Abstract
Early life is a critical period for the progressive establishment of immunity in response to environmental stimuli; the impact of airborne challenges on this process is not well defined. In a longitudinal fashion, we determined the effect of episodic house dust mite (HDM) aerosol and ozone inhalation, both separately and combined, on peripheral blood immune cell phenotypes and cytokine expression from 4-25 weeks of age in an infant rhesus monkey model of childhood development. Immune profiles in peripheral blood were compared with lung lavage at 25 weeks of age. Independent of exposure, peripheral blood cell counts fluctuated with chronologic age of animals, while IFNγ and IL-4 mRNA levels increased over time in a linear fashion. At 12 weeks of age, total WBC, lymphocyte numbers, FoxP3 mRNA and IL-12 mRNA were dramatically reduced relative to earlier time points, but increased to a steady state with age. Exposure effects were observed for monocyte numbers, as well as CCR3, FoxP3, and IL-12 mRNA levels in peripheral blood. Significant differences in cell surface marker and cytokine expression were detected following in vitro HDM or PMA/ionomycin stimulation of PBMC isolated from animals exposed to either HDM or ozone. Lavage revealed a mixed immune phenotype of FoxP3, IFNγ and eosinophilia in association with combined HDM plus ozone exposure, which was not observed in blood. Collectively, our findings show that airborne challenges during postnatal development elicit measureable cell and cytokine changes in peripheral blood over time, but exposure-induced immune profiles are not mirrored in the lung.
Keywords: infant, peripheral blood, cytokine, house dust mite, ozone
Introduction
The neonatal growth period in primate species is characterized by extensive maturation of both the immune system and the lung, representing a significant developmental window of susceptibility to environmental perturbation (Holt and Jones, 2000; Holt et al., 2005). Early life insults may influence individual health outcomes, a notion based upon the “Barker hypothesis,” or “fetal origins of adult disease” theory, which was established from observational studies on fetal nutrient deficiencies found to be associated with adult conditions such as coronary artery disease, diabetes, and hypertension (Barker, 1997). Progressive tracking of cellular trajectories in neonates to assess the influence of inhaled environmental challenges to the immune system elicited by non-infectious antigens (allergens), toxicants, and microbes may provide critical clues on disease mechanisms that persist throughout an individual's lifetime. Understanding the chronology of immune cell development in the context of environmental perturbation could also lead to functional diagnostics at key checkpoints during neonatal maturation.
During fetal and neonatal development, the repertoire of immune cells localized within systemic and mucosal compartments of the body may be considered moveable landscapes. Even with steady progress in the understanding of immunity in the human neonate, substantial knowledge gaps remain. Human longitudinal studies have focused on cord and neonatal blood, describing the evolution of immune cell populations through the first year of life and demonstrating unique functionality that may contribute towards enhanced neonatal susceptibility to disease (Erkeller-Yuksel et al., 1992; Beck and Lam-Po-Tang, 1994; Comans-Bitter et al., 1997; Chipeta et al., 1998; de Vries et al., 2000; Gasparoni et al., 2003; Takahata et al., 2004; Hartel et al., 2005). Neonatal adaptive immunity is compromised, in part, by immature dendritic cell populations that limit establishment of robust T cell memory responses and differentiation of Th1 cell populations (Upham et al., 2006; Naderi et al., 2009). Variations in microRNA expression in neonates relative to adults also appear to play a role in age-dependent differences in T cell cytokine production and activation-dependent signaling (Weitzel et al., 2009; Palin et al., 2013). The functional transition of lymphocyte populations during infancy is thought to mediated by a layering process, consisting of overlapping fetal and adult cell populations originating from distinct hematopoietic precursors (Mold et al., 2010). Despite the known immune limitations in infants that increase vulnerability to i nfectious disease, adult-level T cell responses can develop under certain conditions, such as Bacillus Calmette–Guérin vaccination and cytomegalovirus infection (Vekemans et al., 2001; Hussey et al., 2002; Marchant et al., 2003). Collectively, cellular and molecular features of the immature neonatal immune system may contribute towards a unique response to environmental exposures.
It is not well understood how and when specific environmental exposures can alter immune cell constituents during early life in humans. We hypothesized that airborne exposures during the neonatal growth period can result in detectable alterations in the peripheral blood immune cell and cytokine profile that are dependent upon chronological age. To test this hypothesis within the context of disease development, we utilized a previously reported infant rhesus monkey model of childhood allergic airways disease, in which episodic exposure to a combination of house dust mite (HDM) aerosol and ozone produces multiple hallmark features of human asthma, including airways hyperresponsiveness, airways remodeling, and airways eosinophilia (Schelegle et al., 2001; Miller et al., 2003). The combined allergen and air pollutant model in infant monkeys was based upon multiple human epidemiologic studies demonstrating increased prevalence of childhood asthma in association with early life air pollutant exposures (recently reviewed in (Milligan et al., 2016)). In this current study, we progressively measured the impact of episodic exposure to HDM aerosol and ozone, either individually or in combination, on the peripheral blood immune cell and cytokine profile during the first 6 months of life in the rhesus monkey. We also addressed whether peripheral blood profiles elicited by HDM and/or ozone exposures were predictive of immune cell and cytokine profiles in the rhesus monkey lung at 6 months of age.
Materials and Methods
Animals
Male rhesus macaque (Macaca mulatta) monkeys were housed indoors at the California National Primate Research Center under high efficiency particulate air (HEPA) filtered air conditions within three days following birth. Animals were reared under nursery conditions without dams, and were fed a standardized diet of infant formula, Purina monkey chow and supplemental produce. All animals were inoculated with diptheria, tetanus, and acellular pertussis vaccine (Infanrix; Darby Drug Co, Westbury, NY) at 2 weeks of age to mimic childhood vaccination schedules. All animal procedures were approved by the University of California, Davis, Institutional Animal Care and Use Committee.
Exposure Protocol
The study design is illustrated in Figure 1. Starting at 4 weeks of age, twenty-four monkeys were exposed to 11 cycles of filtered air, HDM aerosol, ozone, or HDM aerosol + ozone (Table 1). For ozone animal groups, each cycle consisted of ozone exposure for 5 days (0.5ppm at 8h/day), followed by 9 days of filtered air. For HDM animal groups, cycle consisted of HDM aerosol on days 3-5 of each cycle, following by 9 days of filtered air (Greer Laboratories, Inc. Lenoir, NC). Animals groups not exposed to ozone remained in HEPA filtered air throughout each cycle. Details of HDM and ozone exposure methodology for this study were previously reported (Schelegle et al., 2003; Moore et al., 2012). Ozone was generated using a Sanders model 25 ozonizer (Eltze, Germany) and the concentration was monitored using a Dasibi 1003-AH ozone analyzer (Dasibi Environmental Corporation, Glendale, CA).
Figure 1.

House dust mite (HDM) and ozone exposure protocol. Infant monkeys received Der p1 and Der p2 antigens via subcutaneous (SQ) injection at 2, 4, 8, and 16 weeks of age. Intranasal (IN) HDM was administered at 3, 5, and 9 weeks of age for HDM aerosol groups only. Beginning at 4 weeks of age, animals were exposed to 11 cycles of HDM aerosol, ozone, or HDM aerosol + ozone as indicated by arrows. Animals were necropsied at 25 weeks of age, 3-4 days following the last HDM aerosol or ozone exposure. Blood collection time points are indicated below the exposure regimen.
Table 1. Animal Group Treatments.
| Treatment Group | HDM SQ | HDM IN | HDM Aerosol | Ozone |
|---|---|---|---|---|
| Filtered Air (n=6) | x | |||
| HDM (n=6) | x | x | x | x |
| Ozone (n=6) | x | |||
| HDM + Ozone (n=6) | x | x | x | x |
At 2, 4, and 8 weeks of age, all animals received subcutaneous injections of 0.5 ug Der p1 and 0.25 ug Der p2 purified from Dermatophagoides peteronyssinus (Indoor Biotechnologies, Inc., Charlottesville, VA) in Imject aluminum hydroxide adjuvant (10 mg, Thermo Fisher Scientific, Rockford, IL). At 16 weeks of age, all animals also received 1 ug Der p1 and 0.5 ug Der p2 in Imject adjuvant. HDM aerosol animal groups were also treated with intranasal HDM at weeks 3, 5, and 9 (whole HDM extract, 49 ug per dose, Greer Laboratories, Inc. Lenoir, NC).
Blood samples were obtained at alternate cycles, with collection taking place immediately after completion of HDM aerosol on the third day (or equivalent time point for filtered air or ozone alone animal groups). Complete blood counts for whole blood samples were measured using a Beckman Coulter analyzer (Beckman Coulter Inc., Miami, FL). All animals were necropsied at 25 weeks of age, 72-96 hours following the last HDM aerosol or ozone exposure. Animals were euthanized by an intravenous overdose of sodium pentobarbital.
Flow Cytometry Analysis
Whole blood samples were immunostained with mouse anti-human CD2 fluorescein isothiocyanate (FITC; clone RPA-2.1, Beckman Coulter), CD45 Peridinin chlorophyll protein (PerCP; clone TU116, Beckman Coulter); CD3 Allophycocyanin (APC; clone SP34, BD Biosciences, San Jose, CA) and CD19 Phycoerythrin (PE; clone J4.119, NIH NHP Reagent Resource (http://www.nhpreagents.org/NHP/default.aspx)). Four-color analysis was performed on a FACSCalibur (BD Biociences), acquiring 30,000–50,000 events per sample and analyzed with CELLQuest software (BD Biosciences).
Quantitative RT-PCR Analysis
RNA was isolated from whole blood using the RiboPure-Blood kit (Applied Biosystems, Foster city, CA). RNA was extracted from lavage cells using TRIzol reagent (Invitrogen, Carlsbad, CA). Reverse transcription was performed with random hexamer primers and MultiScribe Reverse Transcriptase (Applied Biosystems, Foster City, CA). Taqman primer/probe combinations specific for human CD3 epsilon, IL-4, IL-12, FoxP3, IL-2, IL-13, IL-17, IL-6, IFNγ and GAPDH were utilized (Applied Biosystems, Foster City, CA). All reagents were tested and confirmed to detect rhesus monkey mRNA targets. Progressive dilution of purified cDNA plasmids for each human gene (Origene, Rockville, MD) was used to generate standards curves and allow absolute quantitation of copy numbers as previously described (Maniar-Hew et al., 2013). Quantitative RT-PCR analysis was performed using Applied Biosystems 7900 Sequence Detection Systems. Copy numbers were reported relative to GAPDH endogenous control values.
PBMC Culture
Peripheral blood mononuclear cells (PBMC) were prepared as previously described (Miller et al., 2009). PBMC were cultured with HDM or PMA + ionomycin in serum-free AIM-V media (Invitrogen) at a concentration of 1 ×106 cells/mL at 37°C in 5% CO2. The HDM lot used with PBMC cultures was identical to that used for aerosol exposures in this study. RNA was analyzed by quantitative RT-PCR as described above.
Lavage Differentials
Lung lavage was obtained at necropsy by flushing the right caudal lobe with phosphate buffered saline. Lavage samples were cytocentrifuged and stained with a modified Wright stain (Diff-Quik; Thermo Fisher Scientific). The frequency of each cell type was determined by counting 300 cells per sample by light microscopy.
Statistical Analysis
1-way or 2-way repeated measures ANOVA with a 95% confidence interval were used where appropriate, with multiple comparison corrections performed and post-tests used to evaluate differences between individual groups. Pearson correlation analysis was performed on peripheral blood leukocyte numbers and peripheral blood cellular marker/cytokine gene expression values that were natural log transformed. Pearson correlation coefficients between paired data sets were calculated, along with two-tailed p values. Comparisons were considered to be significant at the p<0.05 level. All data are reported as mean ± SEM. All analysis were performed with Prism 6 software (GraphPad Software, La Jolla, CA).
Results
We have previously reported that a 6 month exposure regimen consisting of episodic HDM aerosol in combination with ozone resulted in amplification of eosinophilic inflammation, mucous cell volume, as well as increased reactivity in airways of infant rhesus monkeys, relative to HDM or ozone alone (Schelegle et al., 2003). To assess whether the developmental trajectory of peripheral blood immune profiles during such an exposure regimen might be predictive of an allergic airways phenotype, we longitudinally measured cell and cytokine phenotypes in a cohort of infant rhesus monkeys experimentally exposed to HDM aerosol and ozone for 6 months, both individually and in combination. From 4-24 weeks of age, monkeys underwent 11 cycles of a 14 day protocol consisting of sequential days of HDM aerosol and/or ozone followed by a 9 day recovery in filtered air to mimic episodic environmental exposures (Figure 1). It is notable that all animals, including controls, were housed under filtered air conditions for the entirety of the study when not undergoing experimental exposures. Blood samples were collected every 4 weeks, at the completion of either an HDM aerosol or ozone exposure period (Figure 1).
Peripheral Blood Cell Numbers During Postnatal Development Are Relatively Static
Independent of exposure, CBC values did not exhibit a linear trajectory relative to chronologic age within the time period of evaluation; the slope of each cell population was not significant as compared to the null hypothesis (slope=0.00; Figure E1). Significant age-dependent fluctuations were observed in total white blood cell counts (WBC), lymphocyte numbers and monocyte numbers for all animal groups. Total WBC decreased at 12 weeks of age relative to 8, 16, and 20-week time points (Figure 2A). Similarly, lymphocyte numbers were significantly lower at 12 weeks of age compared with all other time points sampled (Figure 2B). Age-dependent changes in blood monocyte numbers were limited to a reduction at 12 weeks relative to 20 weeks of age (Figure 2C). Circulating eosinophil or neutrophil numbers did not significantly change with increasing chronologic age during the 24-week evaluation period (Figure 2 D, E).
Figure 2.
Peripheral blood leukocyte numbers as a function of chronologic age and exposure. (A) Total white blood cell (WBC) (B) lymphocytes (C) monocytes, (D) eosinophils and (E) neutrophils in blood samples collected as shown in Figure 1. Each column represents the mean+/-SE values, n=6. Horizontal lines indicate significant age-dependent differences between time points by multiple comparison 2-way ANOVA with Tukey's post-test. Exposure dependent effects at individual time points are listed in Table 2. *p<0.05, **p<0.01, ***P<0.001, ****p<0.0001.
Whole blood samples were evaluated by flow cytometry (FACS) to further define the reduction in lymphocyte populations at 12 weeks of age. Frequency of CD45+CD3+ T cells within the total leukocyte population for all animal groups significantly increased from 33.2 ± 1.3% at 12 weeks to 43.6 ± 0.5% at 18 weeks of age (Figure 3A), which is consistent with the observed rise in total lymphocyte numbers at both 16 and 20 weeks relative to 12 weeks of age (Figure 2B). Comparatively, frequency of CD45+CD19+ cells representing the B cell pool showed no significant difference at 12 weeks (21.6 ± 1.5%) relative to 18 weeks (23.8 ± 1.6%) of age for all animal groups (Figure 3B). We were unable to evaluate blood samples by FACS at earlier time points because of restrictions on blood volume based upon body weight of infant monkeys.
Figure 3.
Peripheral blood T and B cell frequency as a function of chronologic age and exposure. (A) Frequency of CD45+CD3+ and (B) CD45+CD19+ cells in whole blood samples collected at 12 and 18 weeks of age as shown in Figure 1. Each column represents the mean+/-SE values, n=6. Horizontal lines indicates significant age-dependent difference between time points by multiple comparison 2-way ANOVA with Tukey's post-test. ***P<0.001.
HDM aerosol and/or ozone exposure did not have an overall effect on total WBC, lymphocyte, eosinophil or neutrophil numbers, regardless of sampling time point. Blood monocyte numbers were reduced in ozone and combined HDM + ozone exposure animal groups relative to filtered air controls, but the effect was only observed at 4 weeks of age (Table 2). Although age was not a significant source of variation for blood eosinophil numbers, a trend towards interaction between age and exposure by two-way ANOVA was observed (p=0.0549; Figure 2D).
Table 2. Effect of exposure on immune parameters as function of age.
Significant effect of exposure on the listed immune parameter (based upon data in Fig 2 and 4) at indicated ages for animals treated with HDM aerosol and/or ozone. Significance between exposure groups indicated by 2-way ANOVA with Tukey's post hoc test.
| Weeks of Age | Parameter | Treatment Group | Control Group | Change Relative to Control Group | Significance |
|---|---|---|---|---|---|
| 4 | Monocyte Number | Ozone | Filtered Air | ↓ | * |
| HDM + Ozone | Filtered Air | ↓ | * | ||
| 4 | FoxP3 | Ozone | Filtered Air | ↑ | * |
| 8 | CCR3 | HDM + Ozone | HDM | ↓ | * |
| 12 | CCR3 | HDM | Filtered Air | ↑ | **** |
| HDM | Ozone | ↑ | * | ||
| HDM + Ozone | HDM | ↓ | **** | ||
| 16 | IL -12 | HDM + Ozone | Filtered Air | ↑ | * |
| 24 | CCR3 | Ozone | Filtered Air | ↑ | **** |
| HDM + Ozone | Ozone | ↓ | **** | ||
| HDM + Ozone | DHM | ↓ | * |
p<0.05,
p<0.0001.
Molecular Immune Parameters in Peripheral Blood Are Dependent Upon Chronologic Age and Exposure
Based upon our initial findings by CBC analysis and FACS, we evaluated mRNA in peripheral blood longitudinally, focusing on T lymphocyte markers, cytokines and CCR3. mRNA levels for CD3, FoxP3, CCR3, IFNγ, IL-4, IL-12 and IL-2 in the first blood sample at 4 months of age were significantly reduced relative to the 24 week blood sample (Figure 4, A-F, Figure E2). As with peripheral blood lymphocyte numbers, mRNA levels for FoxP3 and IL12 were reduced at 12 weeks relative to values obtained at 8 weeks. IL-13 and IL-17 mRNA expression levels were very low throughout the 24 week exposure period and showed no significant association with chronologic age (Figure 4 H, I). Independent of exposure, linear regression analysis for CD3, FoxP3, IFNγ, and IL-4 mRNA resulted in significant slope values as compared with the null hypothesis, whereas CCR3, IL-6, IL-12, IL-13, IL-17 and IL-2mRNA levels fluctuated throughout the 24 week exposure period (Figure E3). IFNγ and IL-4 mRNA expression correlated with age; CD3 expression displayed a trend at p=0.05 (Figure E3).
Figure 4.
Peripheral blood cellular marker/cytokine gene expression as a function of chronologic age and exposure. (A) CD3 (B) FoxP3 (C) CCR3 (D) IFNγ (E) IL-4 (F) IL-12 (G) IL-6 (H) IL-13 and (I) IL-17 mRNA measured as copy number normalized to 104 GAPDH housekeeping gene copies in blood samples collected as shown in Figure 1. Each column represents the mean+/-SE values, n=6. Horizontal lines indicate significant differences between week of age time points by multiple comparison 2-way ANOVA with Tukey's post-test. Exposure dependent effects at individual time points are listed in Table 2. *p<0.05, **p<0.01, ***P<0.001, ****p<0.0001.
Of the molecular immune parameters evaluated in peripheral blood, only FoxP3, IL-12 and CCR3 mRNA levels were significantly associated with exposure (Table 1). FoxP3 mRNA levels in ozone exposed animals relative to filtered air controls at 4 weeks of age, whereas IL-12 mRNA levels were increased in the combined HDM + ozone animal group relative to filtered air controls at 16 weeks of age. CCR3 mRNA levels varied with type of exposure and age; HDM or ozone alone resulted in increased CCR3 relative to filtered air at 12 (HDM) and 24 (ozone) weeks of age. In contrast, the combined HDM + ozone animal group showed significantly less CCR3 mRNA expression in peripheral blood at 8, 12 and 24 weeks relative to HDM alone.
To further assess for associations between peripheral blood cellular and cytokine parameters evaluated in this study, we conducted Pearson correlation analysis for all animal groups (Table E1). A highly significant association was found between lymphocyte and eosinophil numbers. Of the molecular markers evaluated in this study, only eosinophils significantly correlated with CCR3 mRNA. Lymphocyte numbers highly correlated with CD3 and FoxP3 mRNA, and monocyte numbers highly correlated with IL-6 mRNA.
PBMC Display Intrinsic Exposure Dependent Gene Expression Following In Vitro Culture with HDM or PMA/Ionomycin
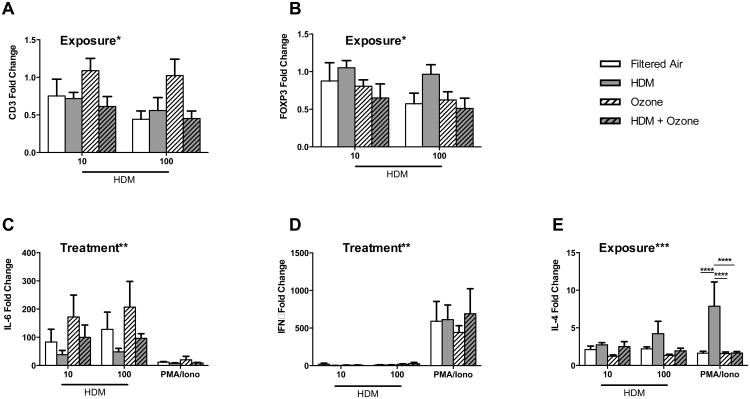
Because analysis of exposure dependent effects by in vivo peripheral blood sampling resulted in detection of a limited number of exposure dependent parameters at specific time points, we assessed whether HDM and/or ozone exposure generated an intrinsic change that could be measured by direct responsiveness to allergen or indirect stimulation with PMA/ionomycin treatment. To address this question, PBMC collected at necropsy (25 weeks of age, 72-96 hrs post HDM aerosol) were cultured with HDM or PMA/ionomycin in vitro and subsequently evaluated for multiple immune markers. A significant exposure-dependent effect was observed for mRNA expression of CD3 and FoxP3 (Figure 5 A, B). CD3 expression was down-regulated compared to media controls in all exposure groups, with the exception of the ozone animal group. FoxP3 mRNA levels were similarly down-regulated compared to media controls in all exposure groups, with the exception of the HDM only group. HDM in vitro significantly increased IL-6 expression but did not promote IFNγ expression, despite significant induction of this cytokine by PMA/ionomycin (Figure 5 C, D); no significant exposure-dependent effects were observed for either cytokine. PMA/ionomycin-induced IL-4 mRNA expression in vitro was significantly impacted by prior in-vivo allergen exposure, with peripheral blood from animals treated with HDM alone yielding the highest amount of IL-4 mRNA as compared with other exposure groups (Figure 5E).
Figure 5.
Intrinsic HDM recall response with exposure. PBMC were collected at necropsy, 3-4 days post final HDM aerosol exposure. Cells were treated in vitro for 24 hours with HDM at 10 or 100ug/ml, or PMA +ionomycin, followed by quantitative RT-PCR analysis. (A) CD3 (B) FoxP3 (C) IL-6 (D) IFNγ and (E) IL-4 mRNA copy number was normalized to GAPDH housekeeping gene copy number. Data are expressed as copy number fold change over unstimulated media control values. Each column represents the mean+/-SE values for 3-6 animals. Exposure* or Treatment* indicates significant overall effects by 2-way ANOVA. Horizontal lines indicate significance by 2-way ANOVA with Tukey's post-test for between exposure group comparisons. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Exposure Effects in Lung Lavage Cell Populations are not Comparable with Peripheral Blood
We also assessed the impact of HDM and/or ozone on lung cell populations relative to peripheral blood cell populations, evaluating samples collected at necropsy. At 25 weeks of age, 3-4 days following completion of the final exposure cycle, significant differences in peripheral blood populations were limited to reduced eosinophil frequency in animals following ozone exposure relative to filtered air controls (Figure 6A). There was no apparent effect of exposure on total WBC, or lymphocyte, monocyte, or neutrophil frequency in peripheral blood collected at 25 week time point.
Figure 6.
Comparison of peripheral blood and lung lavage leukocyte populations at 6 months of age: effect of exposure. (A) Peripheral blood total white blood cell (WBC) number, and lymphocyte, eosinophil, monocyte, and neutrophil frequency at 6 months of age in animals exposed to HDM aerosol and/or ozone or filtered air control. (B) Lung lavage total cell number, and lymphocyte, eosinophil, monocyte, macrophage, and neutrophil frequency at 6 months of age in animals exposed to HDM aerosol and/or ozone or filtered air control. Lavage and blood samples were collected at 25 weeks of age (necropsy), 3-4 days following the last HDM and/or ozone exposure. Each point represents an individual animal, with 5-6 animals/group. Mean+/-SE is indicated for each exposure group. Horizontal bars indicate significance by 1-way ANOVA with Tukey's post-test for between exposure group comparisons. *p<0.05, **p<0.01
Total lavage cell numbers were comparable between animal groups, but frequency of lymphocytes, eosinophils and macrophages was significantly affected by exposure (Figure 6B). Relative to ozone or HDM alone animal groups, combined HDM and ozone exposure increased lymphocyte and eosinophil frequency in lavage, with a corresponding decline in macrophage frequency. Ozone alone also elicited a significant increase in lavage eosinophils relative to filtered air control animals. Although there appeared to be an inverse relationship between peripheral blood and lavage eosinophils with ozone exposure at 25 weeks of age, correlative analysis for individual animals was not found to be statistically significant.
Combined HDM and Ozone Exposure Results in a Mixed IFNγ/FoxP3 Profile in Lung Lavage
In peripheral blood at 6 months of age, we observed exposure dependent effects for IL-12 and IL-13 mRNA. IL-13 mRNA was in elevated in animals that received ozone exposure as compared to filtered air or combined exposure groups. No significant exposure dependent differences were observed for IL-4, IL-6, FNγ, IL-17, CD3, FoxP3, or CCR3 mRNA in peripheral blood (Figure 7A).
Figure 7.
Comparison of peripheral blood and lung cell/cytokine molecular markers at 6 months of age: effect of exposure. (A) Peripheral blood IL-4, IL-6, IFNγ, CD3, FoxP3, IL-12, IL-13, and CCR3 mRNA expression at 6 months of age in animals exposed to HDM aerosol and/or ozone or filtered air control. (B) Lung lavage cell IL-4, IL-6, IFNγ, CD3, FoxP3, IL-12, IL-13, and CCR3 mRNA expression at 6 months of age in animals exposed to HDM aerosol and/or ozone or filtered air control. Copy number is normalized to GAPDH housekeeping gene copy number. Lavage and blood samples were collected at 25 weeks of age (necropsy), 3-4 days following the last HDM and/or ozone exposure. Each point represents an individual animal, with 5-6 animals/group. Mean+/-SE is indicated for each exposure group. Horizontal bars indicate significance by 1-way ANOVA with Tukey's post-test for between exposure group comparisons. Exposure* indicates significant overall exposure dependent effects by 1-way ANOVA, with no significance by post-test. *p<0.05, **p<0.01.
In a comparative fashion, lavage cells were also evaluated for exposure dependent mRNA expression at 6 months of age (Figure 7B). Significant differences in CD3, IFNγ, and FoxP3 mRNA levels were observed with exposure, with increased IFNγ and CD3 in lavage from the combined HDM + ozone animal groups. FoxP3 mRNA was increased in lavage from the combined HDM + ozone animal group compared to filtered air or HDM alone; a similar trend was observed for IL-4 and IL-12, although this did not reach statistical significance. Exposure was not a significant source of variation for CCR3 although it appeared highest in the combined HDM + ozone animal group. Exposure dependent effects were not observed for IL-13, IL-17, or IL-6 mRNA expression in lavage.
Discussion
A strong correlation exists between environmental exposures and persistent respiratory symptoms in children, but the underlying cellular and molecular immune mechanisms remain to be elucidated in the human population (Bernstein et al., 2004). To address this gap in knowledge, we conducted our longitudinal study to determine whether repeated episodes of allergen and/or air pollutant exposure during rhesus monkey neonatal development would result in detectable changes in systemic immune profiles over the course of a 6 month evaluation period. We performed a progressive analysis of developmental fluctuations in both frequency and number of immune cells, in conjunction with cell surface marker/cytokine gene expression in peripheral blood. HDM, ozone or combined HDM + ozone did not appear to produce a prolonged effect on the majority of peripheral blood immune parameters evaluated in this study; rather, detectable changes elicited by exposure were primarily limited to specific time points during the exposure regimen. Cell and cytokine profiles in lung lavage collected at the 6 month sampling time point were significantly changed with exposure to either ozone or HDM + ozone, but were not comparable to findings in peripheral blood. Overall, these data may have important implications in the interpretation of blood samples collected from pediatric populations, both with respect to the transient nature of immune profiles during early development as well as distinction between systemic versus mucosal compartments.
Independent of exposure, we observed age-dependent fluctuations for multiple parameters measured during the 6 month evaluation period, highlighting that immune development does not necessarily follow a linear trajectory relative to age. For example, there was a significant reduction in total WBC and lymphocyte numbers (Figure 2 A,B), as well as FoxP3 and IL-12 mRNA at 12 weeks of age (Figure 4B, F) in peripheral blood from filtered air control animals, suggesting an important checkpoint during development in the rhesus monkey. While we do not yet understand the significance of 12 weeks with respect to immune development in the rhesus monkey, nursery-raised neonatal animals (including animals in this study) undergo weaning from formula to laboratory monkey chow during this growth period.
Of the 10 cytokines we evaluated in peripheral blood, only IL-4 and IFNγ significantly correlated with chronologic age, along with a trend towards positive correlation for CD3 expression (Figure E3). In humans, total lymphocyte numbers and CD3+ cell counts appear to be relatively stable until 1-2 years of age, followed by a steady decline into adolescence (Shearer et al., 2003). Consistent with findings in human infants, regression analysis of total lymphocyte numbers in rhesus monkey infants in our study did not support a linear relationship with chronologic age. However, Pearson correlation did show a significant association of CD3 copy number with lymphocyte numbers (Table E1); this would suggest that either T cells make a greater contribution to the total lymphocyte population over time or expression levels for CD3 on individual cells progressively increase with chronologic age. Multiple correlations between cytokine/cellular markers associated with Th1, Th2, Treg and Th17 cells were observed in, suggesting that the T cell profile during immune development is heterogeneous in phenotype. While protein expression may differ from mRNA profiles, our observation of very low peripheral blood IL-17 mRNA throughout the first six months of life is consistent with a recent report demonstrating that peripheral blood Th17 cells in nursery-reared infant rhesus monkeys are infrequent until approximately 12 months of age (Ardeshir et al., 2014).
Identification of exposure biomarkers in peripheral blood utilizing a highly sensitive method such as quantitative RT-PCR would readily allow translation to parallel studies in human children, as only small quantities of blood are generally available for analysis. While we did not detect global changes in peripheral blood that were predictive of a specific exposure regimen, a subset of parameters at defined time points were significantly associated with exposure (Table 2). CCR3 expression is particularly relevant as a Th2 biomarker for our studies because this chemokine receptor is highly expressed on eosinophils. Of the parameters identified in this manner, CCR3 mRNA expression in peripheral blood was most frequently found to vary with exposure, with combined HDM + ozone resulting in significantly reduced levels relative to HDM alone at 8, 12 and 24 weeks (Table 1). Comparatively, exposures consisting of HDM or ozone alone resulted in increased CCR3 mRNA in peripheral blood collected at 12 or 24 weeks, respectively. We did not find increased CCR3 mRNA in lung lavage for all animals within the combined HDM + ozone exposure group (Figure 7B), but eosinophil frequency was significantly increased (Figure 6B). We speculate that the observed reduction in CCR3 mRNA in peripheral blood from HDM + ozone animals may be due to eosinophil recruitment into the airways following exposure. Consistent with our findings, Ullmann and colleagues reported that peripheral blood eosinophil counts in severe asthmatic children does not correlate with the high degree of airways eosinophilia in this population (Ullmann et al., 2013).
Given the potential for trafficking and depletion of cell populations from peripheral blood immediately following exposure, we also assessed for intrinsic exposure effects in vitro using blood obtained several days after the final allergen/ozone challenge at 6 months of age, with the rationale that antigen-specific immune populations might replete the periphery. Using this strategy, we found that in vitro culture with HDM modulated expression of multiple parameters in an exposure-dependent fashion (Figure 5), which was not observed in the initial assessment of peripheral blood samples collected at this time point (Figure 7), suggesting that timing of analysis relative to exposure can substantially impact upon cellular markers and cytokine synthesis. It is notable that lung lavage from combined HDM + ozone exposure at this time point displayed an immune profile consisting of increased lymphocytes and eosinophils, as well as IFNγ and FoxP3 mRNA (Figure 7B). Similarly, pediatric severe asthmatics are also characterized by eosinophilic airways inflammation but do not have a distinct Th1 or Th2 immune profile (Fitzpatrick et al., 2010; Bossley et al., 2012).
Our study is unique in its ability to utilize a carefully controlled exposure system in an outbred primate laboratory animal model with a similar developmental pattern to that of humans. Rhesus monkeys display postnatal alveolar growth curves that are nearly identical to that of humans, and also exhibit prolonged alveolar growth into early adulthood (Hyde et al., 2007; Herring et al., 2014). Further, HDM is a common allergen clinically associated with allergic asthma and ozone is one of six criteria pollutants for which National Ambient Air Quality Standards are set by the United States Environmental Protection Agency, therefore the environmental exposure relevance to human subjects is high. An important caveat to our findings in this study is that neonatal development is accelerated in rhesus monkeys relative human infants. With respect to sexual maturation, it can be estimated that the rhesus monkey development is approximately 4 times that of humans, although motor abilities are 7-10 times that of humans. As such, the childhood developmental period assessed in our study might be extrapolated to pediatric populations that are 2-3 years of age (Golub and Gershwin, 1984). An additional limitation of this study is that only male rhesus monkeys were evaluated; recent studies in aging monkey populations suggest that gender does impact upon lung structure, with female animals demonstrating increased loss of alveoli as compared with male counterparts (Herring et al., 2013).
It has been proposed that environmental airborne exposures during early life may contribute to childhood asthma and other allergic diseases in the pediatric population (Holt et al., 2005; Asher et al., 2006). Peripheral blood biomarkers recognized as being reflective of changes occurring in the lung following environmental airborne exposures could be incorporated into parallel studies in pediatric populations, as it has been speculated that variable clinical asthma phenotypes might be due to differences in underlying immune response profiles (Heaton et al., 2005). A number of human birth cohorts have tracked asthmatic children longitudinally, primarily assessing for allergy, lung function and other respiratory symptoms (Sears et al., 2003; Stern et al., 2008). In support of a transitory immune phenotype during early childhood and association with a disease phenotype, McLoughlin and colleagues reported that increased CD4+ cells with a T regulatory cell phenotype were associated with reduced incidence of allergic sensitization at one year of age; conversely, a similar T helper subset was positively associated with allergic sensitization and eczema at two years of age (McLoughlin et al., 2012). While birth cohort studies are imperative for understanding pathogenesis of chronic lung disease in human subjects, identification of specific periods of susceptibility in this population is challenging given the diversity of potential environmental exposures, particularly if shifts in immune profiles take place within narrow developmental windows.
In conclusion, we show in a primate model of infant development that establishment of the peripheral blood immune profile during early life is a process that does not exclusively follow a linear trajectory with increasing chronologic age. Our findings suggest that dynamic changes in immune cell populations with exposure are limited to small subsets, whereas most of the repertoire remains relatively static. Nevertheless, exposure-dependent changes were readily detectable within specific developmental time points for the infant rhesus monkey. It can be speculated that a comparable process takes place in very young children, and as such, may necessitate more frequent or consistent sampling of study subjects in birth cohorts to ascertain whether an observed phenotype is due to environment or simply the outcome of a specific period in development. Future studies that focus on putative “windows of susceptibility” in our animal model system with an emphasis on evaluating the lung immune phenotype may identify key molecular mechanisms for both HDM and ozone that ultimately lead to persistent airways disease elicited with maturity.
Supplementary Material
Highlights.
Allergen and air pollutant exposures alter the immune profile of infant monkeys.
Exposure effects were observed for CCR3, FoxP3 and IL-12 mRNA in peripheral blood.
The lung immune profile following exposure was not comparable to peripheral blood.
Acknowledgments
We would like to acknowledge the contributions of Sarah Davis, Paul-Michael Sosa, Sona Santos, Louise Olsen, and Brian Tarkington for technical support during this study. Dr. Candice Clay provided critical comments and editorial assistance on the manuscript.
Grant Funding: This publication was supported by grants ES011617, ES000628, HL081286, HL097087, OD011107, ES007059 from the National Institutes of Health and STAR Grant 832947 from the Environmental Protection Agency.
Footnotes
Author Contributions: L.A.M. conceived the study and served as a co-investigator and principal investigator for grants that that supported the research. C.M.C, J.H.F., and J.E.G. contributed to data collection and analysis for the manuscript. C.M.C., E.S.S., D.M.H. and L.A.M. reviewed the data analysis for the manuscript. C.M.C. and L.A.M. wrote the manuscript. J.H.F., J.E.G., E.S.S., and D.M.H. critically reviewed the manuscript. All authors provided significant intellectual contributions to the study and approved the final version of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ardeshir A, Narayan NR, Mendez-Lagares G, Lu D, Rauch M, Huang Y, Van Rompay KK, Lynch SV, Hartigan-O'Connor DJ. Breastfed and bottle-fed infant rhesus macaques develop distinct gut microbiotas and immune systems. Science translational medicine. 2014;6:252ra120. doi: 10.1126/scitranslmed.3008791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher MI, Montefort S, Bjorksten B, Lai CK, Strachan DP, Weiland SK, Williams H Group, I.P.T.S. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–743. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- Barker DJP. Maternal nutrition, fetal nutrition, and disease in later life. Nutrition. 1997;13:807–813. doi: 10.1016/s0899-9007(97)00193-7. [DOI] [PubMed] [Google Scholar]
- Beck R, Lam-Po-Tang PR. Comparison of cord blood and adult blood lymphocyte normal ranges: a possible explanation for decreased severity of graft versus host disease after cord blood transplantation. Immunology and cell biology. 1994;72:440–444. doi: 10.1038/icb.1994.65. [DOI] [PubMed] [Google Scholar]
- Bernstein JA, Alexis N, Barnes C, Bernstein IL, Bernstein JA, Nel A, Peden D, Diaz-Sanchez D, Tarlo SM, Williams PB. Health effects of air pollution. J Allergy Clin Immunol. 2004;114:1116–1123. doi: 10.1016/j.jaci.2004.08.030. [DOI] [PubMed] [Google Scholar]
- Bossley CJ, Fleming L, Gupta A, Regamey N, Frith J, Oates T, Tsartsali L, Lloyd CM, Bush A, Saglani S. Pediatric severe asthma is characterized by eosinophilia and remodeling without T(H)2 cytokines. J Allergy Clin Immunol. 2012;129:974–982 e913. doi: 10.1016/j.jaci.2012.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipeta J, Komada Y, Zhang XL, Deguchi T, Sugiyama K, Azuma E, Sakurai M. CD4+ and CD8+ cell cytokine profiles in neonates, older children, and adults: increasing T helper type 1 and T cytotoxic type 1 cell populations with age. Cell Immunol. 1998;183:149–156. doi: 10.1006/cimm.1998.1244. [DOI] [PubMed] [Google Scholar]
- Comans-Bitter WM, de Groot R, van den Beemd R, Neijens HJ, Hop WC, Groeneveld K, Hooijkaas H, van Dongen JJ. Immunophenotyping of blood lymphocytes in childhood. Reference values for lymphocyte subpopulations. J Pediatr. 1997;130:388–393. doi: 10.1016/s0022-3476(97)70200-2. [DOI] [PubMed] [Google Scholar]
- de Vries E, de Bruin-Versteeg S, Comans-Bitter WM, de Groot R, Hop WC, Boerma GJ, Lotgering FK, van Dongen JJ. Longitudinal survey of lymphocyte subpopulations in the first year of life. Pediatr Res. 2000;47:528–537. doi: 10.1203/00006450-200004000-00019. [DOI] [PubMed] [Google Scholar]
- Erkeller-Yuksel FM, Deneys V, Yuksel B, Hannet I, Hulstaert F, Hamilton C, Mackinnon H, Stokes LT, Munhyeshuli V, Vanlangendonck F, et al. Age-related changes in human blood lymphocyte subpopulations. J Pediatr. 1992;120:216–222. doi: 10.1016/s0022-3476(05)80430-5. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick AM, Higgins M, Holguin F, Brown LA, Teague WG. The molecular phenotype of severe asthma in children. J Allergy Clin Immunol. 2010;125:851–857 e818. doi: 10.1016/j.jaci.2010.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparoni A, Ciardelli L, Avanzini A, Castellazzi AM, Carini R, Rondini G, Chirico G. Age-related changes in intracellular TH1/TH2 cytokine production, immunoproliferative T lymphocyte response and natural killer cell activity in newborns, children and adults. Biol Neonate. 2003;84:297–303. doi: 10.1159/000073638. [DOI] [PubMed] [Google Scholar]
- Hartel C, Adam N, Strunk T, Temming P, Muller-Steinhardt M, Schultz C. Cytokine responses correlate differentially with age in infancy and early childhood. Clin Exp Immunol. 2005;142:446–453. doi: 10.1111/j.1365-2249.2005.02928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton T, Rowe J, Turner S, Aalberse RC, de Klerk N, Suriyaarachchi D, Serralha M, Holt BJ, Hollams E, Yerkovich S, Holt K, Sly PD, Goldblatt J, Le Souef P, Holt PG. An immunoepidemiological approach to asthma: identification of in-vitro T-cell response patterns associated with different wheezing phenotypes in children. Lancet. 2005;365:142–149. doi: 10.1016/S0140-6736(05)17704-6. [DOI] [PubMed] [Google Scholar]
- Herring MJ, Avdalovic MV, Quesenberry CL, Putney LF, Tyler NK, Ventimiglia FF, St George JA, Hyde DM. Accelerated structural decrements in the aging female rhesus macaque lung compared with males. Am J Physiol Lung Cell Mol Physiol. 2013;304:L125–134. doi: 10.1152/ajplung.00226.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring MJ, Putney LF, Wyatt G, Finkbeiner WE, Hyde DM. Growth of alveoli during postnatal development in humans based on stereological estimation. Am J Physiol Lung Cell Mol Physiol. 2014 doi: 10.1152/ajplung.00094.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt PG, Jones CA. The development of the immune system during pregnancy and early life. Allergy. 2000;55:688–697. doi: 10.1034/j.1398-9995.2000.00118.x. [DOI] [PubMed] [Google Scholar]
- Holt PG, Upham JW, Sly PD. Contemporaneous maturation of immunologic and respiratory functions during early childhood: implications for development of asthma prevention strategies. J Allergy Clin Immunol. 2005;116:16–24. doi: 10.1016/j.jaci.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Hussey GD, Watkins ML, Goddard EA, Gottschalk S, Hughes EJ, Iloni K, Kibel MA, Ress SR. Neonatal mycobacterial specific cytotoxic T-lymphocyte and cytokine profiles in response to distinct BCG vaccination strategies. Immunology. 2002;105:314–324. doi: 10.1046/j.1365-2567.2002.01366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde DM, Blozis SA, Avdalovic MV, Putney LF, Dettorre R, Quesenberry NJ, Singh P, Tyler NK. Alveoli increase in number but not size from birth to adulthood in rhesus monkeys. Am J Physiol Lung Cell Mol Physiol. 2007;293:L570–L579. doi: 10.1152/ajplung.00467.2006. [DOI] [PubMed] [Google Scholar]
- Maniar-Hew K, Clay CC, Postlethwait EM, Evans MJ, Fontaine JH, Miller LA. Innate immune response to LPS in airway epithelium is dependent on chronological age and antecedent exposures. Am J Respir Cell Mol Biol. 2013;49:710–720. doi: 10.1165/rcmb.2012-0321OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant A, Appay V, Van Der Sande M, Dulphy N, Liesnard C, Kidd M, Kaye S, Ojuola O, Gillespie GM, Vargas Cuero AL, Cerundolo V, Callan M, McAdam KP, Rowland-Jones SL, Donner C, McMichael AJ, Whittle H. Mature CD8(+) T lymphocyte response to viral infection during fetal life. J Clin Invest. 2003;111:1747–1755. doi: 10.1172/JCI17470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin RM, Calatroni A, Visness CM, Wallace PK, Cruikshank WW, Tuzova M, Ly NP, Ruiz-Perez B, Kattan M, Bloomberg GR, Lederman H, Gern JE, Gold DR. Longitudinal relationship of early life immunomodulatory T cell phenotype and function to development of allergic sensitization in an urban cohort. Clin Exp Allergy. 2012;42:392–404. doi: 10.1111/j.1365-2222.2011.03882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LA, Gerriets JE, Tyler NK, Abel K, Schelegle ES, Plopper CG, Hyde DM. Ozone and allergen exposure during postnatal development alters the frequency and airway distribution of CD25+ cells in infant rhesus monkeys. Toxicol Appl Pharmacol. 2009;236:39–48. doi: 10.1016/j.taap.2008.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LA, Plopper CG, Hyde DM, Gerriets JE, Pieczarka EM, Tyler NK, Evans MJ, Gershwin LJ, Schelegle ES, Van Winkle LS. Immune and airway effects of house dust mite aeroallergen exposures during postnatal development of the infant rhesus monkey. Clin Exp Allergy. 2003;33:1686–1694. doi: 10.1111/j.1365-2222.2003.01812.x. [DOI] [PubMed] [Google Scholar]
- Milligan KL, Matsui E, Sharma H. Asthma in Urban Children: Epidemiology, Environmental Risk Factors, and the Public Health Domain. Curr Allergy Asthma Rep. 2016;16:33. doi: 10.1007/s11882-016-0609-6. [DOI] [PubMed] [Google Scholar]
- Mold JE, Venkatasubrahmanyam S, Burt TD, Michaelsson J, Rivera JM, Galkina SA, Weinberg K, Stoddart CA, McCune JM. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science. 2010;330:1695–1699. doi: 10.1126/science.1196509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BD, Hyde D, Miller L, Wong E, Frelinger J, Schelegle ES. Allergen and ozone exacerbate serotonin-induced increases in airway smooth muscle contraction in a model of childhood asthma. Respiration. 2012;83:529–542. doi: 10.1159/000336835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub MS, Gershwin ME. Standardized neonatal assessment in the rhesus monkey. In: N MW, P JT, editors. Research in Perinatal Medicine. Perinatology Press; Ithaca, NY: 1984. pp. 55–138. [Google Scholar]
- Naderi N, Pourfathollah AA, Alimoghaddam K, Moazzeni SM. Cord blood dendritic cells prevent the differentiation of naive T-helper cells towards Th1 irrespective of their subtype. Clinical and experimental medicine. 2009;9:29–36. doi: 10.1007/s10238-008-0020-2. [DOI] [PubMed] [Google Scholar]
- Palin AC, Ramachandran V, Acharya S, Lewis DB. Human neonatal naive CD4+ T cells have enhanced activation-dependent signaling regulated by the microRNA miR-181a. J Immunol. 2013;190:2682–2691. doi: 10.4049/jimmunol.1202534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelegle ES, Gershwin LJ, Miller LA, Fanucchi MV, Van Winkle LS, Gerriets JP, Walby WF, Omlor AM, Buckpitt AR, Tarkington BK, Wong VJ, Joad JP, Pinkerton KB, Wu R, Evans MJ, Hyde DM, Plopper CG. Allergic asthma induced in rhesus monkeys by house dust mite (Dermatophagoides farinae) Am J Pathol. 2001;158:333–341. doi: 10.1016/S0002-9440(10)63973-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelegle ES, Miller LA, Gershwin LJ, Fanucchi MV, Van Winkle LS, Gerriets JE, Walby WF, Mitchell V, Tarkington BK, Wong VJ, Baker GL, Pantle LM, Joad JP, Pinkerton KE, Wu R, Evans MJ, Hyde DM, Plopper CG. Repeated episodes of ozone inhalation amplifies the effects of allergen sensitization and inhalation on airway immune and structural development in Rhesus monkeys. Toxicol Appl Pharmacol. 2003;191:74–85. doi: 10.1016/s0041-008x(03)00218-7. [DOI] [PubMed] [Google Scholar]
- Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, Cowan JO, Herbison GP, Silva PA, Poulton R. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349:1414–1422. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- Shearer WT, Rosenblatt HM, Gelman RS, Oyomopito R, Plaeger S, Stiehm ER, Wara DW, Douglas SD, Luzuriaga K, McFarland EJ, Yogev R, Rathore MH, Levy W, Graham BL, Spector SA, Pediatric ACTG. Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J Allergy Clin Immunol. 2003;112:973–980. doi: 10.1016/j.jaci.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Stern DA, Morgan WJ, Halonen M, Wright AL, Martinez FD. Wheezing and bronchial hyper-responsiveness in early childhood as predictors of newly diagnosed asthma in early adulthood: a longitudinal birth-cohort study. Lancet. 2008;372:1058–1064. doi: 10.1016/S0140-6736(08)61447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahata Y, Nomura A, Takada H, Ohga S, Furuno K, Hikino S, Nakayama H, Sakaguchi S, Hara T. CD25+CD4+ T cells in human cord blood: an immunoregulatory subset with naive phenotype and specific expression of forkhead box p3 (Foxp3) gene. Exp Hematol. 2004;32:622–629. doi: 10.1016/j.exphem.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Ullmann N, Bossley CJ, Fleming L, Silvestri M, Bush A, Saglani S. Blood eosinophil counts rarely reflect airway eosinophilia in children with severe asthma. Allergy. 2013;68:402–406. doi: 10.1111/all.12101. [DOI] [PubMed] [Google Scholar]
- Upham JW, Rate A, Rowe J, Kusel M, Sly PD, Holt PG. Dendritic cell immaturity during infancy restricts the capacity to express vaccine-specific T-cell memory. Infect Immun. 2006;74:1106–1112. doi: 10.1128/IAI.74.2.1106-1112.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vekemans J, Amedei A, Ota MO, D'Elios MM, Goetghebuer T, Ismaili J, Newport MJ, Del Prete G, Goldman M, McAdam KP, Marchant A. Neonatal bacillus Calmette-Guerin vaccination induces adult-like IFN-gamma production by CD4+ T lymphocytes. Eur J Immunol. 2001;31:1531–1535. doi: 10.1002/1521-4141(200105)31:5<1531::AID-IMMU1531>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Weitzel RP, Lesniewski ML, Haviernik P, Kadereit S, Leahy P, Greco NJ, Laughlin MJ. microRNA 184 regulates expression of NFAT1 in umbilical cord blood CD4+ T cells. Blood. 2009;113:6648–6657. doi: 10.1182/blood-2008-09-181156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.