Abstract
The composition of an infant’s gut microbiome can impact their immediate and long-term health. Bifdobacteria play a major role in structuring the gut microbiome of breastfed infants due to their ability to consume oligosaccharides found in human milk. However, recent studies have revealed that bifidobacteria are often absent in the gut microbiome of breastfed infants in some locations. This lack of colonization may be due either to differences in the environmental conditions in the gastrointestinal tract of uncolonized infants which prohibit the growth of bifidobacteria or a dearth of sources from which infants may acquire these specialized bacterial species. Potential mechanisms by which these broad factors may lead to lower colonization of infants by bifidobacteria are discussed herein. Environmental conditions which may select against bifidobacteria include low rates/duration of breastfeeding, milk glycan composition, and antimicrobial use. Routes of colonization by bifidobacteria which may be disrupted include maternal transfer via vaginal birth, fecal-oral routes, or via breast milk itself. A careful contemplation of the conditions experienced by bifidobacteria over human evolutionary history may lead to further hypotheses as to the causative factors of the differential colonization by this foundation genus in some contemporary locations.
Introduction
Breastfeeding benefits human infants in numerous ways. One realm in which breast milk exerts an influence is in the development of the infant intestinal microbiome. Infants transition from near sterility in the womb to a sudden embrace by the microbial diversity of their new ex utero environment and yet possess a gut microbiota temporarily distinct from that of adults who encounter many of the same sources of inoculum. The guts of breastfed infants are typically dominated by bifidobacteria, unlike those of adults [1, 2]. Bifidobacteria are gram-positive anaerophilic bacteria commonly used as probiotics and are beneficial to infants in a number of ways. Infants with bifidobacteria-dominated gastrointestinal tracts are more resistant to colonization by pathogens, respond better to some vaccines, and possess better-functioning gut barriers [3–6]. Bifidobacteria also appear to simultaneously enhance immune surveillance and reduce inflammation [6–9]. Infant-type bifidobacteria present during weaning may guide the immune system towards tolerance during the introduction of new foods and their associated antigens, potentially influencing the development of allergic diseases [10–13]. For these public health reasons, among other motivations, bifidobacterial levels have been studied in infants across the globe.
Interestingly, it seems that not all infants have large amounts of bifidobacteria in their stool [14]. Comparisons of worldwide datasets (Norway [15], Sweden [16], Canada [17], Italy [18], Switzerland [19], Bangladesh [4], the USA [20], Malawi, and Finland [21]) show that the gut microbiomes of healthy breastfed infants in some populations had lower amounts of bifidobacteria than others. While further study is needed on the importance of bifidobacterial colonization in infants from such diverse contexts, given the apparent benefits of their presence, identifying the cause(s) of this phenomenon and developing potential solutions is of interest. The Dutch microbiologist Lourens Baas Becking once famously hypothesized that when it came to microbial biogeography “Everything is everywhere, but the environment selects” [22]. Proposed mechanisms for the differential bifidobacterial abundance phenomenon may be broken down into two broad categories mirroring Bass Becking’s statement: either the gut environments of some infants are differentially selective (against bifidobacteria), or there are higher barriers to bifidobacteria getting into infants in some places than others (bifidobacteria are, in fact, not “everywhere”). Using this conceptual framework, we will discuss various hypotheses for how bifidobacteria are acquired by infants, and how the gut microbiota is shaped in ways that may impact the immediate and future health of an infant.
Environmental Selection in the Gut
Many factors that influence the gut microbiome of infants fall under the general umbrella of selection-based determination, including the antimicrobial ingredients of breast milk (lysozyme, lactoferrin, and antibodies), the infant immune system, and infant exposure to antimicrobials [23–25]. Antibiotic use in particular has been recently shown to impact the infant microbiome, with some studies indicating that antibiotic exposure lowers bifidobacterial levels [26–29]. However, of the potential environmental conditions exerting selective pressure on the gut microbiota, diet is perhaps the most apparent, both in adults and infants [17, 30, 31]. Breast milk has been the principal source of nutrition for infants over human evolutionary history, and formula feeding leads to disruptions in the typical pattern of microbiota development in infants [30, 32]. The mechanism by which breast milk influences the microbiota is of translational interest, as current diet-based means to alter microbial ecosystems in a targeted manner are poorly developed. Breast milk contains macronutrient concentrations of oligosaccharides and glycoconjugates, collectively known as human milk glycans (HMGs) [33]. These carbohydrates pass undigested through the infant small intestine, and, once in the colon, they act as prebiotics supporting the establishment of bifidobacteria [34].
Select bifidobacterial species are uniquely equipped to fully consume the complex HMGs found in breast milk [35]. For example, Bifidobacterium longum subsp. infantis colonized premature infants better than Bifidobacterium animalis subsp. lactis when given concurrently with human milk, likely due to the capacity of B. longum subsp. infantis to consume a wider variety of HMGs [36, 37]. The structural complexity of HMGs serves as a barrier to other microbes which cannot successfully compete with bifidobacteria specialized for growth on these substrates. It should also be noted that not all breast milk contains the same mixture of HMGs. For example, maternal secretor status can influence the amount and type of fucosylated oligosaccharides present, which impacts the microbial community structure [20]. In this way, a breast milk diet is selective for a narrow set of bacterial species. The selective pressures of breast milk are strong enough that it is the cessation of breastfeeding, rather than the introduction of complementary foods, that allows for a community-wide shift in microbial composition [38]. Given this knowledge, it is plausible that cultural differences in the duration or rates of breastfeeding between locations may lead to differential colonization by bifidobacteria due to the availability of these selective growth substrates.
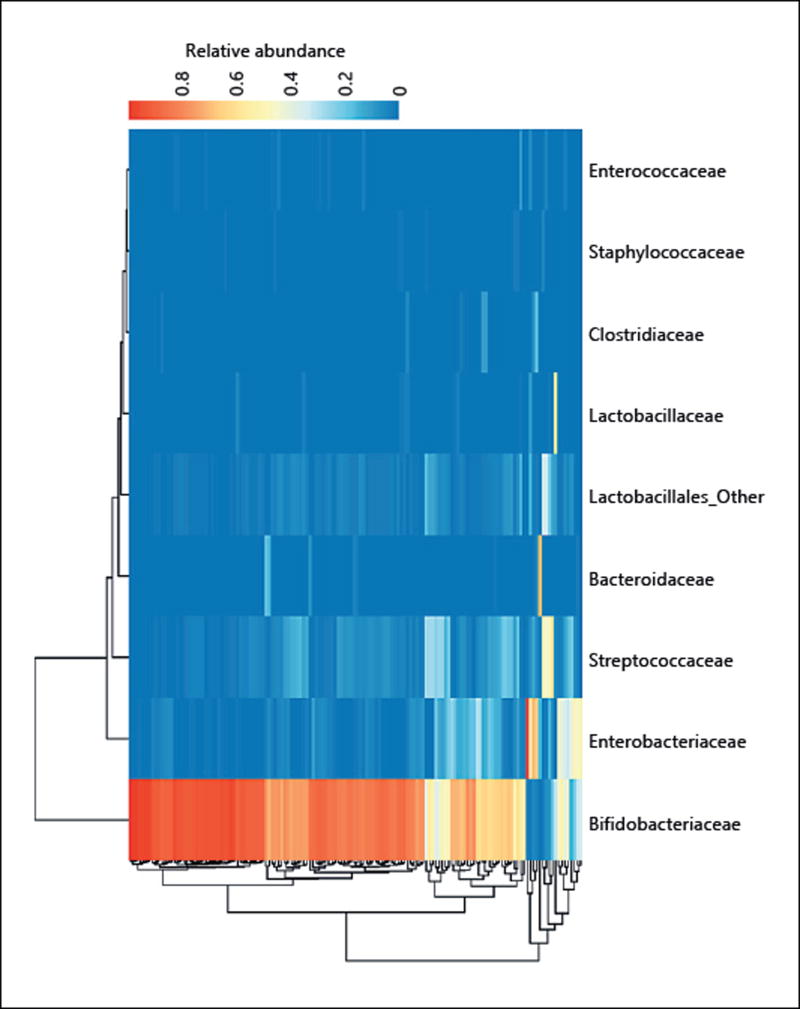
The context of other species present in any given infant is also an important selective factor in shaping the final community and thus infant health. Microbes do not exist in isolation but in consortia with large numbers of different taxa, which cooperate and compete in a dizzying array of interactions. Bacteroides and bifidobacteria, for example, have strain level metabolic interactions which depend on carbon source availability [39, 40]. They can both consume at least some HMGs, though select bifidobacteria have been shown to outcompete Bacteroides for these growth substrates [41]. In the absence of HMG-consuming bifidobacteria, however, Bacteroides species may dominate the infant gut microbiome and expose the infant to increased amounts of lipopolysaccharide types which are linked to downstream autoimmune disease [42]. In addition, Bacteroides degradation products of sialylated milk oligosaccharides have been shown to promote the growth of potentially pathogenic Enterobacteriaceae in in vitro studies, suggesting the presence of a “cross-feeding” effect [43–45]. This outgrowth of Enterobacteriaceae may also induce gut inflammation [45]. Several studies have previously observed a trade-off between the abundance of bifidobacteria and Proteobacteria in infants, which may partially be the result of the import-and-degrade strategy of HMG consumption of some bifidobacteria, which, unlike Bacteroides, do not leave behind degradation products for future proteobacterial consumption [4, 20, 37, 46] (Fig. 1). Some bifidobacteria do externally degrade glycans, as Bifidobacterium bifidum deploys external glycosyl hydrolases which have been shown to promote cross-feeding [47].
Fig. 1.
Trade-off between the abundances of bifidobacteria and Bacteroides in the gut of breastfed infants: the gut microbial community of infants in Bangladesh (adapted from Huda et al. [4]). Samples in rows are clustered by microbiome euclidean distance using a complete agglomeration method. Samples are colored in each column according to the relative abundance of the microbe on the x-axis label in that sample (1 = 100% abundance). Bifidobacteria dominate the gut microbiome of most infants and appear to be mutually exclusive with Enterobacteriaceae and Bacteroidaceae.
The metabolic end products of bifidobacterial metabolism (i.e. lactate and acetate) can also feed and influence the rest of the microbial community [48]. Acetate is also known to be protective against some pathogens [3]. Acetate and lactate also have the secondary effect of lowering the pH of the fecal environment, which is in and of itself a major selective factor, including via specific inhibition of Bacteroides species [49–51]. Taken together, this evidence suggests that colonization of breastfed infants by HMG-consuming bifidobacteria averts an alternative pattern of microbiota establishment which may include overexposure to Bacteroides endotoxin, cross-feeding of potentially pathogenic Enterobacteriaceae, and the induction of proinflammatory cytokines – all of which impact infant health.
Acquisition of Species: Bacterial Migration and Transmission
In comparison to environmental selection, the transmission of microbial species to the infant gastrointestinal tract is more difficult to measure. Many studies show overlap between operational taxonomic units in putative source environments and the infant gut microbiome, but this does not show directionality of transfer, nor does it rule out a third common source for the other two environments. However, there is existing evidence to support several possible routes of transfer to at least some infants.
The initial source of microbes in infants is often thought to be from the mother. The placenta is near sterile and likely contributes little to the gut microbiome in the first days of life [52]. Transfer from the mother’s vaginal canal during birth is the first major source of inoculum [53]. Cesarean section birth limits exposure to possible inoculation both via maternal stool during birth and via vaginal contact, and, as a result, cesarean section-born infants often possess distinct gut microbial assemblages which are occasionally lower in bifidobacteria [17, 32, 54]. Bifidobacteria have been detected in the vagina, although specialized HMG degraders such as B. longum subsp. infantis appear to be rare in that environment, and, where found, their presence may be due to fecal contamination [55–59]. The mother’s intestinal microbiota is a likely source of some bifidobacteria for the infant, both during and after the birthing process, and several studies have proven strain congruence among isolates from mother’s and infant’s feces [55, 60]. The skin of mothers and other caretakers may also be a vector for the early transfer of intestinal microbes [61]. Other potential sources of microbes include siblings, pets, and the built environment [62–64].
Breast milk contains microbes, including bifidobacteria, but their origin and potential impact on colonization remains unclear [65–68]. Reverse flow of milk during nursing indicates likely contaminating transfer of external microbes into the mammary gland, complicating inference of transfer directionality [69]. A so-called “enteromammary” pathway has also been postulated whereby the mother’s immune system gathers microbes from the mother’s gastrointestinal tract and, without killing them, transfers them to be expressed from the mammary gland to the infant during suckling at the breast [70, 71]. This hypothesis remains speculative and has the disadvantage that the absolute number of microbes transferred in such a system would likely be relatively low in comparison to inoculation from other sources (i.e., feces). Whether an elaborate system for transferring what were likely common infant intestinal microbes would be advantageous in the “environment of evolutionary adaptedness ” [72] (the ancestral conditions under which the proposed enteromammary pathway putatively arose) or whether a simple fecal-oral route would suffice is an open question.
The environment of evolutionary adaptedness was likely more microbially intensive than is typical in developed nations today, given the lack of hygiene, absence of man-made antimicrobials, and more regular exposure to the diverse array of “outdoor” microbes that go hand-in-hand with a hunter-gatherer or, later, pastoral lifestyle [73]. However, anaerophilic HMG-degrading specialist bifidobacteria were and are not likely to be ubiquitous in all putative source environments for intestinal microbes. Indeed, bifidobacteria have been rare in the gastrointestinal tract of human adults in the traditionally living people studied so far [74]. Indeed, Bifidobacterium abundance in adults appears to correlate with the consumption of dairy in the population, as the genus is absent from the Hadza hunter-gatherers (no access to dairy) while being prevalent only in the urban populations of the remote Nicobarese tribe (the subpopulation which has access to dairy) [75, 76]. However, it is often difficult to disentangle the effect of dairy with that of simply living in higher-density populations with more opportunity for horizontal acquisition of microbes from other individuals. The ultimate source of infant-type bifidobacteria, for the moment, remains unidentified.
Conclusion
The past century has seen drastic shifts in both the selective pressures experienced by the human gut microbiota and the opportunities for microbe transmission. The possibility for undesirable side effects of these changes has recently become increasingly clear. Lack of exposure to commensal species, such as bifidobacteria, was unlikely to be an issue often faced by our ancestors. Human physiology may have co-evolved with “expected” exposure to bifidobacteria during the life stages concurrent with breastfeeding, and their presence may provide important developmental cues and protection from disease. Because bifidobacteria are an important foundation species that unlock a key carbon source and facilitate an entire metabolic network leading to adaptive development of the infant gut microbiota, their disappearance may lead to sequelae of public health importance [71, 77, 78]. The “hygiene hypothesis” encompasses this idea and attempts to connect diseases such as diabetes, inflammatory bowel disease, autism, allergies, atopy, metabolic syndrome, and chronic inflammatory bowel diseases with microbial dysbiosis [reviewed in 11, 71, 79].
To design interventions that remedy putative Bifidobacterium -related microbial dysbiosis early in life and avoid its potential undesirable outcomes, one must first understand the reasons behind the undercolonization of infants by bifidobacteria in some locations. If the causative mechanism falls under the “environmental selection” umbrella, the solution would need to shift conditions in the gut, such as eliminating the presence of antimicrobials or promoting bifidobacterial growth through targeted HMG-like prebiotics. If the cause is instead that many infants are simply never exposed to the appropriate bifidobacteria, application of an HMG-consuming Bifidobacterium -containing probiotic may be a simple solution. However, no amount of prebiotic can enrich a taxa that is not present, and no amount of administered live bifidobacterial cells can establish colonization of a species in an environment that is nonpermissive for its growth. Transnational comparisons of breastfed infant gut microbial communities, combined with the appropriate metadata, may be useful to disentangle the relevant contributing factors and clarify what drives local bifidobacterial colonization patterns. Such studies may best be conducted in the form of ongoing “microbial community observatories” as suggested by Charbonneau et al. [80]. Ultimately, the combination of multiple types of expertise, such as anthropology, epidemiology, microbiology, chemistry, medicine, and public health, will be necessary to address this developing phenomenon.
Acknowledgments
We kindly thank Steven Frese for his assistance with Figure 1. This work was supported, in part, by funding from National Institutes of Health awards R01AT007079 and R01AT008759 and the Peter J. Shields Endowed Chair in Dairy Food Science (D.A.M.). Z.T.L. is supported by an Alfred P. Sloan Foundation’s Microbiology of the Built Environment postdoctoral fellowship.
Footnotes
Disclosure Statement
D.A.M. is a co-founder of Evolve Biosystems, a company focused on diet-based manipulation of the gut microbiota. Evolve BioSystems played no role in the design, execution, interpretation, or publication of this study.
References
- 1.Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khonsari S, Suganthy M, Burczynska B, et al. A comparative study of bifidobacteria in human babies and adults. Biosci Microbiota. 2016;35:97–103. doi: 10.12938/bmfh.2015-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukuda S, Toh H, Hase K, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543–547. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 4.Huda MN, Lewis ZT, Kalanetra KM, et al. Stool microbiota and vaccine responses of infants. Pediatrics. 2014;134:e362–e372. doi: 10.1542/peds.2013-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romond M-B, Colavizza M, Mullié C, et al. Does the intestinal bifidobacterial colonisation affect bacterial translocation? Anaerobe. 2008;14:43–48. doi: 10.1016/j.anaerobe.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Chichlowski M, De Lartigue G, German JB, et al. Bifidobacteria isolated from infants and cultured on human milk oligosaccharides affect intestinal epithelial function. J Pediatr Gastroenterol Nutr. 2012;55:321–327. doi: 10.1097/MPG.0b013e31824fb899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheil B, MacSharry J, O’Callaghan L, et al. Role of interleukin (IL-10) in probiotic-mediated immune modulation: an assessment in wild-type and IL-10 knock-out mice. Clin Exp Immunol. 2006;144:273–280. doi: 10.1111/j.1365-2249.2006.03051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanabe S, Kinuta Y, Saito Y. Bifidobacterium infantis suppresses proinflammatory interleukin-17 production in murine splenocytes and dextran sodium sulfate-induced intestinal inflammation. Int J Mol Med. 2008;22:181–185. [PubMed] [Google Scholar]
- 9.Preising J, Philippe D, Gleinser M, et al. Selection of bifidobacteria based on adhesion and anti-inflammatory capacity in vitro for amelioration of murine colitis. Appl Environ Microbiol. 2010;76:3048–3051. doi: 10.1128/AEM.03127-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young SL, Simon MA, Baird MA, et al. Bifidobacterial species differentially affect expression of cell surface markers and cytokines of dendritic cells harvested from cord blood. Clin Diagn Lab Immunol. 2004;11:686–690. doi: 10.1128/CDLI.11.4.686-690.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown EM, Arrieta M-C, Finlay BB. A fresh look at the hygiene hypothesis: how intestinal microbial exposure drives immune effector responses in atopic disease. Semin Immunol. 2013;25:378–387. doi: 10.1016/j.smim.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Kirjavainen PV, Arvola T, Salminen SJ, Isolauri E. Aberrant composition of gut microbiota of allergic infants: a target of bifidobacterial therapy at weaning? Gut. 2002;51:51–55. doi: 10.1136/gut.51.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enomoto T, Sowa M, Nishimori K, et al. Effects of bifidobacterial supplementation to pregnant women and infants in the prevention of allergy development in infants and on fecal microbiota. Allergol Int. 2014;63:575–585. doi: 10.2332/allergolint.13-OA-0683. [DOI] [PubMed] [Google Scholar]
- 14.Tannock GW, Lee PS, Wong KH, et al. Why don’t all infants have bifidobacteria in their stool? Front Microbiol. 2016;7:834. doi: 10.3389/fmicb.2016.00834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avershina E, Ola S, Oien T, et al. Major fecal microbiota shifts in composition and diversity with age in a geographically restricted cohort of mothers and their children. FEMS Microbiol Ecol. 2014;87:280–290. doi: 10.1111/1574-6941.12223. [DOI] [PubMed] [Google Scholar]
- 16.Abrahamsson TR, Jakobsson HE, Andersson AF, et al. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin Exp Allergy. 2014;44:842–850. doi: 10.1111/cea.12253. [DOI] [PubMed] [Google Scholar]
- 17.Azad MB, Konya T, Maughan H, et al. Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ. 2013;185:385–394. doi: 10.1503/cmaj.121189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roos S, Dicksved J, Tarasco V, et al. 454 pyrosequencing analysis on faecal samples from a randomized DBPC trial of colicky infants treated with Lactobacillus reuteri DSM 17938. PLoS One. 2013;8:e56710. doi: 10.1371/journal.pone.0056710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jost T, Lacroix C, Braegger CP, Chassard C. New insights in gut microbiota establishment in healthy breast fed neonates. PLoS One. 2012;7:e44595. doi: 10.1371/journal.pone.0044595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis ZT, Totten SM, Smilowitz JT, et al. Maternal fucosyltransferase 2 status affects the gut bifidobacterial communities of breastfed infants. Microbiome. 2015;3:1–21. doi: 10.1186/s40168-015-0071-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grześkowiak Ł, Collado MC, Mangani C, et al. Distinct gut microbiota in southeastern African and northern European infants. J Pediatr Gastroenterol Nutr. 2012;54:812–816. doi: 10.1097/MPG.0b013e318249039c. [DOI] [PubMed] [Google Scholar]
- 22.de Wit R, Bouvier T. “Everything is everywhere, but, the environment selects,” what did Baas Becking and Beijerinck really say? Environ Microbiol. 2006;8:755–758. doi: 10.1111/j.1462-2920.2006.01017.x. [DOI] [PubMed] [Google Scholar]
- 23.Microbiomes H, Halden RU. Lessons learned from probing for impacts of triclosan and triclocarban on human microbiomes. mSphere. 2014;1:1–4. doi: 10.1128/mSphere.00089-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zivkovic AM, Lewis ZT, German JB, Mills DA. Establishment of a milk-oriented microbiota (MOM) in early life: how babies meet their MOMs. Funct Food Rev. 2013;5:3–12. [Google Scholar]
- 25.van Best N, Hornef MW, Savelkoul PHM, Penders J. On the origin of species: factors shaping the establishment of infant’s gut microbiota. Birth Defects Res Part C Embryo Today. 2015;105:240–251. doi: 10.1002/bdrc.21113. [DOI] [PubMed] [Google Scholar]
- 26.Korpela K, Salonen A, Virta LJ, et al. Association of early-life antibiotic use and protective effects of breastfeeding: role of the intestinal microbiota. JAMA Pediatr. 2016;170:750–757. doi: 10.1001/jamapediatrics.2016.0585. [DOI] [PubMed] [Google Scholar]
- 27.Yassour M, Vatanen T, Siljander H, et al. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci Transl Med. 2016;8:343ra81. doi: 10.1126/scitranslmed.aad0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bokulich NA, Chung J, Battaglia T, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016;8:343ra82. doi: 10.1126/scitranslmed.aad7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azad M, Konya T, Persaud R, et al. Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study. BJOG. 2015;123:983–993. doi: 10.1111/1471-0528.13601. [DOI] [PubMed] [Google Scholar]
- 30.Liu Z, Roy NC, Guo Y, et al. Human breast milk and infant formulas differentially modify the intestinal microbiota in human infants and host physiology in rats. J Nutr. 2016;146:191–199. doi: 10.3945/jn.115.223552. [DOI] [PubMed] [Google Scholar]
- 31.Muegge BD, Kuczynski J, Knights D, et al. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science. 2011;332:970–974. doi: 10.1126/science.1198719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madan JC, Hoen AG, Lundgren SN, et al. Association of cesarean delivery and formula supplementation with the intestinal microbiome of 6-week-old infants. JAMA Pediatr. 2016;170:212–219. doi: 10.1001/jamapediatrics.2015.3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.German JB, Freeman SL, Lebrilla CB, Mills DA. Human milk oligosaccharides: evolution, structures and bioselectivity as substrates for intestinal bacteria. Nestle Nutr Work Ser Pediatr Progr. 2008;62:205–222. doi: 10.1159/000146322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kunz C, Rudloff S, Baier W, et al. Oligosaccharides in human milk: structural, functional, and metabolic aspects. Annu Rev Nutr. 2000;20:699–722. doi: 10.1146/annurev.nutr.20.1.699. [DOI] [PubMed] [Google Scholar]
- 35.Garrido D, Dallas DC, Mills DA. Consumption of human milk glycoconjugates by infant-associated bifidobacteria: mechanisms and implications. Microbiology. 2013;159:649–664. doi: 10.1099/mic.0.064113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LoCascio RG, Desai P, Sela DA, et al. Broad conservation of milk utilization genes in Bifidobacterium longum subsp. infantis as revealed by comparative genomic hybridization. Appl Environ Microbiol. 2010;76:7373–7381. doi: 10.1128/AEM.00675-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Underwood MA, Kalanetra KM, Bokulich NA, et al. A comparison of two probiotic strains of bifidobacteria in premature infants. J Pediatr. 2013;163:1585–1591. doi: 10.1016/j.jpeds.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bäckhed F, Roswall J, Peng Y, et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. 2015;17:690–703. doi: 10.1016/j.chom.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 39.Rios-Covian D, Arboleya S, Hernandez-Barranco AM, et al. Interactions between Bifidobacterium and Bacteroides species in cofermentations are affected by carbon sources, including exopolysaccharides produced by bifidobacteria. Appl Environ Microbiol. 2013;79:7518–7524. doi: 10.1128/AEM.02545-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Falony G, Calmeyn T, Leroy F, De Vuyst L. Coculture fermentations of Bifidobacterium species and Bacteroides thetaiotaomicron reveal a mechanistic insight into the prebiotic effect of inulin-type fructans. Appl Environ Microbiol. 2009;75:2312–2319. doi: 10.1128/AEM.02649-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marcobal A, Barboza M, Sonnenburg ED, et al. Bacteroides in the infant gut consume milk oligosaccharides via mucus-utilization pathways. Cell Host Microbe. 2011;10:507–514. doi: 10.1016/j.chom.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vatanen T, Kostic AD, d’Hennezel E, et al. Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell. 2016;165:1551. doi: 10.1016/j.cell.2016.05.056. [DOI] [PubMed] [Google Scholar]
- 43.Charbonneau MR, O’Donnell D, Blanton LV, et al. Sialylated milk oligosaccharides promote microbiota-dependent growth in models of infant undernutrition. Cell. 2016;164:1–13. doi: 10.1016/j.cell.2016.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frese SA, Parker K, Calvert CC, Mills DA. Diet shapes the gut microbiome of pigs during nursing and weaning. Microbiome. 2015;3:28. doi: 10.1186/s40168-015-0091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang Y-L, Chassard C, Hausmann M, et al. Sialic acid catabolism drives intestinal inflammation and microbial dysbiosis in mice. Nat Commun. 2015;6:8141. doi: 10.1038/ncomms9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang M, Li M, Wu S, et al. Fecal microbiota composition of breast-fed infants is correlated with human milk oligosaccharides consumed. J Pediatr Gastroenterol Nutr. 2015;60:825–833. doi: 10.1097/MPG.0000000000000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Egan M, Motherway MO, Kilcoyne M, et al. Cross-feeding by Bifidobacterium breve UCC2003 during co-cultivation with Bifidobacterium bifidum PRL2010 in a mucinbased medium. BMC Microbiol. 2014;14:282. doi: 10.1186/s12866-014-0282-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Vuyst L, Leroy F. Cross-feeding between bifidobacteria and butyrate-producing colon bacteria explains bifdobacterial competitiveness, butyrate production, and gas production. Int J Food Microbiol. 2011;149:73–80. doi: 10.1016/j.ijfoodmicro.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 49.Ogawa K, Ben RA, Pons S, et al. Volatile fatty acids, lactic acid, and pH in the stools of breast-fed and bottle-fed infants. J Pediatr Gastroenterol Nutr. 1992;15:248–252. doi: 10.1097/00005176-199210000-00004. [DOI] [PubMed] [Google Scholar]
- 50.Lauber CL, Hamady M, Knight R, Fierer N. Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl Environ Microbiol. 2009;75:5111–5120. doi: 10.1128/AEM.00335-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walker AW, Duncan SH, Leitch ECM, et al. pH and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within microbial communities from the human colon. Appl Environ Microbiol. 2005;71:3692–3700. doi: 10.1128/AEM.71.7.3692-3700.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lauder AP, Roche AM, Sherrill-Mix S, et al. Comparison of placenta samples with contamination controls does not provide evidence for a distinct placenta microbiota. Microbiome. 2016;4:29. doi: 10.1186/s40168-016-0172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107:11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martin R, Makino H, Cetinyurek Yavuz A, et al. Early-life events, including mode of delivery and type of feeding, siblings and gender, shape the developing gut microbiota. PLoS One. 2016;11:e0158498. doi: 10.1371/journal.pone.0158498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mikami K, Takahashi H, Kimura M, et al. Influence of maternal bifidobacteria on the establishment of bifidobacteria colonizing the gut in infants. Pediatr Res. 2009;65:669–674. doi: 10.1203/PDR.0b013e31819ed7a8. [DOI] [PubMed] [Google Scholar]
- 56.Makino H, Kushiro A, Ishikawa E, et al. Transmission of intestinal Bifidobacterium longum subsp. longum strains from mother to infant, determined by multilocus sequencing typing and amplified fragment length polymorphism. Appl Environ Microbiol. 2011;77:6788–6793. doi: 10.1128/AEM.05346-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Makino H, Kushiro A, Ishikawa E, et al. Mother-to-infant transmission of intestinal bifidobacterial strains has an impact on the early development of vaginally delivered infant’s microbiota. PLoS One. 2013;8:e78331. doi: 10.1371/journal.pone.0078331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burton JP, Dixon JL, Reid G. Detection of Bifidobacterium species and Gardnerella vaginalis in the vagina using PCR and denaturing gradient gel electrophoresis. 2003;81:61–63. doi: 10.1016/s0020-7292(02)00408-3. [DOI] [PubMed] [Google Scholar]
- 59.Chaban B, Links MG, Jayaprakash TP, et al. Characterization of the vaginal microbiota of healthy Canadian women through the menstrual cycle. Microbiome. 2014;2:23. doi: 10.1186/2049-2618-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fanaro S, Chierici R, Guerrini P, Vigi V. Intestinal microflora in early infancy: composition and development. Acta Paediatr Suppl. 2003;91:48–55. doi: 10.1111/j.1651-2227.2003.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 61.Adlerberth I, Wold AE. Establishment of the gut microbiota in Western infants. Acta Paediatr. 2009;98:229–238. doi: 10.1111/j.1651-2227.2008.01060.x. [DOI] [PubMed] [Google Scholar]
- 62.Lax S, Smith D, Shogan BD, et al. Longitudinal analysis of microbial interaction between humans and the indoor environment. Science. 2014;345:1048–1052. doi: 10.1126/science.1254529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Song SJ, Lauber C, Costello EK, et al. Cohabiting family members share microbiota with one another and with their dogs. eLIFE. 2013;2:e00458. doi: 10.7554/eLife.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Konya T, Koster B, Maughan H, et al. Associations between bacterial communities of house dust and infant gut. Environ Res. 2014;131:25–30. doi: 10.1016/j.envres.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 65.Perez PF, Doré J, Leclerc M, et al. Bacterial imprinting of the neonatal immune system: lessons from maternal cells? Pediatrics. 2007;119:e724–e732. doi: 10.1542/peds.2006-1649. [DOI] [PubMed] [Google Scholar]
- 66.Fernández L, Langa S, Martín V, et al. The human milk microbiota: origin and potential roles in health and disease. Pharmacol Res. 2013;69:1–10. doi: 10.1016/j.phrs.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 67.Urbaniak C, Burton JP, Reid G. Breast, milk and microbes: a complex relationship that does not end with lactation. Womens Health (Lond) 2012;8:385–398. doi: 10.2217/whe.12.23. [DOI] [PubMed] [Google Scholar]
- 68.Martín R, Jiménez E, Heilig H, et al. Isolation of bifidobacteria from breast milk and assessment of the bifidobacterial population by PCR-denaturing gradient gel electrophoresis and quantitative real-time PCR. Appl Environ Microbiol. 2009;75:965–969. doi: 10.1128/AEM.02063-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ramsay DT, Kent JC, Owens RA, Hartmann PE. Ultrasound imaging of milk ejection in the breast of lactating women. Pediatrics. 2004;113:361–367. doi: 10.1542/peds.113.2.361. [DOI] [PubMed] [Google Scholar]
- 70.Jeurink PV, van Bergenhenegouwen J, Jiménez E, et al. Human milk: a source of more life than we imagine. Benef Microbes. 2013;4:17–30. doi: 10.3920/BM2012.0040. [DOI] [PubMed] [Google Scholar]
- 71.Scholtens PAMJ, Oozeer R, Martin R, et al. The early settlers: intestinal microbiology in early life. Annu Rev Food Sci Technol. 2012;3:425–447. doi: 10.1146/annurev-food-022811-101120. [DOI] [PubMed] [Google Scholar]
- 72.Bowlby J. Attachment and Loss. New York: Basic Books; 1969. [Google Scholar]
- 73.Schnorr SL. The diverse microbiome of the hunter-gatherer. Nature. 2015;518:S14. doi: 10.1038/518S14a. [DOI] [PubMed] [Google Scholar]
- 74.Obregon-Tito AJ, Tito RY, Metcalf J, et al. Subsistence strategies in traditional societies distinguish gut microbiomes. Nat Commun. 2015;6:6505. doi: 10.1038/ncomms7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schnorr SL, Candela M, Rampelli S, et al. Gut microbiome of the Hadza hunter-gatherers. Nat Commun. 2014;5:3654. doi: 10.1038/ncomms4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Anwesh M, Kumar KV, Nagarajan M, et al. Elucidating the richness of bacterial groups in the gut of Nicobarese tribal community – perspective on their lifestyle transition. Anaerobe. 2016;39:68–76. doi: 10.1016/j.anaerobe.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 77.Biasucci G, Benenati B, Morelli L, et al. Cesarean delivery may affect the early biodiversity of intestinal bacteria. J Nutr. 2008;138:1796S–1800S. doi: 10.1093/jn/138.9.1796S. [DOI] [PubMed] [Google Scholar]
- 78.Sassone-Corsi M, Raffatellu M. No vacancy: how beneficial microbes cooperate with immunity to provide colonization resistance to pathogens. J Immunol. 2015;194:4081–4087. doi: 10.4049/jimmunol.1403169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bäckhed F, Fraser CM, Ringel Y, et al. Defining a healthy human gut microbiome: current concepts, future directions, and clinical applications. Cell Host Microbe. 2012;12:611–622. doi: 10.1016/j.chom.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 80.Charbonneau MR, Blanton LV, DiGiulio DB, et al. A microbial perspective of human developmental biology. Nature. 2016;535:48–55. doi: 10.1038/nature18845. [DOI] [PMC free article] [PubMed] [Google Scholar]