Abstract
There is an unmet need for identifying new clinical biomarkers in chronic Graft-versus-Host-disease (cGVHD) suitable for diagnosis and disease monitoring. Circulating autoantibodies represent an ongoing immune response and suggest a pathogenic role for B cells in cGVHD. Autoantibodies could be useful markers of cGVHD disease activity, severity, or organ specificity; however, their clinical utility is not established. The focus of this study was to determine the incidence and associations of a broad array of clinical autoantibodies with cGVHD manifestations in a large patient cohort characterized by NIH criteria. A panel of 21 circulating antibodies commonly used in clinical medicine was tested in 280 cGVHD patients (70% severe) enrolled in a cross-sectional prospective natural history study. Median cGVHD duration was two years. Patients with circulating autoantibodies (62%) had significantly higher levels of IgM (P < 0.0001), IgG (P < 0.0001), and IgA (P = 0.001), elevated uric acid (P = 0.008) and total protein (P = 0.0004), and higher numbers of CD31 (P = 0.002), CD41 (P = 0.001), CD81 (P = 0.023) T cells, and CD191 B cells (P < 0.0001). Multiple antibodies were detected in 35% of patients. Prior rituximab therapy (n = 66) was associated with reduced presence of autoantibodies (48 vs. 66% P = 0.01). Only oral cGVHD was significantly associated with presence of autoantibodies in this study (P = 0.028). No significant associations were found between cGVHD activity and severity, and presence of autoantibodies. Circulating autoantibodies are common in patients with advanced cGVHD. Their presence is associated with better quantitative immunologic reconstitution but does not have utility as a clinical biomarker of cGVHD.
Introduction
Chronic graft-versus-host disease (cGVHD) remains a serious late complication of allogeneic hematopoietic stem cell transplantation (HCT) [1–4]. The clinical presentations of cGVHD are similar to autoimmune disorders such as scleroderma, systemic lupus erythematosus (SLE), Sjogren’s syndrome and rheumatoid arthritis [5–7]. These autoimmune disorders are significantly associated with antibody production leading to target tissue damage, immune complex formation and tissue deposition [8–10]. Both allo- and auto antibodies are commonly observed in cGVHD, but their role in the pathogenesis of cGVHD still remains unclear [11–14]. Antibodies may be present before first clinical presentation of cGVHD [15] similar to autoimmune diseases [16] and anti-HY allo antibodies have been significantly associated with the development of cGVHD [17]. Antibodies may also reflect the presence and intensity of the autoimmune response in cGVHD [18]. Nucleic acid components of DNA- and RNA- autoantigens are released due to tissue damage and apoptosis in graft-versus-host reactions. After binding to these antigens antibodies may serve as a stimulus for activation of autoreactive B cells, complement fixation, immune complex formation and engagement of Fc and Toll like receptors (TLR) [19]. In addition, deficient clearance of the damaged patterns (DAMPS) can lead to accumulation and chronic activation of the innate immunity [20]. Besides, the presence of circulating antibodies, a disturbance of B-cell homeostasis with prolonged reconstitution of B cells, accumulation of atypical B cells due to an excess of B-cell activation factor (BAFF) and over-activation of B-cells were described in patients with cGVHD [21–24]. Anti-CD20 B cell depletion has been tested in prophylaxis and treatment of cGVHD with mixed success [25–32].
There is a prominent unmet need for developing clinically useful biomarkers for cGVHD diagnosis and disease monitoring. However, in spite of evidence of their frequent detection in patients, the biological significance and role of autoantibodies in cGVHD is not defined [33]. The 2005 NIH consensus project provided new classification of cGVHD diagnosis and staging [2,34]. This classification leads to better disease characterization, stricter diagnostic definitions of cGVHD, and separation from acute GVHD. Using these standardized and more detailed cGVHD criteria may enhance the ability to detect significant associations between circulating auto antibodies and disease manifestations. Here, we investigated a broad spectrum of autoantibodies for their potential utility in defining cGVHD activity, severity and organ specificity in a large cohort of cGVHD patients with wide spectrum of organ involvement described by NIH criteria.
Methods
Study conduct
Patients enrolled in the natural history study of clinical and biological factors determining outcomes in cGVHD (NCI protocol clinicaltrials.gov identifier: NCT00331968) from October 2004 to May 2013 were included in this analysis. This protocol provides a one-time week-long evaluation during which all patients undergo comprehensive assessment of cGVHD by the multidisciplinary team at the NIH Clinical Center. Peripheral blood samples were analyzed for presence of a panel of autoantibodies which are commonly used in clinical medicine. Patients were subdivided in two groups—autoantibody positive and autoantibody negative, based on absolute values or titers. Activity of cGVHD was defined as clinician decision to intensify systemic therapy and recently validated by this group, as reported by Grkovic et al. [35]. Intensity of systemic therapy at study entry was defined as no therapy, mild (single agent prednisone 0.5 mg/kg/day), moderate (prednisone ≥ 0.5 mg/kg/day and/or any single agent/modality), and high (2 or more agents/modalities ± prednisone ≥ 0.5 mg/kg/day) [36]. The study protocol was approved by the NCI IRB and all patients signed written informed consent.
Laboratory assessments
Blood samples were submitted to the Department of Laboratory Medicine, Clinical Center, NIH. Evaluation of immune status was performed by detecting CD3+, CD4+, CD8+ T cells, CD19+ B cells, and NK cells (CD56+CD3−). Serum levels of IgG, IgM, and IgA immunoglobulins were quantified by nephelometry using Beckman Coulter IMMAGE (Beckman Coulter Inc., Brea, CA) and Siemens Dimension Vista (Siemens Diagnostics, Tarrytown, NY). The patient’s serum was screened for the 21 autoantibodies listed in Supporting Information Table I with details of methodologies for antibody assessment. Antibody testing was performed by ELISA and/or immunoflouresence. For immunoassay-based methods used to screen antibodies patient values were compared to the relevant reference interval as provided by the manufacturer. In house ELISA antibody testing was performed on the DSX ELISA processing system. (Dynex Technologies, Chantilly, VA). Patients positive for ENA were further screened for anti-SmRNP, Smith, SSA, and SSB autoantibodies.
Statistical analysis
Factors which were reported as a continuous parameter, or which could be essentially considered as if continuous, were compared between two groups, positive and negative for autoantibodies, using a Wilcoxon rank sum test. Ordered categorical parameters were compared between the two groups using a Cochran-Armitage test for trend. Dichotomous parameters were compared between the two groups using Fisher’s exact test. Unordered categorical parameters were compared between two groups using Mehta’s modification to Fisher’s exact test.
For univariate analysis the association of different autoantibodies with cGVHD characteristics such as activity, severity, organ specificity, duration of cGVHD with time after HCT, and intensity of immunosuppression were selected. Following an initial screening by univariate methods, multivariable logistic regression analysis was used to identify a set of factors which could jointly impact the particular parameter under consideration. Covariates with a P-value <0.05 were entered into the multivariate analysis. All P-values reported are two tailed, and presented without any formal adjustment for multiple comparisons. In view of the number of tests performed, P-values such that P < 0.005 could be considered statistically significant while 0.005 < P < 0.05 would represent strong trends. Titers of autoantibodies are shown in medians. Analyses were performed using R, SPSS 20.0 (IBM Company, Chicago, IL), and SAS version 9.3 (SAS Institute, Cary, NC).
Results
Patient characteristics
Two-hundred eighty patients with cGVHD, median age of 46 years (range, 4–70) at a median of 36 (range, 4–297) months after HCT were included in the study. The clinical characteristics of the patients are shown in Table I. Patients were subdivided in two groups, positive with autoantibodies (n = 174) and negative, without antibodies (n = 106). No significant differences in transplant characteristics were observed between these two groups. Chronic GVHD assessment was conducted according to the NIH consensus criteria, cGVHD patient characteristics are shown in Table II. All patients were in remission of their original hematologic disease. The majority of patients (70%) had severe cGVHD. 33% had de novo onset cGVHD. Median time to onset of cGVHD was 7 months. The most frequent organ manifestations of cGVHD at study entry were skin (78%), eyes (77%), and lung involvement (75%), and severe disease (NIH scores 2–3) was present in 61%, 43%, and 35% of these organs, respectively. Over ninety percent of patients had multiorgan involvement, identified as more than two organs. The median duration of cGVHD was 2 years (range, 0.4–222 months) (Table II).
TABLE I.
Patient Characteristics
| Patients n = 280 (%) | Antibody positive n = 174 (%) | Antibody negative n = 106 (%) | |
|---|---|---|---|
| Median age in years, range | 46 (4–70) | 46 (4–69) | 45 (5–70) |
| Patient | |||
| Male | 158 (56) | 100 (57) | 58 (55) |
| Female | 122 (44) | 74 (43) | 48 (45) |
| Gender matcha F/M | 56 (20) | 30 (17) | 26 (25) |
| Diagnosisa | |||
| Acute leukemia | 127 (45) | 78 (45) | 49 (46) |
| CML | 38 (14) | 26 (15) | 12 (11) |
| Malignant lymphoma | 81 (29) | 46 (26) | 35 (33) |
| MM | 15 (5) | 12 (6) | 3 (3) |
| Nonmalignant | 16 (6) | 10 (6) | 6 (6) |
| Otherb | 3 (1) | 2 (1) | 1 (1) |
| Conditioning regimen | |||
| Myeloablative | 153 (55) | 94 (54) | 59 (56) |
| RIC | 127 (45) | 80 (46) | 47 (44) |
| TBI | 105 (38) | 59 (34) | 46 (46) |
| Stem cell donors | |||
| Related | 173 (62) | 110 (63) | 63 (59) |
| Unrelated | 107 (38) | 64 (37) | 43 (41) |
| HLA-identical | 228 (81) | 143 (82) | 85 (80) |
| HLA-mismatcheda | 45 (16) | 27 (16) | 18 (17) |
| Stem cell source | |||
| Bone marrow | 52 (19) | 29 (17) | 23 (22) |
| PBSC | 218 (78) | 138 (79) | 80 (75) |
| Cord blood | 10 (4) | 7 (4) | 3 (3) |
| Median time form HSCT, months, range | 36 (4–297) | 36 (4–258) | 36 (5–297) |
N, number; GVHD, graft-versus-host disease; TBI, total body irradiation; RIC, reduced intensity conditioning; PBCS, peripheral blood stem cells.
Missing values.
Other diseases include sarcoma, CML-chronic myeloid leukemia, MM, multiple myeloma.
None of the comparisons were significant different.
TABLE II.
Chronic Graft-Versus-Host Disease Characteristics
| All patients n = 280 (%) | Positive n = 174 (%) | Negative n = 106 (%) | |
|---|---|---|---|
| Severity at enrollment | |||
| Mild | 4 (1) | 3 (2) | 1 (1) |
| Moderate | 80 (29) | 54 (31) | 26 (24) |
| Severe | 196 (70) | 117 (67) | 79 (75) |
| Onset type of cGVHD | |||
| de novo | 91 (33) | 53 (30) | 38 (36) |
| Quiescent | 79 (28) | 48 (28) | 31 (29) |
| Progressive | 110 (39) | 73 (42) | 37 (35) |
| Organ involvement | |||
| Skin | 217 (78) | 134 (77) | 83 (78) |
| Score 2–3 | 172 (61) | 105 (60) | 67 (63) |
| Eyes | 216 (77) | 132 (76) | 84 (79) |
| Score 2–3 | 121 (43) | 74 (43) | 47 (44) |
| Oral mucosa | 184 (66) | 123 (71)b | 61 (58) |
| Score 2–3 | 40 (14) | 30 (17) | 10 (9) |
| Liver | 135 (48) | 91 (52) | 44 (42) |
| Score 2–3 | 46 (16) | 30 (17) | 16 (15) |
| Lungs | 210 (75) | 122 (70) | 88 (83) |
| Score 2–3 | 99 (35) | 54 (31) | 45 (42) |
| GI | 122 (44) | 66 (38) | 56 (35) |
| Score 2–3 | 33 (12) | 20 (11) | 13 (12) |
| Genital | 80 (29) | 49 (28) | 31 (29) |
| Score 2–3 | 61 (22) | 38 (22) | 23 (22) |
| Joint and Fascia | 168 (60) | 102 (59) | 66 (62) |
| Score 2–3 | 108 (39) | 61 (35) | 47 (44) |
| ≤ 2 organs | 25 (9) | 17 (10) | 8 (8) |
| > 2 organs | 255 (91) | 157 (90) | 98 (92) |
| Activity of cGVHDa | |||
| Active | 119 (42) | 71 (41) | 48 (45) |
| Nonactive | 159 (58) | 102 (59) | 57 (54) |
| Treatment of cGVHD | |||
| Lines for prior therapy | 4 (0–9) | 4 (0–9) | 4 (0–9) |
| Intensity of ISa | |||
| None | 53 (19) | 39 (22) | 14 (13) |
| Mild | 16 (6) | 8 (5) | 8 (8) |
| Moderate | 99 (35) | 66 (38) | 33 (31) |
| High | 110 (39) | 60 (34) | 50 (47) |
| Anti-CD20 for cGVHD | 66 (24) | 32 (18) | 34 (32) |
| Median time to onset of cGVHD, months (range) | 7 (1.6–267) | 7 (1.6–144) | 7 (1.7–267) |
| Median duration of cGVHD in months (range) | 24 (0.4–222) | 23 (0.4–222) | 26 (0.7–210) |
| Duration of cGVHD till study enrolment | |||
| ≤1year | 78 (28) | 45 (26) | 33 (31) |
| >1year | 202 (72) | 129 (74) | 73 (69) |
N, number; GI, gastrointestinal; IS, immunosuppressive.
Some patients with activity of cGVHD (n = 2) and intensity of immunosuppression (n = 2) have missing data.
Statistical significance between the groups (P < 0.05).
Prevalence of autoantibodies
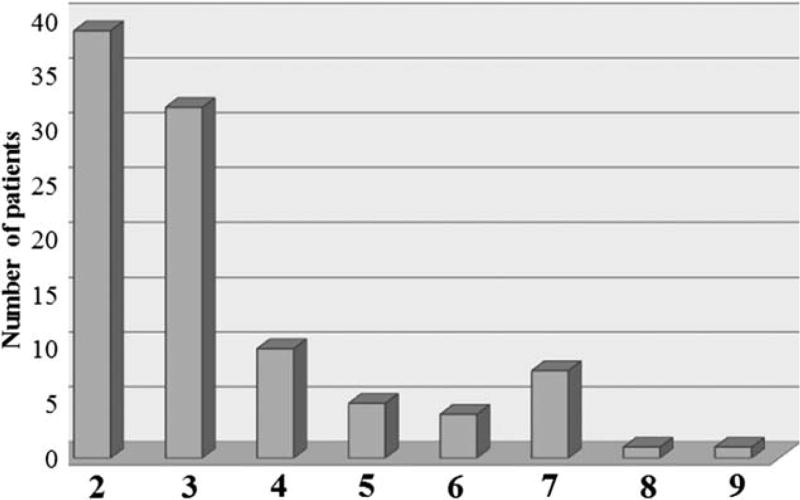
Autoantibodies were identified in 62% (n = 174) of patients and multiple antibodies were detected in 35% of patients (n = 61) Fig. 1. According to onset of cGVHD, the distribution of autoantibodies was 30% in de novo, 28% in quiescent and 42% in progressive cGVHD (NS) (Table II). The most frequent antibodies were ANA (29%) and RF (13%). Furthermore, 74% of patients who had antibodies exhibited a longer duration of cGVHD (>1 year) (NS). Patients with circulating autoantibodies had significantly higher levels of IgM, IgG, and IgA, elevated uric acid and total protein, and higher numbers of CD3+, CD4+, CD8+ T cells, and CD19+ B cells (Table III).
Figure 1.
Distribution of multiple autoantibodies in patients with cGVHD. Each bar represents the number of patients with numbers of autoantibodies (≥ 2).
TABLE III.
Univariate Associations Between Laboratory Parameters and Autoantibodies, in Medians
| Parameter (units) | Positive n = 174 | Negative n = 106 | P |
|---|---|---|---|
| Uric acid (mg/dl) | 4.7 | 4.2 | 0.0082 |
| Total protein (g/dl) | 6.5 | 6.1 | 0.0004 |
| IgM (mg/dl) | 74 | 38 | <0.0001 |
| IgG (mg/dl) | 762 | 514 | <0.0001 |
| IgA (mg/dl) | 66 | 40 | 0.0012 |
| C4 (mg/dl) | 26 | 28 | 0.045 |
| CD3+ × 106 | 948 | 663 | 0.0023 |
| CD4+ × 106 | 454 | 222 | 0.0014 |
| CD8+ × 106 | 383 | 308 | 0.023 |
| CD19+ × 106 | 189 | 43 | <0.0001 |
| CD56+ × 106 | 181 | 160 | 0.17 |
| CRP | 1.3 | 1.6 | 0.38 |
| CPK | 57 | 48 | 0.046 |
| ESR | 17.5 | 14 | 0.29 |
| WBC | 7.3 | 7.12 | 0.68 |
| Eosinophils | 0.09 | 0.07 | 0.1 |
CRP-C, reactive protein; CPK, creatine phosphokinase; ESR, erythrocyte sedimentation rate; WBC, white blood count; Ig, immunoglobulin; C4, complement 4.
Autoantibodies according to NIH severity and activity
According to the cGVHD NIH global severity score, autoantibodies were present in 60% with severe (117/196) and in 68% (54/80) with moderate cGVHD (P = 0.27) (Table II). We further compared overall antibody incidence, individual autoantibodies, and their titers to severity of cGVHD. Among those with autoantibodies, no association between overall incidence of autoantibodies and severity of cGVHD was detected (Supporting Information Table II). Active cGVHD was present in 43% (n = 119/280) of patients and within this subset, circulating autoantibodies were present in 60% (n = 71/119), (Table II). No significant relationship between overall incidence of autoantibodies and activity of cGVHD was observed (Supporting Information Table III).
Circulating autoantibodies in cGVHD patients with different organ manifestations
Next, we studied individual organ involvements with the aim to identify organ-specific antibodies. Only oral cGVHD showed significantly higher frequency of overall autoantibodies (P = 0.028) mainly ANA (79% vs. 60%, P = 0.0023, ANA titer 1.3U vs 0.7U, P = 0.0008). No significant association between overall incidence of autoantibodies or titers with skin, liver, lungs or eye cGVHD was observed. Significantly higher titer of RF was seen in patients (n = 108) with moderate to severe joints and fascia involvement (20 vs. 15 IU/ml, P = 0.003).
Presence of autoantibodies according to duration and therapy of cGVHD
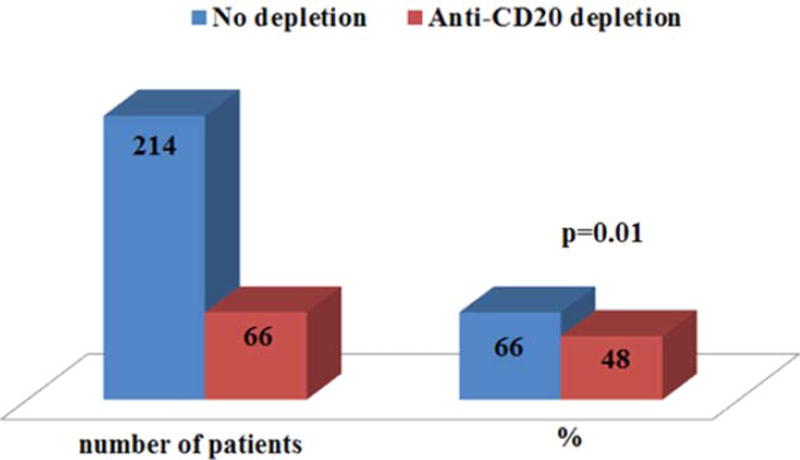
When short (≤1 year) and longer (>1 year) cGVHD durations were compared, a trend towards higher incidence of ACA G titer (2.6 vs. 0.74 GPL, P = 0.028) and RF (15% vs. 6%, P = 0.07) were detected in the longer duration cGVHD group. When separated by intensity of systemic therapy (0–4), 74% of patients without any systemic therapy at the time of evaluation had one or more antibodies, compared to 26% of those on some form of systemic treatment for cGVHD (P = 0.06). No difference was seen between previous lines of immunosuppressive therapy for cGVHD; however, patients who received prior anti-CD20 antibody therapy for cGVHD had significantly lower incidence of autoantibodies (48% vs. 66%, P = 0.01), as seen in Fig. 2.
Figure 2.
Presence of autoantibodies according to B cell depletion therapy. The left blue bar represent patients without B cell depletion, in red are patients who received anti-CD20 therapy. On the right side the percentages of patients positive for autoantibodies according to the B cell depletions. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Multivariable analysis
The following variables (uric acid, total protein, IgG, IgM, IgA, C4, CD3+, CD4+, CD8+ T cells, CD19+ B cells, cGVHD of oral mucosa, liver, GI, lung involvement, and intensity of immunosuppression) were considered for inclusion in a multivariable model for predicting whether a patient had any positive antibodies or not. The above mentioned laboratory parameters were significantly different in univariate analysis (Table III) with a P < 0.05. The final multivariable model showed that higher levels of IgG and IgM, and less GI and lung cGVHD involvement were independently associated with the presence of autoantibodies (Table IV).
TABLE IV.
Multivariable Analysis of Parameters Associated with Presence of Circulating Autoantibodies
| Factor | HR | 95% CI | P |
|---|---|---|---|
| IgM | 1.008 | 1.002–1.0013 | 0.0061 |
| IgG | 1.001 | 1.001–1.002 | 0.0001 |
| Lung | 0.428 | 0.251–0.728 | 0.0017 |
| GI | 0.434 | 0.254–0.742 | 0.0023 |
Ig, immunoglobulin M, G; GI, cGVHD of gastrointestinal tract; HR, hazard ratio; CI, confidence interval.
Discussion
The primary focus of this study was to determine the prevalence and potential role of autoantibodies as disease markers in a large cohort of patients with cGVHD characterized by NIH criteria. The dysregulated humoral immunity in cGVHD can be comparable to autoimmune diseases (AID), where the pathogenic role of autoantibodies has been confirmed. Furthermore, higher titers of autoantibodies may be useful for monitoring diseases activity in AID [37].
A substantial proportion of cGVHD patients demonstrated autoantibodies (62%) but no significant association with severity, activity, and clinical characteristics of cGVHD was found. In our study, transplant characteristics and duration of cGVHD were similar between autoantibody positive and autoantibody negative groups. In accordance with our analysis, where patients with long-lasting cGVHD have a trend towards higher incidence of autoantibodies, Fujii et al. reported increased frequency of anti-dsDNA antibody and ANA in patients with late (≥ 9 months after HCT) compared with early onset (<9) cGVHD, suggesting a predominant immune activation and accumulation of autoantibodies in the later stage of the disease [18]. Additionally in his analysis, BAFF level was elevated, which is known to rescue autoreactive B-cells resulting in expansion of anti-DNA antibodies [38]. In this study patients with autoantibodies had a significantly better immune reconstitution regarding overall higher T-, B cells and serum immunoglobulin numbers.
The detection of autoantibodies was more frequently found in patients with oral cGVHD involvement and less in GI and lung cGVHD involvement. The latter may suggest direct pathogenic role of autoantibodies with germinal center formation and immunoglobulin deposition in affected tissues as recently described by Flynn et al. [39]. Of note, ANA was the primary autoantibody detected in patients with oral cGVHD. Positive ANA was described previously in six studies including 293 cGVHD patients that did not apply NIH diagnosis and staging criteria. In these studies the median prevalence of ANA positivity was 38% (range 22% to 82%) [5,13,40–43] compared with 29% in our cGVHD cohort. However, ANA positivity can be seen in 15% of healthy adults and 8% of children, usually at a low titer [44]. Also, ANA is more frequent in individuals >60 years and women (25%). In contrast to published data on 102 patients [13,40,43], the incidence of ds-DNA in our study was only 3% compared to 15% (range, 3–31%). Anti-phospholipid antibodies (ACA M and G) are found in patients with localized systemic sclerosis and in SLE [45], in our study ACA IgM was significantly elevated in patients with moderate cGVHD, whereas ACA IgG was only found in patients with GI cGVHD. Anti-SSA (Ro) and anti-SSB (La) are highly specific for Sjögren’s syndrome and are seen in approximately one-third of patients with systemic sclerosis predominantly with sicca manifestations, and up to 60% in patients with SLE. In our cGVHD cohort, the majority of patients positive for SAA had sicca. RF can be found in a wide variety of clinical settings and, in rheumatoid arthritis, high titer of RF correlate with severe erosive joint disease [46]. From 34 previously reported cGVHD patients, 9% (range, 8–10) were positive for RF what is similar to our results [13,40,43]. In our cohort, other autoantibodies were much less common.
Here we tested a large number of well characterized cGVHD patients using NIH criteria for diagnosis and staging who had a wide variety of disease manifestations [35]. However, this study does have limitations. The absence of longitudinal analysis limits further interpretation of the association of the antibodies and cGVHD. Autoantibodies can be also present prior to the development of autoimmune diseases [16] and are described in post-allogeneic patients without cGVHD [15]. Our antibody panel excluded alloantibodies which have been associated with development of cGVHD [12,17]. Finally, it is challenging to draw conclusions on the role of single autoantibody higher titers due to their overall low incidence.
In conclusion, autoantibodies are very common in cGVHD reflecting the dysregulated humoral immunity in these patients and their levels correlate with better quantitative immunological reconstitution. However, autoantibodies show no relationship to activity, severity of cGVHD and have very limited organ specificity, so at this stage they should not be used as clinically useful markers in cGVHD patients. Further research should elucidate the biological role of these antibodies in cGVHD.
Supplementary Material
Footnotes
Additional Supporting Information may be found in the online version of this article.
Conflict of interest: Nothing to report
Preliminary data of this manuscript were presented at the Annual Meeting of the European Group for Blood and Marrow Transplantation (EBMT) in March 2014.
References
- 1.Baird K, Pavletic SZ. Chronic graft versus host disease. Curr Opin Hematol. 2006;13:426–435. doi: 10.1097/01.moh.0000245689.47333.ff. [DOI] [PubMed] [Google Scholar]
- 2.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease. I. diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Socie GRJ. Current issues in chronic graft-versus-host disease. Blood. 2014;124:374–384. doi: 10.1182/blood-2014-01-514752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee SJ, Vogelsang G, Flowers MED. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9:215–233. doi: 10.1053/bbmt.2003.50026. [DOI] [PubMed] [Google Scholar]
- 5.Rouquette-Gally AMBD, Prost AC, Gluckman E, Abuaf N, Combrisson A. Autoimmunity in 28 patients after allogeneic bone marrow transplantation: Comparison with Sjogren’s syndrome and scleroderma. Br J Haematol. 1987;66:45–47. doi: 10.1111/j.1365-2141.1987.tb06888.x. [DOI] [PubMed] [Google Scholar]
- 6.Sherer YSY. Autoimmune diseases and autoimmunity post-bone marrow transplantation. Bone Marrow Transplant. 1988;22:873–881. doi: 10.1038/sj.bmt.1701437. [DOI] [PubMed] [Google Scholar]
- 7.Roquette-Gally A, Boyeldieu D, Prost A, Gluckman E. Autoimmunity after allogeneic bone marrow transplantation: A study of 53 long-term-surviving patients. Transplantation. 1988;22:238–240. doi: 10.1097/00007890-198808000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Eggert M, Zettl UK, Neeck G. Autoantibodies in autoimmune diseases. Curr Pharm Des. 2010;16:1634–1643. doi: 10.2174/138161210791164144. [DOI] [PubMed] [Google Scholar]
- 9.Tan E. Autoantibodies, autoimmune disease, and the birth of immune diagnostics. J Clin Invest. 2010;122:3835–3836. doi: 10.1172/JCI66510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watts RA. Autoantibodies in autoimmune diseases. Medicine. 2002;30:2–6. [Google Scholar]
- 11.Zorn E, Miklos DB, Floyd BH, et al. Minor histocompatibility antigen DBY elicits a coordinated B and T cell response after allogeneic stem cell transplantation. J Exp Med. 2004;199:1133–1142. doi: 10.1084/jem.20031560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miklos DBKH, Zorn E, Hochberg EP, Guo L, Mattes-Ritz A. Antibody response to DBY minor histocompatibility antigen is induced after allogeneic stem cell transplantation and in healthy female donors. Blood. 2004;103:353–359. doi: 10.1182/blood-2003-03-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patriarca F, Skert C, Sperotto A, et al. The development of autoantibodies after allogeneic stem cell transplantation is related with chronic graft-vs-host disease and immune recovery. Exp Hematol. 2006;34:389–396. doi: 10.1016/j.exphem.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 14.Kier PPE, Bakos S, Kalhs P, et al. Autoantibodies in chronic GVHD: High prevalence of antinucleolar antibodies. Bone Marrow Transplant. 1990;6:93–96. [PubMed] [Google Scholar]
- 15.Dighiero G, Intrator L, Cordonnier C, et al. High levels of anti-cytoskeleton autoantibodies are frequently associated with chronic GVHD. Br J Haematol. 1987;67:301–305. doi: 10.1111/j.1365-2141.1987.tb02351.x. [DOI] [PubMed] [Google Scholar]
- 16.Arbuckle MRMM, Rubertone MV, Scofield RH, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 17.Miklos DB, Kim HT, Miller KH, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005;105:2973–2978. doi: 10.1182/blood-2004-09-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujii H, Cuvelier G, She K, et al. Biomarkers in newly diagnosed pediatric extensive chronic graft-versus-host disease: A report from the Children’s Oncology Group. Blood. 2008;111:3276–3285. doi: 10.1182/blood-2007-08-106286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolland S, Garcia-Sastre A. Vicious circle: Systemic autoreactivity in Ro52/TRIM21-deficient mice. J Exp Med. 2009;206:1647–1651. doi: 10.1084/jem.20091507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jounai N, Kobiyama K, Takeshita F, Ishii K. Recognition of damage-associated molecular patterns related to nucleic acids during inflammation and vaccination. Front Cell Infect Microbiol. 2013;2:1–13. doi: 10.3389/fcimb.2012.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarantopoulos S, Stevenson KE, Kim HT, et al. Altered B-cell homeostasis and excess BAFF in human chronic graft-versus-host disease. Blood. 2009;113:3865–3874. doi: 10.1182/blood-2008-09-177840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allen JL, Fore MS, Wooten J, et al. B cells from patients with chronic GVHD are activated and primed for survival via BAFF-mediated pathways. Blood. 2012;120:2529–2536. doi: 10.1182/blood-2012-06-438911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarantopoulos S, Stevenson KE, Kim HT, et al. High levels of B-cell activating factor in patients with active chronic graft-versus-host disease. Clin Cancer Res. 2007;13:6107–6114. doi: 10.1158/1078-0432.CCR-07-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuzmina Z, Greinix HT, Weigl R, et al. Significant differences in B-cell subpopulations characterize patients with chronic graft-versus-host disease-associated dysgammaglobulinemia. Blood. 2011;117:2265–2274. doi: 10.1182/blood-2010-07-295766. [DOI] [PubMed] [Google Scholar]
- 25.Bates JS, Engemann AM, Hammond JM. Clinical utility of rituximab in chronic graft-versus-host disease. Annals Pharmacotherapy. 2009;43:316–321. doi: 10.1345/aph.1L386. [DOI] [PubMed] [Google Scholar]
- 26.Kharfan-Dabaja MA, Mhaskar AR, Djulbegovic B, et al. Efficacy of rituximab in the setting of steroid-refractory chronic graft-versus-host disease: A systematic review and meta-analysis. Biol Blood Marrow Transplant. 2009;15:1005– 1013. doi: 10.1016/j.bbmt.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 27.van Dorp S, Resemann H, te Boome L, et al. The immunological phenotype of rituximab-sensitive chronic graft-versus-host disease: A phase II study. Haematologica. 2011;96:1380–1384. doi: 10.3324/haematol.2011.041814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arai S, Sahaf B, Narasimhan B, et al. Prophylactic rituximab after allogeneic transplantation decreases B-cell alloimmunity with low chronic GVHD incidence. Blood. 2012;119:6145–6154. doi: 10.1182/blood-2011-12-395970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohty M, Marchetti N, El-Cheikh J, et al. Rituximab as salvage therapy for refractory chronic GVHD. Bone Marrow Transplant. 2008;41:909–911. doi: 10.1038/bmt.2008.12. [DOI] [PubMed] [Google Scholar]
- 30.Cutler C, Miklos D, Kim HT, et al. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood. 2006;108:756–762. doi: 10.1182/blood-2006-01-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaja F, Bacigalupo A, Patriarca F, et al. Treatment of refractory chronic GVHD with rituximab: A GITMO study. Bone Marrow Transplant. 2007;40:273–277. doi: 10.1038/sj.bmt.1705725. [DOI] [PubMed] [Google Scholar]
- 32.Kim SJ, Lee JW, Jung CW, et al. Weekly rituximab followed by monthly rituximab treatment for steroid-refractory chronic graft-versus-host disease: Results from a prospective, multicenter, phase II study. Haematologica. 2010;95:1935–1942. doi: 10.3324/haematol.2010.026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spies-Weisshart B, Schilling K, Böhmer F, et al. Lack of association of platelet-derived growth factor (PDGF) receptor autoantibodies and severity of chronic graft-versus-host disease (GvHD) J Cancer Res Clin Oncol. 2013;139:1397–1404. doi: 10.1007/s00432-013-1451-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baird K, Steinberg SM, Grkovic L, et al. National institutes of health chronic graft-versus-host disease staging in severely affected patients: Organ and global scoring correlate with established indicators of disease severity and prognosis. Biol Blood Marrow Transplant. 2013;19:632–639. doi: 10.1016/j.bbmt.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grkovic L, Baird K, Steinberg SM, et al. Clinical laboratory markers of inflammation as determinants of chronic graft-versus-host disease activity and NIH global severity. Leukemia. 2012;26:633–643. doi: 10.1038/leu.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitchell SA, Jacobsohn D, Thormann Powers KE, et al. A multicenter pilot evaluation of the National Institutes of Health chronic graft-versus-host disease (cGVHD) therapeutic response measures: Feasibility, interrater reliability, and minimum detectable change. Biol Blood Marrow Transplant. 2011;17:1619–1629. doi: 10.1016/j.bbmt.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sinico RA, Radice A, Corace C, et al. Value of a new automated fluorescence immunoassay (EliA) for PR3 and MPO-ANCA in monitoring disease activity in ANCA-associated systemic vasculitis. Ann NY Acad Sci. 2005;1050:185–192. doi: 10.1196/annals.1313.019. [DOI] [PubMed] [Google Scholar]
- 38.Ota M, Duong B, Torkamani A, et al. Regulation of the B cell receptor repertoire and self-reactivity by BAFF. J Immunol. 2010;185:4128–4136. doi: 10.4049/jimmunol.1002176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flynn R, Du J, Veenstra RG, et al. Increased T follicular helper cells and germinal center B cells are required for cGVHD and bronchiolitis obliterans. Blood. 2014;123:3988–3998. doi: 10.1182/blood-2014-03-562231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lister J, Messner H, Keystone E, et al. Autoantibody analysis of patients with graft versus host disease. J Clin Lab Immunol. 1987;24:19–23. [PubMed] [Google Scholar]
- 41.Chan EYL, Lie JW, Lau AK, et al. Autoantibody formation after allogeneic bone marrow transplantation: Correlation with the reconstitution of CD51 B cells and occurrence of graft-versus-host disease. Pathology. 1997;29:184–188. doi: 10.1080/00313029700169834. [DOI] [PubMed] [Google Scholar]
- 42.Quaranta S, Shulman H, Ahmed A, et al. Autoantibodies in human chronic graft-versus-host disease after hematopoietic cell transplantation. Clin Immunol. 1999;91:106–116. doi: 10.1006/clim.1998.4666. [DOI] [PubMed] [Google Scholar]
- 43.Wechalekar A, Cranfield T, Sinclair D, Ganzckowski M. Occurrence of autoantibodies in chronic graft vs. host disease after allogeneic stem cell transplantation. Clin Lab Haematol. 2005;27:247–249. doi: 10.1111/j.1365-2257.2005.00699.x. [DOI] [PubMed] [Google Scholar]
- 44.Thompson D, Bird HA. Acute phase response. In: Isenberg DA, Maddison PJ, Woo P, Glass D, Breedveld FC, editors. What is the acute phase response and how is it assessed? Vol. 3. Oxford Textbook of Rheumatology; 2004. pp. 473–479. [Google Scholar]
- 45.Cefle A, Inanc M, Sayarlioglu M, et al. Pulmonary hypertension in systemic lupus erythematosus: Relationship with antiphospholipid antibodies and severe disease outcome. Rheumatol Int. 2011;31:183–189. doi: 10.1007/s00296-009-1255-2. [DOI] [PubMed] [Google Scholar]
- 46.Bukhari MLM, Harrison BJ, Scott DG, et al. Rheumatoid factor is the major predictor of increasing severity of radiographic erosions in rheumatoid arthritis: Results from the Norfolk Arthritis Register Study, a large inception cohort. Arthritis Rheum. 2002;46:906–912. doi: 10.1002/art.10167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.