Abstract
The structure of an intermediate in the initiation to elongation transition of Escherichia coli RNA polymerase has been visualized through region-specific DNA cleavage by the hydroxyl radical reagent FeBABE. FeBABE was tethered to specific sites of the σ70 subunit and incorporated into two specialized paused elongation complexes that obligatorily retain the σ70 initiation subunit and are targets for modification by lambdoid phage late gene antiterminators. The FeBABE cleavage pattern reveals structures similar to open complex, except for notable changes to region 3 of σ70 that might reflect the presence of stably bound transcript. Binding of the antiterminator protein Q displaces the reactivity of FeBABE conjugated to region 4 of σ70, suggesting that σ70 subunit rearrangement is a step in conversion of RNAP to the antiterminating form.
The σ70 subunit of Escherichia coli RNA polymerase (RNAP) both recognizes promoter elements and mediates important contacts with regulatory proteins. In the holoenzyme complex with core enzyme, σ70 binds the −35 and −10 elements of the predominant class of bacterial promoters: region 4 of σ70 recognizes the −35 element (1, 2) as double-stranded (ds)DNA, and region 2 of σ70 recognizes the −10 element as both dsDNA and single-stranded (ss)DNA (2–6). At certain promoters that depend on positive activation, such as those targeted by the transcription activators AdaA (7), λcI (8, 9), PhoB (10), and FNR (11), σ70 region 4 also is required for the function of the activator, almost certainly through a direct contact that facilitates binding or function of holoenzyme.
In a role somewhat reminiscent of its function in transcription activation, σ70 is required for a unique regulatory pathway directed by a transcription antiterminator, the bacteriophage λ gene Q protein (Qλ), as well as Q proteins of related phages (12). Like some activators, Q binds to DNA just upstream or overlapping the upstream portion of RNAP (13), positioning Q and RNAP for contact. However, Qλ acts not on RNAP at the promoter but instead on a paused elongation complex containing (for λ) 16–17 nucleotides of RNA transcript (14). Pausing is induced by an analogue of the −10 element, displaced (for λ) 12 bp downstream from the −10 element of the promoter and recognized as nontemplate ssDNA by region 2 of σ70 (3, 15, 16). The paused complex is unique, having characteristics of both open complex (presence of σ70) and elongation complex [presence of stable nascent RNA (16)]. The σ70-induced paused state is the essential substrate that Q protein binds in converting RNAP to the antiterminating form by becoming a subunit of the elongating complex (17).
Because σ70 is an essential component of the paused complex, it could be a target for recognition by Q, in addition to its role in recognizing the pause-inducing sequence. For Qλ, there is evidence for this interaction. First, σ70 is required for modification of RNAP by Qλ at the pause site, even if RNAP is stopped by substrate deprivation at the same site (13, 16). Second, Qλ interacts with region 4 of σ70 in the two-hybrid assay and in vitro binding assays (18). Third, Qλ activates transcription in an artificial construct requiring Qλ-σ70 region 4 contact (B. Nickels and A. Hochschild, personal communication). Fourth, AsiA, a protein that binds region 4 of σ70, interferes with Qλ activity (M.T.M. and J.W.R., unpublished results). Finally, in the paused complexes, the Q DNA-binding element is in a position analogous to binding sites of transcription activators that contact σ70 region 4 in open complex (19).
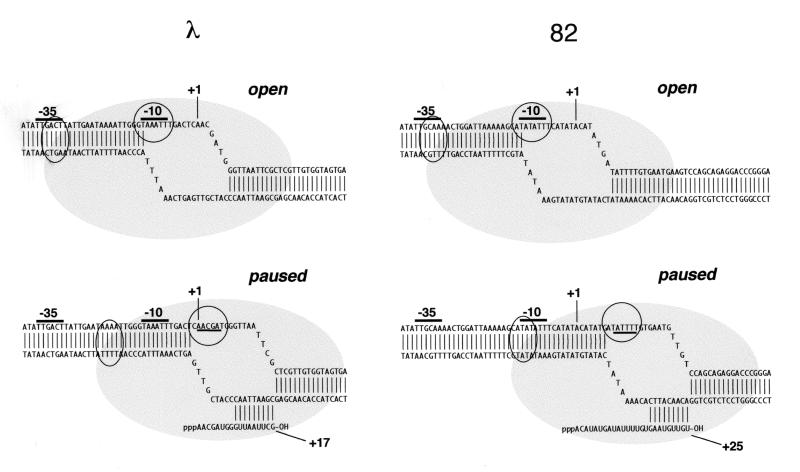
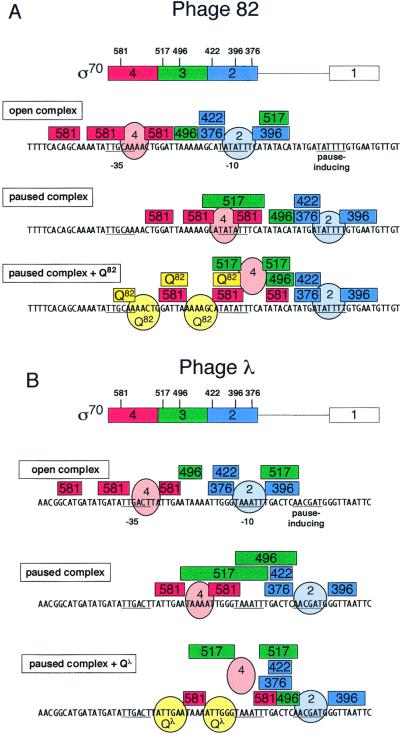
The position of σ70 region 4 in the paused complex, as well as its potential role in establishing or stabilizing the pause, has been unknown. There is no sequence that matches the −35 consensus at an appropriate spacing from the pause-inducing −10 analogue typical of promoters (16–18 bp; see Fig. 1) (20, 21); however, σ70 region 4 might well bind a sequence that matches the −35 consensus at some different spacing in the paused complex. In fact, for λ, there are several good matches to the −35 consensus at spacings different from 16–18 bp.
Figure 1.
Open and paused complexes of late promoters of phages λ and 82. Structures were described (39); the 82 sequence is the variant described. Circles and ovals represent, respectively, the assigned positions of σ70 regions 2 and 4. Paused complexes are shown in the forward position (38).
Understanding the structure of the paused complex, particularly the position of σ70 region 4 and the potential effect of Q on this structure, may reveal important intermediates in the modification of RNAP by Q protein. To examine the disposition of σ70 regions, we have used the chemical cleavage reagent FeBABE [iron (S)-1-(p-bromoacetamidobenzyl)-EDTA] tethered to single cysteines in the conserved regions of σ70 (22, 23). On addition of hydrogen peroxide and ascorbate, FeBABE serves as a localized source of hydroxyl radicals that cleave adjacent DNA and thus mark the vicinity of the tethered site. Attaching FeBABE to specific positions in a DNA-binding protein thus establishes the proximity of this attachment site to cleaved sites on DNA (24). Previously, a set of such conjugates was used to map the position of the conserved regions of σ70 in the open complex; each conjugate produces a characteristic pattern of cleavage (22, 23, 25). By noting positions of cleavage in the paused complex and comparing them to open complex, we can discern the disposition of σ on the DNA and relate this to the structure of the open complex.
We show that in the late gene promoter paused complex of either λ or the related phage 82, σ70 region 4 occupies just the position expected if there were a −35 element at the correct spacing from the −10 analogue, arguing that region 4 is positioned by the holoenzyme structure, possibly in conjunction with a general DNA-binding activity. Most important, we show that Qλ or Q82 modifies the conformation of σ70 region 4 in the paused complex, apparently displacing it downstream by the equivalent of about half a turn of DNA; this displaced complex may be an important intermediate in the modification of RNAP by Q. Finally, we used an FeBABE conjugate of Q82 to produce a positive footprint that localizes Q82 in the ternary Q82/DNA/RNAP complex.
Materials and Methods
Proteins.
E. coli RNAP core, Q82, Qλ, σ70, and σS were purified as described (3, 26).
Construction and Purification of Single Cysteine Q82.
A single cysteine was introduced by PCR into the third codon of a Q82 gene that also carried a C-terminal 6-His tag, and the modified protein was made by overexpression in BL21 cells (Promega). After cell lysis in 6 M guanidine HCl and 50 mM Tris, pH 8.0, clarified supernatant was supplemented with Ni-NTA agarose and incubated at 4° for 1 h; agarose beads were collected by centrifugation and washed with 50 mM Tris, pH 8/500 mM NaCl/8 M urea, and then with 50 mM Hepes, pH 7.9/500 mM NaCl/2 M urea. Protein was eluted with 50 mM Mops, pH 7.9/50 mM EDTA/2 M urea and frozen in aliquots. For conjugation, an aliquot of Q82 was supplemented to 1 mM with FeBABE and incubated 1 h at room temperature. The reaction was stopped by quenching with an equal volume of 1 M Tris, pH 8, and the protein was dialyzed into 10 mM Mops, pH 7.9/0.1 mM EDTA/1 M KCl. Conjugation was verified by Western blotting with a monoclonal antibody to FeBABE (27).
Plasmids and DNA.
Templates for in vitro transcription and footprinting were obtained from pM650 (for λ pR′) and ppM416 (for phage 82 pR′) (28). For 3′ labeling, an EcoRI end at position +89 for λ pR′ (pMT650) or +87 for phage 82 pR′ (pM416) was filled with α-32P-dATP by the Klenow fragment of Pol I. For 5′ labeling, an oligonucleotide that annealed upstream of the promoter (145 bp from +1 of λ pR′ and 101 bp from +1 of phage 82 pR′) was phosphorylated with γ-[32P]ATP and T4 polynucleotide kinase and used for PCR. Labeled DNA was purified by electrophoresis in nondenaturing polyacrylamide followed by elution as described (29).
In Vitro Transcription.
For pausing experiments, RNAP (25 nM) was incubated with 1 nM biotinylated DNA in transcription buffer (10 mM Tris⋅HCl, pH 8.0/50 mM potassium glutamate/0.1 mM EDTA/1 mM DTT/10 mM MgCl2) for 15 min at 37° to form open complex. Streptavidin-coated paramagnetic beads were added, and open complex was separated from the free enzyme by magnetic partitioning of the coated beads. Open complexes were washed three times with wash buffer (10 mM Tris⋅HCl, pH 8.0/50 mM potassium glutamate/0.1 mM EDTA/1 mM DTT/10 mM MgCl2/100 μg/ml heparin) and resuspended in transcription buffer (10 mM Hepes, pH 7.9/50 mM potassium glutamate/0.1 mM EDTA/1 mM DTT/10 mM MgCl2) supplemented with 800 nM of the appropriate σ70 or an equal volume of σ70 storage buffer (10 mM Tris⋅HCl, pH 8.0/250 mM NaCl/1 mM EDTA/1 mM DTT/50% glycerol). Transcription was initiated by addition of 200 μM ATP, GTP, and CTP, and 50 μM UTP containing 0.5 μCi/μl α-32P-UTP. Samples (20 μl) were removed at intervals during incubation at 37° into 100 μl of transcription stop buffer (0.6 M Tris 8.0/12 mM EDTA/0.2%SDS/50 μg/ml tRNA). Samples were extracted with an equal volume of phenol/chloroform/isoamyl alcohol and precipitated with 2.5 volumes ethanol. RNA was resuspended in formamide loading buffer (10 mM Tris 8.0/10 mM EDTA/97% formamide/0.1% SDS/0.1% xylene cyanol/0.1% bromophenol blue) and separated on a 15% 7 M urea polyacrylamide gel. Radioactive RNA was detected with a PhosphorImager (Molecular Dynamics).
FeBABE Conjugation.
Single cysteine mutants of σ70 were conjugated with FeBABE, and its presence was verified by antibody, as described (27).
FeBABE DNA Footprinting.
Core RNAP was incubated with 5-fold molar excess of each FeBABE-σ70 on ice for 30 min to form holoenzyme. Open complex was formed by incubating 50 nM RNAP and 1 nM end-labeled DNA in 10 mM Hepes, pH 7.9/50 mM potassium glutamate/0.1 mM EDTA for 15 min at 37°C. A sample was removed and added to a 20× stock of ascorbate and hydrogen peroxide, to produce final concentrations of 5 mM each. After incubation at 37°C for 10 min, reaction was stopped with one volume quench buffer (0.1 M thiourea/1.0 M Tris, pH 8.0/0.1 mg/ml calf thymus DNA/0.2% SDS/10 mM EDTA). To produce paused complex, open complexes were walked to +15 for λ or +25 for 82 by the addition of an initiating oligoribonucleotide to 100 μM (ApApC for λ or ApC for 82) and 1 mM ATP, GTP, and CTP. A sample was removed and treated with ascorbate and hydrogen peroxide. Qλ was added to 500 nM or Q82 to 200 nM. The elongation factor NusA also was added to 150 nM for Qλ, because this stabilizes Qλ interaction with the paused complex (17). A sample was removed and treated identically to the open complex. Quenched FeBABE samples were precipitated with 2 volumes 95% ethanol and dissolved in formamide loading buffer (10 mM Tris 8.0/10 mM EDTA/97% formamide/0.1% SDS/0.1% xylene cyanol/0.1% bromophenol blue), separated on a 6 or 8% denaturing gel, dried, and visualized with a PhosphorImager.
Results
The Same σ70 Molecule Directs Initiation and Pausing.
We proposed a mechanism of pausing in which holoenzyme moves from promoter to pause site without dissociation of σ70 (16). An alternative possibility is that σ70 dissociates after initiation, allowing a different σ70 molecule to bind the RNAP core and induce the pause. We provide evidence here that in fact the same σ70 molecule mediates initiation and pausing during a round of transcription, thereby establishing the origin of the paused complexes we characterize below.
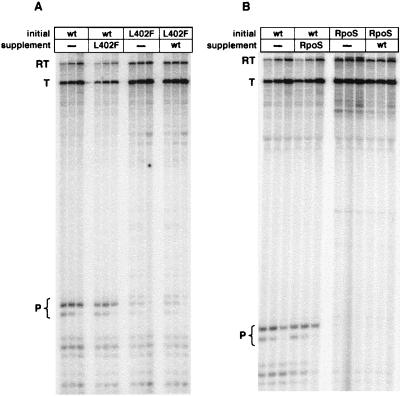
To determine whether the σ molecule present in the open complex also induces the pause, we used σs of variant pausing behavior to distinguish the initiating σ molecule from others available in the reaction. Previously, we isolated mutants altering σ70, such as L402F, that fail to respond to the pause-inducing signal (30). In the holoenzyme, these altered σs form stable open complex, although the stability is somewhat diminished compared with wild type. A second source of variant σ was the stationary phase σ RpoS; we found previously that RpoS holoenzyme responds poorly to the pause-inducing signal (M.T.M. and J.W.R., unpublished results).
For the analysis, we first formed open complexes with DNA immobilized on magnetic beads, using wild-type holoenzyme, holoenzyme containing the L402F mutant of σ70, or holoenzyme containing RpoS. After washing to remove unbound RNAP and σ, we initiated and transcribed in the presence of a large molar excess of variant σ. If the σ that induces the pause comes from the open complex, then the pausing characteristics of the enzyme should not be affected by exogenous σ. This is exactly what we observed (Fig. 2). When wild-type holoenzyme forms open complex the pausing characteristics are wild-type, whether the mutant σ is added or not (Fig. 2A). When the L402F mutant σ70 is used to form open complex, the pausing characteristics of the mutant enzyme are observed, even in the presence an 800-fold molar excess of wild-type σ. RpoS gives the same result (Fig. 2B). We conclude that the σ molecule present in open complex persists to induce the pause.
Figure 2.
Effect of variant σ added in excess in solution on pausing in vitro. (A) Open complex was formed with RNAP containing either wild-type σ70 (lanes 1–6) or σ70 L402F (lanes 7–12); after washing, excess σ70 L402F (lanes 4–6) or wild-type σ70 (lanes 10–12) was added, as described. Reactions were sampled at 1, 2, and 5 min for each set of three. (B) As for A, except that the supplement was σS. RT, readthrough; T, terminated; P, paused.
FeBABE Mapping of σ70 in Open and Paused Complexes.
We used FeBABE to map the position of the conserved σ70 subunit domains in both open and paused complexes of the late promoters (pR′) of phages 82 and λ. Previously, the same FeBABE conjugates were used to map σ70 in open complex of the lacUV5 and galP1 promoters (22, 23, 25); in general, our results for the open complex agree very well with these.
For simplicity of manipulation, we used a variant of the phage 82 early transcribed region that allows stopping by nucleotide deprivation at the natural pause site at +25 and that lacks a potentially confusing pause at +15. The greater distance of the 82 paused complex from the promoter allows its image to be better resolved from the open complex than that of λ. For λ, we used the +15 complex, which is functionally equivalent to the natural +16 complex (13, 31). KMnO4 footprinting was used to verify the movement of RNAP (data not shown). Although promoter clearance is not complete, enough RNAP progressed to the pause to allow us to map regions of the DNA in close proximity to the conserved regions of σ70.
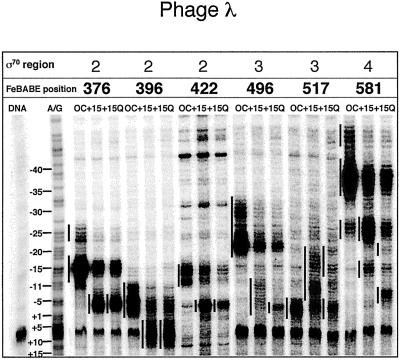
We first describe the activity of FeBABE conjugated to the conserved regions 2 and 4, which recognize, respectively, the −10 and −35 promoter elements; in these conjugates, cysteines replace amino acid 396 in conserved region 2.1 and amino acid 581 in region 4.2. All cleavage was measured on the nontemplate strand, which is a better substrate than the template strand for region 2 conjugates (22).
Conserved Region 2 of σ70.
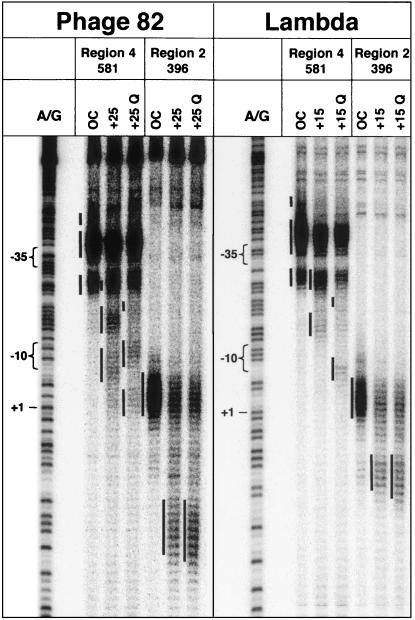
In the open complex at phage 82 pR′, the region 2 conjugate to amino acid 396 cleaves the nontemplate strand from position −7 to about +3 or +4 (Figs. 3 and 4), with a small amount of cleavage around position −9. When RNAP is allowed to move to the pause site, a new pattern of reactivity appears between nucleotides +14 and +22, just the positions predicted if region 2 is positioned at the pause-inducing sequence (+9 to +14), as it is at the promoter −10 sequence (−12 to −7). Addition of Q82 to the phage 82 complex paused at +25 induces a slight change, shifting the new reactivity downstream about 1 bp to position +23. In the open complex at the λ pR′ promoter, the 396 FeBABE conjugate cleaves the nontemplate strand in the region from −6 to about +1 with additional cleavage at position −9 (Figs. 3 and 5). In the paused complex, this conjugate cleaves DNA from about +8 to about +13 (see especially Fig. 3), again as expected from the position of the pause-inducing sequence. Qλ appears to extend this reactivity downstream about 1–2 nt, like Q82 at its cognate promoter.
Figure 3.
Cleavage of DNA in open, paused, and Q-modified complexes by FeBABE conjugates in σ70 regions 2 and 4. DNA was labeled at the 3′ end of the nontemplate strand. An A/G ladder is shown for reference; assigned regions of cleavage are designated by adjacent vertical bars.
Figure 4.
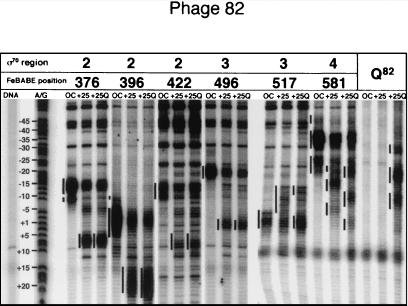
Cleavage of DNA in phage 82 complexes by a panel of FeBABE conjugates to regions 2, 3, and 4 of σ70, and to Q82. DNA was labeled at the 3′ end of the nontemplate strand. FeBABE was attached to the N terminus of Q82, as described in the text.
Figure 5.
Cleavage of DNA in phage λ complexes by a panel of FeBABE conjugates to regions 2, 3, and 4 of σ70. DNA was labeled at the 5′ end of the nontemplate strand, and the image was flipped for comparison to Figs. 3 and 4.
Some RNAP remains in open complex at the promoter when complexes are provided NTP substrate to advance to the pause site, according to both FeBABE reactivity (these experiments) and KMnO4 reactivity (data not shown). As expected, neither Q affects the reactivity of any FeBABE conjugate of RNAP that remains in open complex.
Conserved Region 4 of σ70.
In the open complex of phage 82 pR′, the region 4 conjugate to amino acid 581 cleaves DNA strongly in the region of the −35 element, from −40 to −31 and −27 to −23 (Figs. 3 and 4), with a third region of reactivity from about −48 to −43. At the +25 pause, new reactivity is observed in the regions from −19 to −15 and −10 to −5. This is the position predicted if region 4 is positioned relative to region 2 as it would be in open complex, despite the absence of a −35 consensus. Q82 has a striking effect on the region 4 reactivity, displacing it downstream about 5 bp (Figs. 3 and 4). New reactivity is observed in regions from positions −22 to −18, −12 to −7, and −4 to +2, and the reactivity due to the paused complexes disappears.
FeBABE at amino acid 581 cuts DNA in the λ pR′ open complex similarly (Figs. 3 and 5): there is strong cleavage from position −40 to position −34, with additional cleavage around −27 to −24 and in a far upstream segment from −48 to about −44. In the paused complex, new reactivity appears from about −17 to about −12 and from about −28 to −23, where it overlaps the downstream segment of open complex reactivity, again where region 4 should be if a −35 element were optimally positioned relative to region 2. Like Q82, Qλ strongly affects reactivity of the region 4 conjugate, inducing new reactivity downstream, at positions −22 to −19 and −8 to −4, and reducing reactivity in the pause-specific regions. (These changes, particularly in the segment where open and paused complex reactivity overlap at −28 to −23, are most evident in Fig. 5.) Thus Q82 and Qλ induce similar changes in their target complexes.
Reactivity of Additional σ70 Conjugates.
To confirm and extend these results, we used two additional conjugates in region 2, at amino acids 376 (region 2.1) and 422 (region 2.3), as well as two conjugates in the intervening region 3, at amino acids 496 and 517 (Figs. 4, 5, and 7). The region 2.1 and 2.3 conjugates react similarly, cutting behind the −10 consensus in open complex, in good agreement with previous results for open complexes (22). Some extraneous background from the 422 conjugate could be resolved by comparison of open and paused complexes. The activity of these two conjugates in paused complexes mirrors open complex but is displaced an appropriate amount downstream; thus, regions 2 and 4 appear to move coordinately as the paused complex is formed.
Figure 7.
Model of the disposition of σ70 regions 2, 3, and 4, Q82, and Qλ in open and paused complexes of phage late gene promoters. Rectangular boxes span regions of reactivity by FeBABE conjugates to σ70 residues of the enclosed number, or to Q82. Ovals and spheres designate positions of protein regions. Region 4 of σ70 in the presence of Q cannot be presumed to bind DNA and is shown displaced. (A) λ; (B) phage 82.
Region 3 conjugates behave differently from those of regions 2 and 4. Their reactivity in open complexes reproduces previous results but does not move in coordination with regions 2 and 4 as the pause develops. For the 496 conjugate, reactivity moves somewhat farther than the displacement of the pause-inducing sequence would predict for λ, by about a half a turn of DNA, and about the same distance for 82 as for λ, despite the different displacement of the pause-inducing sequence. A more striking exception is the 517 conjugate in region 3, which reacts as reported for open complex, but appears to undergo a retrograde movement as RNAP moves to the paused position. It is an interesting prospect that these disjunctive movements of region 3 reflect a significant change in the conformation of σ70 correlated with appearance of the initial portion of the transcript.
Reactivity of the region 3 conjugates is affected by Q, although less than region 4 conjugates. For the 517 conjugate, both Q82 and Qλ protect the DNA from cleavage in the upstream regions, and, most evidently for Q82, enhance or focus the downstream peak of reactivity around the start site (Figs. 4 and 5). For the 496 conjugate, addition of Qλ also protects the upstream region and concentrates reactivity in the downstream segment; however, Q82 has no visible effect on the 496 conjugate-mediated cleavage. Possibly the distinct relative positions of Q and RNAP in the two promoters accounts for this difference.
Reactivity of a Q82 FeBABE Conjugate.
A particular advantage of the positive footprint provided by tethered cleavage reagents is the ability to detect the proximity to DNA of one protein in a large complex containing other DNA-binding proteins. Thus, we have used an FeBABE conjugate of Q82 to locate the Q-binding region in the paused complex, which contains about eight polypeptides and has a mass of about 500 kDa; previous attempts to use traditional protection footprinting in the paused complex were ambiguous. Q82 has no natural cysteines, so that a cysteine could be placed at the N terminus to allow conjugation of a single FeBABE.
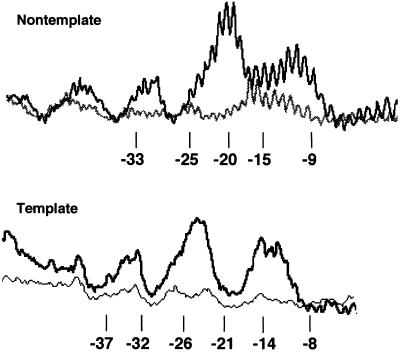
The conjugated Q82 cleaves DNA in the region between the −35 element and the −10 element in the paused complex, consistent with previous determinations of the binding region of three lambdoid phage Q proteins (31). On the nontemplate strand, three regions of cleavage are observed: −32 to −29, −23 to −18, and −14 to −9 (Figs. 4 and 6). On the template strand, cleavage is displaced slightly upstream, as expected, appearing in the three regions −34 to −31, −26 to −21, and −15 to −12 (Fig. 6). These segments partly coincide with cleavage due to the σ70 region 4 conjugate to amino acid 581, thus identifying regions of DNA free to react with hydroxyl radicals in the presence of both of these polypeptides.
Figure 6.
Profile of Fe-BABE-Q82 cleavage of both DNA strands in paused complexes. Lighter lower traces are of unreacted DNA.
Discussion
Positive footprinting with FeBABE conjugates of σ70 and Q82 provides a detailed view of the disposition of protein domains in the paused transcription complex that is bound by lambdoid phage Q proteins. The paused complex is similar in structure to open complex but features a significant difference that could be associated with the presence of a transcript in the early paused complex; we generally confirm previous studies of open complex structure according to FeBABE reactivity. The restructuring of σ70 domains as Q binds the complex may reflect steps in the pathway that leads to incorporation of Q protein into the mature transcription elongation complex. Fig. 7 summarizes the loci of cleavage and the inferred positions of regions of σ70 and Q proteins.
Open Complexes.
In general, the open promoter complexes at the λ and 82 late gene promoters match those of other promoters in their pattern of FeBABE reactivity. As proposed (32), this consistency supports the existence of a common open complex structure even at promoters as divergent as the strong phage promoter λ pR′ and the activator-regulated promoter galP1, which lacks a −35 element altogether. The region 2 conjugates, taken together, cleave DNA in an extended segment from about −16 to +2 (Fig. 7 A and B). Notably, the actual −10 element remains uncleaved, implying that it is bound by a protein that leaves only surrounding DNA free to react with hydroxyl radicals. This is consistent with the function of region 2 in promoter recognition and open complex formation to make base-specific interactions with the nontemplate strand (3, 4).
The region 3 conjugates at amino acids 496 and 517 cut DNA between the −10 and −35 promoter elements and around the start site, respectively (Fig. 7 A and B). For 82, the 496 conjugate cleavage pattern is concentrated in a single peak near −20, whereas for λ it is somewhat diffuse and extends upstream, more nearly as reported for lacUV5 and galP1 (22, 23, 25). Region 3 of free σ70 is protease sensitive (33, 34), suggesting a lack of structure and possible mobility that might allow it to be positioned according to the details of the promoter. The activity of FeBABE conjugated to amino acid 517 to cleave around the start site in both open complexes is consistent with the ability of the initiating nucleotide to crosslink to a region between Glu-508 and Met-561 (35); the orientation of crosslinking defined this region of σ as part of the “5′ face” of the nucleotide binding site.
The region 4 conjugates cleave both open complexes in two segments that straddle the −35 region, although there is significant cleavage as far upstream as −47. Region 4 thus binds and protects bases in the −35 element (Fig. 7), allowing hydroxyl radical to cut adjacent DNA.
Paused Complexes.
As anticipated from their dependence on both a pause-inducing sequence that matches the promoter −10 consensus and the presence and function of σ70, the paused complexes of both promoters are substantially similar to open complex in FeBABE reactivity. In the paused complex, positions of reactivity are displaced by an amount appropriate to the position of the pause-inducing sequence, with the exception of region 3, as described below. Region 2 is placed directly over the pause-inducing sequence in both λ and 82, just as for open complex. More interesting, despite the absence of a recognizable −35 element, region 4 also is placed as in the open complex, at a position appropriate to the nearly optimal 18-bp spacing between −10 and −35 elements at a promoter. Presumably, region 4 is not making base-specific contacts with the DNA but instead is held in the appropriate position by holoenzyme structure. Possibly the helix-turn-helix DNA-binding motif of region 4 also makes nonspecific, but energetically favorable, contacts with any DNA sequence. This same behavior of region 4 was observed in FeBABE cleavage at a promoter, galP1, that lacks a −35 element (23). We note that FeBABE reactivity provides definitive proof that σ70 is present in the paused complex.
Whereas regions 2 and 4 follow the pause-inducing sequence downstream to replicate their position in the open complex, region 3 gives an interesting and unexpected pattern of cleavage. The region 3 position 496 reactivity moves downstream with conjugates of regions 2 and 4, but not coordinately, instead staying near the start site for both paused complexes, despite their different overall positions. More remarkably, the position of the 517 conjugate appears to move backwards, changing entirely with respect to regions 2 and 4. Conceivably, this movement is related to the presence of an RNA stably bound in the transcription complex at the pause. Because the segment 508–561 in region 3 of σ70 makes up the 5′ face of the active site, it might be displaced by RNA exiting the polymerase. Perhaps the position of region 3 in the paused complex represents an intermediate in promoter escape that is trapped at the pause. The site of 496 reactivity in the paused complex appears near the transcription starting position for both λ and 82 templates, even though the paused complexes are displaced 10 bp on the templates. This raises the possibility that this locus of region 3 remains associated with DNA after initiation.
The Q-Modified Complex.
Q protein has distinct and significant effects on the conformation of σ70 subunits in the paused complex. Whereas the paused complex itself is a near replicate of open complex with respect to positioning of the major DNA-binding domains, Q binding alters this structure, most obviously causing FeBABE bound to region 4 to react about a half turn of DNA closer to region 2. For λ at least, the Q-binding site overlaps the default position of region 4 in the paused complex, suggesting that interaction with Q repositions region 4 in the complex to allow Q to bind simultaneously to its DNA site and to region 4. There is no evidence that region 4 moves relative to core; the path of DNA in open complex is unknown and might well change on Q binding. Nor is it known that the entire σ70 region 4 domain moves, because only repositioning of the bound FeBABE is shown. However, the appearance of reactivity from both σ70 and Q82 at the original apparent binding site of σ70 region 4 when Q is added to the paused complex suggests substantial repositioning of the domain.
Q has lesser but possibly significant effects on the reactivity of FeBABE conjugates in regions 2 and 3. Reactivity from position 396 of region 2, at the forward boundary of the pause-inducing sequence, is advanced one or two nucleotides. Because position 396 reacts more than two turns of DNA from the site where Q82 is believed to bind (see below), such influence likely is transmitted via conformational changes in the entire complex. For the conjugates in region 3 (496 and 517), Q tends to concentrate reactivity in a peak around the start site; one interpretation is that binding of Q fixes this site of region 3 and prevents its wandering along a broader segment of DNA. Possibly the repositioning of region 4 by Q displaces region 3 downstream. Previous footprinting of Qλ in the paused complex revealed Q-dependent changes in methidiumpropyl-EDTA⋅Fe(II) (MPE) reactivity around the pause-inducing sequence, from about −2 to +6, near the site of region 2 (13).
Reactivity of FeBABE Q82.
FeBABE conjugated to the N terminus of Q82 gives three regions of cleavage in the paused complex, with the middle region approximately twice as intense as the outside two regions. This position of cleavage agrees well with the interval where Q proteins of phages λ, 82, and 21 map by MPE footprinting on free DNA (13, 31). Qλ is believed to bind its site as a dimer (36), and we suggest that a dimer of phage Q82 bound to the paused complex explains cleavage in three sites (see Fig. 7): each monomer cleaves DNA on either side of its binding site, and overlapping cleavage by both monomers in the middle gives a peak of about double intensity. The apparent Q82-binding sites are positions −31 to −25 (AAAACTG) and −19 to −13 (AAAAGCA), with a possible consensus AAAANYR. In this model, the Q dimer would bind a directly repeated sequence, like the λ cII activator (37). Despite the fact that Q82 and Qλ bind paused complexes displaced by 8 bp, the sites protected by Qλ from ⋅OH cleavage in free DNA match these putative Q82-binding sites quite closely (36).
Structure of the Paused and Modified Complex.
These and other data provide the following view of formation of the paused complex and its modification by Q. During initial elongation, the σ70 molecule present in open complex persists to bind the −10-like pause-inducing sequence, trapping RNAP. In the paused complex, region 4 of σ70 is positioned about 18 bp upstream of region 2, as in a promoter, despite the apparent lack of a −35 element. Although RNAP remains bound to the pause-inducing sequence, RNAP continues elongation, possibly by winding (scrunching) DNA into the enzyme. After 3 or 4 nucleotides are added (to +16 or +17 for λ and to +25 for phage 82), increasing strain stops elongation and pauses the complex, which tends to backtrack until the catalytic center is at an appropriate distance from the pause-inducing −10 homologue (≈+12 for λ and ≈+20 for phage 82). As we describe elsewhere (38), RNAP escapes the pause either by Gre factor-induced cleavage and resynthesis to the stall point or by slow isomerization forward; eventually σ70 is released from the pause-inducing sequence, and elongation succeeds from the forward position.
Binding of Q to the paused complex, which is believed to occur only in the forward position (38), both accelerates release of RNAP into elongation and leads to modification of the enzyme. Either the conformation of the paused RNAP or σ70 itself (or both) must be recognized directly by Qλ (and possibly Q82) in the complex, because RNAP stalled at the pause site in the absence of the σ70 subunit is not modified by Qλ (13, 16), despite the presence of a tight Qλ-binding site in the DNA. The ensuing conformational alteration of σ70 region 4 by Q could result from direct contact between Q and σ70, for which there is further evidence in the case of Qλ. Restructuring of σ70 may be a step in the modification pathway that weakens binding of σ70 to the pause-inducing sequence, thus releasing RNAP from the pause. Presumably, Q is positioned in this intermediate to establish a contact with core subunits that persists through elongation.
Acknowledgments
We thank G. Gussin, R. Landick, K. Murakami, A. Hochschild, and members of the laboratory for reading the manuscript. Supported by National Institutes of Health Grants GM21941 (to J.W.R.) and GM25909 (to C.F.M.).
Abbreviations
- RNAP
RNA polymerase
- ds
double-stranded
- ss
single-stranded
Footnotes
This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected on April 27, 1999.
References
- 1.Gardella T, Moyle H, Susskind M M. J Mol Biol. 1989;206:579–590. doi: 10.1016/0022-2836(89)90567-6. [DOI] [PubMed] [Google Scholar]
- 2.Siegele D A, Hu J C, Walter W A, Gross C A. J Mol Biol. 1989;206:591–604. doi: 10.1016/0022-2836(89)90568-8. [DOI] [PubMed] [Google Scholar]
- 3.Marr M T, Roberts J W. Science. 1997;276:1258–1260. doi: 10.1126/science.276.5316.1258. [DOI] [PubMed] [Google Scholar]
- 4.Roberts C W, Roberts J W. Cell. 1996;86:495–501. doi: 10.1016/s0092-8674(00)80122-1. [DOI] [PubMed] [Google Scholar]
- 5.Waldburger C, Gardella T, Wong R, Susskind M M. J Mol Biol. 1990;215:267–276. doi: 10.1016/s0022-2836(05)80345-6. [DOI] [PubMed] [Google Scholar]
- 6.McKane M, Malone C, Gussin G N. Biochemistry. 2001;40:2023–2031. doi: 10.1021/bi0019085. [DOI] [PubMed] [Google Scholar]
- 7.Landini P, Bown J A, Volkert M R, Busby S J. J Biol Chem. 1998;273:13307–13312. doi: 10.1074/jbc.273.21.13307. [DOI] [PubMed] [Google Scholar]
- 8.Kuldell N, Hochschild A. J Bacteriol. 1994;176:2991–2998. doi: 10.1128/jb.176.10.2991-2998.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li M, Moyle H, Susskind M M. Science. 1994;263:75–77. doi: 10.1126/science.8272867. [DOI] [PubMed] [Google Scholar]
- 10.Kim S K, Makino K, Amemura M, Nakata A, Shinagawa H. Mol Gen Genet. 1995;248:1–8. doi: 10.1007/BF02456607. [DOI] [PubMed] [Google Scholar]
- 11.Hu J C, Gross C A. Mol Gen Genet. 1985;199:7–13. doi: 10.1007/BF00327502. [DOI] [PubMed] [Google Scholar]
- 12.Roberts J W, Yarnell W, Bartlett E, Guo J, Marr M, Ko D C, Sun H, Roberts C W. Cold Spring Harbor Symp Quant Biol. 1998;63:319–325. doi: 10.1101/sqb.1998.63.319. [DOI] [PubMed] [Google Scholar]
- 13.Yarnell W S, Roberts J W. Cell. 1992;69:1181–1189. doi: 10.1016/0092-8674(92)90639-t. [DOI] [PubMed] [Google Scholar]
- 14.Grayhack E J, Yang X J, Lau L F, Roberts J W. Cell. 1985;42:259–269. doi: 10.1016/s0092-8674(85)80121-5. [DOI] [PubMed] [Google Scholar]
- 15.Ring B Z, Roberts J W. Cell. 1994;78:317–324. doi: 10.1016/0092-8674(94)90300-x. [DOI] [PubMed] [Google Scholar]
- 16.Ring B Z, Yarnell W S, Roberts J W. Cell. 1996;86:485–493. doi: 10.1016/s0092-8674(00)80121-x. [DOI] [PubMed] [Google Scholar]
- 17.Yarnell W S, Roberts J W. Science. 1999;284:611–615. doi: 10.1126/science.284.5414.611. [DOI] [PubMed] [Google Scholar]
- 18.Sun H. Ph.D. thesis. Ithaca, NY: Cornell University; 2000. [Google Scholar]
- 19.Busby S, Ebright R H. Mol Microbiol. 1997;23:853–859. doi: 10.1046/j.1365-2958.1997.2771641.x. [DOI] [PubMed] [Google Scholar]
- 20.Hawley D K, McClure W R. Nucleic Acids Res. 1983;11:2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lisser S, Margalit H. Nucleic Acids Res. 1993;21:1507–1516. doi: 10.1093/nar/21.7.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Owens J T, Chmura A J, Murakami K, Fujita N, Ishihama A, Meares C F. Biochemistry. 1998;37:7670–7675. doi: 10.1021/bi980188n. [DOI] [PubMed] [Google Scholar]
- 23.Bown J A, Owens J T, Meares C F, Fujita N, Ishihama A, Busby S J, Minchin S D. J Biol Chem. 1999;274:2263–2270. doi: 10.1074/jbc.274.4.2263. [DOI] [PubMed] [Google Scholar]
- 24.Greiner D P, Miyake R, Moran J K, Jones A D, Negishi T, Ishihama A, Meares C F. Bioconjugate Chem. 1997;8:44–48. doi: 10.1021/bc9600731. [DOI] [PubMed] [Google Scholar]
- 25.Colland F, Fujita N, Kotlarz D, Bown J A, Meares C F, Ishihama A, Kolb A. EMBO J. 1999;18:4049–4059. doi: 10.1093/emboj/18.14.4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hager D A, Jin D J, Burgess R R. Biochemistry. 1990;29:7890–7894. doi: 10.1021/bi00486a016. [DOI] [PubMed] [Google Scholar]
- 27.Traviglia S L, Datwyler S A, Meares C F. Biochemistry. 1999;38:4259–4265. doi: 10.1021/bi983016z. [DOI] [PubMed] [Google Scholar]
- 28.Marr M T. Ph.D. thesis. Ithaca, NY: Cornell University; 2000. [Google Scholar]
- 29.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 30.Ko D C, Marr M T, Guo J, Roberts J W. Genes Dev. 1998;12:3276–3285. doi: 10.1101/gad.12.20.3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yarnell W. Ph.D. thesis. Ithaca, NY: Cornell University; 1993. [Google Scholar]
- 32.Siebenlist U, Simpson R B, Gilbert W. Cell. 1980;20:269–281. doi: 10.1016/0092-8674(80)90613-3. [DOI] [PubMed] [Google Scholar]
- 33.Gribskov M, Burgess R R. Gene. 1983;26:109–118. doi: 10.1016/0378-1119(83)90180-4. [DOI] [PubMed] [Google Scholar]
- 34.Severinova E, Severinov K, Fenyo D, Marr M, Brody E N, Roberts J W, Chait B T, Darst S A. J Mol Biol. 1996;263:637–647. doi: 10.1006/jmbi.1996.0604. [DOI] [PubMed] [Google Scholar]
- 35.Severinov K, Fenyo D, Severinova E, Mustaev A, Chait B T, Goldfarb A, Darst S A. J Biol Chem. 1994;269:20826–20828. [PubMed] [Google Scholar]
- 36.Bartlett E. Ph.D. thesis. Ithaca, NY: Cornell University; 1998. [Google Scholar]
- 37.Wulff D, Rosenberg M. Lambda II. Plainview, NY: Cold Spring Harbor Lab. Press; 1983. pp. 53–73. [Google Scholar]
- 38.Marr M T, Roberts J W. Mol Cell. 2000;6:1275–1285. doi: 10.1016/s1097-2765(00)00126-x. [DOI] [PubMed] [Google Scholar]
- 39.Kainz M, Roberts J. Science. 1992;255:838–841. doi: 10.1126/science.1536008. [DOI] [PubMed] [Google Scholar]