure and expression of the human haptoglobin locus. EMBO J. 1985 Jan;4(1):119–126. doi: 10.1002/j.1460-2075.1985.tb02325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Bowman B. H., Kurosky A. Haptoglobin: the evolutionary product of duplication, unequal crossing over, and point mutation. Adv Hum Genet. 1982;12:189-261, 453-4. doi: 10.1007/978-1-4615-8315-8_3. [DOI] [PubMed] [Google Scholar]
- Ciliberto G., Dente L., Cortese R. Cell-specific expression of a transfected human alpha 1-antitrypsin gene. Cell. 1985 Jun;41(2):531–540. doi: 10.1016/s0092-8674(85)80026-x. [DOI] [PubMed] [Google Scholar]
- Ciliberto G., Raugei G., Costanzo F., Dente L., Cortese R. Common and interchangeable elements in the promoters of genes transcribed by RNA polymerase iii. Cell. 1983 Mar;32(3):725–733. doi: 10.1016/0092-8674(83)90058-2. [DOI] [PubMed] [Google Scholar]
- Colantuoni V., Pirozzi A., Blance C., Cortese R. Negative control of liver-specific gene expression: cloned human retinol-binding protein gene is repressed in HeLa cells. EMBO J. 1987 Mar;6(3):631–636. doi: 10.1002/j.1460-2075.1987.tb04801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Onofrio C., Colantuoni V., Cortese R. Structure and cell-specific expression of a cloned human retinol binding protein gene: the 5'-flanking region contains hepatoma specific transcriptional signals. EMBO J. 1985 Aug;4(8):1981–1989. doi: 10.1002/j.1460-2075.1985.tb03881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington G. J., Wilson D. R., Lachman L. B. Monocyte-conditioned medium, interleukin-1, and tumor necrosis factor stimulate the acute phase response in human hepatoma cells in vitro. J Cell Biol. 1986 Sep;103(3):787–793. doi: 10.1083/jcb.103.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgin S. C. DNAase I-hypersensitive sites of chromatin. Cell. 1981 Dec;27(3 Pt 2):413–415. doi: 10.1016/0092-8674(81)90381-0. [DOI] [PubMed] [Google Scholar]
- Gabrielli F., Aden D. P., Carrel S. C., von Bahr C., Rane A., Angeletti C. A., Hancock R. Histone complements of human tissues, carcinomas, and carcinoma-derived cell lines. Mol Cell Biochem. 1984 Nov;65(1):57–66. [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hammer R. E., Krumlauf R., Camper S. A., Brinster R. L., Tilghman S. M. Diversity of alpha-fetoprotein gene expression in mice is generated by a combination of separate enhancer elements. Science. 1987 Jan 2;235(4784):53–58. doi: 10.1126/science.2432657. [DOI] [PubMed] [Google Scholar]
- Knowles B. B., Howe C. C., Aden D. P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980 Jul 25;209(4455):497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- Kushner I., Gewurz H., Benson M. D. C-reactive protein and the acute-phase response. J Lab Clin Med. 1981 Jun;97(6):739–749. [PubMed] [Google Scholar]
- Kushner I. The phenomenon of the acute phase response. Ann N Y Acad Sci. 1982;389:39–48. doi: 10.1111/j.1749-6632.1982.tb22124.x. [DOI] [PubMed] [Google Scholar]
- Maeda N. Nucleotide sequence of the haptoglobin and haptoglobin-related gene pair. The haptoglobin-related gene contains a retrovirus-like element. J Biol Chem. 1985 Jun 10;260(11):6698–6709. [PubMed] [Google Scholar]
- Maeda N., Yang F., Barnett D. R., Bowman B. H., Smithies O. Duplication within the haptoglobin Hp2 gene. Nature. 1984 May 10;309(5964):131–135. doi: 10.1038/309131a0. [DOI] [PubMed] [Google Scholar]
- Oliviero S., DeMarchi M., Bensi G., Raugei G., Carbonara A. O. A new restriction fragment length polymorphism in the haptoglobin gene region. Hum Genet. 1985;70(1):66–70. doi: 10.1007/BF00389461. [DOI] [PubMed] [Google Scholar]
- Oliviero S., DeMarchi M., Carbonara A. O., Bernini L. F., Bensi G., Raugei G. Molecular evidence of triplication in the haptoglobin Johnson variant gene. Hum Genet. 1985;71(1):49–52. doi: 10.1007/BF00295668. [DOI] [PubMed] [Google Scholar]
- Raugei G., Bensi G., Colantuoni V., Romano V., Santoro C., Costanzo F., Cortese R. Sequence of human haptoglobin cDNA: evidence that the alpha and beta subunits are coded by the same mRNA. Nucleic Acids Res. 1983 Sep 10;11(17):5811–5819. doi: 10.1093/nar/11.17.5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie D. G., Fuller G. M. Hepatocyte-stimulating factor: a monocyte-derived acute-phase regulatory protein. Ann N Y Acad Sci. 1983 Jun 27;408:490–502. doi: 10.1111/j.1749-6632.1983.tb23268.x. [DOI] [PubMed] [Google Scholar]
- Robson E. B., Polani P. E., Dart S. J., Jacobs P. A., Renwick J. H. Probable assignment of the alpha locus of haptoglobin to chromome 16 in man. Nature. 1969 Sep 13;223(5211):1163–1165. doi: 10.1038/2231163a0. [DOI] [PubMed] [Google Scholar]
- Simmers R. N., Stupans I., Sutherland G. R. Localization of the human haptoglobin genes distal to the fragile site at 16q22 using in situ hybridization. Cytogenet Cell Genet. 1986;41(1):38–41. doi: 10.1159/000132193. [DOI] [PubMed] [Google Scholar]
- Sollazzo M., Frank R., Cesareni G. High-level expression of RNAs and proteins: the use of oligonucleotides for the precise fusion of coding-to-regulatory sequences. Gene. 1985;37(1-3):199–206. doi: 10.1016/0378-1119(85)90273-2. [DOI] [PubMed] [Google Scholar]
- Vannice J. L., Taylor J. M., Ringold G. M. Glucocorticoid-mediated induction of alpha 1-acid glycoprotein: evidence for hormone-regulated RNA processing. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4241–4245. doi: 10.1073/pnas.81.14.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk B., Wasylyk C., Chambon P. Short and long range activation by the SV40 enhancer. Nucleic Acids Res. 1984 Jul 25;12(14):5589–5608. doi: 10.1093/nar/12.14.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. The 5' ends of Drosophila heat shock genes in chromatin are hypersensitive to DNase I. Nature. 1980 Aug 28;286(5776):854–860. doi: 10.1038/286854a0. [DOI] [PubMed] [Google Scholar]
- Yang F., Brune J. L., Baldwin W. D., Barnett D. R., Bowman B. H. Identification and characterization of human haptoglobin cDNA. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5875–5879. doi: 10.1073/pnas.80.19.5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza S. L., Frain M., Mornet E., Sala-Trépat J. M., Lucotte G. Polymorphisms of human albumin gene after DNA restriction by HaeIII endonuclease. Hum Genet. 1984;67(1):48–51. doi: 10.1007/BF00270557. [DOI] [PubMed] [Google Scholar]
- vander Straten A., Herzog A., Jacobs P., Cabezón T., Bollen A. Molecular cloning of human haptoglobin cDNA: evidence for a single mRNA coding for alpha 2 and beta chains. EMBO J. 1983;2(6):1003–1007. doi: 10.1002/j.1460-2075.1983.tb01534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]