Abstract
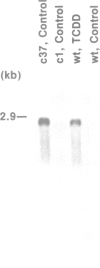
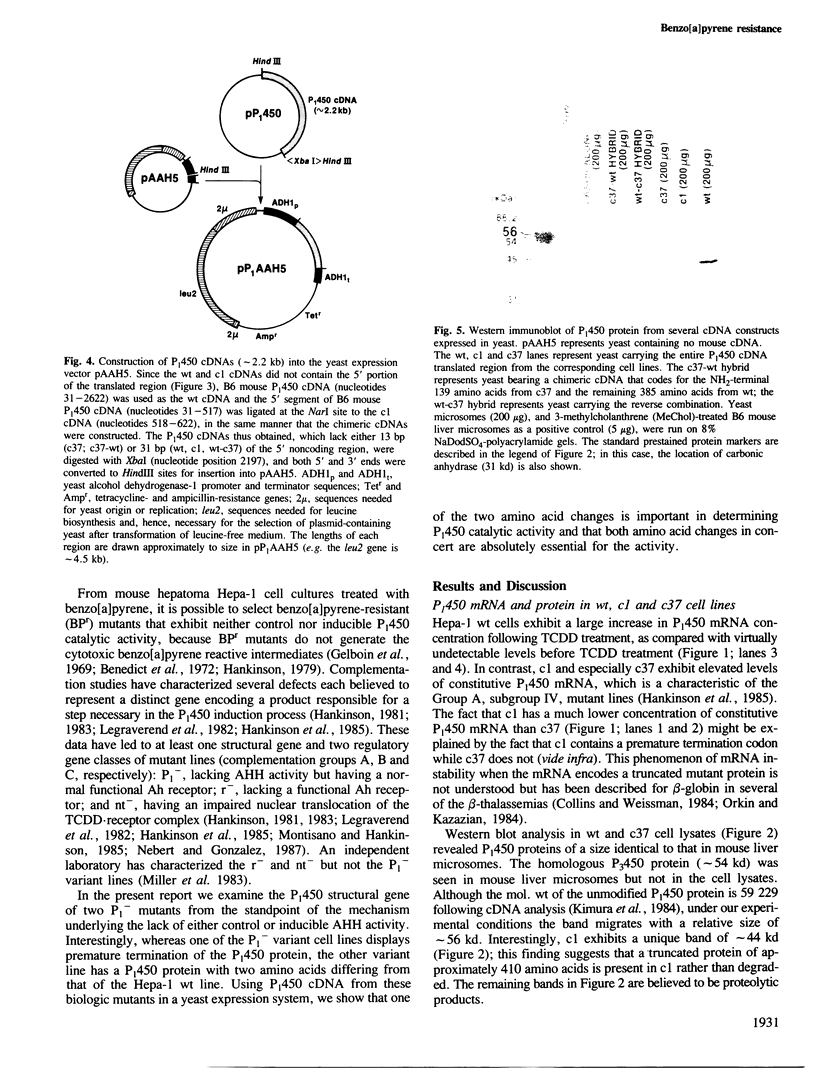
Two benzo[a]pyrene-resistant mutant clones (c1 and c37) of the mouse hepatoma Hepa-1 wild-type (wt) cell line were examined for their lack of P(1)450 [aryl hydrocarbon (benzo[a]pyrene) hydroxylase (AHH)] activity. From lambda gt11 cDNA libraries, the nearly full-length P(1)450 cDNAs of wt, c1 and c37 were isolated and sequenced. The c1 cDNA was found to have a single mutation leading to premature termination of the protein after Asn-414; a rapidly migrating band corresponding to this truncated protein was found on Western immunoblots. The c37 cDNA was found to have two point mutations, leading to Leu-118----Arg-118 and Arg-245----Pro-245, but otherwise to encode the normal (524-residue) protein; the mature protein was confirmed by Western blot analysis. P(1)450 cDNA from wt, c1 and c37 and chimeric cDNAs between wt and c37 were inserted into the expression vector pAAH5 and expressed in Saccharomyces cerevisiae strain 50.L4. The Leu-118----Arg-118 mutation alone was found to have negligible effect on AHH activity, while the Arg-245----Pro-245 mutation alone leads to a 2- to 3-fold decrease in enzyme activity. The two mutations together totally abrogate AHH activity. The biologic mutant c37 provides the first evidence for the importance of Arg-245, and the complementary function of Leu-118, in normal P(1)450 enzymic function. This alteration in a single amino acid from arginine to proline might block electron flow directly, or change secondary structure of the protein, such that normal monooxygenation of benzo[a]pyrene cannot occur.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesnik M., Atchison M. Genes for cytochrome P-450 and their regulation. CRC Crit Rev Biochem. 1986;19(3):247–305. doi: 10.3109/10409238609084657. [DOI] [PubMed] [Google Scholar]
- Ammerer G. Expression of genes in yeast using the ADCI promoter. Methods Enzymol. 1983;101:192–201. doi: 10.1016/0076-6879(83)01014-9. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict W. F., Gielen J. E., Nebert D. W. Polycyclic hydrocarbon-produced toxicity, transformation, and chromosomal aberrations as a function of aryl hydrocarbon hydroxylase activity in cell cultures. Int J Cancer. 1972 Mar 15;9(2):435–451. doi: 10.1002/ijc.2910090223. [DOI] [PubMed] [Google Scholar]
- Benedict W. F., Gielen J. E., Owens I. S., Niwa A., Bebert D. W. Aryl hydrocarbon hydroxylase induction in mammalian liver cell culture. IV. Stimulation of the enzyme activity in established cell lines derived from rat or mouse hepatoma and from normal rat liver. Biochem Pharmacol. 1973 Nov 1;22(21):2766–2769. doi: 10.1016/0006-2952(73)90138-x. [DOI] [PubMed] [Google Scholar]
- Bernhard H. P., Darlington G. J., Ruddle F. H. Expression of liver phenotypes in cultured mouse hepatoma cells: synthesis and secretion of serum albumin. Dev Biol. 1973 Nov;35(1):83–96. doi: 10.1016/0012-1606(73)90008-0. [DOI] [PubMed] [Google Scholar]
- Black S. D., Coon M. J. P-450 cytochromes: structure and function. Adv Enzymol Relat Areas Mol Biol. 1987;60:35–87. doi: 10.1002/9780470123065.ch2. [DOI] [PubMed] [Google Scholar]
- Callen D. F., Wolf C. R., Philpot R. M. Cytochrome P-450 mediated genetic activity and cytotoxicity of seven halogenated aliphatic hydrocarbons in Saccharomyces cerevisiae. Mutat Res. 1980 Jan;77(1):55–63. doi: 10.1016/0165-1218(80)90120-2. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Collins F. S., Weissman S. M. The molecular genetics of human hemoglobin. Prog Nucleic Acid Res Mol Biol. 1984;31:315–462. doi: 10.1016/s0079-6603(08)60382-7. [DOI] [PubMed] [Google Scholar]
- Conney A. H. Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons: G. H. A. Clowes Memorial Lecture. Cancer Res. 1982 Dec;42(12):4875–4917. [PubMed] [Google Scholar]
- Deininger P. L. Random subcloning of sonicated DNA: application to shotgun DNA sequence analysis. Anal Biochem. 1983 Feb 15;129(1):216–223. doi: 10.1016/0003-2697(83)90072-6. [DOI] [PubMed] [Google Scholar]
- Gelboin H. V., Huberman E., Sachs L. Enzymatic hydroxylation of benzopyrene and its relationship to cytotoxicity. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1188–1194. doi: 10.1073/pnas.64.4.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez F. J., Mackenzie P. I., Kimura S., Nebert D. W. Isolation and characterization of full-length mouse cDNA and genomic clones of 3-methylcholanthrene-inducible cytochrome P1-450 and P3-450. Gene. 1984 Sep;29(3):281–292. doi: 10.1016/0378-1119(84)90057-x. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Tukey R. H., Nebert D. W. Structural gene products of the Ah locus. Transcriptional regulation of cytochrome P1-450 and P3-450 mRNA levels by 3-methylcholanthrene. Mol Pharmacol. 1984 Jul;26(1):117–121. [PubMed] [Google Scholar]
- Hankinson O., Andersen R. D., Birren B. W., Sander F., Negishi M., Nebert D. W. Mutations affecting the regulation of transcription of the cytochrome P1-450 gene in the mouse Hepa-1 cell line. J Biol Chem. 1985 Feb 10;260(3):1790–1795. [PubMed] [Google Scholar]
- Hankinson O. Dominant and recessive aryl hydrocarbon hydroxylase-deficient mutants of mouse hepatoma line, Hepa-1, and assignment of recessive mutants to three complementation groups. Somatic Cell Genet. 1983 Jul;9(4):497–514. doi: 10.1007/BF01543050. [DOI] [PubMed] [Google Scholar]
- Hankinson O. Evidence that Benzo(a) pyrene-resistant, aryl hydrocarbon hydroxylase-deficient variants of mouse hepatoma line, Hepa-1, are mutational in origin. Somatic Cell Genet. 1981 Jul;7(4):373–388. doi: 10.1007/BF01542983. [DOI] [PubMed] [Google Scholar]
- Hankinson O. Single-step selection of clones of a mouse hepatoma line deficient in aryl hydrocarbon hydroxylase. Proc Natl Acad Sci U S A. 1979 Jan;76(1):373–376. doi: 10.1073/pnas.76.1.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel D. I., Whitlock J. P., Jr Regulation of cytochrome P1-450 gene transcription by 2,3,7, 8-tetrachlorodibenzo-p-dioxin in wild type and variant mouse hepatoma cells. J Biol Chem. 1984 May 10;259(9):5400–5402. [PubMed] [Google Scholar]
- Jerina D. M. The 1982 Bernard B. brodie Award Lecture. Metabolism of Aromatic hydrocarbons by the cytochrome P-450 system and epoxide hydrolase. Drug Metab Dispos. 1983 Jan-Feb;11(1):1–4. [PubMed] [Google Scholar]
- Kimura S., Gonzalez F. J., Nebert D. W. The murine Ah locus. Comparison of the complete cytochrome P1-450 and P3-450 cDNA nucleotide and amino acid sequences. J Biol Chem. 1984 Sep 10;259(17):10705–10713. [PubMed] [Google Scholar]
- Käppeli O. Cytochromes P-450 of yeasts. Microbiol Rev. 1986 Sep;50(3):244–258. doi: 10.1128/mr.50.3.244-258.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legraverend C., Hannah R. R., Eisen H. J., Owens I. S., Nebert D. W., Hankinson O. Regulatory gene product of the Ah locus. Characterization of receptor mutants among mouse hepatoma clones. J Biol Chem. 1982 Jun 10;257(11):6402–6407. [PubMed] [Google Scholar]
- Miller A. G., Israel D., Whitlock J. P., Jr Biochemical and genetic analysis of variant mouse hepatoma cells defective in the induction of benzo(a)pyrene-metabolizing enzyme activity. J Biol Chem. 1983 Mar 25;258(6):3523–3527. [PubMed] [Google Scholar]
- Montisano D. F., Hankinson O. Transfection by genomic DNA of cytochrome P1-450 enzymatic activity and inducibility. Mol Cell Biol. 1985 Apr;5(4):698–704. doi: 10.1128/mcb.5.4.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert D. W., Adesnik M., Coon M. J., Estabrook R. W., Gonzalez F. J., Guengerich F. P., Gunsalus I. C., Johnson E. F., Kemper B., Levin W. The P450 gene superfamily: recommended nomenclature. DNA. 1987 Feb;6(1):1–11. doi: 10.1089/dna.1987.6.1. [DOI] [PubMed] [Google Scholar]
- Nebert D. W. Genetic differences in microsomal electron transport: the Ah locus. Methods Enzymol. 1978;52:226–240. doi: 10.1016/s0076-6879(78)52026-0. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Gonzalez F. J. P450 genes: structure, evolution, and regulation. Annu Rev Biochem. 1987;56:945–993. doi: 10.1146/annurev.bi.56.070187.004501. [DOI] [PubMed] [Google Scholar]
- Negishi M., Jensen N. M., Garcia G. S., Nebert D. W. Structural gene products of the murine Ah complex. Differences in ontogenesis and glucosamine incorporation between liver microsomal cytochromes P1-450 and P-448 induced by polycyclic aromatic compounds. Eur J Biochem. 1981 Apr;115(3):585–594. [PubMed] [Google Scholar]
- Oeda K., Sakaki T., Ohkawa H. Expression of rat liver cytochrome P-450MC cDNA in Saccharomyces cerevisiae. DNA. 1985 Jun;4(3):203–210. doi: 10.1089/dna.1985.4.203. [DOI] [PubMed] [Google Scholar]
- Okey A. B., Bondy G. P., Mason M. E., Kahl G. F., Eisen H. J., Guenthner T. M., Nebert D. W. Regulatory gene product of the Ah locus. Characterization of the cytosolic inducer-receptor complex and evidence for its nuclear translocation. J Biol Chem. 1979 Nov 25;254(22):11636–11648. [PubMed] [Google Scholar]
- Okey A. B., Bondy G. P., Mason M. E., Nebert D. W., Forster-Gibson C. J., Muncan J., Dufresne M. J. Temperature-dependent cytosol-to-nucleus translocation of the Ah receptor for 2,3,7,8-tetrachlorodibenzo-p-dioxin in continuous cell culture lines. J Biol Chem. 1980 Dec 10;255(23):11415–11422. [PubMed] [Google Scholar]
- Orkin S. H., Kazazian H. H., Jr The mutation and polymorphism of the human beta-globin gene and its surrounding DNA. Annu Rev Genet. 1984;18:131–171. doi: 10.1146/annurev.ge.18.120184.001023. [DOI] [PubMed] [Google Scholar]
- Pelkonen O., Nebert D. W. Metabolism of polycyclic aromatic hydrocarbons: etiologic role in carcinogenesis. Pharmacol Rev. 1982 Jun;34(2):189–222. [PubMed] [Google Scholar]
- Poland A., Glover E., Kende A. S. Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J Biol Chem. 1976 Aug 25;251(16):4936–4946. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods L. F., Wiseman A. Benzo(a)pyrene hydroxylase from Saccharomyces cerevisiae. Substrate binding, spectral and kinetic data. Biochim Biophys Acta. 1980;613(1):52–61. doi: 10.1016/0005-2744(80)90191-6. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]