Abstract
Introduction
Non-central nervous system (non-CNS) rhabdoid tumors tend to present at a young age and have an extremely aggressive course, with dismal overall survival rates. Inactivation of the tumor suppressor gene SMARCB1 has been shown in rhabdoid tumors regardless of anatomic location, suggesting a common genetic basis. We retrospectively analyzed our institutional experience with non-CNS rhabdoid tumors to determine overall survival and prognostic variables.
Methods
We reviewed records of pediatric patients (age <22y) with non-CNS rhabdoid tumor at our institution between 1980 and 2014. Variables evaluated for correlation with survival included: age > or <1.5 years (median) at diagnosis, M1 status, and radiation therapy. The log-rank test was used to compare Kaplan-Meier probability distributions with P values adjusted for multiple testing using the false discovery rate approach.
Results
Nineteen consecutive patients (10 female) with histologically verified rhabdoid tumor were identified. Mean age at diagnosis was 3.2 years (median 1.5y, range 1.3mo–21.8y). Primary tumors were located in the kidney (n=10), head and neck (n=5), and in the liver, thigh, mediastinum and retroperitoneum (n=1 each). SMARCB1 expression was absent in all 10 patients tested. Eight patients had distant metastases at diagnosis. Median overall survival was 1.2 years. Age greater than the median and radiation therapy were associated with better outcome, with a median overall survival of 2.7 years (P=0.049 and P=0.003, respectively).
Conclusion
Survival rates for rhabdoid tumor remain poor, but prognosis is better in older children, regardless of primary tumor location. Because of its rarity, clinical trials with present agents are difficult to conduct. Further progress will require a focus on therapies targeted at tumor biology rather than anatomic location for non-CNS rhabdoid tumors.
Keywords: Rhabdoid tumor, SMARCB1, pediatric cancer
1. Introduction
Malignant rhabdoid tumors (MRTs) are a rare and highly aggressive group of pediatric tumors, accounting for about 2% of renal tumors in childhood [1]. During the first National Wilms’ Tumor Study (NWTS), these tumors were identified in the kidney as a rhabdomyosarcomatoid variant of Wilms’ tumor [2]; however, since 1981 these tumors have been recognized as a distinct pathologic entity [3]. Between 10–15% of patients with MRTs present with primary CNS disease known as atypical teratoid/rhabdoid tumors (AT/RT) [4, 5]. Although MRTs were initially described as arising from the kidney and have been well described in the CNS, other cases have been identified in various locations, including the liver, lung, and soft tissues [6, 7]. MRTs, regardless of the anatomic site, tend to present at a young age and have an extremely aggressive course with dismal overall survival rates estimated near 23% [5]. In addition to poor overall survival, MRTs in comparison to other pediatric cancers have a high tendency to metastasize early [8]. The tissue of origin of MRTs remains unclear [5, 8]; however, molecular analyses have shown few genetic changes other than the common inactivating mutation of the tumor suppressor SMARCB1 (also known as hSNF5, INI1 and BAF47) in chromosome band 22q11.2, regardless of their anatomic location, suggesting their common genetic basis [7, 9–13]. Due to their rarity, there is no standardized treatment protocol for MRTs [7] and poor outcomes are common, despite intense chemotherapy and radiotherapy regimens [12]. As such, surgical resection remains central to treatment, and prognostic variables of age, surgery and adjunctive therapies have been evaluated in several studies with varied results [5, 8, 14]. At our institution, the pediatric surgery service typically treats rhabdoid tumors that arise in non-central nervous system (non-CNS) anatomic sites. To better characterize the clinical course and outcome of pediatric and adolescent patients with non-CNS rhabdoid tumors, we analyzed our institutional experience in treating these tumors over a 35-year period, in order to investigate overall survival rates and identify relevant prognostic indicators.
2. Methods
After obtaining institutional review board approval, our institutional database was searched for all patients younger than 22 years of age treated for malignant rhabdoid tumor or atypical teratoid/rhabdoid tumor (AT/RT) between January 1980 and July 2015. The medical records of these patients were reviewed for age at diagnosis, age at diagnosis relative to the full cohort’s median age at diagnosis, M1 metastatic status, location of primary tumor (renal or extra-renal), surgical intervention, adjuvant therapies received, and histologic information including SMARCB1 status. These variables were analyzed for associations with overall survival. The log-rank test was used to compare Kaplan-Meier survival probability distributions, with P values adjusted for multiple testing using the false discovery rate approach. P values of less than 0.05 were considered statistically significant. All statistical analyses were performed using R software (version 3.2.3, R Project for Statistical Computing, Vienna, Austria; www.r-project.org).
3. Results
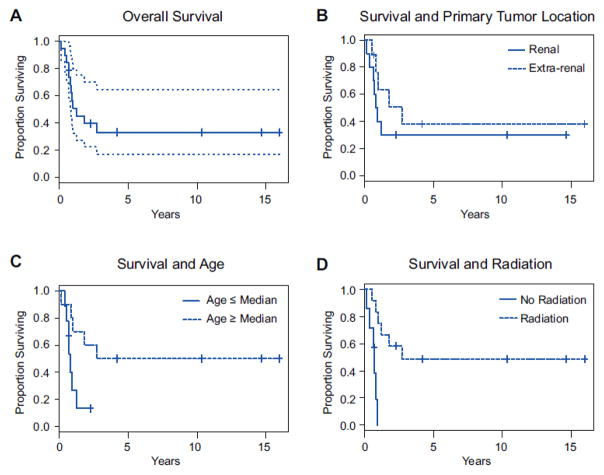
Nineteen patients (10 female, 9 male) who received treatment at our institution for primary or metastatic non-CNS rhabdoid tumors were identified, with an average age at diagnosis of 3.2 years (median 1.5 y; range, 1.3 mo – 21.8 y). Of these 19 patients, 7 underwent surgery for the primary tumor at other institutions. The anatomic locations of the primary tumors were the kidney (n=10), head and neck (n=5), and the liver, thigh, mediastinum, and retroperitoneum (n=1 each). Histopathologic assessment of SMARCB1 expression was negative in all 10 patients tested. Metastases were detected at diagnosis in 8 patients, of whom 5 had primary tumors in the kidney; the remaining patients each had a primary tumor in the mediastinum, liver, and left thigh. Patients had metastases in the lung (n=4), brain (n=2), thymus (n=1), and both lung and retroperitoneum (n=1). One patient was diagnosed with a synchronous primary tumor (primitive neuroectodermal tumor of the brain). Surgical margin data were available for review for 17 patients, of whom 8 had R0 resections, 4 had R1 resection, 1 had an R2 resection, and 5 patients only had biopsies performed (Table 1). Median follow-up for all patients was 11.8 months (range, 1.7 mo –16 y). The median follow-up period was 4.2 years (range, 8 mo –16 y) for survivors and 9.8 months (range, 1.7 mo – 2.7 y) for patients who died of disease. Neoadjuvant chemotherapy was given to 8 patients, and adjuvant radiotherapy was administered to 12. Median overall survival was 1.2 years. Only age greater than the median was associated with better outcome, with a median overall survival of 2.7 years (P=0.049). Radiotherapy administration, as part of the multimodal treatment, appeared to be statistically significant with median overall survival of 2.7 years (P=0.002) (Figure 1). However, given our limited sample size, we would caution against a global conclusion based on this P value and we cannot consider radiotherapy to be an independent predictive factor until larger studies have been completed. No survival benefit was observed in association with location of primary tumor or metastatic disease status at diagnosis (Table 2).
Table 1.
Patient Demographics and Disease Characteristics
| Pt | Gender | Age at Dx | Alive | Location of Tumor | Mets at Dx | Location of Metastasis | Neoadjuvant Chemotherapy | Radiation | Loss of SMARCB1 | Resection status | Follow up time |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 6.2 mo | No | Kidney | Yes | Brain | No | Yes | -- | 2 | 1.2 y |
| 2 | M | 3.9 y | No | Mediastinum | Yes | Lung | Yes | No | -- | Biopsy | 10 mo |
| 3 | M | 2.5 mo | No | Kidney | No | -- | No | No | -- | 0 | 4.6 mo |
| 4 | F | 4.9 mo | No | Head/Neck | No | -- | Yes | Yes | Yes | Biopsy | 6.4 mo |
| 5 | M | 1.5 y | No | Kidney | Yes | Lung | Yes | No | -- | Biopsy | 1.7 mo |
| 6 | M | 1.8 y | Yes | Kidney | No | -- | No | Yes | -- | 0 | 10.3 y |
| 7 | F | 8.4 mo | No | Kidney | Yes | Lung | Yes | No | -- | 0 | 7.8 mo |
| 8 | M | 9 mo | Yes | Kidney | Yes | Thymus | Yes | Yes | Yes | 1 | 2.3 y |
| 9 | M | 3.2 y | No | Head/Neck | No | -- | No | Yes | Yes | 1 | 2.7 y |
| 10 | F | 5 mo | No | Kidney | Yes | Brain | No | No | Yes | 1 | 8.4 mo |
| 11 | M | 2.2 y | No | Head/Neck | No | -- | No | Yes | Yes | Biopsy | 11.8 mo |
| 12 | F | 1.1 y | Yes | Liver | Yes | Lung, Retroperitoneum | Yes | No | -- | Biopsy | 8.2 mo |
| 13 | F | 1.3 mo | No | Kidney | No | -- | No | No | Yes | 0 | 11 mo |
| 14 | F | 7.7 y | Yes | Thigh | Yes | Lung | Yes | Yes | Yes | 0 | 4.1 y |
| 15 | M | 3.6 mo | No | Kidney | No | -- | No | Yes | Yes | 0 | 9.6 mo |
| 16 | F | 6.5 y | Yes | Head/Neck | No | -- | Yes | Yes | Yes | 0 | 3.5 y |
| 17 | F | 6.6 y | Yes | Head/Neck | No | -- | No | Yes | -- | 0 | 16.0 y |
| 18 | M | 21.8 y | No | Retroperitoneum | No | -- | No | Yes | Yes | Unknown | 1.8 y |
| 19 | F | 1.5 y | Yes | Kidney | No | -- | No | Yes | -- | 1 | 14.7 y |
Figure 1. Kaplan-Meier Survival Analyses.
(A) Overall survival for the entire cohort. Dotted lines show the 95% confidence interval. (B) Survival stratified by the presence of a renal or extrarenal primary tumor at presentation (P=0.321). (C) Survival stratified by age above or below the median age of 1.5 years (P=0.049). (D) Survival of patients treated with and without adjuvant radiation therapy (P=0.002).
Table 2.
Variables evaluated for correlation with improved survival.
| Variable | P value* |
|---|---|
| Age ≥ Median | 0.049 |
| Location of Primary Tumor | 0.46 |
| Metastasis at Diagnosis | 0.53 |
| Radiation Therapy | 0.002 |
Adjusted for multiple testing using the false discovery rate approach.
4. Discussion
MRTs do not arise in any unique anatomic location; thus, there is no uniform staging system or treatment protocols for these patients. Currently, patients are treated based on protocols classified by the tumor’s site of origin [8]. Rhabdoid tumors, regardless of location, continue to have a terrible prognosis. As a rare, aggressive malignancy, there is a dire need for the development of new adjunctive therapies to complement surgical intervention. Surgical treatment of non-CNS MRTs is initially guided by the location. However, preoperative diagnosis is not always possible, as non-CNS MRTs are frequently mistaken for other more common tumors that arise in the location in which they are found [15]. For MRTs presenting in the kidney, the initial management strategy follows that of Wilms tumor. Biopsy of the primary tumor is usually not carried out prior to removal, to avoid rupture of the tumor capsule and consequent spillage of tumor cells [16].
Various chemotherapeutic regimens are used in treating MRTs, including combinations of actinomycin D, carboplatin, cisplatin, cyclophosphamide, doxorubicin, etoposide, ifosfamide, methotrexate, and vincristine [1, 7, 8, 14, 17]. While multi-agent regimens are often used, and prior studies have shown that chemotherapy can reduce tumor volume [1], only the inclusion of actinomycin D or doxorubicin in drug regimens has been associated with reductions in the risk of death in a population of non-CNS rhabdoid tumors [14, 17]. In an analysis of patients enrolled in studies conducted by the Société International d’Oncologie Pédiatrique (SIOP) and Gesellschaft für Pädiatrische Onkologie und Hämatologie (GPOH), patients who received a preoperative regimen of doxorubicin-intensified actinomycin D and vincristine achieved a better response than patients who received actinomycin D and vincristine without doxorubicin [17]. Although chemotherapy plays an essential role in treatment of MRT, our analysis of the 8 (42%) patients who received multidrug chemotherapy regimens, neoadjuvant chemotherapy was not a prognostic indicator for survival. Continuing with our evaluation of adjuvant treatment options, we assessed survival benefit of radiotherapy. Eleven patients (58%) received radiation as part of their multimodal treatment. While the dosage and irradiated field varied between our patients, radiation therapy appeared to result in a statistically significant difference in survival (P=0.002); however, as mentioned previously, larger studies are necessary to definitively show this as a prognostic indicator in survival. Thus, we would conclude that radiotherapy might benefit patients over 1.5 years of age, as others have previously shown [5]. Further studies investigating the utility of radiotherapy in this older age group are clearly warranted.
Studies reviewing patients within the NWTS and trials of the SIOP Renal Tumor Study Group have shown older age at diagnosis portends better survival for renal rhabdoid tumors [1, 5]. Additionally, separate review of extra-renal, extra-CNS rhabdoid tumors also showed older age at diagnosis giving a better prognosis to MRT patients[14]. Our study examining non-CNS rhabdoid tumors similarly shows that age over 1.5 years at time of diagnosis, regardless of anatomic location, has higher overall survival (P=0.049). While this is encouraging, a limitation of our study is the small sample size, which can be attributed to the rarity of this malignancy. National and international collaborative investigations are warranted to enroll a large cohort to further characterize rhabdoid tumors from numerous anatomic locations.
The discovery of a common inactivating SMARCB1 mutation across all MRTs was a promising step towards directing the search for desperately needed therapeutic options for patients with these tumors. Absence of immunohistochemical staining for SMARCB1 has already proved to be clinically valuable in confirming the MRT diagnosis regardless of anatomic location [18]. On review of pathology reports of our patients, one was diagnosed with a synchronous primitive neuroectodermal tumor (PNET). While possible diagnoses of metastatic MRT and AT/RT were both considered, after histologic and immunohistochemical analysis, a consensus diagnosis of PNET was made. This patient, however, did not have SMARCB1 testing conducted due to the year the patient was diagnosed. Had SMARCB1 testing been conducted, this may have helped elucidate the diagnosis and further assist with treatment selection.
Research has implicated the inactivation of SMARCB1 in the regulation of cyclin D1/CDK4 activation and cell cycle arrest. Restoration of SMARCB1 expression in deficient cells results in suppression of cyclin D1 and consequent G1 cell cycle arrest [19, 20]. With this background, as well as in vivo studies showing the relation of cyclin D1 in tumorigenesis of rhabdoid tumors [21, 22], research efforts have progressed to the bedside with a phase 1 multicenter clinical trial exploring CDK4 inhibition in patients with MRT (ClinicalTrials.gov Identifier: NCT01747876). Aside from the loss of SMARCB1 in MRTs, prior studies that included SNP and exome analysis noted very little variation in MRT genomes[13, 23–25]. Recent studies by Chun et al. found clustering of CG island promoter methylation into two groups that correlated with age at diagnosis, which may provide a biological reason for the apparent survival benefit among older patients[26]. Similar to non-CNS rhabdoid tumors, recent investigations in transcriptomic and epigenetic organization of CNS AT/RTs suggests that these tumors can be subclassified into three distinct molecular subgroups with different preferred locations in the brain, suggesting they may originate from different precursor cells while maintaining their common loss of SMARCB1[27].
Another potential target, EZH2, which encodes a catalytic subunit of the polycomb repressive complex 2 (PRC2), has similarly been recognized in MRTs. Elevated EZH2 expression has been observed in primary SMARCB1-deficient tumors and pharmacological inhibition of EZH2 has been shown to induce antiproliferative effects specifically in MRT cell lines with SMARCB1 deletion [28]. Trials examining the safety and efficacy of EZH2 inhibitors in patients with MRTs are currently under way. Given the dismal prognosis of MRTs despite aggressive chemotherapy regimens, the continued exploration of rational targeted therapies is especially important to achieve progress in managing this disease group.
Acknowledgments
This research was supported in part by the U.S. National Institutes of Health/National Cancer Institute (#P30 CA008748).
Abbreviations
- AT/RT
atypical teratoid/rhabdoid tumor
- GPOH
Gesellschaft für Pädiatrische Onkologie und Hämatologie (Society for Pediatric Oncology and Hematology)
- MRT
malignant rhabdoid tumor
- NWTS
National Wilms Tumor Study
- PNET
primitive neuroectodermal tumor
- PRC
polycomb repressive complex
- SIOP
Société International d’Oncologie Pédiatrique (International Society of Pediatric Oncology)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van den Heuvel-Eibrink MM, van Tinteren H, Rehorst H, Coulombe A, Patte C, de Camargo B, et al. Malignant rhabdoid tumours of the kidney (MRTKs), registered on recent SIOP protocols from 1993 to 2005: a report of the SIOP Renal Tumour Study Group. Pediatr Blood Cancer. 2011;56:733–7. doi: 10.1002/pbc.22922. [DOI] [PubMed] [Google Scholar]
- 2.Beckwith JB, Palmer NF. Histopathology and prognosis of Wilms tumors: results from the First National Wilms’ Tumor Study. Cancer. 1978;41:1937–48. doi: 10.1002/1097-0142(197805)41:5<1937::aid-cncr2820410538>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 3.Haas JE, Palmer NF, Weinberg AG, Beckwith JB. Ultrastructure of malignant rhabdoid tumor of the kidney. A distinctive renal tumor of children. Hum Pathol. 1981;12:646–57. doi: 10.1016/s0046-8177(81)80050-0. [DOI] [PubMed] [Google Scholar]
- 4.Rorke LB, Packer RJ, Biegel JA. Central nervous system atypical teratoid/rhabdoid tumors of infancy and childhood: definition of an entity. J Neurosurg. 1996;85:56–65. doi: 10.3171/jns.1996.85.1.0056. [DOI] [PubMed] [Google Scholar]
- 5.Tomlinson GE, Breslow NE, Dome J, Guthrie KA, Norkool P, Li S, et al. Rhabdoid tumor of the kidney in the National Wilms’ Tumor Study: age at diagnosis as a prognostic factor. J Clin Oncol. 2005;23:7641–5. doi: 10.1200/JCO.2004.00.8110. [DOI] [PubMed] [Google Scholar]
- 6.Sevenet N, Lellouch-Tubiana A, Schofield D, Hoang-Xuan K, Gessler M, Birnbaum D, et al. Spectrum of hSNF5/INI1 somatic mutations in human cancer and genotype-phenotype correlations. Hum Mol Genet. 1999;8:2359–68. doi: 10.1093/hmg/8.13.2359. [DOI] [PubMed] [Google Scholar]
- 7.Uwineza A, Gill H, Buckley P, Owens C, Capra M, O’Sullivan C, et al. Rhabdoid tumor: the Irish experience 1986–2013. Cancer Genet. 2014;207:398–402. doi: 10.1016/j.cancergen.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 8.Reinhard H, Reinert J, Beier R, Furtwangler R, Alkasser M, Rutkowski S, et al. Rhabdoid tumors in children: prognostic factors in 70 patients diagnosed in Germany. Oncol Rep. 2008;19:819–23. [PubMed] [Google Scholar]
- 9.Biegel JA, Tan L, Zhang F, Wainwright L, Russo P, Rorke LB. Alterations of the hSNF5/INI1 gene in central nervous system atypical teratoid/rhabdoid tumors and renal and extrarenal rhabdoid tumors. Clin Cancer Res. 2002;8:3461–7. [PubMed] [Google Scholar]
- 10.Versteege I, Sevenet N, Lange J, Rousseau-Merck MF, Ambros P, Handgretinger R, et al. Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer. Nature. 1998;394:203–6. doi: 10.1038/28212. [DOI] [PubMed] [Google Scholar]
- 11.Biegel JA, Zhou JY, Rorke LB, Stenstrom C, Wainwright LM, Fogelgren B. Germ-line and acquired mutations of INI1 in atypical teratoid and rhabdoid tumors. Cancer Res. 1999;59:74–9. [PubMed] [Google Scholar]
- 12.Kim KH, Roberts CW. Mechanisms by which SMARCB1 loss drives rhabdoid tumor growth. Cancer Genet. 2014;207:365–72. doi: 10.1016/j.cancergen.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee RS, Stewart C, Carter SL, Ambrogio L, Cibulskis K, Sougnez C, et al. A remarkably simple genome underlies highly malignant pediatric rhabdoid cancers. J Clin Invest. 2012;122:2983–8. doi: 10.1172/JCI64400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horazdovsky R, Manivel JC, Cheng EY. Surgery and actinomycin improve survival in malignant rhabdoid tumor. Sarcoma. 2013;2013:315170. doi: 10.1155/2013/315170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagner LM, Garrett JK, Ballard ET, Hill DA, Perry A, Biegel JA, et al. Malignant rhabdoid tumor mimicking hepatoblastoma: a case report and literature review. Pediatr Dev Pathol. 2007;10:409–15. doi: 10.2350/06-08-0155.1. [DOI] [PubMed] [Google Scholar]
- 16.Davidoff AM. Surgical Management of Wilms Tumor. In: Fischer JE, editor. Fischer’s Mastery of Surgery. 6. Philadelphia: Lippincott Williams Wilkins; 2012. pp. 1973–82. [Google Scholar]
- 17.Furtwangler R, Nourkami-Tutdibi N, Leuschner I, Vokuhl C, Niggli F, Kager L, et al. Malignant rhabdoid tumor of the kidney: significantly improved response to pre-operative treatment intensified with doxorubicin. Cancer Genet. 2014;207:434–6. doi: 10.1016/j.cancergen.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Hollmann TJ, Hornick JL. INI1-deficient tumors: diagnostic features and molecular genetics. Am J Surg Pathol. 2011;35:e47–63. doi: 10.1097/PAS.0b013e31822b325b. [DOI] [PubMed] [Google Scholar]
- 19.Versteege I, Medjkane S, Rouillard D, Delattre O. A key role of the hSNF5/INI1 tumour suppressor in the control of the G1-S transition of the cell cycle. Oncogene. 2002;21:6403–12. doi: 10.1038/sj.onc.1205841. [DOI] [PubMed] [Google Scholar]
- 20.Betz BL, Strobeck MW, Reisman DN, Knudsen ES, Weissman BE. Re-expression of hSNF5/INI1/BAF47 in pediatric tumor cells leads to G1 arrest associated with induction of p16ink4a and activation of RB. Oncogene. 2002;21:5193–203. doi: 10.1038/sj.onc.1205706. [DOI] [PubMed] [Google Scholar]
- 21.Tsikitis M, Zhang Z, Edelman W, Zagzag D, Kalpana GV. Genetic ablation of Cyclin D1 abrogates genesis of rhabdoid tumors resulting from Ini1 loss. Proc Natl Acad Sci U S A. 2005;102:12129–34. doi: 10.1073/pnas.0505300102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith ME, Cimica V, Chinni S, Jana S, Koba W, Yang Z, et al. Therapeutically targeting cyclin D1 in primary tumors arising from loss of Ini1. Proc Natl Acad Sci U S A. 2011;108:319–24. doi: 10.1073/pnas.0913297108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasselblatt M, Isken S, Linge A, Eikmeier K, Jeibmann A, Oyen F, et al. High-resolution genomic analysis suggests the absence of recurrent genomic alterations other than SMARCB1 aberrations in atypical teratoid/rhabdoid tumors. Genes Chromosomes Cancer. 2013;52:185–90. doi: 10.1002/gcc.22018. [DOI] [PubMed] [Google Scholar]
- 24.Jackson EM, Sievert AJ, Gai X, Hakonarson H, Judkins AR, Tooke L, et al. Genomic analysis using high-density single nucleotide polymorphism-based oligonucleotide arrays and multiplex ligation-dependent probe amplification provides a comprehensive analysis of INI1/SMARCB1 in malignant rhabdoid tumors. Clin Cancer Res. 2009;15:1923–30. doi: 10.1158/1078-0432.CCR-08-2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKenna ES, Sansam CG, Cho YJ, Greulich H, Evans JA, Thom CS, et al. Loss of the epigenetic tumor suppressor SNF5 leads to cancer without genomic instability. Mol Cell Biol. 2008;28:6223–33. doi: 10.1128/MCB.00658-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chun HJ, Lim EL, Heravi-Moussavi A, Saberi S, Mungall KL, Bilenky M, et al. Genomewide profiles of extra-cranial malignant rhabdoid tumors reveal heterogeneity and dysregulated developmental pathways. Cancer Cell. 2016;29:394–406. doi: 10.1016/j.ccell.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johann PD, Erkek S, Zapatka M, Kerl K, Buchhalter I, Hovestadt V, et al. Atypical teratoid/rhabdoid tumors are comprised of three epigenetic subgroups with distinct enhancer landscapes. Cancer Cell. 2016;29:379–93. doi: 10.1016/j.ccell.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Knutson SK, Warholic NM, Wigle TJ, et al. Durable tumor regression in genetically altered malignant rhabdoid tumors by inhibition of methyltransferase EZH2. Proc Natl Acad Sci U S A. 2013;110:7922–7. doi: 10.1073/pnas.1303800110. [DOI] [PMC free article] [PubMed] [Google Scholar]