Abstract
Acetylcholine contributes to accurate performance of some navigation, but details of its contribution to the underlying brain signals are not fully understood. Selective damage to medial septal cholinergic neurons generally has little effect on landmark-based navigation, or the underlying neural representations of location and directional heading in visual environments. In contrast, the loss of cholinergic neurons disrupts navigation based on path integration, but no studies have tested whether these path integration deficits are associated with disrupted head direction (HD) cell activity. We therefore evaluated HD cell responses to visual cue rotations in a familiar arena, and during navigation between familiar and novel arenas, following muscarinic receptor blockade with systemic atropine. Atropine treatment reduced the peak firing rate of HD cells, but failed to significantly affect other HD cell firing properties. Atropine also failed to significantly disrupt the dominant landmark control of the HD signal, even though we used a procedure that challenged this landmark control. In contrast, atropine disrupted HD cell stability during navigation between familiar and novel arenas, where path integration normally maintains a consistent HD cell signal across arenas. These results suggest that acetylcholine contributes to path integration, in part, by facilitating the use of idiothetic cues to maintain a consistent representation of directional heading.
Keywords: acetylcholine, atropine, head direction, orientation, navigation
The ability to perceive location and directional heading within an environment is necessary for accurate navigation. In the rodent brain, place cells provide a neural representation of location, and head direction (HD) cells provide a neural representation of directional heading (O’Keefe & Dostrovsky, 1971; Taube, Muller, & Ranck, 1990a). Allothetic cues, or landmarks, dominantly control both of these representations when animals navigate within a familiar environment, as long as the animal perceives the landmark to be stable (Goodridge & Taube, 1995). However, in situations where familiar landmarks cannot be detected – such as when animals walk to a novel arena or when the animal is in complete darkness – an idiothetic cue-based mechanism (path integration) is capable of maintaining the stability of place cell and HD cell activity (McNaughton et al., 1996; Muller & Kubie, 1987; Stackman, Golob, Bassett, & Taube, 2003; Taube & Burton, 1995; Yoder, Clark, Brown, et al., 2011). The flexible use of allothetic and idiothetic cues to control spatial representations suggests that some type of mechanism enables the switch between information sources.
A candidate mechanism for switching between allothetic and idiothetic cues is the cholinergic system. Neural signals representing allothetic cues appear to gain control of place cell and HD cell signals via the postsubiculum (Calton et al., 2003; Goodridge & Taube, 1997; Yoder, Clark, & Taube, 2011; Yoder, Peck, & Taube, 2015), which receives direct cholinergic input from the medial septum (Gaykema, Luiten, Nyakas, & Traber, 1990). It is possible that this cholinergic input faciliates the use of allothetic cues, given its apparent modulation of attention to visual stimuli in associative learning tasks (M. G. Baxter, Holland, & Gallagher, 1997). If true, we would expect the removal of medial septal cholinergic neurons to disrupt some aspects of visuo-spatial performance. However, selective damage to these cholinergic cells with 192 IgG-saporin has largely failed to impair performance on navigational tasks (M. G. Baxter et al., 1996; M. G. Baxter & Gallagher, 1996b; M. G. Baxter, Bucci, Gorman, Wiley, & Gallagher, 1995; Berger-Sweeney et al., 1994; Chappell, McMahon, Chiba, & Gallagher, 1998; Dornan et al., 1996; Dwyer, Servatius, & Pang, 2007; Janis, Glasier, Fulop, & Stein, 1998; Kirby & Rawlins, 2003; McMahon, Sobel, & Baxter, 1997; Pang, Nocera, Secor, & Yoder, 2001; Shen, Barnes, Wenk, & McNaughton, 1996; Walsh, Herzog, Gandhi, Stackman, & Wiley, 1996). Acetylcholine’s limited role in navigational performance is consistent with physiological studies. For example, complete medial septal lesion or inactivation, which effectively removed cholinergic input to hippocampal and parahippocampal regions, only mildly affected hippocampal place cells’ representation of location (Leutgeb & Mizumori, 1999; Mizumori, Barnes, & McNaughton, 1989). Similarly, systemic muscarinic receptor blockade with scopolamine only mildly reduced the precision and reproducibility of place cell representations (Brazhnik, Muller, & Fox, 2003). Further, muscarinic receptor blockade failed to significantly disrupt thalamic HD cells’ responses to cue rotation when the animal navigated within a familiar visual environment (Sandoval, McDaniel, Murawski, Doerr, & Calton, 2008). In contrast to the limited role of acetylcholine in visual navigation, idiothetic cue-based navigation in darkness was markedly impaired following selective damage to medial septal cholinergic cells (Hamlin, Windels, Boskovic, Sah, & Coulson, 2013; Martin & Wallace, 2007). Idiothetic cue-based navigation is thought to involve entorhinal grid cell signals (Moser, Kropff, & Moser, 2008), and the periodicity of grid cell firing was disrupted following systemic muscarinic receptor blockade (Newman et al., 2014). The available evidence thus suggests that the use of allothetic cues for navigation occurs independent of acetylcholine, but the use of idiothetic cues requires acetylcholine.
Acetylcholine’s contribution to path integration performance may involve the HD signal. Idiothetic cue-based navigation was impaired following damage/inactivation of the vestibular system, damage to the dorsal tegmental nucleus, and in animals with dysfunctional semicircular canals or otolith organs (Frohardt, Bassett, & Taube, 2006; Wallace, Hines, Pellis, & Whishaw, 2002; Yoder, Goebel, et al., 2015). All of these manipulations also disrupted the HD cell signal (Bassett, Tullman, & Taube, 2007; Muir et al., 2009; Stackman, Clark, & Taube, 2002; Stackman & Taube, 1997; Valerio & Taube, 2016; Yoder & Taube, 2009), suggesting the HD signal is involved in path integration performance. Specifically, the HD signal appears to serve as an “error correction” mechanism which updates, or “resets,” to account for heading error that occurred during a previous journey (Valerio & Taube, 2012). Because this error correction requires both allothetic and idiothetic information, it is possible that the postsubiculum is a crucial node where both types of information are integrated (Yoder, Clark, & Taube, 2011). Acetylcholine may modulate this integration by facilitating the influence of idiothetic cues on the HD signal. Based on these studies, we hypothesized that the removal of acetylcholine input to the postsubiculum would disrupt HD signal stability based on idiothetic cues.
No previous studies have tested whether acetylcholine contributes to HD cell stability in tasks that favor the use of idiothetic cues. Here, we used systemic atropine injection to disrupt acetylcholine’s influence on the HD signal. We then evaluated allothetic cue processing by using a series of cue removal and cue rotation sessions that challenged the dominant landmark control of HD cell activity. In a separate group of rats, we evaluated idiothetic cue processing by requiring the animal to locomote between a familiar and a novel environment, where self-movement cues normally facilitate a consistent HD signal representation across both environments (Taube & Burton, 1995).
Method
Subjects
The rotation test included 16 rats, and the dual chamber test included 13 rats (female, age 4–9 mo, 250–360 g, Long-Evans; Harlan Laboratories, Boston, MA). All rats were group housed preoperatively and individually housed postoperatively within the same colony room on a 12:12-h light-dark cycle. All rats received food and water ad libitum. Despite numerous studies on the HD signal function in female rats, no studies have demonstrated any variability in the HD signal that is consistent with estrous cycle sensitivity; we therefore did not evaluate the estrous cycle at the time of recording. All procedures involving live animals were approved by the Dartmouth Institutional Animal Care and Use Committee.
Materials and Procedure
Electrodes
Electrodes were constructed as described previously (Kubie, 1984). Briefly, a microdrive was constructed from ten 25-μm Teflon-insulated nichrome wires (California Fine Wire, Grover Beach, CA) encased in a 26-gauge stainless steel cannula. Each electrode in the array had a tip impedance of ~2 MΩ. Dental acrylic was used to encase the cannula, wires, and connector and to hold the heads of three custom drive screws. Tips of the drive screws were threaded into custom-built plastic cuffs, which were later cemented to the skull as described below. These cuffs provided a fixed base into which the screws were incrementally turned to advance the electrodes through the brain.
Presurgical Training
Presurgical training occurred in a gray wooden cylinder (diameter = 76 cm, height = 50 cm) containing a white cue card along the inside wall that covered ~100° of the wall’s surface. The position of the cue card remained constant throughout presurgical training and HD cell screening procedures. The cylinder floor was covered by gray photographic backdrop paper. A black curtain extending from the ceiling to the floor surrounded the arena (diameter = 2.44 m) to discourage animals from using visual cues other than the white cue card. An overhead speaker controlled by a white noise generator was used to prevent the use of auditory cues. For 1 wk before surgical procedures, rats were trained to forage for 20-mg sucrose pellets (Bio-Serv, Frenchtown, NJ) that dropped onto the cylinder floor at pseudorandom intervals (mean interval = 30 s). Behavior was qualitatively evaluated during the pretraining procedure, which involved placing food-restricted animals into the apparatus and pseudo-randomly dispensing sugar pellets from above using a motorized feeder. Animals typically demonstrated signs of anxiety (thigmotaxis, freezing, defecation) at the beginning, but these signs disappeared over the course of several training sessions as the animal learned to search for food rewards. Pretraining was terminated when the animal reliably searched for food, and no longer showed signs of anxiety. All rats demonstrated proficiency in foraging for the sucrose pellets by the end of training.
Surgery
Rats were anesthetized with a ketamine-xylazine cocktail (90 and 10 mg/kg, respectively) and positioned in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA) with bregma and lambda in the same horizontal plane. The scalp was retracted and a small hole (~1 mm) was drilled through the skull above the anterodorsal thalamic nucleus (ADN). Additional holes were drilled in the frontal, parietal, and occipital bones to provide anchor points for miniature screws (Small Parts, Miramar, FL). Each electrode bundle was sterilized and coated, except for the tips, with polyethylene glycol (Carbowax) before being implanted just dorsal to the ADN (1.5 mm posterior, 1.3 mm lateral, 3.7 mm ventral to bregma). For one animal, the electrode was positioned dorsal to lateral mammillary nucleus (4.55 mm posterior, 1.1 mm lateral, 8.7 mm ventral to bregma), as described previously (Yoder et al., 2015). With the electrode bundle in position, the drive screw/cuff assemblies were fastened to the skull and jeweler’s screws with Grip cement (Dentsply International, Milford, DE). The scalp was sutured around the electrode drive, and the animal was allowed to recover 1 wk before screening and recording procedures commenced. Buprenorphine (0.015 mg/kg) was administered as a postoperative analgesic.
HD Cell Screening and Signal Processing
Animals were placed into the same cylinder that was used for training and each electrode was evaluated for cellular activity while the rat foraged for food pellets. The electrical signal from each electrode passed through a 10-channel headstage containing a unity gain operational amplifier. A flexible cable connected the headstage to an overhead commutator, which then connected to a patch panel. From the patch panel, electrical signals were amplified (x 20,000; P5 series; Grass, West Warwick, RI) and band-pass filtered (300 – 10,000 Hz) before auditory and visual display on a loudspeaker and oscilloscope (model 2214; Tektronix, Beaverton, OR), respectively. A dual time and amplitude window discriminator (model DDIS-1; BAK Electronics, Mount Airy, MD) was used to isolate single-unit spikes from background noise and to generate a square-wave TTL pulse upon spike detection. An overhead video camera (SONY XC-711; Tokyo, Japan) was used to monitor the animal’s HD at 60 Hz by detecting the positions of one red and one green light-emitting diode (LED) that were attached to the animal’s headstage and separated by 11 cm. Signals generated by the window discriminator at the occurrence of single-unit spikes and the x-y coordinates of the concurrent LED positions were acquired by a computer (Macintosh G4) running LabVIEW software (version 5.0; National Instruments, Austin, TX). Data were analyzed with LabVIEW.
Drug Injection and Recording Procedure
Landmark Rotation
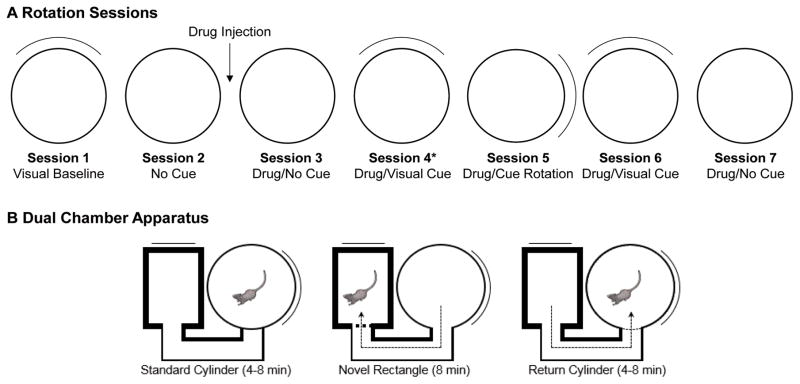
We evaluated the effects of muscarinic receptor blockade on the HD signal by subjecting rats to two different tests. A single i.p. injection of atropine sulfate (50mg/ml/kg body weight) was chosen because this dose is sufficient to disrupt spatial performance and cholinergic function (for discussion, see Whishaw, 1985). The first test was designed to challenge the animals’ attention to visual landmarks, and comprised a series of seven 8-min recording sessions within a cylinder (Fig 1A). Session 1 (referred to as visual baseline) was a baseline session with the cue card located at the “standard” position in the cylinder; at the end of session 1, the rat was removed from the arena and placed in an opaque disorientation box. The researcher then replaced the floor paper to remove olfactory cues and then disoriented the animal by slowly turning the box while walking around the arena. The animal was then placed into the cylinder for session 2 (no-cue session), where the cue card was removed from the cylinder in order to encourage navigation based on path integration (the overhead lights remained on throughout the session). At the end of session 2, the animal was placed in the disorientation box and given an intraperitoneal injection of normal saline (1ml/kg body weight) or atropine sulfate. After 15-min confinement to the disorientation box (which was sufficient time for atropine to produce spatial impairments (Whishaw, 1985), the floor paper was changed and the animal was placed into the cylinder for session 3 (drug/no-cue session). With the animal continuing to forage for food pellets within the cylinder, session 4 (drug/visual session) began when the experimenter returned the cue card to its original position in the cylinder. The floor paper was not changed between sessions 3 and 4. At the end of session 4, the rat was removed from the cylinder and placed in the disorientation box. The cue card was rotated 90° CW or CCW and the floor paper was changed prior to session 5 (drug/cue rotation). The rat was then disoriented, the floor paper changed, and the cue card was returned to its original position for session 6 (drug/cue return session). The rat was then disoriented, the cue was removed, and the floor paper was replaced prior to session 7 (second drug/no-cue session).
Figure 1.
Recording procedures used with the cylinder and dual-chamber apparatus. A, The animal was removed from the cylinder and placed in an opaque holding chamber following the first recording session. The floor paper was then changed, the cue card removed, and the animal was disoriented prior to the second session. At the end of the second session, the animal received an IP injection of saline or atropine sulfate, and placed into the holding chamber for 15 min. The animal was then replaced in the cylinder without disorientation. Following session 3, the cue card was inserted while the animal continued to navigate within the cylinder. For all subsequent sessions, the animal was removed and placed into the holding chamber, the floor paper was changed, and the animal was disoriented before the next session.
Dual-Chamber Apparatus
A different group of rats was used for the dual-chamber apparatus test (see Fig. 1B), which used the same apparatus described previously (Stackman et al., 2003; Taube & Burton, 1995; Yoder, Peck, et al., 2015). The dual-chamber apparatus includes a cylindrical chamber (diam. = 76 cm, height = 43.5 cm) connected to a rectangular chamber (51 × 68.5 cm) by an alleyway (width = 15 cm). The cylinder’s cue card was in the 3 o’clock position and the rectangle’s cue card was in the 12 o’clock position, as viewed from the overhead camera. This configuration places the cue card in the rectangle to be 90° rotated relative to the cue card in the cylinder (in room coordinates).
The dual-chamber apparatus procedure began with a Familiar Cylinder recording session (8 min) in the cylinder, after which the door was opened and the rat was permitted to enter the alleyway. The cylinder door was then closed to prevent the rat from returning to the cylinder. A Novel Rectangle session (16 min) then occurred while the rat was confined to the rectangle and/or alleyway. At the end of the Novel Rectangle session, the cylinder door was opened to permit the animal to return to the familiar cylinder. The door was then closed, at which point the Return Cylinder session (4 min) began.
Data Analysis
For all recording sessions, HD was determined by calculating the angle between the x-y coordinates of the two LEDs within a 256 × 256-pixel field. The rats HDs were sorted into sixty 6° bins along with the concurrent spikes. The average firing rate as a function of HD was calculated by dividing the total number of spikes by the amount of time in each bin. Each cell’s preferred firing direction, henceforth referred to simply as “preferred direction,” was defined as the HD bin at which the cell showed the highest mean firing rate.
HD Cell Firing Properties
Firing properties were calculated from the firing rate × HD tuning curve using a triangular model and raw data, as described previously (Stackman & Taube, 1998; Taube et al., 1990a). These firing properties include 1) observed peak firing rate (PFR): the maximal y-coordinate of a single directional bin within the tuning curve; 2) background firing rate (BFR): the mean firing rate in all directions 18° away from the x-intercept of each triangle leg; 3) preferred firing direction: the x-coordinate of the bin with maximal firing rate; 4) directional firing range: the difference, in degrees, between the x-coordinates of the base of the triangle legs.
Anticipatory time interval (ATI)
The ATI was calculated for each HD cell using a time–shift analysis, as reported previously (Blair & Sharp, 1995). Cellular activity during angular head velocities >60°/s in either direction were used for ATI analyses. Cells that were recorded simultaneously with other cells on the same electrode (with overlapping directional firing ranges) were excluded from the ATI analysis.
Directional information content (IC)
The IC was calculated as described previously (Stackman & Taube, 1998). IC = Σpi (λi/λ) log2(λi/ λ), where pi is the probability of the head pointing in the ith bin, λi is the firing rate when the head is pointed within the ith bin, and λ is the overall mean firing rate of the cell for all bins. A directional information content score of 0 indicates no relation between HD and firing rate, and a value above 1 indicates a strong relationship between HD and firing rate. In cases in which multiple HD cells were recorded on the same electrode, directional IC scores were not calculated because λ is potentially biased by the spikes of additional cell(s).
HD Cell Shift Analysis
We compared the tuning curves obtained from both enclosures in order to determine the amount of angular shift exhibited by a HD cell between recording sessions (or between chambers), as described previously (Taube, Muller, & Ranck, 1990b). Briefly, the firing rate vs. HD function for one recording session was shifted in 6° steps and compared with the HD function from another recording session. The amount of shift that produced the maximal correlation between curves was considered to be the amount of preferred direction shift between the two sessions. For recording sessions during which multiple HD cells were recorded simultaneously, the average shift of the preferred directions across cells was used for statistical calculations. This averaging method was used because all available data indicate that when multiple HD cells are recorded simultaneously, the preferred direction for all HD cells rotate in register (Taube et al., 1990b), and we did not want to bias our analyses to sessions in which multiple cells were recorded.
HD Cell Drift Analysis
Some HD cells were “unstable,” or failed to maintain a consistent preferred direction, within a recording session. In these cases, the amount of preferred direction drift was evaluated by calculating the animal’s HD at times when the cell’s firing rate reached 80% of the maximum for that session, plotted across time for the entire recording session. The slope of this HD × firing rate × time plot (°/sec) then served as a measure of the rate at which the cell’s preferred direction drifted, as described previously (Yoder & Taube, 2009).
Statistical Analyses
One goal of the present study was to determine whether the firing properties of HD cells were affected by atropine. We first calculated each HD cell’s firing properties from the standard recording sessions before and after the drug injection: session 1 (pre-injection) and session 4 (post injection). We then calculated the change (Δ) in each firing property between sessions, and compared the mean Δ values between groups. Mean Δ values were compared between groups with separate t-tests, with adjusted df when Levene’s test revealed variance heterogeneity, or with Mann-Whitney U tests when a Shapiro-Wilk test indicated that the distribution was not normally distributed (SPSS; IBM, Armonk, NY or Statview, SAS, Cary, NC).
Preferred direction shifts were evaluated with circular statistics (Oriana; Kovach Computing, Anglesey, UK). The mean angle of the distribution, μ, is referred to as the “mean shift.” The length of the associated vector, r, serves as a measure of concentration. The concentration parameter, κ, was compared between groups to determine whether atropine influenced the variability of preferred direction shifts.
Histology
Rats were anesthetized with sodium pentobarbital (150mg/kg), and one or more electrode tip locations were marked with an ion deposit by passing constant anodal current (15μA, 20sec) through several electrode wire(s). Rats were then perfused through the heart with normal saline followed by 10% formalin. Brains were then placed in 10% formalin containing 2% potassium ferrocyanide for 24–48 h to produce a Prussian blue reaction at the electrode tip locations. After this post-fixation period, brains were placed in 20% sucrose for cryoprotection before they were sectioned at 30μm on a cryostat. Brain sections containing the ADN were mounted on gelatin-coated microscope slides. Brain tissue was then stained with thionin and covered with glass prior to examination under a light microscope.
Results
The sequence of cue manipulations used here is notably more complex than that of previous HD cell studies. Previous studies have typically used five recording sessions (standard, 90° cue rotation, standard, dark/no cue, and standard), with the animal removed from the arena and the floor paper changed between every session. In response to these manipulations, the preferred direction is typically influenced strongly by the position of the visual cue card; for example, see (Yoder, Peck, et al., 2015). We challenged this dominant landmark control of HD cell activity in the present study by using several sequential recording sessions where the cue card was absent (sessions 2 and 3), and the cue was replaced while the animal remained in the cylinder. Thus, as intended, the response of control HD cells in terms of their preferred directions, showed more variability to these manipulations than in previous studies. The increased variability of HD cell preferred directions in control animals suggests that the present recording sequence was successful at challenging the visual cue card’s influence on the HD signal.
HD Cell Firing Properties
Table 1 shows the firing properties calculated from HD cells recorded during sessions 1 (pre-injection) and 4 (post injection). Interestingly, the mean peak and background firing rates were significantly greater in atropine rats than in controls for session 1, peak firing rate: t(31.4) = 3.50, p < .01 (df adjusted for unequal variances); background firing rate: t(21.3) = 3.15, p < .01 (df adjusted for unequal variances). In both cases, however, the ranges of peak firing rates showed considerable overlap. This difference may have resulted from a greater number of cells with high peak firing rates, as well as the somewhat greater upper limit on the range of firing rates for the atropine group.
Table 1.
HD Cell Firing Characteristics
| Control Rats | Session | |||
|---|---|---|---|---|
|
| ||||
| 1 (pre-injection) | 4 (post-injection) | |||
|
| ||||
| Parameter | Mean (SEM) | Range | Mean (SEM) | Range |
| Peak Firing Rate (Hz) | 26.0 (4.12) | 6.78–76.7 | 25.0 (3.57) | 6.19–65.5 |
| Background Firing Rate (Hz) | .39 (.06) | .02–1.04 | .73 (.23) | .03–4.29 |
| Directional Firing Range (°) | 101.0 (6.48) | 66.3–159.7 | 99.8 (5.47) | 67.6–148.7 |
| Directional Information Content (bits) | 1.52 (.14) | .55–2.72 | 1.38 (.17) | .45–2.76 |
| Anticipatory Time Interval (ms) | 49.0 (5.34) | 6.64–97.2 | 61.2 (8.33) | .69–133.7 |
|
| ||||
| Atropine Rats | Session | |||
|
| ||||
| 1 (pre-injection) | 4 (post-injection) | |||
|
| ||||
| Parameter | Mean (SEM) | Range | Mean (SEM) | Range |
|
| ||||
| Peak Firing Rate (Hz) | 55.24 (7.29)* | 6.99–125.9 | 36.5 (6.11)† | 8.32–113.6 |
| Background Firing Rate (Hz) | 1.50 (.35)* | .12–5.3 | 1.51 (.30) | .09–4.06 |
| Directional Firing Range (°) | 107.6 (9.75) | 64.9–230.9 | 124.8 (11.1) | 20.1–220.5 |
| Directional Information Content (bits) | 1.20 (.11) | .31–2.07 | 1.03 (.09) | .37–1.95 |
| Anticipatory Time Interval (ms) | 47.8 (5.12) | 16.3–100.3 | 57.8 (6.00) | 12.4–89.2 |
p < .05, relative to control group
p < .05, relative to Session 1
Table 2 shows the change in firing properties resulting from systemic drug injection. Only the change in peak firing rate was significantly different between groups, t(26.0) = 2.69, p = .012 (df adjusted for unequal variances). The peak firing rate was lower in session 4 compared to session 1 for the atropine group, but not for the control group. It is possible that this reduction occurred because the directional firing range was somewhat (albeit not significantly) broader in the atropine group. Even a small decrease in the stability of the cell’s preferred direction – caused by drift of the preferred direction within a session – could lead to a decrease in the cell’s peak firing rate. However, the mean absolute drift of the preferred direction within session 4 was not significantly different between groups, control mean .108 ± .03°/sec; atropine mean = .107 ± .02°/sec; Mann-Whitney U = 184, p = .665. A second possibility is that each HD cell in the atropine group exhibited a relatively broad range of preferred directions. We therefore calculated the standard deviation of the range in which the cell’s firing rate exceeded 80% of the maximum rate for that session. However, the standard deviation values were not significantly different between groups, control mean = 44.9 ± 3.26°; atropine mean = 42.78 ± 2.98°; t(38) = .488, p = .628. Thus, atropine appeared to reduce the peak firing rate of HD cells without disrupting the cell’s stability within a recording session.
Table 2.
Mean (SEM) Change in HD Cell Firing Characteristics from Session 1 to Session 4
|
|
|||||
|---|---|---|---|---|---|
| Condition (n) | ΔPFR | ΔBG | ΔATI | ΔDFR | ΔIC |
| Control (20) | −1.01 (2.46) | .329 (.232) | 11.7 (6.09) | −1.22 (2.65) | −.144 (.116) |
| Atropine (19) | −16.2 (5.09) | .036 (.235) | 3.99 (6.94) | 20.1 (11.2) | −.219 (.120) |
| t-test p value | < .01 | .38 | .41 | .06 | .66 |
Landmark Rotation Test
We evaluated the stability of the preferred direction across sessions by comparing the angular distance between the preferred direction values from each HD cell in sessions 1 and 4, which were separated by two ‘no cue’ sessions and the saline/drug injection. Most control cells (12 out of 15, or 80%) had similar preferred directions in sessions 1 and 4, although the preferred direction of several cells shifted randomly following the no cue sessions, μ = .15 ± 13.4° (see example in Fig. 2, left; overall distribution in Fig. 3B, left). Despite the fact that many cells in the atropine group had greater directional firing ranges than control cells, most of the atropine cells (12 out of 16, or 75%) had preferred directions that also shifted very little between sessions 1 and 4, although several cells shifted their preferred directions randomly, μ = −5.04 ± 12.4° (see example in Fig. 2, right; overall distribution in Fig. 3B, right). The distribution of preferred direction shifts did not differ significantly between groups, Watson-Williams F(1, 29) = .073, p = .79. Muscarinic receptor blockade thus failed to disrupt HD cells’ stable representation of directional heading across recording sessions, even though the sessions were separated by two recording sessions where no visual cue card was available.
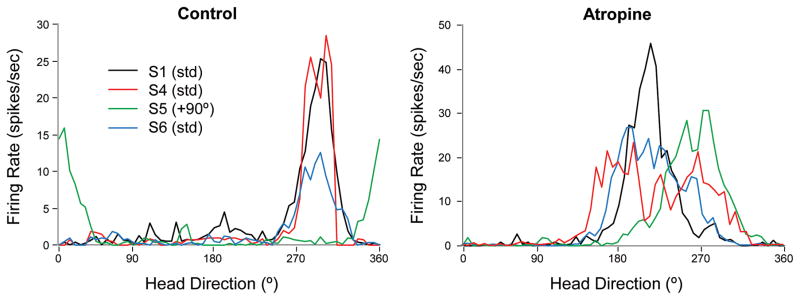
Figure 2.
Representative tuning curves for HD cells in control (left) and atropine (right) conditions. For HD cells in both groups, the preferred direction remained consistent across standard recording sessions and shifted in the direction of the cue card during the rotation session. However, atropine treatment resulted in a significantly decreased peak firing rate and somewhat broader tuning curves, although this increased directional firing range was not significant.
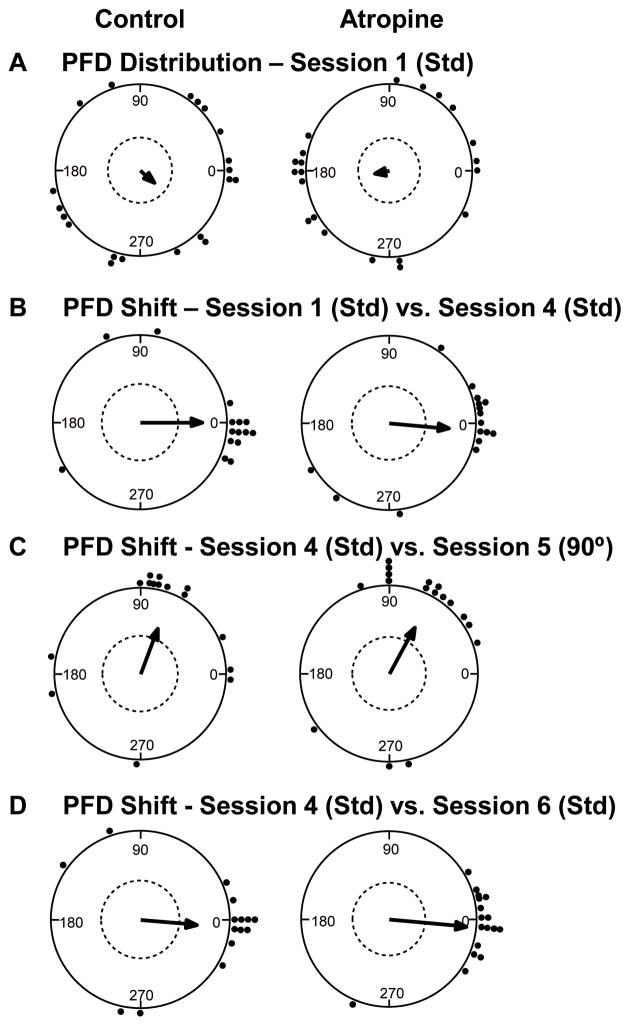
Figure 3.
HD cell response to cue card manipulations in the cylinder. A) The preferred directions of HD cells in both control (left) and atropine (right) groups initially showed a random distribution during the baseline recording session. B) The preferred direction remained relatively stable and well aligned with the cue card between standard (Std) sessions for both control and atropine groups. C) 90° rotation of the cue card resulted in a similar shift of the preferred direction for HD cells in both groups. D) The preferred firing direction returned to the original alignment for both groups, after the cue card was returned to its original position during the subsequent standard recording session.
Recording session 5 tested the HD cells’ response to 90° cue rotation. Perfect landmark control of the HD signal is indicated by a 90° shift of the preferred direction, relative to the previous recording session where the cue was located in the standard position. Most control HD cells were strongly influenced by the position of the cue card, although some cells shifted randomly (as expected, given the insertion of the cue card while the animal was in the cylinder at the beginning of session 4). Overall, the control group showed an under-rotation, μ = 69.3 ± 17.4° (see example in Fig. 2, left; overall distribution in Fig. 3C, left). The atropine HD cells also showed an under-rotation, μ = 61.7 ± 14.8° (see example in Fig. 2, right; overall distribution in Fig. 3C, right). These shifts did not differ significantly between groups, Watson-Willams F(1, 30) = 0.114, p = .74. Thus, systemic atropine does not appear to have had a significant effect on HD cells’ response to 90° cue rotation.
Perfect landmark control of the HD signal would also be reflected by a 0° shift of the preferred direction in session 6, relative to session 4. Overall, the preferred direction of control HD cells remained relatively consistent across standard recording sessions, μ = −5.46 ± 15.0° (see example in Fig. 3, left; overall distribution in Fig. 3D, left). However, the distribution of preferred direction shifts was somewhat more variable than in previous studies, suggesting the present rotation sequence successfully challenged the visual cue card’s control of the HD signal for some cells. The atropine group also showed a relatively consistent response to the landmark position, μ = −5.64 ± 7.0° (see example in Fig. 2, right; overall distribution in Fig. 3D, right). The distributions of preferred direction shifts did not differ significantly between groups, Watson-Willams F(1, 30) = 0.0, p = .99. Thus, systemic atropine failed to significantly affect HD cells’ response to the visual cue card.
A potential issue with the present experiment is the tendency for atropine-treated animals to exhibit hyperactivity (Watanabe, Watanabe, & Hagino, 1978), which could affect HD cell activity and/or attention to visual cues. We therefore evaluated the animals’ movement during the first standard recording session post-injection (session 4). Atropine treatment was associated with significantly greater distances than control rats. The control group traveled mean = 1,942.7 ± 206.4 cm (range = 812.9 – 3,683.1 cm) during the recording session, whereas the atropine group traveled mean = 3,276.0 ± 383.8 cm (range = 1,534.4 – 5,659.0 cm). The distance was significantly greater for the atropine group than the control group, t(29) = 3.00, p = .006. The greater distance traveled, however, was not associated with a greater number of head turns. For the control group, the mean number of head turns, defined as ≥10 continuous samples (.6 sec) with angular velocity > 0 deg/sec in one direction, was mean = 29.7 ± 2.64 (range = 15.7 – 52.5). The mean number of head turns for the atropine group was mean = 32.4 ± 2.09 (range = 16.2 – 46.4). These values were not significantly different, t(29) = .81, p = .43. Thus, although atropine-treated rats moved considerably more than control rats during the recording session (in terms of total distance traveled), this hyperactivity was not associated with an increase in the number of head turns, or with altered HD cell stability within the recording session.
No-cue Recording Sessions
Many HD cells appeared to show reduced directionality during the no-cue sessions, relative to visual cue sessions. This reduced directionality appears to have resulted from a “drifting” preferred direction during the recording session, and comparison of the preferred direction shift between cue and no-cue sessions is therefore inappropriate. We therefore evaluated the drift of the cell’s preferred direction within each no-cue session, as a measure of the HD signal’s reliance on other cues such as path integration. Separate t-tests were used to compare the drift values between groups, within each of the three no-cue recording sessions. As with the preferred direction shift analyses, we averaged the drift values when multiple HD cells were recorded simultaneously from the same rat.
Control HD cells exhibited an absolute preferred direction drift of mean = .079 ± .018°/sec during the first no-cue session, session 2 (pre-injection). The atropine group showed a similar amount of drift in session 2, mean = .075 ± .023°/sec. These values were not significantly different between groups, t(31) = −.15, p = .88. Session 3 (post-injection) revealed a slight increase in the preferred direction drift for HD cells in animals that received saline injection, mean = .112 ± .030°/sec, as well as in those animals that received atropine injection, mean = .146 ± .035°/sec. These values did not differ significantly between groups, t(30) = .720, p = .48, suggesting that atropine failed to affect the stability of HD cell activity when no visual cue card was present. For the no-cue session following the rotation sequence (session 7), the preferred direction drift for the control group was mean = .105 ± .027°/sec. For the atropine group, the preferred direction drift was mean = .237 ± .083°/sec. These values did not differ significantly between groups, t(30) = 1.35, p = .19. Thus, muscarinic receptor blockade failed to significantly disrupt HD cell stability in a cue-poor visual environment.
Dual-Chamber Apparatus
Several previous studies have demonstrated that ADN and postsubiculum HD cell preferred directions are generally stable as animals navigate from the familiar to the novel enclosure within the dual-chamber apparatus (Golob & Taube, 1999; Stackman et al., 2003; Taube & Burton, 1995). We recently found that HD cells in the lateral mammillary nuclei respond similarly, as rats navigated within the same dual chamber apparatus used in previous studies (Yoder et al., 2015). We therefore combined the previously reported HD cells recorded from the ADN (n=7; Taube & Burton, 1995) and lateral mammillary nuclei (n=4; Yoder, Peck, et al., 2015) as a control group, for comparison to data from atropine-treated animals. We note that these control animals did not receive intraperitoneal injection, which is different from the atropine animals. Intraperitoneal injection is associated with a stress response that could have influenced HD cell function. However, the injection occurred 15 min before the start of the familiar cylinder session (4–8 min), during which time HD cell activity (e.g., peak firing rate, directional firing range, PFD stability, background firing rate) was similar to that of uninjected animals. The total time between injection and traversal of the dual chamber apparatus was therefore 19–23 min, which may have been sufficient to eliminate stress-induced changes from the drug injection on HD cell activity during path integration. Further, the atropine rats did not exhibit signs of anxiety (thigmotaxis, defecation, stretch attend posture, etc.) after injection, and readily entered the novel alley and arena. The behavioral characteristics of anxiety were therefore absent, suggesting the stress associated with intraperitoneal injection did not persist during the following recording session. Nevertheless, it is important to consider the possible influence of the atropine injection procedure on HD cell activity for the atropine group.
HD cell activity was initially recorded while the rat was confined to the cylinder containing a prominent white cue card, and the preferred direction exhibited during this session was used as a reference for all comparisons. Following the Familiar Cylinder recording session, the door was opened to allow the rat to exit the cylinder and walk through the alley to the novel rectangle.
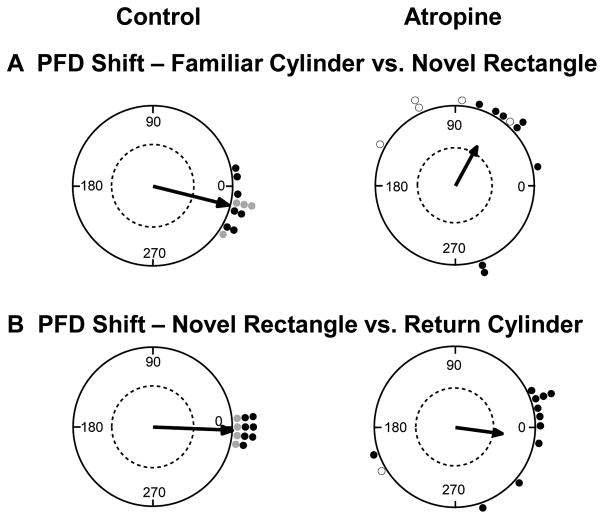
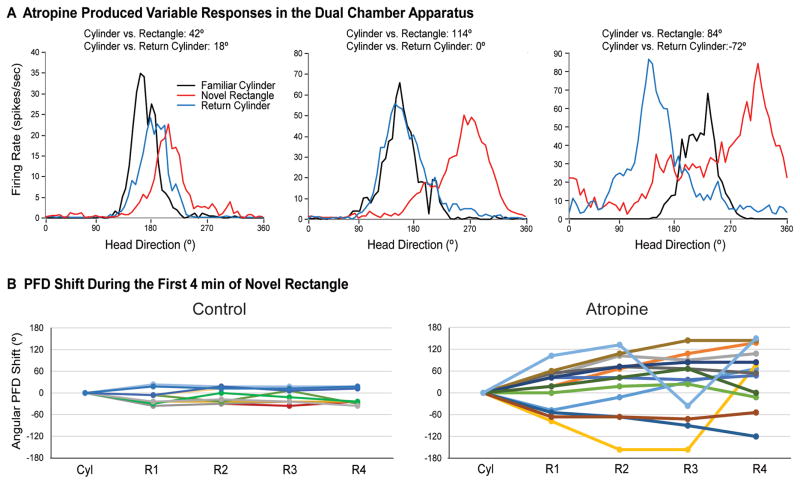
For control HD cells (n=11), the preferred directions remained relatively stable between the Familiar Cylinder and Novel Rectangle sessions, μ(11) = −14.2 ± 4.9°, |range| = 0–36°, κ = 12.7 (Fig. 4A, left). The atropine group showed a more variable response, with five of the 13 HD cells having a shift in the preferred direction shift of > 90° in the rectangle (discussed in detail below). Nevertheless, we included these five cells to calculate the mean preferred direction shift for the atropine group, μ(13) = 60.1 ± 19.1°, |range| = 12–150°, κ = 1.24 (Fig. 4A, right; cells that shifted > 90° within the session are indicated with open points). Note that the distribution of shifts for the atropine group had low concentration. The distributions of preferred direction shifts were significantly different between groups, Watson-Williams F(1,22) = 14.9, p < .01. Thus, the control group of HD cells maintained a consistent preferred direction across the familiar and novel arenas, even though the cue card was transposed 90° CCW in the novel arena, relative to the familiar arena. In contrast, atropine interestingly caused a significant preferred direction shift toward the cue card, along with increased variability.
Figure 4.
Preferred firing direction (PFD) shifts in the dual chamber apparatus. A) Control HD cells (left) showed a relatively consistent preferred direction as the animal walked from the cylinder to the novel rectangle, suggesting the HD signal was maintained by path integration. In contrast, most HD cells in the atropine group (right) shifted toward the visual cue card (90° CCW), suggesting path integration failed to maintain the HD signal between arenas, and that the cue card in the rectangle may have driven the shift in the cells’ PFDs. B) Upon return to the familiar cylinder, control HD cells (left) realigned to the cue card in the cylinder. Atropine HD cells (right) also realigned to the cue card, albeit with less precision than the control group. Dashed line indicates .05 significance criterion. Open circles represent HD cells that shifted > 90° within the recording session. For the control group, black points represent HD cells recorded from the ADN (Taube & Burton, 1995) and gray points represent HD cells recorded from the lateral mammillary nuclei (Yoder et al., 2015).
The Return Cylinder session began when the animal exited the novel rectangle and walked back through the alley to the cylinder. Relative to the preferred direction exhibited during the first recording session in the cylinder, control cells showed a mean shift of μ(11) = −2.2 ± 2.2°, |range| = 0–12°, κ = 59.9 (Fig. 4B, left), demonstrating dominant reliance on the visual cue card. For the atropine group, isolation of single-unit activity was lost for one cell, but the remaining cells showed a small mean shift in the preferred direction of μ(12) = −8.11 ± 18.7°, |Range| = 0–162°, κ = 1.34 (Fig. 4B, right). Note that the distribution of shifts for the atropine group had a much lower concentration, compared to control cells. The distribution of preferred direction shifts for the atropine cells was not significantly different compared to the control cells, Watson-Williams F(1,21) = 0.109, p = .75. Thus, atropine failed to disrupt HD cells’ reliance on a visual landmark across recording sessions, although the atropine group showed considerably more variability than the control group.
The atropine group’s response to the novel rectangle varied across cells. First, upon entry to the novel rectangle, some cells in the atropine group shifted their preferred direction in the direction of the visual cue card, and then shifted to an intermediate amount when the animal returned to the cylinder (i.e., the cell’s preferred direction shifted to a value in between its initial orientation in the cylinder and its orientation in the rectangle) (Fig. 5A, left). Other cells were strongly influenced by the cue card in the novel rectangle, and then realigned accurately during the return cylinder session (Fig. 5A, center). A third group of cells shifted toward the rotated cue card in the novel rectangle, but then over-rotated when the animal returned to the cylinder (Fig. 5A, right). Importantly, five of the 13 cells (31%) in the atropine group showed considerably broader tuning curves during the Novel Rectangle session, relative to the Familiar Cylinder session. This increased directional firing range appears to have been caused by a drift or shift of the preferred direction of > 90° during the recording session (Fig. 5B, right), which contrasts with the relative stability for all control cells (Fig. 5B, left). This instability disappeared for four of the five unstable cells when the animals returned to the cylinder; only one HD cell showed a preferred direction shift or drift > 60° in the Return Cylinder session. Any of these responses could be expected if self-movement information is unable to dominantly control HD cell activity during navigation into the novel environment. However, it is unclear whether the broader tuning curves in the novel rectangle resulted from impaired path integration or from another factor, given that HD cells also had broader tuning curves during the standard recording session following atropine injection (reported above for the cue rotation procedure). Thus, atropine treatment appears to have disrupted the use of self-movement cues to maintain a consistent preferred direction between familiar and novel environments, and may reduce HD cells’ robust representation of head direction.
Figure 5.
Atropine treatment produced variable effects on HD cell activity during performance in the dual chamber apparatus. A) Some cells were influenced by the visual cue card, and then shifted to an intermediate direction upon return to the cylinder (left); other cells were influenced by the cue card and then returned precisely to the original alignment upon return (center); other cells drifted or were otherwise unstable within the rectangle, resulting in broad tuning curves (right). B) Temporal dynamics of HD cell activity in the novel rectangle. Control HD cells (left) remained relatively stable across the cylinder (Cyl) and the first four min (R1–R4) of the novel rectangle. In contrast, the preferred direction of many cells in the atropine group (right) drifted during the first four min of the rectangle session, relative to the cylinder.
Overall, muscarinic receptor blockade had the greatest effect on the use of idiothetic cues to maintain the HD signal during navigation into a novel environment. Atropine also produced greater variability in the landmark control of HD cells when the animals returned to the familiar cylinder. These results suggest that the familiar geometry and/or cue position had a greater influence on HD cell activity than path integration.
Discussion
We conducted two tests to evaluate acetylcholine’s contribution to HD cell function. A series of recording sessions that involved cue removal and cue rotation revealed that systemic muscarinic receptor blockade had little effect on the landmark control of HD cells when the animal was located in the same arena. However, systemic muscarinic receptor blockade impaired idiothetic cue control of the HD signal during navigation between familiar and novel arenas of the dual-chamber apparatus. These results are consistent with behavioral studies that demonstrate an important role for acetylcholine in path integration, but not in landmark-based navigation.
Landmark-Based Navigation
Many studies of learning in rodents have used landmark-based navigation as a model memory system to evaluate the effects of brain damage or pharmaceutical manipulations. Two such tasks are the radial arm maze (Olton & Samuelson, 1976) and the Morris water maze (Morris, 1981), both of which require animals to find goal locations defined by their position(s) relative to distal visual landmarks. One brain mechanism that appears to contribute to this type of navigation is the HD signal, which provides a neural representation of the animal’s heading within the environment. Indeed, damage to the postsubiculum, which is thought to contribute to landmark processing and convey the HD signal to higher brain regions, impairs performance on both of these tasks (Taube, Kesslak, & Cotman, 1992). Performance on these tasks also appears to involve acetylcholine, as systemic muscarinic receptor blockade disrupts performance on both mazes (Decker & Gallagher, 1987; Whishaw, 1985). This cholinergic involvement was originally thought to depend on the septohippocampal projection, but selective damage to medial septal cholinergic cells with 192 IgG-saporin, which effectively eliminates cholinergic input to the hippocampus, failed to disrupt spatial learning or produced only mild impairments (see Parent & Baxter, 2004 for review). Another possibility is that the effects of systemic atropine occur at the postsubiculum, given that it is the first point in the ascending HD cell circuit known to receive cholinergic input from the basal forebrain (Gaykema et al., 1990). Because the postsubiculum is necessary for the landmark control of the HD signal in ADN and lateral mammillary nuclei (Goodridge and Taube, 1997; Yoder et al., 2011b; Yoder, Peck, et al., 2015), this site of action may disrupt landmark control of HD cells in ADN. However, our results suggest that muscarinic receptor activation, whether in the postsubiculum or elsewhere, is not necessary for landmark control of HD cells. Thus, along with the findings of behavioral studies, the lack of an atropine effect on landmark control of the HD signal suggests that muscarinic receptor antagonists impair landmark-based navigation via brain regions other than the postsubiculum.
A candidate brain region for atropine’s influence on landmark-based navigation is the entorhinal cortex. Muscarinic receptor blockade with systemic scopolamine or inactivation of the medial septum, which eliminates acetylcholine input to many regions including the entorhinal cortex, disrupted the firing pattern of many entorhinal grid cells (Brandon et al., 2011; Koenig et al., 2011; Newman et al., 2014). However, the directional selectivity of many HD cells and grid × HD cells was not affected with these manipulations, suggesting a crucial cholinergic contribution to the grid component of entorhinal grid cell activity, but not to the directional component. This interpretation is also consistent with the view that the HD signal contributes to the activity of grid cells, but other signals are also necessary for the grid pattern of activity. One possibility is that acetylcholine contributes to grid cell function via hippocampal theta rhythm (Dannenberg, Hinman, & Hasselmo, 2016), which is abolished by medial septal lesion and reduced by damage to cholinergic or GABAergic cells (Lee, Chrobak, Sik, Wiley, & Buzsaki, 1994; Winson, 1978; Yoder & Pang, 2005). The disrupted grid cell function following medial septal inactivation could therefore have resulted from the removal of cholinergic or non-cholinergic input to entorhinal cortex, or the disruption of theta rhythm.
Path Integration
The use of idiothetic cues for navigation depends on the medial septal cholinergic system. Rodents are able to use idiothetic cues to determine their current position and to return directly to a starting point (Mittelstaedt and Mittelstaedt, 1980). In terms of the directional component of path integration, this ability may involve the HD signal, given that idiothetic information is sufficient to maintain HD signal stability when the animal locomotes in complete darkness or from a familiar environment into a novel environment, where no familiar visual cues are available (Stackman et al., 2003; Taube & Burton, 1995; Yoder, Clark, Brown, et al., 2011). Involvement of the medial septal cholinergic system in idiothetic cue processing is indicated by impaired homing performance after fornix damage or injection of 192 IgG-saporin into the medial septum (Martin & Wallace, 2007; Whishaw, Hines, & Wallace, 2001). One possibility is that acetylcholine’s contribution to self-movement cue processing is limited to vestibular signaling in the hippocampal formation, given the reduction of muscarinic receptors following bilateral vestibular damage (Aitken et al., 2016). However, the present results suggest that acetylcholine is also important for idiothetic cue maintenance of the HD signal, as atropine disrupted preferred direction stability during navigation into a novel environment.
A caveat to this interpretation is that path integration may differentially depend on cholinergic function when the animal is performing exploratory versus goal-directed behaviors. Navigating from the familiar cylinder to the novel rectangle in the dual-chamber task is purely exploratory, motivated only by curiosity. Atropine disrupted the use of self-movement cues to maintain HD signal stability between chambers, suggesting acetylcholine is necessary for path integration during exploratory navigation. In contrast, the no-cue recording sessions cannot be considered purely exploratory, given that the rats were searching for food within a familiar arena. However, these no-cue sessions cannot be considered purely goal-directed, given that the location of the reward was not consistent within the trial. Regardless, atropine failed to disrupt HD signal stability within the no-cue trials, suggesting that acetylcholine may not be necessary for the use of path integration within a familiar environment devoid of prominent visual cues. Alternatively, it’s possible that visual cues other than the cue card – such as the overhead food dispenser – facilitated HD cell stability, given that the overhead lights remained on during these sessions. Yet another possibility is that atropine disrupted the grid cell signal in the medial entorhinal cortex, given that systemic muscarinic receptor blockade is known to interfere with the grid signal (Newman et al., 2014). Because the grid cell signal is thought to be important for path integration (Moser et al., 2008), disruption of it could have led to the impaired spatial updating of the HD signal as the animals locomoted to the novel chamber. Finally, it is worth noting that the impaired path integration in the dual-chamber apparatus could have been produced by peripheral actions of atropine, given that the drug was administered systemically. However, we feel that this possibility is unlikely, given that the effect of atropine on HD cells was limited to the dual-chamber apparatus. Overall, the present results suggest that acetylcholine may differentially contribute to path integration in goal directed versus exploratory tasks, or in novel vs. familiar environments.
Acetylcholine’s influence on the HD signal may involve the medial septal projection to postsubiculum or hippocampus. The medial septum projects to various navigation-related brain structures, including the retrosplenial cortex, entorhinal cortex, postsubiculum, and hippocampus (Gaykema et al., 1990). Damage to the retrosplenial or entorhinal cortex disrupted idiothetic cue-based navigation (Cooper & Mizumori, 1999; Van Cauter et al., 2013; Whishaw, Maaswinkel, Gonzalez, & Kolb, 2001). In contrast, retrosplenial cortex lesions only mildly disrupted HD signal stability in darkness, and entorhinal cortex lesions failed to disrupt thalamic HD signal stability in darkness (Clark, Bassett, Wang, & Taube, 2010; Clark & Taube, 2011). Further, both of these lesions failed to impair HD signal stability during navigation between the familiar and novel arenas of the dual-chamber apparatus. Because the HD signal remained stable following retrosplenial or entorhinal cortex lesions, these regions are unlikely to be crucial for acetylcholine’s influence on the HD signal. In contrast, both of the other two regions, the postsubiculum and hippocampus, contribute to navigation based on idiothetic cues and are necessary for HD signal stability in the dual-chamber apparatus (Golob & Taube, 1999; Valerio, Clark, Yoder, & Taube, 2011; Yoder, Peck, et al., 2015) but see (Bett, Wood, & Dudchenko, 2012). Thus, although acetylcholine influences many brain regions, only the postsubiculum and hippocampus are known to be involved in HD signal stability and performance on idiothetic cue-based navigation tasks.
Conclusion
We evaluated the effects of systemic muscarinic receptor blockade on HD cell activity in several environmental conditions, in order to determine whether acetylcholine facilitates the flexible switch between allothetic and idiothetic cues for navigation. In a series of cue rotation/cue removal sessions that challenged the use of allothetic cues, muscarinic receptor blockade only mildly affected HD cell activity. However, muscarinic receptor blockade produced a marked deficit in HD cell stability based on idiothetic cues during navigation from a familiar to a novel environment. These results suggest that the behavioral effects of cholinergic lesions, the greatest of which occur during idiothetic cue-based navigation, may involve deficits in the stability of the HD signal.
Acknowledgments
This research was supported by grant #NS053907 from NINDS.
Footnotes
These data were previously presented in an honors thesis (J. Chan) and at the annual meeting of the Society for Neuroscience.
Contributor Information
Ryan M. Yoder, Dartmouth College
Jeremy H.M. Chan, Dartmouth College
Jeffrey S. Taube, Dartmouth College
References
- Aitken P, Benoit A, Zheng Y, Philoxene B, Le Gall A, Denise P, … Smith PF. Hippocampal and striatal M1 -muscarinic acetylcholine receptors are down-regulated following bilateral vestibular loss in rats. Hippocampus. 2016;26:1509–1514. doi: 10.1002/hipo.22651. [DOI] [PubMed] [Google Scholar]
- Bassett JP, Tullman ML, Taube JS. Lesions of the tegmentomammillary circuit in the head direction system disrupt the head direction signal in the anterior thalamus. J Neurosci. 2007;27:7564–7577. doi: 10.1523/JNEUROSCI.0268-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, Bucci DJ, Sobel TJ, Williams MJ, Gorman LK, Gallagher M. Intact spatial learning following lesions of basal forebrain cholinergic neurons. Neuroreport. 1996;7:1417–1420. doi: 10.1097/00001756-199605310-00019. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Gallagher M. Intact spatial learning in both young and aged rats following selective removal of hippocampal cholinergic input. Behav Neurosci. 1996a;110:460–467. doi: 10.1037//0735-7044.110.3.460. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Gallagher M. Neurobiological substrates of behavioral decline: models and data analytic strategies for individual differences in aging. Neurobiol Aging. 1996b;17:491–495. doi: 10.1016/0197-4580(96)00011-5. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Holland PC, Gallagher M. Disruption of decrements in conditioned stimulus processing by selective removal of hippocampal cholinergic input. J Neurosci. 1997;17:5230–5236. doi: 10.1523/JNEUROSCI.17-13-05230.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, Bucci DJ, Gorman LK, Wiley RG, Gallagher M. Selective immunotoxic lesions of basal forebrain cholinergic cells: effects on learning and memory in rats. Behav Neurosci. 1995;109:714–722. doi: 10.1037//0735-7044.109.4.714. [DOI] [PubMed] [Google Scholar]
- Berger-Sweeney J, Heckers S, Mesulam MM, Wiley RG, Lappi DA, Sharma M. Differential effects on spatial navigation of immunotoxin-induced cholinergic lesions of the medial septal area and nucleus basalis magnocellularis. J Neurosci. 1994;14:4507–4519. doi: 10.1523/JNEUROSCI.14-07-04507.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bett D, Wood ER, Dudchenko PA. The postsubiculum is necessary for spatial alternation but not for homing by path integration. Behav Neurosci. 2012;126:237–248. doi: 10.1037/a0027163. [DOI] [PubMed] [Google Scholar]
- Blair HT, Sharp PE. Anticipatory head direction signals in anterior thalamus: evidence for a thalamocortical circuit that integrates angular head motion to compute head direction. J Neurosci. 1995;15:6260–6270. doi: 10.1523/JNEUROSCI.15-09-06260.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon MP, Bogaard AR, Libby CP, Connerney MA, Gupta K, Hasselmo ME. Reduction of theta rhythm dissociates grid cell spatial periodicity from directional tuning. Science. 2011;332:595–599. doi: 10.1126/science.1201652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazhnik ES, Muller RU, Fox SE. Muscarinic blockade slows and degrades the location-specific firing of hippocampal pyramidal cells. J Neurosci. 2003;23:611–621. doi: 10.1523/JNEUROSCI.23-02-00611.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calton JL, Stackman RW, Goodridge JP, Archey WB, Dudchenko PA, Taube JS. Hippocampal place cell instability after lesions of the head direction cell network. J Neurosci. 2003;23:9719–9731. doi: 10.1523/JNEUROSCI.23-30-09719.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell J, McMahon R, Chiba A, Gallagher M. A re-examination of the role of basal forebrain cholinergic neurons in spatial working memory. Neuropharmacol. 1998;37:481–487. doi: 10.1016/s0028-3908(98)00032-x. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Bassett JP, Wang S, Taube JS. Impaired head direction cell representation in the anterodorsal thalamus after lesions of the retrosplenial cortex. J Neurosci. 2010;30:5289–5302. doi: 10.1523/JNEUROSCI.3380-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BJ, Taube JS. Intact landmark control and angular path integration by head direction cells in the anterodorsal thalamus after lesions of the medial entorhinal cortex. Hippocampus. 2011;21:767–782. doi: 10.1002/hipo.20874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper BG, Mizumori SJ. Retrosplenial cortex inactivation selectively impairs navigation in darkness. Neuroreport. 1999;10:625–630. doi: 10.1097/00001756-199902250-00033. [DOI] [PubMed] [Google Scholar]
- Dannenberg H, Hinman JR, Hasselmo ME. Potential roles of cholinergic modulation in the neural coding of location and movement speed. J Physiol Paris. 2016;110:52–64. doi: 10.1016/j.jphysparis.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker MW, Gallagher M. Scopolamine-disruption of radial arm maze performance: modification by noradrenergic depletion. Brain Res. 1987;417:59–69. doi: 10.1016/0006-8993(87)90179-x. 0006-8993(87)90179-X [pii] [DOI] [PubMed] [Google Scholar]
- Dornan WA, McCampbell AR, Tinkler GP, Hickman LJ, Bannon AW, Decker MW, Gunther KL. Comparison of site-specific injections into the basal forebrain on water maze and radial arm maze performance in the male rat after immunolesioning with 192 IgG saporin. Behav Brain Res. 1996;82:93–101. doi: 10.1016/s0166-4328(97)81112-6. [DOI] [PubMed] [Google Scholar]
- Dwyer TA, Servatius RJ, Pang KC. Noncholinergic lesions of the medial septum impair sequential learning of different spatial locations. J Neurosci. 2007;27:299–303. doi: 10.1523/JNEUROSCI.4189-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckerman DA, Gordon WA, Edwards JD, MacPhail RC, Gage MI. Effects of scopolamine, pentobarbital, and amphetamine on radial arm maze performance in the rat. Pharmacol Biochem Behav. 1980;12:595–602. doi: 10.1016/0091-3057(80)90194-x. [DOI] [PubMed] [Google Scholar]
- Frohardt RJ, Bassett JP, Taube JS. Path integration and lesions within the head direction cell circuit: comparison between the roles of the anterodorsal thalamus and dorsal tegmental nucleus. Behav Neurosci. 2006;120:135–149. doi: 10.1037/0735-7044.120.1.135. [DOI] [PubMed] [Google Scholar]
- Galani R, Lehmann O, Bolmont T, Aloy E, Bertrand F, Lazarus C, … Cassel JC. Selective immunolesions of CH4 cholinergic neurons do not disrupt spatial memory in rats. Physiol Behav. 2002;76:75–90. doi: 10.1016/s0031-9384(02)00674-1. [DOI] [PubMed] [Google Scholar]
- Gaykema RPA, Luiten PGM, Nyakas C, Traber J. Cortical projection patterns of the medial septum-diagonal band complex. J Comp Neurol. 1990;293:103–124. doi: 10.1002/cne.902930109. [DOI] [PubMed] [Google Scholar]
- Golob EJ, Taube JS. Head direction cells in rats with hippocampal or overlying neocortical lesions: evidence for impaired angular path integration. J Neurosci. 1999;19:7198–7211. doi: 10.1523/JNEUROSCI.19-16-07198.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodridge JP, Taube JS. Preferential use of the landmark navigational system by head direction cells in rats. Behav Neurosci. 1995;109:49–61. doi: 10.1037//0735-7044.109.1.49. [DOI] [PubMed] [Google Scholar]
- Goodridge JP, Taube JS. Interaction between the postsubiculum and anterior thalamus in the generation of head direction cell activity. J Neurosci. 1997;17:9315–9330. doi: 10.1523/JNEUROSCI.17-23-09315.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin AS, Windels F, Boskovic Z, Sah P, Coulson EJ. Lesions of the basal forebrain cholinergic system in mice disrupt idiothetic navigation. PLoS One. 2013;8:e53472. doi: 10.1371/journal.pone.0053472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler DJ, Olton DS, Wenk GL, Coyle JT. Lesions in nucleus basalis magnocellularis and medial septal area of rats produce qualitatively similar memory impairments. J Neurosci. 1985;5:866–873. doi: 10.1523/JNEUROSCI.05-04-00866.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga Y, Iwasaki T. Effects of cholinergic and monoaminergic antagonists and tranquilizers upon spatial memory in rats. Pharmacol Biochem Behav. 1984;20:205–207. doi: 10.1016/0091-3057(84)90243-0. [DOI] [PubMed] [Google Scholar]
- Janis LS, Glasier MM, Fulop Z, Stein DG. Intraseptal injections of 192 IgG saporin produce deficits for strategy selection in spatial-memory tasks. Behav Brain Res. 1998;90:23–34. doi: 10.1016/s0166-4328(97)00078-8. [DOI] [PubMed] [Google Scholar]
- Kelsey JE, Landry BA. Medial septal lesions disrupt spatial mapping ability in rats. Behav Neurosci. 1988;102:289–293. doi: 10.1037//0735-7044.102.2.289. [DOI] [PubMed] [Google Scholar]
- Kelsey JE, Vargas H. Medial septal lesions disrupt spatial, but not nonspatial, working memory in rats. Behav Neurosci. 1993;107:565–574. doi: 10.1037//0735-7044.107.4.565. [DOI] [PubMed] [Google Scholar]
- Kirby BP, Rawlins JN. The role of the septo-hippocampal cholinergic projection in T-maze rewarded alternation. Behav Brain Res. 2003;143:41–48. doi: 10.1016/s0166-4328(03)00005-6. [DOI] [PubMed] [Google Scholar]
- Koenig J, Linder AN, Leutgeb JK, Leutgeb S. The spatial periodicity of grid cells is not sustained during reduced theta oscillations. Science. 2011;332:592–595. doi: 10.1126/science.1201685. [DOI] [PubMed] [Google Scholar]
- Kubie JL. A driveable bundle of microwires for collecting single-unit data from freely-moving rats. Physiol Behav. 1984;32:115–118. doi: 10.1016/0031-9384(84)90080-5. [DOI] [PubMed] [Google Scholar]
- Lee MG, Chrobak JJ, Sik A, Wiley RG, Buzsaki G. Hippocampal theta activity following selective lesion of the septal cholinergic system. Neurosci. 1994;62:1033–1047. doi: 10.1016/0306-4522(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Mizumori SJ. Excitotoxic septal lesions result in spatial memory deficits and altered flexibility of hippocampal single-unit representations. J Neurosci. 1999;19:6661–6672. doi: 10.1523/JNEUROSCI.19-15-06661.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MM, Wallace DG. Selective hippocampal cholinergic deafferentation impairs self-movement cue use during a food hoarding task. Behav Brain Res. 2007;183:78–86. doi: 10.1016/j.bbr.2007.05.026. S0166-4328(07)00286-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon RW, Sobel TJ, Baxter MG. Selective immunolesions of hippocampal cholinergic input fail to impair spatial working memory. Hippocampus. 1997;7:130–136. doi: 10.1002/(SICI)1098-1063(1997)7:2<130::AID-HIPO2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA, Gerrard JL, Gothard K, Jung MW, Knierim JJ, … Weaver KL. Deciphering the hippocampal polyglot: the hippocampus as a path integration system. J Exp Biol. 1996;199:173–185. doi: 10.1242/jeb.199.1.173. [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, Rawlins JN, Steward O, Olton DS. Medial septal area lesions disrupt theta rhythm and cholinergic staining in medial entorhinal cortex and produce impaired radial arm maze behavior in rats. J Neurosci. 1982;2:292–302. doi: 10.1523/JNEUROSCI.02-03-00292.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumori SJ, Barnes CA, McNaughton BL. Reversible inactivation of the medial septum: selective effects on the spontaneous unit activity of different hippocampal cell types. Brain Res. 1989;500:99–106. doi: 10.1016/0006-8993(89)90303-x. [DOI] [PubMed] [Google Scholar]
- Morris RG. Spatial localization does not require the presence of local cues. Learn Mem. 1981;12:239–260. [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Moser EI, Kropff E, Moser MB. Place cells, grid cells, and the brain’s spatial representation system. Ann Rev Neurosci. 2008;31:69–89. doi: 10.1146/annurev.neuro.31.061307.090723. [DOI] [PubMed] [Google Scholar]
- Muir GM, Brown JE, Carey JP, Hirvonen TP, Della Santina CC, Minor LB, Taube JS. Disruption of the head direction cell signal after occlusion of the semicircular canals in the freely moving chinchilla. J Neurosci. 2009;29:14521–14533. doi: 10.1523/JNEUROSCI.3450-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RU, Kubie JL. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J Neurosci. 1987;7:1951–1968. doi: 10.1523/JNEUROSCI.07-07-01951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EL, Climer JR, Hasselmo ME. Grid cell spatial tuning reduced following systemic muscarinic receptor blockade. Hippocampus. 2014;24:643–655. doi: 10.1002/hipo.22253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- Okaichi H, Oshima Y, Jarrard LE. Scopolamine impairs both working and reference memory in rats: a replication and extension. Pharmacol Biochem Behav. 1989;34:599–602. doi: 10.1016/0091-3057(89)90565-0. 0091-3057(89)90565-0 [pii] [DOI] [PubMed] [Google Scholar]
- Olton DS, Samuelson RJ. Remembrance of Places Passed: Spatial Memory in Rats. J Exp Psych: Animal Behav Proc. 1976;2:97–116. [Google Scholar]
- Olton DS, Walker JA, Gage FH. Hippocampal connections and spatial discrimination. Brain Research. 1978;139:295–308. doi: 10.1016/0006-8993(78)90930-7. [DOI] [PubMed] [Google Scholar]
- Pang KC, Nocera R, Secor AJ, Yoder RM. GABAergic septohippocampal neurons are not necessary for spatial memory. Hippocampus. 2001;11:814–827. doi: 10.1002/hipo.1097. [DOI] [PubMed] [Google Scholar]
- Parent MB, Baxter MG. Septohippocampal acetylcholine: involved in but not necessary for learning and memory? Learn Mem. 2004;11:9–20. doi: 10.1101/lm.69104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval K, McDaniel KM, Murawski NJ, Doerr CE, Calton JL. Combined blockade of serotonergic and muscarinic transmission disrupts the anterior thalamic head direction signal. Behav Neurosci. 2008;122:1226–1235. doi: 10.1037/a0013138. [DOI] [PubMed] [Google Scholar]
- Shen J, Barnes CA, Wenk GL, McNaughton BL. Differential effects of selective immunotoxic lesions of medial septal cholinergic cells on spatial working and reference memory. Behav Neurosci. 1996;110:1181–1186. doi: 10.1037//0735-7044.110.5.1181. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Clark AS, Taube JS. Hippocampal spatial representations require vestibular input. Hippocampus. 2002;12:291–303. doi: 10.1002/hipo.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackman RW, Golob EJ, Bassett JP, Taube JS. Passive transport disrupts directional path integration by rat head direction cells. J Neurophysiol. 2003;90:2862–2874. doi: 10.1152/jn.00346.2003. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Taube JS. Firing properties of head direction cells in the rat anterior thalamic nucleus: dependence on vestibular input. J Neurosci. 1997;17:4349–4358. doi: 10.1523/JNEUROSCI.17-11-04349.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackman RW, Taube JS. Firing properties of rat lateral mammillary single units: head direction, head pitch, and angular head velocity. J Neurosci. 1998;18:9020–9037. doi: 10.1523/JNEUROSCI.18-21-09020.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens R. Scopolamine impairs spatial maze performance in rats. Physiol Behav. 1981;27:385–386. doi: 10.1016/0031-9384(81)90285-7. [DOI] [PubMed] [Google Scholar]
- Taube JS, Burton HL. Head direction cell activity monitored in a novel environment and during a cue conflict situation. J Neurophysiol. 1995;74:1953–1971. doi: 10.1152/jn.1995.74.5.1953. [DOI] [PubMed] [Google Scholar]
- Taube JS, Kesslak JP, Cotman CW. Lesions of the rat postsubiculum impair performance on spatial tasks. Behav Neural Biol. 1992;57:131–143. doi: 10.1016/0163-1047(92)90629-i. [DOI] [PubMed] [Google Scholar]
- Taube JS, Muller RU, Ranck JB., Jr Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J Neurosci. 1990a;10:420–435. doi: 10.1523/JNEUROSCI.10-02-00420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS, Muller RU, Ranck JB., Jr Head-direction cells recorded from the postsubiculum in freely moving rats. II. Effects of environmental manipulations. J Neurosci. 1990b;10:436–447. doi: 10.1523/JNEUROSCI.10-02-00436.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerio S, Clark BJ, Yoder RM, Taube JS. Lesions of the postsubiculum disrupt path integration; Paper presented at Neuroscience; 2011; Washington, DC. 2011. [Google Scholar]
- Valerio S, Taube JS. Path integration: how the head direction signal maintains and corrects spatial orientation. Nat Neurosci. 2012 doi: 10.1038/nn.3215. nn.3215 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerio S, Taube JS. Head Direction Cell Activity Is Absent in Mice without the Horizontal Semicircular Canals. J Neurosci. 2016;36:741–754. doi: 10.1523/JNEUROSCI.3790-14.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cauter T, Camon J, Alvernhe A, Elduayen C, Sargolini F, Save E. Distinct roles of medial and lateral entorhinal cortex in spatial cognition. Cereb Cortex. 2013;23:451–459. doi: 10.1093/cercor/bhs033. [DOI] [PubMed] [Google Scholar]
- Wallace DG, Hines DJ, Pellis SM, Whishaw IQ. Vestibular information is required for dead reckoning in the rat. J Neurosci. 2002;22:10009–10017. doi: 10.1523/JNEUROSCI.22-22-10009.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh TJ, Herzog CD, Gandhi C, Stackman RW, Wiley RG. Injection of IgG 192-saporin into the medial septum produces cholinergic hypofunction and dose-dependent working memory deficits. Brain Res. 1996;726:69–79. [PubMed] [Google Scholar]
- Watanabe H, Watanabe K, Hagino K. The involvement of catecholamine in scopolamine-induced locomotor activation and rotational behavour in mice. Japan J Pharmacol. 1978;28:465–472. doi: 10.1254/jjp.28.465. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ. Cholinergic receptor blockade in the rat impairs locale but not taxon strategies for place navigation in a swimming pool. Behav Neurosci. 1985;99:979–1005. doi: 10.1037//0735-7044.99.5.979. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Hines DJ, Wallace DG. Dead reckoning (path integration) requires the hippocampal formation: evidence from spontaneous exploration and spatial learning tasks in light (allothetic) and dark (idiothetic) tests. Behav Brain Res. 2001;127:49–69. doi: 10.1016/s0166-4328(01)00359-x. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Maaswinkel H, Gonzalez CL, Kolb B. Deficits in allothetic and idiothetic spatial behavior in rats with posterior cingulate cortex lesions. Behav Brain Res. 2001;118:67–76. doi: 10.1016/s0166-4328(00)00312-0. [DOI] [PubMed] [Google Scholar]
- Winson J. Loss of hippocampal theta rhythm results in spatial memory deficit in the rat. Science. 1978;201:160–163. doi: 10.1126/science.663646. [DOI] [PubMed] [Google Scholar]
- Wirsching BA, Beninger RJ, Jhamandas K, Boegman RJ, El-Defrawy SR. Differential effects of scopolamine on working and reference memory of rats in the radial maze. Pharmacol Biochem Behav. 1984;20:659–662. doi: 10.1016/0091-3057(84)90180-1. [DOI] [PubMed] [Google Scholar]
- Yoder RM, Clark BJ, Brown JE, Lamia MV, Valerio S, Shinder ME, Taube JS. Both visual and idiothetic cues contribute to head direction cell stability during navigation along complex routes. J Neurophysiol. 2011;105:2989–3001. doi: 10.1152/jn.01041.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder RM, Clark BJ, Taube JS. Origins of landmark encoding in the brain. Trends Neurosci. 2011;34:561–571. doi: 10.1016/j.tins.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder RM, Goebel EA, Koppen JR, Blankenship PA, Blackwell AA, Wallace DG. Otolithic information is required for homing in the mouse. Hippocampus. 2015;25:890–899. doi: 10.1002/hipo.22410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder RM, Pang KCH. Involvement of GABAergic and cholinergic medial septal neurons in hippocampal theta rhythm. Hippocampus. 2005;15:381–392. doi: 10.1002/hipo.20062. [DOI] [PubMed] [Google Scholar]
- Yoder RM, Peck JR, Taube JS. Visual landmark information gains control of the head direction signal at the lateral mammillary nuclei. J Neurosci. 2015;35:1354–1367. doi: 10.1523/JNEUROSCI.1418-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder RM, Taube JS. Head direction cell activity in mice: robust directional signal depends on intact otolith organs. J Neurosci. 2009;29:1061–1076. doi: 10.1523/JNEUROSCI.1679-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]