Abstract
Purpose
To evaluate the impact of the extension of the radiotherapy field cranially toward para-aortic lymph nodes (EF-RT) in advanced cervical cancer.
Materials and Methods
A systematic search of databases (PubMed, CENTRAL, Clinical Trials) was performed and included studies that were published between 1960 and November 2015 without language restrictions. All randomized clinical trials (RCTs) were analyzed further. All patients must have undergone pelvic radiotherapy and the same systemic therapy in both arms. The primary endpoints were locoregional failure, incidence of distant metastasis, para-aortic failure, and cancer related death. The Mantel-Haenszel method was used in the meta-analysis. The risk of bias analysis was determined using the 7-domain method per the Cochrane Handbook for Systematic Reviews of Interventions V5.1.0. A review of the treatment technique and toxicity was also performed.
Results
A total of 1309 studies were evaluated, 4 RCTs of which met the inclusion criteria; 506 patients were allocated to standard pelvic irradiation, and 494 underwent EF-RT. The risk of bias was considered to be low in nearly 80% of the domains. EF-RT significantly reduced the rate of para-aortic failure (HR 0.35, 95% CI 0.19–0.64; p<0.01) and the incidence of other distant metastases (HR 0.69, 95% CI 0.50–0.96; p=0.03). Locoregional failure and cancer-related death were not significantly altered (OR 1.06 [0.80–1.42]; p=0.67, and 0.68 [0.45–1.01]; p=0.06, respectively). The radiotherapy technique was conventional in 3 studies and conformational in 1 study. In total, 10 treatment-related deaths occurred—4 in pelvic radiation and 6 in EF-RT (OR 2.12 [0.71–6.27]; p=0.18).
Conclusions
EF-RT that targets the para-aortic lymphatic chain reduces distant metastatic events, but its impact on survival is unknown. Future studies should examine the value of EF-RT using modern radiation techniques.
Keywords: cervical cancer, para-aortic radiotherapy, extended-field
INTRODUCTION
Cisplatin-based chemoradiation has been the standard treatment for patients with locally advanced cervical cancer for the past 2 decades (1). Although it is not considered in the FIGO staging system (2), the presence of lymph node metastasis is common in advanced cervical cancer and is well known as an important prognostic factor. Lymph node involvement in the para-aortic region increases progressively according to tumor stage— 5%, 16%, and 25% for stages I, II, and III, respectively (3).
Prior to clinical trials that established combination treatment with chemotherapy and radiotherapy as the standard of care, 2 multi-institutional studies from the European Organization for Research and Treatment of Cancer (EORTC) (4) and the Radiation Therapy Oncology Group (RTOG, protocol 7920) (5–6) evaluated the impact of adding radiotherapy (RT) to the para-aortic field in advanced cervical cancer. The rationale for prophylactic extended-field RT toward the para-aortic node area (EF-RT) was to sterilize micrometastatic disease and mitigate the risk of future distant relapse. Both studies noted a benefit with regard to para-aortic control and distant metastasis, and the RTOG trial also suggested an impact on cancer-specific survival.
Moreover, the RTOG 9001 (7–8) trial demonstrated that pelvic RT, combined with chemotherapy, was superior to extended-field RT (pelvic and para-aortic areas). The reduction in distant metastasis was related to a decrease in the number of combined distal and local failures. Its findings suggested that better pelvic control with chemoradiotherapy limits secondary spread from uncontrolled pelvic disease rather then the treatment of the systemic disease at diagnosis.
The benefits for locoregional control, distant metastasis, and cancer-specific survival were not accompanied by a reduction in para-aortic metastasis, because the para-aortic region can remain a sanctuary of malignant cells. Consequently, the logical approach would be to combine the benefits of systemic therapy for pelvic locoregional control with those of EF-RT for para-aortic control. However, the increased toxicity in RTOG 9210 (9–10) using conventional (2D) chemoradiotherapy was disappointing and prematurely halted research in this field.
Recent developments in radiology (11–12), minimally invasive surgical staging (13), and modern radiotherapy technologies (14–15) have renewed interest in treatments to the para-aortic region.
The aim of this systematic review was to determine the impact of EF-RT in terms of locoregional failure, incidence of distant metastasis, para-aortic failure, and cervical cancer-related deaths, combining the best evidence of published phase III trials. Further, we compiled irradiation techniques and their toxicity-related deaths. Our hypothesis was that the reduction in para-aortic failure improves distal control, especially it occurs with an unaltered locoregional control.
MATERIALS AND METHODS
Identification of relevant studies
Based on the guidelines that are provided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Report (PRISMA) (16), the following inclusion criteria for phase III trials were fulfilled: 1) all patients were treated with pelvic radiotherapy; 2) all patients received the same systemic therapy (or not) in both treatment arms, and 3) allocation to the para-aortic irradiation group was randomized. No specific protocol for EF-RT in the experimental arm was excluded (ie, guided by para-aortic-directed imaging).
Relevant studies were identified in MEDLINE (PUBMED) and CENTRAL (The Cochrane Library) without language restrictions. The method and terms that were used are available in Supplementary Figure 1S. The terms in the systematic search were also used for unpublished material on the Google website. Briefly, the authors performed the following steps: search engine, title evaluation, abstract evaluation, and full-text evaluation. After direct correspondence with the journal editor or authors, there was no article that remained unavailable at any step. Further, the ClinicalTrials database and reference lists of all retrieved articles from the last step (14 papers) were examined to identify other potentially relevant reports.
If the same trial was published more than once, the most actualized information was prioritized, but additional information from previous publications or supplementary materials were also used. We did not obtain data on individual patients for this analysis. The data extraction occurred after registration in the PROSPERO database (CRD42015030034).
Quality assessment
Because no additional study was identified after searching for unpublished RCTs using the internet, all articles were published as full peer-reviewed reports. Studies that were selected for the meta-analysis were evaluated using a 3-level scale (low, unclear, high) with regard to the risk of bias in grading 7 domains of each outcome per the Cochrane Handbook for Systematic Review of Interventions (17). The quality assessment was performed by 3 of the authors.
Statistical analysis
Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated by Mantel-Haenszel method. In cases of heterogeneity, a random effects model was fitted to estimate the pooled OR data. Otherwise, we adopted a fixed effects model. Heterogeneity was examined using Q statistic (Cochran’s Q: the chi-square statistic for the test of heterogeneity and measure of inconsistency). Publication bias was verified by funnel plot. Sensitivity analysis was performed to test the stability and robustness of the results of each study. The influence of individual studies on the summary estimate was determined by plotting the summary estimate in the absence of each study. Statistical analyses were conducted using RevMan 5.0 and R (version 3.2.1) Software. All statistical tests were 2-sided, and we adopted a significance level of 5%.
RESULTS
Included Studies
A total of 1309 articles were screened, resulting in 6 randomized studies with para-aortic RT: EORTC (4), RTOG 7920 (5–6), Osaka University (18), Chang Gung University (12,19), RTOG 9001 (7–8), and a Chinese trial (20). The study by Du et al. (20) was excluded because it compared IMRT versus 3D techniques, but both arms treated para-aortic nodes. RTOG 9001 was excluded because the systemic therapy was used in the control but not EF-RT arm. A total of 1000 patients from 4 RCTs met the inclusion criteria and were analyzed, in which 506 patients were allocated to pelvic irradiation (P) compared with 494 patients to EF-RT. The majority (87.5%) of the patients was treated with documented prophylactic para-aortic irradiation; 12.5% of patients in the RTOG trial were not subjected to para-aortic image staging. Other studies characteristics, including staging and follow-up, are presented in Table 1. The risk of bias was low in more than 80% of the domains in the meta-analysis, as demonstrated in Figure 1 and detailed in Supplementary Figure S2.
Table 1.
Studies Characteristics.
| Study | Patients (n) |
Years of Recruitment |
Inclusion Criteria |
Follow- up Period |
Follow-up Evaluation |
Para- aortic Status (staging) |
Chemotherapy | Uterine Additional Treatment |
|---|---|---|---|---|---|---|---|---|
| EORTC | 441 | 1977 – 1981 | IB – IIB pelvic + and IIB - III | 94% patients more than four years | Clinical (2-monthly for 1 year + 4-monthly for 2 years + 6-monthly thereafter | All negative (100% evaluated) | not used | BT |
| RTOG 7920 | 335 | 1979 – 1986 | IB2 - IIB PAor - | 8 years after date of the last patient recruited | Clinical (3-monthly for 3 years + 6-monthly for 2 years + 12-monthly thereafter) | All negative (63% evaluated) | not used | BT |
| Osaka University | 93 | 1986 – 1990 | IB - IIB PAor- | 57.6 months | Clinical (every month for one year, then every 3 menthos) | All negative (100% evaluated) | not used | BT (38.8 %) Histerectomy (61.2%) |
| Chang Gung University | 129 | 2002 – 2006 | IB - IVA, pelvic +, PAor-(MRI) | 89 months | CT or MRI | 100 % negative (MRI) 11% positive (PET-CT) | Yes (Cisplatin, 6 cycles, weekly) | BT |
BT: brachytherapy. MRI: magnetic resonance image. PET-CT: positron emission tomography. PAor: para-aortic nodes.
Figure 1.
Oncological Outcomes
There was a significant decrease in para-aortic failure (OR 0.35 [0.19 – 0.64], p = 0.0006) (Figure 2A) and incidence of distant metastasis in favor of the EF-RT arm (OR 0.69 [0.50 – 0.96], p = 0.03) (Figure 2B).
Figure 2.
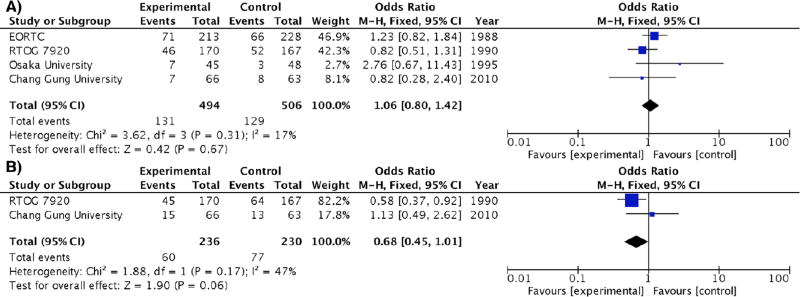
Because the control and experimental arms (EF-RT) received the same pelvic treatment, no difference in pelvic locoregional relapse was observed (OR 1.06 [0.80 – 1.42], p = 0.67) (Figure 3A). Moreover, there was a tendency towards decrease in death from cancer for EF-RT (OR 0.68 [0.45 – 1.01], p = 0.06) (Figure 3B), but only 2 studies (RTOG 7920 and Chang Gung University) provided data on cancer-related deaths. Multiple sensitivity analyses (excluding 1 trial at a time) were performed for each endpoint, and the same trend remained (Supplementary Figure S3). Funnel plots suggested symmetry along the treatment effect axis for all outcomes (Supplementary Figure S4).
Figure 3.
Technique and Toxicity
The para-aortic RT technique was conventional (2D) in 3 studies and conformational (3D) in 1. The total para-aortic dose was 45 Gy for all cases; the fraction dose varied from 1.5 Gy to 1.8 Gy, and the upper limits of the extended field differed between the upper border of L1 and T11. Most cases (over 90%) were administered uterine brachytherapy. The Japanese trial included posthysterectomy patients, for whom the vaginal vault received high-dose-rate brachytherapy, prescribed at 0.5 cm from the surface. Table 2 presents the treatment characteristics.
Table 2.
Radiotherapy Technique.
| EORTC | RTOG 7920 | Osaka University |
Chang Gung University |
|||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Treatment planning | 2D | 2D | 2D | 3D (CT-assisted) | ||||
|
| ||||||||
| Field configuration | Pelvic: AP/PA or four-field (box) | Para-aortic: AP/PA | Pelvic: four-field (box) | Para-aortic: four fields | Pelvic: NR | Para-aortic: AP/PA | Pelvic: four-field (box) | Para-aortic: NR |
|
| ||||||||
| Beam energy | megavoltage | megavoltage | megavoltage (10 MV) | megavoltage (10 MV) | ||||
|
| ||||||||
| Total Pelvic dose | 40 – 50 Gy | 40 – 50 Gy | 50 Gy | 45 Gy | ||||
|
| ||||||||
| Upper pelvic limit | Lower border of L4 | NR | NR | NR | ||||
|
| ||||||||
| Upper para-aortic limit | Upper border of L1 | NR | T11-T10 | NR | ||||
|
| ||||||||
| Total Para-aortic dose (fraction size) | 45 Gy | 45 Gy | 45 Gy | 45 Gy | ||||
| (1.5 – 1.7 Gy) | (1.6 – 1.8 Gy) | (1.8 Gy) | (NR) | |||||
|
| ||||||||
| Brachytherapy boost to point A | Control: 90.8% | Experimental: 89.6 % | 100% | 100 % | Control: 90% | Experimental: 91% | ||
|
| ||||||||
| protocol dose not reported | 30 – 40 Gy (LDR) | RT arm: 30Gy (HDR) | OP arm: 25 – 30 Gy cylinder 0.5cm surface | 6 × 4.3 Gy to point A (HDR) | ||||
CT: computed tomography. AP/PA: antero-posterior/posterior-anterior. NR not reported. HDR: high-dose-rate brachytherapy. LDR: low-dose-rate brachytherapy.
The EORTC and RTOG trials reported an increase in the number of cases with grade 5 toxicity (treatment-related death) due to EF-RT, but it was not statistically significant (OR 2.12 [0.71 – 6.27], p = 0.18) (Supplementary Figure S5). More bone fractures were recorded in the EF-RT arm of the Osaka trial (4 in the control arm and 0 in the experimental arm) but not in the EORTC trial (2 in the control arm and 3 in the experimental arm). Other types of toxicity were primarily gastrointestinal in origin. However, the heterogeneity of the scales limited our compilation of toxicity data.
DISCUSSION
Although CT and RNM are routine components of clinical staging by several centers in cervical cancer, their sensitivity and specificity are disappointing (21,22). Further, PET-CT has a sensitivity of 33% to 36% (23,24) and a high false negative rate of 8% to 12% in advanced cervical cancer (25–27). Several retrospective studies suggested a high rate of upstaging after surgical para-aortic staging, with rates of treatment modification ranging from 18% to 45% (27–31).
However, surgical staging is still not the standard of care in advanced cervical cancer, because some controversies remain. Does prophylactic RT that includes the para-aortic region provide a survival benefit with acceptable morbidity? Also, is the morbidity that is related to the surgery reasonable, and is the delay of RT acceptable? Finally, is adjusting the primary treatment after surgical staging, based on the pathological results, associated with better survival? (27–32). One phase III trial failed to demonstrate any impact on outcome, but it had a small sample size and was terminated prematurely due to significant morbidity that was related to an open surgical approach (33). However, there are 2 ongoing prospective randomized trials (LiLACS and Uterus-11) that are examining the prognostic value of surgical para-aortic staging with minimally invasive surgery (34).
Our study is the first systematic review and meta-analysis that addresses the oncological benefit of EF-RT for the treatment of advanced (stage ≥IB2) cervical cancer. The significant benefit in terms of distant metastasis supports the hypothesis that the para-aortic region is a sanctuary of microscopic disease that further increases in the incidence of distant metastasis. Although para-aortic lymph node involvement is considered to be metastatic disease, this area is usually the first echelon of systemic disease and presents an opportunity for regional treatment. This approach might be justified by the reduction in distant metastases, as noted in our analysis.
As expected, the pooled analysis on pelvic control showed no benefit with EF-RT. This finding contributes to the robustness of our meta-analysis, because it reinforces that para-aortic treatment was the only difference between arms. The recent improvements in pelvic control with image-guided brachytherapy (35) associated with a reduction in toxicity with modern irradiation techniques (36), consequently increase the contribution of distant failures to overall survival and renewing interest in systemic and para-aortic RT trials.
The 2 multi-institutional studies contributed 77.8% of cases that have been published, giving them greater weight in the conclusions. Both trials reported a tendency toward a positive impact of EF-RT in the control of para-aortic nodes and distant metastasis. However, the addition of 11% on 10-year survival in RTOG 7920 (5–6) was not detected in the EORTC trial (4). Because the RTOG trial did not include stage III patients and further showed a survival benefit, we theorized that the sterilization of microscopic para-aortic disease is more effective in a patient with a higher likelihood of pelvic control.
Although they were excluded from our meta-analysis, other studies had notable results. The RTOG 9001 trial compared the addition of cisplatin-based chemotherapy to pelvic radiotherapy (7,8). The researchers used as the standard arm the best treatment combination in the RTOG 7920 (5–6) trial, which consisted of pelvic and para-aortic RT without chemotherapy. The outcomes—disease-free survival, locoregional recurrence, distant relapse, and overall survival—were clearly better in the chemoradiation arm. The magnitude of the benefit due to the addition of systemic therapy might have overshadowed the para-aortic treatment. Notably, the prevalence of para-aortic node failure was reduced in the para-aortic arm, even without chemotherapy. This study was a landmark in understanding the importance of chemoradiotherapy in cervical cancer. However, it did not demonstrate a definitive value of EF-RT.
Regarding the toxicity and efficacy of para-aortic irradiation in combination with chemotherapy, a phase II trial (RTOG 9210) (9) reported an unacceptably high rate (17%) of grade 4 late toxicity—5/29 cases (10). Although extended field chemoradiation was believed to cause the reported toxicity, the altered fractionation (twice-daily) and obsolete abdominal 2D radiotherapy technique probably had an influence on the high incidence of side effects.
Moreover, the RT techniques of the 3 studies (EORTC, RTOG 7920 and Osaka University) in the meta-analysis are now considered to be obsolete. The high incidence of grade 5 toxicity and bone fractures likely occurred due to the 2D planning and parallel opposed para-aortic field techniques. Conversely, some recent studies have suggested tolerable toxicity from EF-RT with intensity-modulated RT, with a 3.9% rate of grade 3 gastrointestinal and no grade 4 or 5 events from a Pittsburgh trial (37) and 6.5% rate of late gastrointestinal toxicity from a Boston series (36).
Results on acute toxicity from the Uterus 11 phase III trial have been recently reported (38). It included 236 patients who had EF-RT (97.5% with concomitant chemotherapy) after positive surgical para-aortic lymphadenectomy. Of all cases, 60% received intensity-modulated RT and the remaining received 3D technique. The treatment was well tolerated overall, with no grade ≥3 genitourinary or gastrointestinal toxicity.
Based on the results of the Chemoradiotherapy for Cervical Cancer Meta-analysis Collaboration (CCCMAC), the survival benefit of chemotherapy in a combination treatment correlated with an increase in locoregional disease-free survival (absolute 5-year benefit of 9%) and metastases-free survival (absolute 5-year benefit of 7%) (1). Further, the planned radiotherapy dose after grouping the trials (high-dose group: >45 Gy + brachytherapy versus low-dose group: < 45 Gy + brachytherapy) provided a significant overall survival benefit after adding chemotherapy for the high-dose (HR 0.78 [0.68 – 0.89]) but not low-dose group (HR 0.93 [0.70 – 1.24]). These findings suggest that the greatest benefit of chemotherapy with regard to overall survival is also a consequence of the increase in pelvic locoregional control (essential in cervical cancer), rather than a decrease in microscopic systemic disease. Conversely, in our meta-analysis, EF-RT directly reduced the rate of distal failures, without affecting the pelvis.
The main limitations of our study were the lack of information on individual patients, preventing us from drawing valuable conclusions on specific subgroups. Another concern was the absence of trials that directly compared pelvic versus EF-RT with standard cisplatin chemotherapy in both arms, because it would have provided important insights into the additional benefits of para-aortic RT. More detailed information of the pelvic lymph node status in both groups (with or without EF-RT), would also add more reliability to the results, since an occult unbalance in the proportions of positive nodes could translate in differences in loco-regional or distant control, however it was not reported in these trials. Finally, older radiotherapy techniques likely achieved the proximity to abdominal organs at risk (OARs), which might have caused the limiting high-grade toxicity events, such as bone fractures.
Based on our analysis, the para-aortic lymphatic chain is central in the systemic dissemination of cervical cancer. Also, similar results and analogies from other trials support our thesis. Our conclusions are consistent with those of Stehman et al. (39), who performed a multivariate analysis of 3 Gynecologic Oncology Group trials that opened recruitment between 1977–1984 (GOG 24, 56, and 59) and suggested that para-aortic involvement (with or without pelvic involvement) is the strongest predictor (regression coefficient 2.37, p<0.01) of recurrence, most commonly in distant sites. An analogous situation was also recently demonstrated in breast cancer: the extended regional field effected a significant reduction in the incidence of distant metastases. The 2 well-designed trials by EORTC and Canada detected a survival benefit as a result of the relapse of distant metastases (40–41), even with adjuvant chemotherapy.
A precise recommendation of elective irradiation (prophylactic) or irradiation that is based on positive findings by surgical-radiological evaluation (therapeutic) can not be drawn with the current data. However, the potential impact of para-aortic irradiation on survival in our study, with the lower morbidity of modern RT techniques, warrants a reconsideration of EF-RT.
We conclude that EF-RT reduces systemic (para-aortic and nonpara-aortic) failures. The value of EF-RT should be further examined in modern trials with chemotherapy and modern radiotherapy techniques.
Supplementary Material
Highlights.
Distant metastasis events were reduced with para-aortic (PAor) radiotherapy.
Pelvic locoregional control was not altered with the addition of extended-field.
This study favored the idea that the PAor could be a sanctuary of malignant cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interests: None.
References
- 1.Chemoradiotherapy for Cervical Cancer Meta-analysis Collaboration (CCCMAC) Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: individual patient data meta-analysis. Cochrane Database of Systematic Reviews. 2010;(1) doi: 10.1002/14651858.CD008285. Art. No.: CD008285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belhadj H, Berek J, Bermudez A, et al. FIGO staging for carcinoma of the vulva, cervix, and corpus uteri. Int J Gynaecol Obstet. 2014;125:97–8. doi: 10.1016/j.ijgo.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Berman ML, Keys H, Creasman W, et al. Survival and patterns of recurrence in cervical cancer metastatic to periaortic lymph nodes (a Gynecologic Oncology Group study) Gynecol Oncol. 1984;19:8–16. doi: 10.1016/0090-8258(84)90151-3. [DOI] [PubMed] [Google Scholar]
- 4.Haie C, Pejovic MH, Gerbaulet A, et al. Is prophylactic para-aortic irradiation worthwhile in the treatment of advanced cervical carcinoma? Results of a controlled clinical trial of the EORTC radiotherapy group. Radiotherapy and Oncology. 1988;11:101–112. doi: 10.1016/0167-8140(88)90245-9. [DOI] [PubMed] [Google Scholar]
- 5.Rotman M, Choi K, Guze C, et al. Prophylactic irradiation of the para-aortic lymph node chain in stage IIB and bulky stage IB carcinoma of the cervix, initial treatment results of RTOG 7920. Int J Radiat Oncol Biol Phys. 1990;19:513–521. doi: 10.1016/0360-3016(90)90475-y. [DOI] [PubMed] [Google Scholar]
- 6.Rotman M, Pajak TF, Choi K, et al. Prophylactic extended-field irradiation of para-aortic lymph nodes in stages IIB and bulky IB IIA cervical carcinomas. Ten-year treatment results of RTOG 7920. JAMA. 1995;274:387–393. [PubMed] [Google Scholar]
- 7.Morris M, Eifel PJ, Jiandong L, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340(15):1137–43. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 8.Eifel PJ, Winter K, Morris M, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an uptade of Radiation Therapy Oncology Group Trial (RTOG) 90-01. J Clin Oncol. 2004;22:872–880. doi: 10.1200/JCO.2004.07.197. [DOI] [PubMed] [Google Scholar]
- 9.Grigsby PW, Lu JD, Mutch DG, et al. Twice-daily fractionation of external irradiation with brachytherapy and chemotherapy in carcinoma of the cervix with positive para-aortic lymph nodes: phase II study of the Radiation Therapy Oncology Group 92-10. Int J Radiat Oncol Biol Phys. 1998;41(4):817–822. doi: 10.1016/s0360-3016(98)00132-1. [DOI] [PubMed] [Google Scholar]
- 10.Grigsby PW, Heydon K, Mutch DG, et al. Long-term follow-up of RTOG 92-10: cervical cancer with positive para-aortic lymph nodes. Int J Radiat Oncol Biol Phys. 2001;51(4):982–987. doi: 10.1016/s0360-3016(01)01723-0. [DOI] [PubMed] [Google Scholar]
- 11.Havrilesky LJ, Kulasingam SL, Matchar DB, Myers ER. FDG-PET for management of cervical and ovarian cancer. Gynecol Oncol. 2005;97(1):183–91. doi: 10.1016/j.ygyno.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Tsai CS, Lai CH, Chang TC, et al. A prospective randomized trial to study the impact of pretreatment FDG-PET for cervical cancer patients with MRI-detected positive pelvic but negative para-aortic lymphadenopathy. Int J Radiat Oncol Biol Phys. 2010;76(2):477–484. doi: 10.1016/j.ijrobp.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 13.Fine BA, Hempling RE, Piver S, et al. Severe radiation morbidity in carcinoma of the cervix: impact of pre therapy surgical staging and previous surgery. Int J Radiat Oncol Biol Phys. 1995;31(4):717–723. doi: 10.1016/0360-3016(94)00458-7. [DOI] [PubMed] [Google Scholar]
- 14.Portelance L, Chao KSC, Grigsby PW, et al. Intensity-modulated radiation therapy (IMRT) reduces small bowel, rectum and bladder doses in patients with cervical cancer receiving pelvic and para-aortic irradiation. Int J Radiat Oncol Biol Phys. 2001;51(1):261–266. doi: 10.1016/s0360-3016(01)01664-9. [DOI] [PubMed] [Google Scholar]
- 15.Ahmed RS, Kim RY, Duan J, et al. IMRT dose escalation for positive para-aortic lymph nodes in patients with locally advanced cervical cancer while reducing dose to bone marrow and other organs at risk. Int J Radiat Oncol Biol Phys. 2004;60(2):505–512. doi: 10.1016/j.ijrobp.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 16.Liberati A, Altman DG, Tetzlaff J, et al. The PRIMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration; 2011. Available from www.cochrane-handbook.org. [Google Scholar]
- 18.Chatani M, Matayoshi Y, Masaki N, et al. Prophylactic irradiation of para-aortic lymph nodes in carcinoma of the uterine cervix. Strahlenther Onkol. 1995;171:655–660. [PubMed] [Google Scholar]
- 19.Lin SY, Tsai CS, Chang YC, et al. The role of pretreatment FDG-PET in treating cervical cancer patients with enlarged pelvic lymph node(s) shown on MRI: a phase 3 randomized trial with long-term follow-up. Int J Radiat Oncol Biol Phys. 2015;92(3):577–585. doi: 10.1016/j.ijrobp.2015.02.027. [DOI] [PubMed] [Google Scholar]
- 20.Du XL, Sheng XG, Jiang T, et al. Intensity-modulated radiation therapy versus para-aortic field radiotherapy to treat para-aortic lymph node metastasis in cervical cancer: prospective study. Croat Med J. 2010;51:229–236. doi: 10.3325/cmj.2010.51.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amendola MA, Hricak H, Mitchell DG, et al. Utilization of diagnostic studies in the pretreatment evaluation of invasive cervical cancer in the United States: results of intergroup protocol ACRIN 6651/GOG 183. J Clin Oncol. 2005;23:7454–9. doi: 10.1200/JCO.2004.00.5397. [DOI] [PubMed] [Google Scholar]
- 22.Choi HJ, Ju W, Myung SK, Kim Y. Diagnostic performance of computer tomography, magnetic resonance imaging, and positron emission tomography or positron emission tomography/ computer tomography for detection of meta- static lymph nodes in patients with cervical cancer: meta-analysis. Cancer Sci. 2010;101:1471–9. doi: 10.1111/j.1349-7006.2010.01532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramirez PT, Jhingran A, Macapinlac HA, et al. Laparoscopic extraperitoneal para-aortic lymphadenectomy in locally advanced cervical cancer: a prospective correlation of surgical findings with positron emission tomography/ computed tomography findings. Cancer. 2011;117:1928–34. doi: 10.1002/cncr.25739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leblanc E, Gauthier H, Querleu D, et al. Ac- curacy of 18-fluoro-2-deoxy-D-glucose positron emission tomography in the pretherapeutic detection of occult para-aortic node involvement in patients with a locally advanced cervical carcinoma. Ann Surg Oncol. 2011;18:2302–9. doi: 10.1245/s10434-011-1583-9. [DOI] [PubMed] [Google Scholar]
- 25.Boughanim M, Leboulleux S, Rey A, et al. Histologic results of para-aortic lymphadenectomy in patients treated for stage IB2/II cervical cancer with negative [18F]fluorodeoxyglucose positron emission tomography scans in the para-aortic area. J Clin Oncol. 2008;26:2558–61. doi: 10.1200/JCO.2007.14.3933. [DOI] [PubMed] [Google Scholar]
- 26.Gouy S, Morice P, Narducci F, et al. Pro- spective Multicenter study evaluating the survival of patients with locally advanced cervical cancer undergoing laparoscopic para-aortic lymphadenectomy before chemoradiotherapy in the era of positron emission tomography imaging. J Clin Oncol. 2013;31:3026–33. doi: 10.1200/JCO.2012.47.3520. [DOI] [PubMed] [Google Scholar]
- 27.Köhler C, Mustea A, Marnitz S, Schneider A, Chiantera V, Ulrich U, Scharf JP, Martus P, Vieira MA, Tsunoda A. Perioperative morbidity and rate of upstaging after laparoscopic staging for patients with locally advanced cervical cancer: results of a prospective randomized trial. Am J Obstet Gynecol. 2015;213(4):503.e1–7. doi: 10.1016/j.ajog.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 28.Marnitz S, Kohler C, Roth C, Füller J, Hinkelbein W, Schneider A. Is there a benefit of pretreatment laparoscopic transperitoneal sur-gical staging in patients with advanced cervical cancer? Gynecol Oncol. 2005;99:536–44. doi: 10.1016/j.ygyno.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Del Pino M, Fuste P, Pahisa J, et al. Laparoscopic lymphadenectomy in advanced cervical cancer: prognostic and therapeutic value. Int J Gynecol Cancer. 2013;23:1675–83. doi: 10.1097/IGC.0000000000000000. [DOI] [PubMed] [Google Scholar]
- 30.Hasenburg A, Salama JK, Van TJ, Amosson C, Chiu JK, Kieback DG. Evaluation of patients after extraperitoneal lymph node dissection and subsequent radiotherapy for cervical cancer. Gynecol Oncol. 2002;84:321–6. doi: 10.1006/gyno.2001.6528. [DOI] [PubMed] [Google Scholar]
- 31.Denschlag D, Gabriel B, Mueller-Lantzsch C, et al. Evaluation of patients after extraperitoneal lymph node dissection for cervical cancer. Gynecol Oncol. 2005;96:658–64. doi: 10.1016/j.ygyno.2004.08.053. [DOI] [PubMed] [Google Scholar]
- 32.Gold MA, Tian C, Whitney CW, Rose PG, Lanciano R. Surgical versus radiographic determination of para-aortic lymph node metastases before chemoradiation for locally advanced cervical carcinoma: a Gynecologic Oncology Group study. Cancer. 2008;112:1954–63. doi: 10.1002/cncr.23400. [DOI] [PubMed] [Google Scholar]
- 33.Lai CH, Huang KG, Hong JH, et al. Randomized trial of surgical staging (extraperitoneal or laparoscopic) versus clinical staging in locally advanced cervical cancer. Gynecol Oncol. 2003;89:160–7. doi: 10.1016/s0090-8258(03)00064-7. [DOI] [PubMed] [Google Scholar]
- 34.Frumovitz M, Querleu D, Gil-Moreno A, et al. Lymphadenectomy in locally advanced cervical cancer study (LiLACS): phase III clinical trial comparing surgical with radiologic staging in patients with stages IB2-IVA cervical cancer. J Minim Invasive Gynecol. 2014;21:3–8. doi: 10.1016/j.jmig.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pötter R, Georg P, Dimopoulos JCA, et al. Clinical outcome of protocol based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer. Radiotherapy and Oncology. 2011;100:116–123. doi: 10.1016/j.radonc.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poorvu PD, Sadow CA, Townamchai K, et al. Duodenal and other gastrointestinal toxicity in cervical and endometrial cancer treated with extended-field intensity modulated radiation therapy to paraaortic lymph nodes. Int J Radiat Oncol Biol Phys. 2013;85(5):1262–1268. doi: 10.1016/j.ijrobp.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Xu KM, Rajagopalan MS, Kim H, et al. Extended field intensity modulated radiation therapy for gynecologic cancers: Is the risk of duodenal toxicity high? PRO. 2015;5(4):e291–e297. doi: 10.1016/j.prro.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 38.Marnitz S, Martus P, Köhler C, Stromberger C, Asse E, Mallmann P, Schmidberger H, Affonso Júnior RJ, Nunes JS, Sehouli J, Budach V. Role of Surgical Versus Clinical Staging in Chemoradiated FIGO Stage IIB-IVA Cervical Cancer Patients - Acute Toxicity and Treatment Quality of the Uterus-11 Multicenter Phase III Intergroup Trial of the German Radiation Oncology Group and the Gynecologic Cancer Group. Int J Radiat Oncol Biol Phys. 2016;94(2):243–53. doi: 10.1016/j.ijrobp.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 39.Stehman FB, Bundy BN, DiSaia PJ, et al. Carcinoma of the cervix treated with radiation therapy: a multi-variate analysis of prognostic variables in the Gynecologic Oncology Group. Cancer. 1991;67:2776–2785. doi: 10.1002/1097-0142(19910601)67:11<2776::aid-cncr2820671111>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 40.Whelan TJ, Olivetti IA, Parulekar WR, et al. Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373(4):307–316. doi: 10.1056/NEJMoa1415340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poortmans PM, Collette S, Kirkove C, et al. Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med. 2015;373(4):317–327. doi: 10.1056/NEJMoa1415369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.