Abstract
Estrogen receptor alpha (ESR1) is one of the two intracellular receptors for estrogen and is expressed by hepatocytes in the liver. The role of ESR1 in the regulation of toxicant-induced liver injury and compensatory regeneration is not completely clear. We investigated the role of ESR1 in liver regeneration after carbon tetrachloride (CCl4)-induced liver injury using wild type (WT) and ESR1 knockout rats (ESR1-KO). Adult female WT and ESR1-KO rats were treated with 1ml/kg CCl4 and euthanized over a time course of 0–48 hr. Liver Injury measured by serum alanine amino transaminase (ALT) and histopathological analysis showed significantly higher liver injury in ESR1-KO as compared to WT rats. Hematoxylin and eosin (H&E) staining revealed two-fold higher necrosis and significant inflammatory cell infiltration in ESR1-KO rats. Chloracetate esterase staining revealed higher neutrophil infiltration in ESR1-KO rat livers. Interestingly, Proliferating cell nuclear antigen (PCNA) immunohistochemistry showed that in spite of two-fold higher liver injury, the ESR1KO rats had equal liver regeneration as compared to WT rats. Western blot analysis of cyclin D1 and phosphorylated Rb, proteins involved in the initiation of the cell cycle, were significantly higher at all time points in ESR1KO rats. Further analysis revealed faster activation of canonical Wnt/β-catenin and NF-κB signaling in ESR1-KO rats characterized by higher activated β-catenin and phosphorylated p65 at 12 hr after CCl4 treatment. Taken together, these data indicate that ESR1-mediated signaling inhibits liver regeneration by down regulation of Wnt signaling resulting in lower cyclin D1 activation after chemical-induced liver injury.
Introduction
The liver has a remarkable ability to regenerate itself after injury or partial resection. Studies show that the ability of the liver to regenerate is the key determinant of final outcome after injury or tissue resection 1–5. The mechanisms of liver regeneration have been mainly studied using the surgical resection model of partial hepatectomy 4, 6. However, it is known that acute liver injury induced by overdose of drugs such as acetaminophen and experimental chemicals such as carbon tetrachloride (CCl4) is followed by compensatory increase in liver cell proliferation 5, 7–9. In drug/chemical induced injury models there is extensive cell death followed by inflammatory response along with cell proliferation 5. CCl4 is a model toxicant used to study both liver injury and subsequent liver regeneration. Liver injury and recovery from CCl4 dosing follows a classic dose-response up to a threshold dose, past which there is no increase in regeneration and tissue repair 10. Whereas it is known that liver regeneration is a critical determinant of the final outcome of toxicant exposure, the exact mechanisms of liver regeneration after toxic injury remain unclear.
Estrogen is the primary female steroid hormone that signals via binding to its cognate receptors called estrogen receptors, which exist in nuclear (intracellular) and membrane bound forms 11. The nuclear estrogen receptor exists in two isoforms, estrogen receptor alpha (ERα; also called ESR1) and ERβ; (also called ESR2), both of which are members of the NR3 family of transcription factors 11. Estrogen plays an important role in sexual development and reproduction and disruption of ESR1 leads to infertility, small testes in males, and polycystic ovaries in females 12–14. In the liver, ESR1 is expressed exclusively by the hepatocytes, while biliary cells express both ESR1 and ESR2 15. It has been previously shown that estrogen can act as a strong mitogen for hepatocytes, and helps promote regeneration by increasing levels of cyclin D1 mRNA levels in hepatocytes 16. Ovariectomized female rats given estrogen pellets showed a greater liver regeneration after portal branch ligation as compared to untreated ovariectomized female rats 17. Similarly, treatment of tamoxifen, an estrogen antagonist, to rats after PHX shows a decrease in hepatocyte proliferation 18. Interestingly, estrogen-mediated signaling has been theorized to protect against the development of hepatocellular carcinoma (HCC), the primary hepatic malignancy 19–22. It is known that HCC incidence is significantly higher in males than females and estrogen signaling has been hypothesized as the main protective force by inhibiting cellular proliferation 20. Estrogen is also shown to be anti-inflammatory in the liver following injury 19, 23. This is important because inflammation is known to be a major driver of HCC pathogenesis 19, 24, 25. A major component of HCC pathogenesis is tissue injury and subsequent compensatory regeneration, which is missing in the PHX model 4. It is possible that estrogen and ESR1-mediated signaling plays a different role in situations involving injury and regeneration. The exact role of estrogen and ESR1 signaling in regulation of hepatocyte proliferation in general and specifically after toxicant-induced liver injury remains unclear.
We investigated the role of estrogen and ESR1 signaling in liver injury and regeneration after CCl4 induced injury model using the newly developed ESR1 knockout (ESR1-KO) rats. CCl4-induced liver injury is not only a well studied model of chemical induced liver injury and regeneration but also exhibits several components of HCC pathogenesis including liver injury, inflammation, stellate cell activation and compensatory proliferation. Because of the comprehensive nature of CCl4 model it can provides insights into both regeneration and HCC pathogenesis mechanisms and connections between them.
Materials and Methods
Animals and Tissue Preparation
All animal studies were approved by and performed in accordance with the Institutional Animal Care and Use committee (IACUC) at the University of Kansas Medical Center. The generation, genotyping and characterization of the ESR1-KO rats have been described in detail previously 12. The rats used in this study were provided by Dr. Soares laboratory. Two to three-month old female wild type (WT) or ESR1-KO rats (n=3–4 per time point) were treated intraperitoneally with CCl4 (1 ml/kg; Sigma, St. Louis) in a 1:1 mixture with corn oil. Rats were euthanized over a time course of 0 to 48 hr. Liver and blood were collected and processed as previously described 26. Liver and serum samples were used to determine markers of injury and regeneration as described before 27.
Protein Isolation and Western Blot
Proteins were isolated from liver samples for western blot using methods previously described 26. All western blot antibodies were purchased from Cell Signaling Technology (Danvers, MA) except for active β-Catenin (Millipore, Billerica, MA).
Histology and Immunohistochemistry
H&E stained paraffin sections of livers from CCl4-treated WT and ESR1-KO rats were used for percent necrosis scoring as described before 27. Two sections per slide per rat and 3–4 rats per time point per genotype were used for necrosis scoring. Proliferating cell nuclear antigen (PCNA) and hematoxylin and eosin staining were performed using 4 μm thick paraffin embedded liver sections as described previously 26. PCNA positive cells were quantified in 10 high power (400x) fields of liver sections of from at least 3 individual rats.
Neutrophil Staining
Neutrophils were monitored using a chloroacetate esterase stain (Sigma, St. Louis, MO, USA) according to the manufacturer’s suggested methods. Slides were counterstained with hemotoxylin for 2 minutes. Neutrophils were counted in ten 400x fields for quantification.
Statistical Analysis
All bar graphs depict the mean ± standard deviation. To determine statistical significance, one-way ANOVA on the time course within a group was used. For non-parametric analysis the Mann-Whitney U test was used. Finally, the Dunn’s post-hoc test was used to compare samples versus control for non-parametric and Student-Neuman-Keuls for normally distributed data.
Results
Significantly Higher Liver Injury and Increased Inflammation in ESR1-KO
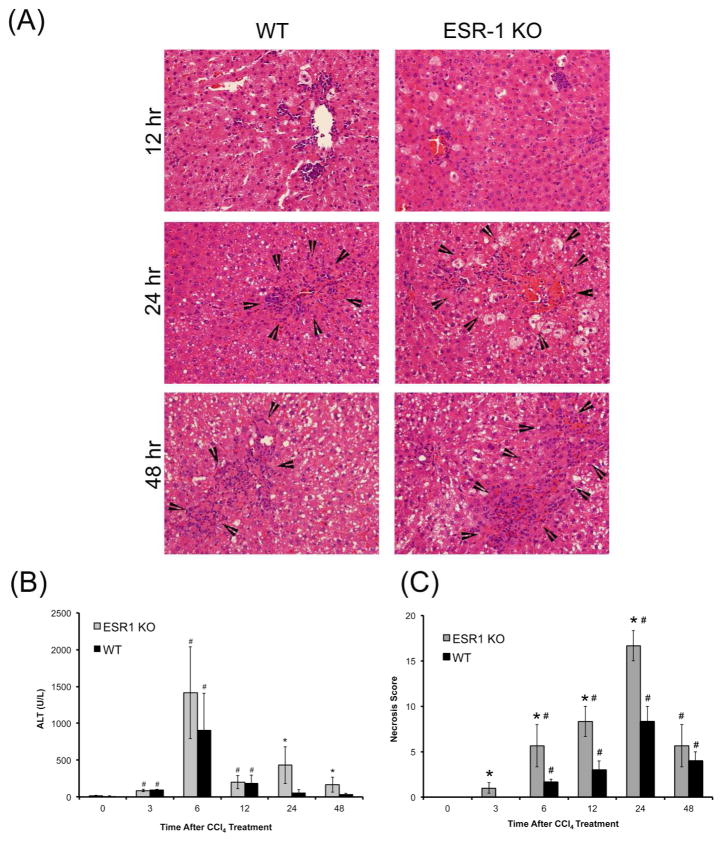
There is no difference in baseline histology of the liver between the WT and ESR1-KO rats (data not shown). Liver injury after CCl4 was measured by serum alanine amino transaminase (ALT) activity and hematoxylin and eosin staining of paraffin embedded liver sections (Figure 1). Hepatocyte balloon degeneration and necrosis were evident between 6 hr to 48 hr in both groups (Figure 1A). Both groups had a peak ALT levels at 6 hr and a subsequent decrease from 12–48 hr. Serum ALT increased in both groups at 6 hr after CCl4 treatment where it was moderately higher in ESR1-KO rats but was not statistically significant (Figure 1B). ALT levels decreased in both groups at 12 to 48 hr but were significantly higher in ESR1-KO rats. Necrosis scoring demonstrated a two-fold higher liver injury and cell death in ESR1-KO livers at 6, 12 and 24 hr (Figure 1C). Increased inflammatory cell foci appeared in both WT and ESR1-KO livers after 12 hr after CCl4 treatment and persisted throughout the time course.
Figure 1.
CCl4-induced liver injury in WT and ESR1-KO rats. (A) Representative photomicrographs (400x) of H&E stained liver sections of WT and ESR1-KO rat liver at 12, 24 and 48 hr after CCl4 treatment. Black arrowheads point to centrilobular necroinflammatory foci. Bar graphs showing serum ALT (B) and (C) percent necrosis in WT and ESR1-KO rats after CCl4 treatment. * denotes significant difference at P≤0.05 between KO and WT, # denoted significant difference at P≤0.05 at that time point from the 0 hr of the respective genotype.
Higher Neutrophil Infiltration in ESR1-KO rats
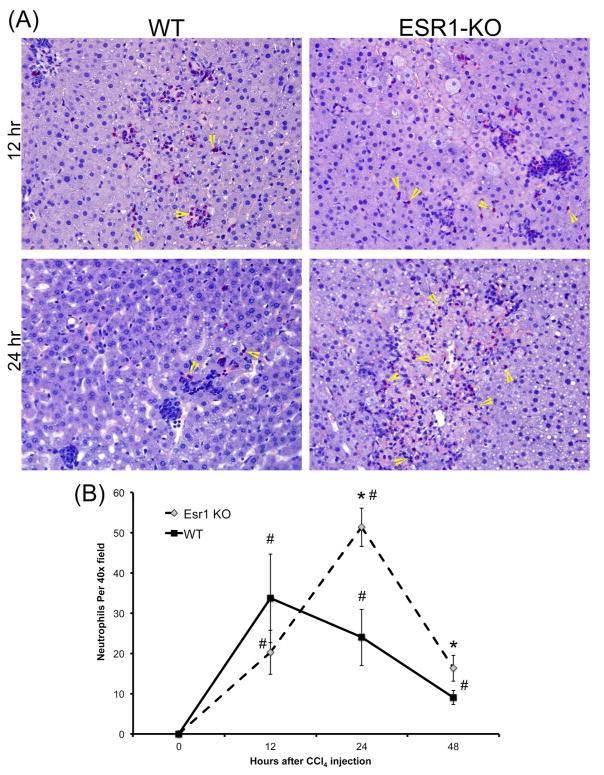
To further evaluate neutrophil infiltration in the liver after CCl4 treatment, we stained liver sections from WT and ESR1-KO rats using chloracetate esterase (CAE) histochemistry (Figure 2A) and counted the number of neutrophils, evident from their lobulated nuclei (Figure 2B). The data indicate that both WT and ESR1-KO rats had significant neutrophil infiltration in the liver after CCl4 treatment. At 12 hr after CCl4, both groups had similar number of neutrophils. However, at 24 and 48 hr, ESR1-KO rats had significantly higher neutrophils congregated in and around the necrotic foci.
Figure 2.
Increased neutrophil infiltration in ESR1-KO rat livers after CCl4 treatment. (A) Representative photomicrographs (400x) of chloracetate esterase (CAE) stained liver sections of WT and ESR1-KO rat liver at 12 and 24 hr after CCl4 treatment. Yellow arrowheads point to neutrophils. (B) Line graph showing neutrophil count of CAE stained liver section of WT and ESR1 rat livers at various time points. * denotes significant difference at P≤0.05 between KO and WT, # denoted significant difference at P≤0.05 at that time point from the 0 hr of the respective genotype.
Stimulated Liver Regeneration in ESR1-KO
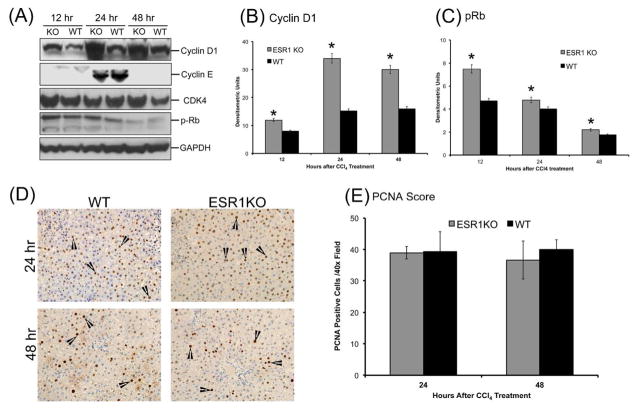
Compensatory liver regeneration was studied using western blot analysis of critical cell cycle proteins such as cyclin D1, Cyclin E, CDK4 and phosphorylated Rb protein. Western blot analysis showed that cyclin D1 protein expression is greater in ESR1-KO rats as compared to WT rats at all time points studied, with peak expression at 24 hr post CCl4 treatment (Figure 3A–B). Cyclin E expression increased in both WT and ESR1-KO livers from 12 to 24 hr and was undetectable at 48 hr. There was no significant difference in Cyclin E expression between WT and ESR1-KO groups at any time point. Protein expression of cyclin dependent kinase 4, or CDK4, was unchanged in the ESR1-KO rats when compared to ESR1-WT rats as shown by western blot. CDK4 phosphorylates its downstream target, the retinoblastoma protein or Rb. Western blot analysis showed that phosphorylation of Rb protein was significantly increased at 12 and 24 hr in ESR1-KO rats when compared to ESR1-WT rats (Figure 3C). Cell proliferation was further studied using PCNA immunohistochemistry (Figure 3D–E). No PCNA positive cells were observed at 12 hr after CCl4 treatment in either group. Equal numbers of PCNA positive cells were observed in WT and ESR1-KO rat livers at 24 and 48 hr. These data indicate that ESR1-KO rats exhibit equal stimulation of compensatory liver regeneration as compared to WT mice despite two-fold higher liver injury.
Figure 3.
Accelerated compensatory cell proliferation in ESR1-KO rats after CCl4 treatment. (A) Western blot analysis of cyclin D1, Cyclin E, CDK4, and phosphorylated Rb proteins at 12, 24 and 48 hr after CCl4 treatment performed using total liver cell extracts of WT and ESR1-KO rat livers. (B–C) Densitometric analysis of Cyclin D1 and pRb blots. (D) Representative photomicrographs of PCNA immunohistochemistry (400x) performed on WT and ESR1-KO rat livers at 24 and 48 hr after CCl4 treatment. Black arrowheads point to cells in S-phase of cell cycle (E) bar graph showing number of PCNA positive cells. * denotes significant difference at P≤0.05 between WT and KO.
Increase in Wnt/β-catenin and p65 signaling in ESR1-KO rats
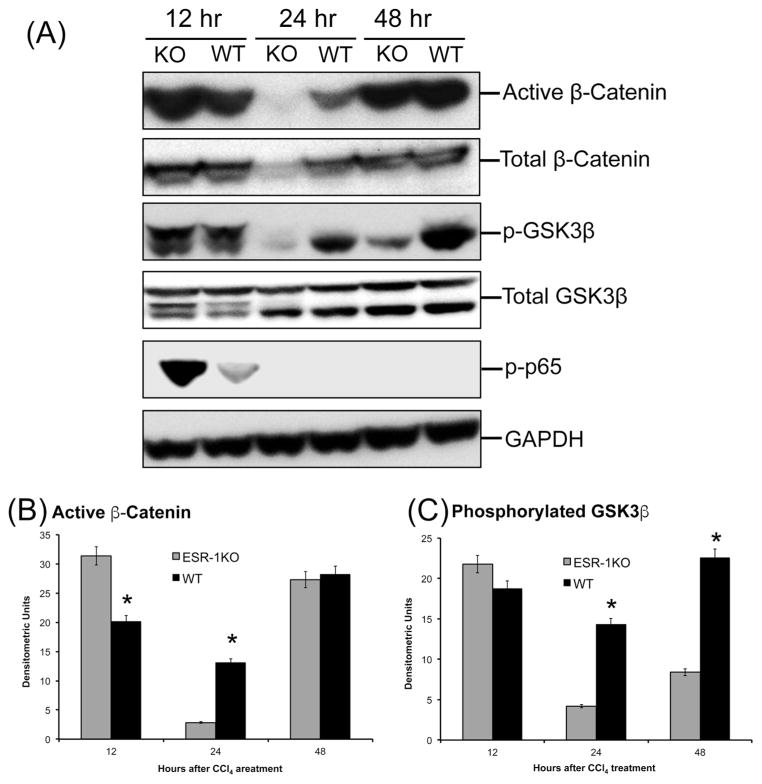
To determine the mechanism behind the stimulated liver regeneration in ESR1-KO rats despite higher injury, we investigated several pathways known to induce cyclin D1 and cell proliferation. Our investigation revealed higher activation of canonical Wnt signaling pathway in ESR1-KO rats. Western blot analysis showed an increase in activated (non-phosphorylated) β-catenin expression at 12 hr after CCl4 administration (Figure 4). Further, phosphorylation of GSK3β, the upstream regulator of β-catenin was also moderately higher in the ESR1-KO rats at 12 hr. Both active β-catenin and p-GSK3β increased in WT rat liver at 24 hr, coinciding with increased cyclin D1 expression and cell proliferation. We also studied expression and activation of p65, which homodimerizes to form active NF-κB signaling molecule There was no difference in total p65 protein (data not shown) but the phosphorylated active form of p65 was significantly higher in the ESR1KO mice at 12 hr after CCl4 treatment (Figure 4A).
Figure 4.
Increased β-catenin activation in ESR1-KO livers after CCl4 treatment. (A) Western blot analysis of total and activated β-catenin, phosphorylated nuclear p65; and total and phosphorylated GSK3β proteins at 12, 24 and 48 hr after CCl4 treatment performed using total liver cell extracts of WT and ESR1-KO rat livers. (B) Bar graphs showing densitometric analysis of the active β-catenin western blot. (C) Bar graphs showing densitometric analysis of the phosphorylated GSK3β western blot. * denotes significant difference at P≤0.05 between WT and KO.
Discussion
Estrogen and estrogen receptor signaling play a central role not only in reproduction but also cell injury and repair. Here we demonstrate that the disruption of ESR1 leads to significantly higher liver injury after CCl4 treatment. However, in spite of higher liver injury, the ESR1-KO rats exhibit rapid increase in compensatory regeneration after liver injury by CCl4. Previous studies on the role of estrogen-induced signaling have revealed contradictory roles in liver cell proliferation. Estrogen and estrogen receptor signaling seems to promote liver regeneration after PHX 28–30 but estrogen receptor signaling also provides protection against development of HCC 20–23, 31 where cell proliferation is an important component. Furthermore, apart from these seemingly contradictory results, the role of ESR1 in liver regeneration after chemical-induced liver injury has not been evaluated.
We investigated the role of estrogen-mediated signaling using the novel ESR1-KO rats and the CCl4 model of liver injury and regeneration. The CCl4 model provides several advantages over the PHX model including development of intrahepatic injury, inflammation and compensatory proliferation, all of which are part of HCC pathogenesis. Our studies indicate that disruption of ESR1-KO rats developed a significantly higher liver injury after CCl4 treatment. The higher injury was accompanied by higher inflammatory response in ESR1-KO rats that in the WT rats. Consistent with these data, we also observed increased neutrophil numbers in ESR1-KO rats after CCl4 administration. This is further supported by higher expression of phosphorylated p65, which indicates higher pro-inflammatory NF-κB signaling. These data are consistent with previous observations that estrogen-mediated signaling via ESR1 is anti-inflammatory 19, 21, 23, 31. Other studies indicate that estrogen may block neutrophil infiltrations and activation 32. Taken together, these data indicate that disruption of ESR1-mediated estrogen signaling results in higher liver injury and increased post-injury inflammatory response after acute exposure to hepatotoxicants.
It is well established that liver injury induced by chemicals results in compensatory liver regeneration 4, 5, 7. It is also known that the compensatory liver regeneration is inhibited by higher liver injury. Very high tissue injury can inhibit tissue repair by various mechanisms including significant cell stress and lack of critical pro-mitogenic signaling 5, 27. Interestingly, we observed that in spite of two-fold higher liver injury compensatory liver regeneration was completely unaffected in the ESR1-KO rats. Our data indicate that disruption of ESR1 may result in removal of estrogen-mediated inhibitory effect of hepatocyte proliferation, which allows the ESR1-KO hepatocytes to enter cell cycle despite high cellular injury. ESR1-KO livers had higher activation of cyclin D1 and pRb, the two critical regulators of cell cycle entry. Interestingly, while expression of the S phase cyclin, cyclin E was upregulated in both WT and ESR1 KO rats, was we did not observe any difference between the genotype. These data indicate that deletion of ESR1 affected mainly the cell cycle entry rather than cell cycle progression following CCl4 administration. This suggests that estrogen-mediated signaling may inhibit cell cycle entry of the hepatocytes. Overall, the faster increase in Cyclin D1 and pRb in ESR1-KO rats resulted in equal compensatory cell proliferation in ESR1-KO rats despite much higher liver injury.
Further studies demonstrated that the higher cyclin D1 protein induction in ESR1-KO rats might be due to increased Wnt/β-catenin and NF-κB signaling. Others and we have previously shown that cyclin D1 is a target gene of β-catenin 33–36 and is activated by canonical Wnt signaling during regeneration after acetaminophen overdose 27. We observed an increase in activated (dephosphorylated) β-catenin in ESR1-KO rat liver much earlier than the WT liver after CCl4 treatment. We also observed a concomitant increase in phosphorylation of GSK3β, which is the inactive form of GSK3β involved in β-catenin activation. Similarly, we observed an increase phosphorylation of p65 indicating increased NF-κB signaling 37–39. NF-κB is also known to stimulate cyclin D1 expression. The exact mechanism(s) by which ESR1 disruption up regulates Wnt signaling remains to be investigated. Previous studies have shown that ESR1 and β-catenin form a regulatory complex 40. It is possible that lack of ESR1 results in increased free β-catenin in hepatocytes. Alternatively, lack of ESR1-mediated signaling may result in increased Wnt signaling that further induces inactivation of GSK3β and activation of β-catenin.
Our study has shown that disruption of ESR1 in rat liver results in significantly higher cell injury but equal cell cycle activation, which is contrary to the role of estrogen and ESR1 in liver regeneration after PHX. One of the reasons for this discrepancy is the inherent difference in the models. In PHX model, where two thirds of the liver is surgically removed and the remaining liver is allowed to regenerate, there is minimal cell death in the regenerating lobes and no inflammation. In contrast, there is significant cell death after CCl4 treatment and cells that are next to the necrotic zone undergo proliferation. Additionally, there is significant inflammation as shown in our studies. It is known that estrogen can inhibit inflammatory signaling and the lack of ESR1 seems to exacerbate the inflammatory response. The inflammatory cells have a dual function in regeneration after toxicant injury. They are involved in phagocytosis of necrotic cell debris. They are also involved in secretion of pro-mitogenic cytokines and growth factors that further stimulate surrounding hepatocytes to proliferate. In our model, the significant inflammatory cell infiltration may be involved in secretion of pro-mitogenic signals, which further induce cell proliferation. Thus the lack of injury and inflammation in PHX model may result in a differential role of estrogen signaling in liver regeneration after PHX.
In summary, our studies indicate that disruption of ESR1 in a model of toxicant induced injury results in higher injury and inflammation. However, disruption of ESR1 removes inhibitory effects of estrogen signaling on compensatory cell proliferation and the dynamics of liver regeneration remains unaffected. These data also partially explain the protective effect of estrogen-mediated signaling on HCC as inflammation is known as a major component of HCC pathogenesis. The ESR1-KO rats are an innovative experimental model and could be further used to determine the mechanisms of hepatic injury and regeneration in the context of chemical cancer pathogenesis.
Acknowledgments
Sources of Funding: This work was supported by NIH grants 8P20 GM103549, 5T32ES007079-34, R01OD01478, R01DK102142, and 1R01DK098414
References
- 1.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–6. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 2.Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michalopoulos GK. Liver regeneration: molecular mechanisms of growth control. FASEB J. 1990;4:176–87. [PubMed] [Google Scholar]
- 4.Apte U. Liver Regeneration: An Introduction. In: Apte U, editor. Liver Regeneration: Basic Mechanisms, Relevant Models and Clinical Applications. 1. New York, NY, USA: Elsevier; 2015. pp. 1–14. [Google Scholar]
- 5.Mehendale HM. Tissue repair: an important determinant of final outcome of toxicant-induced injury. Toxicol Pathol. 2005;33:41–51. doi: 10.1080/01926230590881808. [DOI] [PubMed] [Google Scholar]
- 6.Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol. 2010;176:2–13. doi: 10.2353/ajpath.2010.090675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehendale HM. Role of hepatocellular regeneration and hepatolobular healing in the final outcome of liver injury. A two-stage model of toxicity. Biochem Pharmacol. 1991;42:1155–62. doi: 10.1016/0006-2952(91)90249-5. [DOI] [PubMed] [Google Scholar]
- 8.Mehendale HM, Roth RA, Gandolfi AJ, Klaunig JE, Lemasters JJ, Curtis LR. Novel mechanisms in chemically induced hepatotoxicity. FASEB J. 1994;8:1285–95. doi: 10.1096/fasebj.8.15.8001741. [DOI] [PubMed] [Google Scholar]
- 9.Schultze B, Gerhard H, Schump E, Maurer W. Autoradiographic investigation of proliferation and polyploidization during CCl4-induced liver regeneration in mice (author’s transl) Virchows Arch B Cell Pathol. 1973;14:329–43. [PubMed] [Google Scholar]
- 10.Rao PS, Mangipudy RS, Mehendale HM. Tissue injury and repair as parallel and opposing responses to CCl4 hepatotoxicity: a novel dose-response. Toxicology. 1997;118:181–93. doi: 10.1016/s0300-483x(97)03617-2. [DOI] [PubMed] [Google Scholar]
- 11.Kampa M, Pelekanou V, Notas G, Stathopoulos EN, Castanas E. The estrogen receptor: two or more molecules, multiple variants, diverse localizations, signaling and functions. Are we undergoing a paradigm-shift as regards their significance in breast cancer? Hormones (Athens, Greece) 2013;12:69–85. doi: 10.1007/BF03401288. [DOI] [PubMed] [Google Scholar]
- 12.Rumi MA, Dhakal P, Kubota K, Chakraborty D, Lei T, Larson MA, Wolfe MW, Roby KF, Vivian JL, Soares MJ. Generation of Esr1-knockout rats using zinc finger nuclease-mediated genome editing. Endocrinology. 2014;155:1991–9. doi: 10.1210/en.2013-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest. 2006;116:561–70. doi: 10.1172/JCI27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hewitt SC, Harrell JC, Korach KS. Lessons in estrogen biology from knockout and transgenic animals. Annu Rev Physiol. 2005;67:285–308. doi: 10.1146/annurev.physiol.67.040403.115914. [DOI] [PubMed] [Google Scholar]
- 15.Alvaro D, Alpini G, Onori P, Franchitto A, Glaser SS, Le Sage G, Folli F, Attili AF, Gaudio E. Alfa and beta estrogen receptors and the biliary tree. Molecular and cellular endocrinology. 2002;193:105–8. doi: 10.1016/s0303-7207(02)00103-x. [DOI] [PubMed] [Google Scholar]
- 16.Barone M, Ladisa R, Di Leo A, Spano D, Francioso D, Aglio V, Amoruso A, Francavilla A, Iolascon A. Estrogen-induced proliferation in cultured hepatocytes involves cyclin D1, p21(Cip1) and p27(Kip1) Dig Dis Sci. 2006;51:580–6. doi: 10.1007/s10620-006-3173-4. [DOI] [PubMed] [Google Scholar]
- 17.Kitagawa T, Yokoyama Y, Kokuryo T, Kawai T, Watanabe K, Kawai K, Nagino M. Estrogen promotes hepatic regeneration via activating serotonin signal. Shock (Augusta, Ga) 2009;31:615–20. doi: 10.1097/SHK.0b013e31818ec195. [DOI] [PubMed] [Google Scholar]
- 18.Francavilla A, Polimeno L, DiLeo A, Barone M, Ove P, Coetzee M, Eagon P, Makowka L, Ambrosino G, Mazzaferro V, et al. The effect of estrogen and tamoxifen on hepatocyte proliferation in vivo and in vitro. Hepatology. 1989;9:614–20. doi: 10.1002/hep.1840090417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi L, Feng Y, Lin H, Ma R, Cai X. Role of estrogen in hepatocellular carcinoma: is inflammation the key? J Transl Med. 2014;12:93. doi: 10.1186/1479-5876-12-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimizu I, Kohno N, Tamaki K, Shono M, Huang HW, He JH, Yao DF. Female hepatology: favorable role of estrogen in chronic liver disease with hepatitis B virus infection. World J Gastroenterol. 2007;13:4295–305. doi: 10.3748/wjg.v13.i32.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei T, Chen W, Wen L, Zhang J, Zhang Q, Yang J, Liu H, Chen BW, Zhou Y, Feng X, Yang Q, Bai X, Liang T. G protein-coupled estrogen receptor deficiency accelerates liver tumorigenesis by enhancing inflammation and fibrosis. Cancer Lett. 2016;382:195–202. doi: 10.1016/j.canlet.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Hong EJ, Levasseur MP, Dufour CR, Perry MC, Giguere V. Loss of estrogen-related receptor alpha promotes hepatocarcinogenesis development via metabolic and inflammatory disturbances. Proc Natl Acad Sci U S A. 2013;110:17975–80. doi: 10.1073/pnas.1315319110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei Q, Guo P, Mu K, Zhang Y, Zhao W, Huai W, Qiu Y, Li T, Ma X, Liu Y, Chen X, Han L. Estrogen suppresses hepatocellular carcinoma cells through ERbeta-mediated upregulation of the NLRP3 inflammasome. Lab Invest. 2015;95:804–16. doi: 10.1038/labinvest.2015.63. [DOI] [PubMed] [Google Scholar]
- 24.Sun B, Karin M. Obesity, inflammation, and liver cancer. J Hepatol. 2012;56:704–13. doi: 10.1016/j.jhep.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun L, Beggs K, Borude P, Edwards G, Bhushan B, Walesky C, Roy N, Manley MW, Jr, Gunewardena S, O’Neil M, Li H, Apte U. Bile acids promote diethylnitrosamine-induced hepatocellular carcinoma via increased inflammatory signaling. Am J Physiol Gastrointest Liver Physiol. 2016;311:G91–G104. doi: 10.1152/ajpgi.00027.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borude P, Edwards G, Walesky C, Li F, Ma X, Kong B, Guo GL, Apte U. Hepatocyte-specific deletion of farnesoid X receptor delays but does not inhibit liver regeneration after partial hepatectomy in mice. Hepatology. 2012;56:2344–52. doi: 10.1002/hep.25918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhushan B, Walesky C, Manley M, Gallagher T, Borude P, Edwards G, Monga SP, Apte U. Pro-regenerative signaling after acetaminophen-induced acute liver injury in mice identified using a novel incremental dose model. Am J Pathol. 2014;184:3013–25. doi: 10.1016/j.ajpath.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Francavilla A, di Leo A, Eagon PK, Wu SQ, Ove P, van Thiel DH, Starzl TE. Regenerating rat liver: correlations between estrogen receptor localization and deoxyribonucleic acid synthesis. Gastroenterology. 1984;86:552–7. [PMC free article] [PubMed] [Google Scholar]
- 29.Eagon PK, Porter LE, Francavilla A, DiLeo A, Van Thiel DH. Estrogen and androgen receptors in liver: their role in liver disease and regeneration. Semin Liver Dis. 1985;5:59–69. doi: 10.1055/s-2008-1041758. [DOI] [PubMed] [Google Scholar]
- 30.Umeda M, Hiramoto M, Imai T. Partial hepatectomy induces delayed hepatocyte proliferation and normal liver regeneration in ovariectomized mice. Clinical and experimental gastroenterology. 2015;8:175–82. doi: 10.2147/CEG.S80212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–4. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 32.Miller AP, Feng W, Xing D, Weathington NM, Blalock JE, Chen YF, Oparil S. Estrogen modulates inflammatory mediator expression and neutrophil chemotaxis in injured arteries. Circulation. 2004;110:1664–9. doi: 10.1161/01.CIR.0000142050.19488.C7. [DOI] [PubMed] [Google Scholar]
- 33.Apte U, Singh S, Zeng G, Cieply B, Virji MA, Wu T, Monga SP. Beta-catenin activation promotes liver regeneration after acetaminophen-induced injury. Am J Pathol. 2009;175:1056–65. doi: 10.2353/ajpath.2009.080976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Apte U, Zeng G, Muller P, Tan X, Micsenyi A, Cieply B, Dai C, Liu Y, Kaestner KH, Monga SP. Activation of Wnt/beta-catenin pathway during hepatocyte growth factor-induced hepatomegaly in mice. Hepatology. 2006;44:992–1002. doi: 10.1002/hep.21317. [DOI] [PubMed] [Google Scholar]
- 35.Apte U, Zeng G, Thompson MD, Muller P, Micsenyi A, Cieply B, Kaestner KH, Monga SP. beta-Catenin is critical for early postnatal liver growth. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1578–85. doi: 10.1152/ajpgi.00359.2006. [DOI] [PubMed] [Google Scholar]
- 36.Monga SP. Role and regulation of beta-catenin signaling during physiological liver growth. Gene expression. 2014;16:51–62. doi: 10.3727/105221614X13919976902138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diehl AM. Effect of ethanol on tumor necrosis factor signaling during liver regeneration. Clin Biochem. 1999;32:571–8. doi: 10.1016/s0009-9120(99)00057-0. [DOI] [PubMed] [Google Scholar]
- 38.Diehl AM. Cytokine regulation of liver injury and repair. Immunol Rev. 2000;174:160–71. doi: 10.1034/j.1600-0528.2002.017411.x. [DOI] [PubMed] [Google Scholar]
- 39.Diehl AM, Rai RM. Liver regeneration 3: Regulation of signal transduction during liver regeneration. FASEB J. 1996;10:215–27. doi: 10.1096/fasebj.10.2.8641555. [DOI] [PubMed] [Google Scholar]
- 40.Szotek EL, Narasipura SD, Al-Harthi L. 17beta-Estradiol inhibits HIV-1 by inducing a complex formation between beta-catenin and estrogen receptor alpha on the HIV promoter to suppress HIV transcription. Virology. 2013;443:375–83. doi: 10.1016/j.virol.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]