Abstract
The ubiquitous use of poly- and perfluoroalkyl substances (PFASs) in a variety of industrial and consumer products has resulted in chronic exposure in most industrialized nations, and led to measurable concentrations in blood and other tissues in humans across all life stages; however, behavioral attributes that relate to exposure are not well studied. To further investigate how behavior may relate to PFAS exposure, 37 adults were recruited from central North Carolina. Participants provided blood samples and behavioral questionnaires were administered, asking questions about a variety of household, dietary, and behavioral outcomes. Six PFAAs, including PFHxA (geometric mean: 0.14 ng/mL), PFOA (1.57 ng/mL), PFNA (0.67 ng/mL), PFDA (0.28 ng/mL), PFHxS (3.17 ng/mL) and PFOS (4.96 ng/mL) were detected in > 50% of the samples. Generally, males had higher serum levels than females across all chemicals, and levels were very similar to NHANES levels; however, PFHxS and PFDA levels were higher in our cohort. Several personal characteristics and behaviors were associated with serum PFAS levels. Reported use of filtration devices was associated with lower levels of PFOA (28% lower, p=0.03), but higher levels of PFHxA (122% higher, p=0.04). Serum PFHxS levels were also elevated in individuals that vacuumed less often, and in individuals that reported consuming more microwavable foods. These results suggest that personal behaviors may be important determinants of PFAS exposures.
Keywords: Perfluorinated Chemicals, PFOA, PFOS, Serum, Behavior, Adults
1. Introduction
Poly- and perfluoroalkyl substances (PFASs) are extensively used in a variety of industrial processes and consumer products around the world. These compounds have a base structure consisting of a fluorinated hydrocarbon chain of various lengths, but they differ with respect to the head group. This structure results in unique properties including extreme chemical and thermal stability (perfluoro-chemicals), surfactant properties, and low surface free energy (Fromme et al., 2009). As a result of their physicochemical properties, PFASs are able to repel both moisture and oil and are commonly used in carpets, clothing, and cookware as stain, water, or grease repellents (Zheng et al., 2012; Stahl et al., 2011). PFASs also have exceptional surface performance and are used as polymerization aids, surfactants, and in fire-fighting foams (Fujii et al., 2013).
Within the PFAS family, there are specific subclasses of compounds with different functional groups. In this study, our particular interest focused on the perfluoroalkyl acids (PFAAs). Common examples of PFAAs include perfluorooctanoic acid (PFOA) and perfluorohexanoic acid (PFHxA), perfluorooctane sulfonic acid (PFOS) and perfluorohexane sulfonic acid (PFHxS). These terminal end products are extremely stable once released to the environment, and are the primary driver of PFAS risk concerns at present (Krafft and Riess, 2015). However, nearly all other PFASs can be considered precursors to PFAAs, and these precursors can include the fluorotelomer alcohols (FTOHs) and mono- and di-substituted polyfluoroalkyl phosphates (monoPAPs, diPAPs).
PFASs have triggered increased scientific interest due to their widespread distribution, exposure, and potential toxicity to humans and the environment. PFASs have been measured in human blood worldwide, with the highest levels typically detected in industrialized areas (D’Eon and Mabury, 2007). Humans are exposed to these compounds through several pathways, both directly and indirectly, with ingestion of contaminated food hypothesized to be a primary pathway (Stahl et al., 2011). Drinking water is now also considered a significant source of exposure as recent studies have identified (Stahl et al., 2011; Hu et al., 2016), and the Environmental Protection Agency (USEPA) has established new health advisories for drinking water (USEPA, 2016). Despite measurements of PFASs in food and water, it is not clear if direct exposure via ingestion of compounds in foodstuffs, indirect exposure by precursors in food paper packaging, or other pathways including the inhalation of contaminated air or ingestion of contaminated dust are driving levels of PFASs in human blood (Haug et al., 2011).
Regardless of the source of exposure, PFASs accumulation in humans is evidenced by detectable levels of these man-made chemicals in various tissues. In humans, several PFASs (particularly PFAAs) are likely to accumulate in blood and breast milk, and serum half-lives of stable compounds range considerably, and have been reported at 2.4 years for PFOA, 4.3 years for PFOS, and even up to 25 years for PFHxS (Russell et al., 2015; Olsen et al., 2012; Zhang et al., 2013). Once in the blood, PFAAs have the potential to induce adverse effects in exposed organism (Stahl et al., 2011). Recent studies have suggested that PFAAs may act as endocrine disruptors in human cells, decrease fertility, and interfere with the levels of endogenous hormones (Rosenmai et al., 2013; Bach et al., 2016). Additionally, one study has highlighted the potential for some PFAAs to induce immunosuppression in both human and animal models (Mogensen et al., 2015). Longer-chain PFAAs have also been shown to be significantly negative associated with the rate of antibody response in human adults following diphtheria and tetanus vaccination (Kielsen et al., 2016). This association was further confirmed in antibody studies of children exposed to PFDA and PFOA, where diphtheria antibody concentrations decreased by approximately 25% for each doubling of serum PFAA exposure level (Grandjean et al., 2016).
As data on the potential toxicity and hazard of PFASs continue to be generated, there has been a reflective increase in the level of concern over persistent PFASs in the environment, and a movement towards their phase-out and replacement in products from the U.S. with shorter-chain compounds (Wang et al., 2013). The effects of these changes in chemical use on human exposure are currently unknown. More work needs to be done to characterize the relative importance of exposure pathways for PFASs to ascertain how regulatory shifts are impacting body burdens. Additionally, there is a lack of knowledge around behaviors and personal characteristics that are associated with exposure or steps that could be taken to reduce one’s own exposure. The overall aim of this study was to quantify levels of PFAAs in serum from adults and to determine the types of personal behaviors that may be associated with serum PFAA levels.
2. Materials and Methods
2.1 Study Design
Prior to recruitment and sampling, all protocols were reviewed and approved by the Duke University Institutional Review Board. A convenience sample of young adult volunteers was recruited using advertisements placed throughout the Duke community in the summer and early fall of 2015. Eligible participants were at least 18 years of age and lived off campus, and a total of 40 participants were recruited; however, only 37 provided a blood sample. All participants provided informed consent prior to providing any information or samples. The study consisted of a home visit and blood draw; all samples were collected within two weeks of each other.
2.2 Questionnaires
Research study staff visited each participant’s home to collect environmental samples and to administer a brief questionnaire (Figure S1). designed to collect information on demographics and personal behaviors. Participants provided information on a variety of personal characteristics, including age, sex, race, height, weight, marital status, and education level. Personal behaviors were also examined, and included average frequency of hand washing, vacuuming, dusting, and fast food or microwaveable meal consumption. Questions regarding personal care product use and possession of stain-and water-repellant clothing were also included in the questionnaire. Each participant’s average time spent in specific microenvironments, such as indoors, outside, in public buildings, or on public transportation, was also recorded in the questionnaire. The distribution of answers for each question was assessed and responses were grouped into high or low categories based on distributions as follows: dusting frequency=never vs. at least once per month, vacuuming frequency=once per month or less vs. greater than one time per month, hand washing frequency=less than 5 vs. 5 or more times per day, fast food consumption=once per week or less vs. more than once per week, microwavable meal and popcorn intake=never vs. once per month or more, water filtration use=do not use vs. use, stain or water repellant clothing=own vs. do not own, and smoking=non-smoker vs. past /current smoker. Time spent in various environments (e.g. home, work, etc.) was quite similar across participants and these variables were not included in further analyses.
2.3 Serum Samples
Blood samples (~14mL) were collected by a trained phlebotomist via venipuncture in our laboratory. Serum was isolated, aliquoted and samples were frozen at −20°C until analysis. Serum albumin was analyzed at the Duke University Health System (DUHS) Clinical Laboratories using spectrophotometry techniques. PFAAs were measured in an additional aliquot using methods described below.
2.4 Chemicals
Calibration and internal standard mixtures were purchased from Wellington Laboratories (Guelph, ON, Canada). Specifically, target analytes and internal standards included PFHxS, PFOS, perfluoropentanoate (PFPeA), PFHxA, perfluoroheptanoate (PFHpA), PFOA, perfluorononanoate (PFNA), and PFDA. Formic acid, ammonium hydroxide and ammonium acetate were purchased from Sigma-Aldrich (St. Louis, MO). Oasis HLB columns were purchased from Waters Corporation (Milford, MA). The Luna C18(2) (2.5 µm, 50 × 2 mm) analytical column was purchased from Phenomenex (Torrance, CA, USA). Methanol and water were HPLC grade (Burdick & Jackson, Honeywell, Morris Plains, NJ). Envi-Carb columns (1 mL, 100 mg) were purchased from Supelco (Bellefonte, PA). Mini-UniPrep vials (0.2 µm) were from GE Healthcare Life Sciences (Marlborough, MA). The serum method was validated using Standard Reference Material (SRM) 1957 (National Institute of Standards & Technology, 2016).
2.4 Sample Analyses
Serum samples were analyzed for perfluorinated carboxylates (C4-C10 PFCAs) and perfluorinated sulfonates (C4, C6, C8 PFSAs) only. Extraction methods were modified from Liu et al. (2015). Samples were thawed, and 1 mL of serum was transferred to a 15 mL polypropylene centrifuge tube and spiked with the internal standard mixture (6 ng of MPFAC-MXA). The samples were acidified with 4 mL of 0.1 M formic acid, then vortexed and sonicated for 15 minutes. Extracts were cleaned and concentrated using solid-phase extraction (SPE) techniques with Oasis HLB columns (60 mg, 3 ml). Briefly, the SPE columns were preconditioned with 2 mL methanol followed by 2 mL of 0.1 M formic acid. The sample was loaded and the tube was rinsed (3x) with 500 µL of 0.1 M formic acid. The SPE column was rinsed with 2 mL of 0.1 M formic acid followed by 1 mL of 1% NH4OH in water. Analytes were eluted with 1.0 mL of 1% NH4OH in methanol and reduced to near dryness under a gentle nitrogen gas stream in a 40°C water bath. Extracts were reconstituted in 500 µL of methanol, transferred to a cryovial, and keep at −20°C until analysis. Immediately prior to instrumental analysis, a small aliquot was transferred to a Mini-UniPrep vial, filtered, an equal volume of water was added and vial was vortexed.
2.6 Instrumental Analyses
Extracts were analyzed for PFCAs and PFSAs using negative electrospray ionization liquid chromatography tandem mass spectrometry (LC-MS/MS) techniques. Chromatography was achieved under gradient conditions using a Luna C18(2) column (50 × 2.0 mm, 2.5 µm particle size, Phenomenex, Torrance, CA) preceded by a SecurityGuard Polar-RP (4 × 2.0 mm) guard cartridge. The mobile phases were methanol and water (modified with 2 mM ammonium acetate), flow rate was 300 µL/min, the injection volume was 10 µL, and the column oven was 40°C. Initial conditions were 75:25 water:methanol, increasing to 50:50 in 0.75 min, to 40:60 in 1.0 min, 5:95 in 2.75 min, and held for 0.5 min before decreasing to initial conditions in 0.75 min. Data were acquired under multiple reaction monitoring conditions using optimized parameters (Table S1 in Supporting Information).
2.7 Quality Control
Five laboratory blanks of fetal bovine serum were utilized for quality control. Serum samples were blank-corrected using the average laboratory blank measurement. The method detection limits (MDLs) for each analyte were calculated as three times the standard deviation of the corresponding blanks. Serum samples were normalized to the average extraction volume of 1 mL. Analyte recovery was assessed by spiking the PFAS-MXA mixture into 1 mL of clean fetal bovine serum and extracting using the SPE method described above. Recoveries of the PFCA standards ranged from 90–124% in the 0.6 ng spikes, to 77–94% in the 6 ng spikes. Recoveries of the PFSA standards ranged from 69–97% in the 0.6 ng spikes, to 70–108% in the 6 ng spikes. PFAS levels measured in SRM 1957 were within 30% of those reported by Keller et al (Table S2 in Supporting Information).
2.8 Statistical Analyses
Summary statistics were calculated for all analytes with a detection frequency greater than 80% in serum, and distributions were assessed for normality using Shapiro-Wilk tests. PFAA levels did not meet the conditions for normality, therefore, non-parametric methods were used for statistical analyses or PFAA concentrations were log10 transformed. PFAA measurements below the method detection limit (MDL) were assigned a value equal to MDL/2 for statistical analyses. Because imputing concentrations below the MDL as a constant value could potentially bias statistical analyses (Helsel 2006), we also conducted analyses using random numbers between the 0 and the MDL. Results were quite similar, likely because PFAAs were detected very frequently (results not presented). Serum analytes are reported in concentrations of ng/mL serum.
Associations between PFAAs in serum samples were assessed using Spearman correlations as were relationships between serum PFAAs and albumin, participant age and participant BMI. We assessed potential differences in serum PFAA concentrations between males and females using Kruskal-Wallis rank sum tests. Potential relationships between survey data and PFAA levels were explored using multivariable regression models which adjusted for potential confounding by participant sex, a factor which we anticipated could be related to many of the survey responses and with PFAA measures. These models used log10 transformed PFAA concentrations as outcomes. To facilitate interpretation of results, beta coefficients from these models were exponentiated and represent the multiplicative change in PFAA levels relative to the reference category. Additionally, because some PFAAs are known to bind with high affinity to serum binding proteins (D’eon et al., 2010), sensitivity analyses including albumin as a covariate were conducted to assess possible confounding.
Levels measured in the serum from the study cohort were compared with data from two studies, the National Health and Nutrition Examination Survey (NHANES), and the Canadian Health Measures Survey (CHMS), to assess potential differences. Data from these studies was restricted to the same age range as that of our participants (22 to 34 years of age). The most recent Canadian data was gleaned from the Second Report on Human Biomonitoring for serum samples collected in 2009–2011 (Health Canada, 2013). Data were extracted from the database for the 2011–2012 NHANES participants, the most recent data set that ranged in age from 20–35, and the final cohort (n=460) included 231 females and 229 males. Comparisons between the NHANES and Duke cohorts were run using multivariable regression models which controlled for age and sex.
All statistical analyses were run using SAS version 9.4. Statistical significance was set at p<0.05; however, because our sample size was relatively small, we considered associations with p<0.10 marginally significant and suggestive of relationships.
3. Results
3.1 Population Characteristics
Forty individuals participated in the study and completed the questionnaire; however, only 37 provided a serum sample. The serum cohort included 26 females and 11 males. Ages ranged from 22 to 34 years old, with an average age of 25.8 years. The majority of participants reported non-Hispanic white (81.1%) race. BMI values for each individual were calculated using self-reported height and weight values, and BMIs ranged from 18.6 kg/m2 to 37.6 kg/m2 with an average of 23.2 kg/m2.
3.2 PFAAs in Serum
Six PFAAs were detected >80% of serum samples (Table 1). These included PFHxA, PFHxS, PFOA, PFNA, PFOS, and PFDA. Several (PFHxS, PFOA, PFNA, and PFOS) were present in all of the serum samples, while PFHxA and PFDA were present in 83.8% and 97.3% of the samples, respectively. Geometric means for these six analytes ranged from 0.14–4.96 ng/mL serum. PFHxA was present in serum in the lowest concentrations of all six analytes, with a range of 0.01–1.00 ng/mL and a geometric mean of 0.14 ng/mL. In contrast, PFHxS and PFOS were present in the blood in higher concentrations. PFHxS concentrations ranged from 1.07–12.55 ng/mL serum, with a geometric mean of 3.12 ng/mL serum. PFOS concentrations ranged from 0.39–31.35 ng/mL serum, with a geometric mean of 4.96 ng/mL serum. PFAS concentrations in serum were higher in males compared to females, although this difference was only statistically significant for PFOS (p=0.04) and PFHxS (p<0.001) and suggestive for PFOA (p=0.09) and PFHxA (p=0.10). Correlations between participant age and PFAAs in serum were generally positive; but were only statistically significant for PFDA (rs=0.35; p=0.03). Levels of several PFAAs were significantly correlated with BMI, including PFNA (rs=0.40; p=0.01), PFOS (rs=0.58; p=0.0002) and PFDA (rs=0.40; p=0.02). Other PFAAs were also positively correlated with BMI, but associations did not reach statistical significance.
Table 1.
Levels of PFAAs in serum from 37 young adults.
| Serum (ng/mL), n=37
|
||||
|---|---|---|---|---|
| Congener | MDL | % Detect | GM | Range |
| Perfluoropentanoic acid (PFPeA)
|
0.18 | 0 | NA | NA |
| Perfluorohexanoic acid (PFHxA)
|
0.03 | 83.8 | 0.14 | ND-1.00 |
| Perfluoroheptanoic acid (PFHpA)
|
0.07 | 32.4 | NA | ND-0.30 |
| Perfluorooctanoic acid (PFOA)
|
0.06 | 100 | 1.57 | 0.30–4.07 |
| Perfluorononanoic acid (PFNA)
|
0.02 | 100 | 0.67 | 0.23–4.02 |
| Perfluorodecanoic acid (PFDA)
|
0.04 | 97.3 | 0.28 | ND-1.60 |
| Perfluorohexane sulfonate (PFHxS)
|
0.05 | 100 | 3.12 | 1.07–12.55 |
| Perfluorooctane sulfonate (PFOS) | 0.06 | 100 | 4.96 | 0.39–31.35 |
Abbreviations: % Detect, percent detectable. NA, not available, ND, not detected.
3.3 Correlations within Serum
Several statistically significant correlations were observed between analytes within the cohort’s serum (Table 2). Spearman correlations between serum PFOA, PFNA, PFOS, and PFDA were significant (rs=0.55–0.75; p < 0.001 for all). Longer-chain compounds (i.e. ≥C8) generally were more significantly correlated amongst themselves than the six-carbon chain length PFAAs.
Table 2.
Spearman correlation matrix for PFAAs in serum (Duke Cohort).
| Serum
|
|||||||
|---|---|---|---|---|---|---|---|
| PFHxA | PFHxS | PFOA | PFNA | PFOS | PFDA | ||
| Serum | PFHxA | 1.00 | |||||
| PFHxS | 0.16 | 1.00 | |||||
| PFOA | −0.01 | 0.29^ | 1.00 | ||||
| PFNA | −0.03 | 0.20 | 0.75*** | 1.00 | |||
| PFOS | 0.20 | 0.22 | 0.55*** | 0.60*** | 1.00 | ||
| PFDA | −0.14 | 0.11 | 0.58*** | 0.70*** | 0.72*** | 1.00 | |
Analyses were conducted using analytes in which the detection frequency was > 50%.
< 0.10,
< 0.05,
< 0.01,
< 0.001.
3.4 Comparison with NHANES and CHMS data
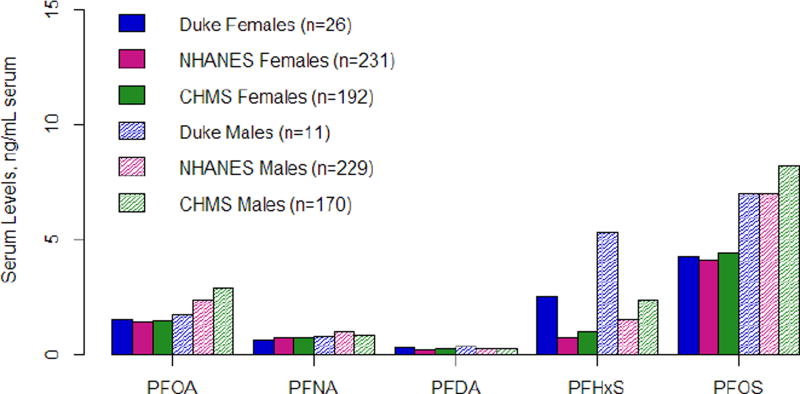
NHANES and CHMS are intended to be representative of the general population in the U.S. and Canada, respectively; therefore, we compared serum levels for five PFAAs that had available data from these biomonitoring programs with levels measured in this study (Figure 1). Serum levels were generally very similar among the three groups; however, PFHxS and PFDA levels were higher in our population compared to both the NHANES and CHMS populations. To determine if levels were significantly different between the Duke cohort and NHANES (all within the U.S.), we used a multivariable regression model (controlling for age and sex). Levels of PFHxS and PFDA were significantly higher in the Duke cohort compared to measurements reported in NHANEs (Table S3). The Duke cohort serum samples had 3.51 (95% CI: 2.63, 4.67; p < 0.001) times as much PFHxS and 1.45 times (95% CI: 1.15, 1.86; p=0.002) as much PFDA as the NHANES cohort.
Figure 1.
Geometric mean concentrations of several PFAAs analytes and a comparison of concentrations reported from the NHANES and CHMS cohorts.
3.5 Serum Questionnaire Results
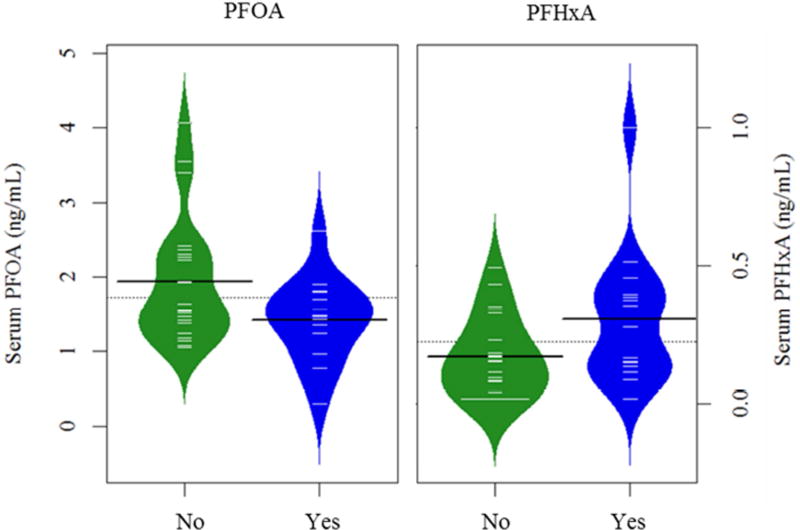
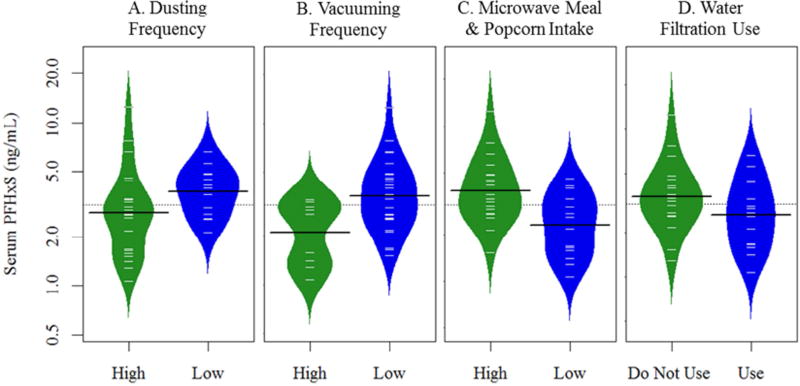
The administered survey included questions regarding personal behaviors that we hypothesized could influence exposure to PFASs. Multivariate models were conducted to examine the influence of these variables on serum PFAA levels (Table 3). After accounting for sex, the use of water filtration devices was positively associated with PFHxA, but negatively associated with PFOA serum levels. Interestingly, participants who used water filtration had 122% (p=0.04) higher PFHxA levels but approximately 30% (p=0.03) lower levels of PFOA in their serum, compared to those who did not use filtration devices (Figure 2). In addition, microwave food intake was positively associated with PFHxS serum concentrations, while vacuuming frequency was negatively associated (Figure 3). For example, after accounting for sex, participants who ate microwave meals or popcorn more frequently had serum PFHxS levels that were 49% higher (p=0.003) than those who ate these foods less frequently. PFHxS levels were 57% higher in participants who reported vacuuming less frequently compared to participants who vacuumed more frequently (p=0.003). Although results were only statistically suggestive, they indicate that PFHxS levels may be higher for individuals who dust less frequently and those who do not use water filtration devices (Table 3 and Figure 3). Interestingly, we observed an inverse association between serum PFOS and stain-repellant or water-proof clothing; those who reported that they did not own water-proof or stain-repellant clothing had PFOS levels that were 86% higher than those who reporting owning these types of clothing (p=0.04; Table 3).
Table 3.
Analysis of personal behaviors and PFAA levels in serum adjusted for sex.
| PFDA | PFHxA | PFHxS | PFNA | PFOA | PFOS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictor | 10β | p- value |
10β | p- value |
10β | p- value |
10β | p- value |
10β | p- value |
10β | p- value |
|
| Hygiene | |||||||||||||
| Dusting Frequency (low) | 1.24 | 0.42 | 1.68 | 0.20 | 1.26 | 0.10 | 1.33 | 0.08 | 1.25 | 0.17 | 1.51 | 0.16 | |
| Vacuuming Frequency (low) | 0.98 | 0.94 | 0.87 | 0.77 | 1.57 | 0.003 | 1.08 | 0.68 | 1.33 | 0.12 | 0.95 | 0.89 | |
| Handwashing Frequency (low) | 1.01 | 0.97 | 0.47 | 0.07 | 1.14 | 0.36 | 1.09 | 0.61 | 1.09 | 0.24 | 1.31 | 0.37 | |
| Diet | |||||||||||||
| Fast Food Consumption (high) | 0.84 | 0.57 | 1.23 | 0.65 | 0.85 | 0.31 | 0.99 | 0.96 | 0.93 | 0.71 | 1.04 | 0.92 | |
| Microwaveable Meals/Popcorn Intake (high) | 1.01 | 0.96 | 1.52 | 0.29 | 1.49 | 0.003 | 1.01 | 0.97 | 1.20 | 0.12 | 1.40 | 0.28 | |
| Water Filtration (users) | 0.85 | 0.53 | 2.22 | 0.04 | 0.79 | 0.08 | 0.81 | 0.18 | 0.72 | 0.03 | 0.73 | 0.27 | |
| Other | |||||||||||||
| Stain/Water Repellant Clothing (do not own) | 1.46 | 0.17 | 0.72 | 0.45 | 0.93 | 0.63 | 1.06 | 0.75 | 1.13 | 0.48 | 1.86 | 0.04 | |
| Smoking (past/current) | 1.39 | 0.32 | 1.65 | 0.32 | 0.82 | 0.25 | 1.25 | 0.28 | 1.4 | 0.09 | 1.44 | 0.32 | |
Exponentiated beta coefficients represent the multiplicative change in the serum concentration relative to the reference group for categorical variables. Reference groups are the opposite frequency category (e.g. high frequency).
Figure 2.
Bean plots showing the distribution of serum PFOA and PFHxA by reported use of water filtration devices (green=No, blue=Yes). Dashed lines indicate the overall mean, black lines depict the median by category of filtration use and individual observations are shown with white dashes.
Figure 3.
Bean plots showing the distribution of serum PFHxS by category of reported dusting (A) and vacuuming frequency (B), consumption of microwave meals or popcorn (C) and the use of water filtration devices (D). Dashed lines indicate the overall mean, black lines depict the median by category and individual observations are shown with white dashes.
3.6 Serum Albumin Sensitivity Analyses
Because some PFAAs are known to bind with high affinity to serum binding proteins (D’eon et al., 2010), we measured serum albumin in 36 of the serum samples (one sample did not have sufficient volume). The arithmetic mean of the normally-distributed albumin samples was 4.6 g/dL (σ = 0.4 g/dL). Albumin levels were found to be significantly correlated with PFHxS levels (rs= 0.34; p = 0.02). Other PFAAs did not follow this correlative trend. The inclusion of albumin in multivariable models had negligible impact on results and accordingly, these results are not reported here.
4. Discussion
Overall, our results indicate that exposures to PFASs are common but variable in our adult population. Even-chain PFCAs were generally detected more frequently in serum than odd-chain compounds, reflective of the heavier usage of even-chain chemicals in commerce as well as degradation pathways of these compounds (Kotthoff et al., 2015). While we did not examine the presence of polyfluorinated substances (PFAA precursors) in the serum, we expect that our internal serum levels are adequately representative of exposure due to the short half-lives of known precursors in the body (Butt et al., 2014). Levels of these PFAAs in the serum were generally similar to those in the U.S. population, but our cohort tended to have slightly lower blood concentrations of PFOS, PFNA, and PFOA, which is consistent with research suggesting that serum levels may be declining (Olsen et al., 2012; Calafat et al., 2007; Kato et al., 2011). Conversely, our cohort’s serum concentrations of PFHxS were elevated compared to the U.S. population, and PFHxA was more frequently detected in this group than in previous studies (Olsen et al., 2012; Calafat et al., 2007; Health Canada, 2013). Our study population differed from the NHANES population in several important ways which may explain patterns. First, data were collected at different points in time. In addition, our study population was general comprised of non-Hispanic white participants, groups which had high levels of several PFASs in serum samples, including PFHxS in NHANES (Calafat et al., 2007).
It is possible that there could be some laboratory bias in our measurement of PFHxS as the measured levels of this compound in our Standard Reference Material 1957 used to validate the serum method appeared to be ~ 30% higher than previously reported concentrations (5.20 ng/g compared to 4.0 ng/g determined in Keller et al., 2010). However, a 30% difference does not seem to be enough to explain the 3.5 time higher levels measured in this cohort compared to NHANES. Elevated PFHxS serum levels have been associated with exposure to aqueous film forming foam (AFFF)-impacted drinking water. A recent study suggested that AFFF use may be associated with elevated levels of PFAAs in drinking water (Hu et al., 2016). Given the longer half-life of PFHxS in serum, this may suggest past exposure to PFHxS in drinking water from sources that were recently contaminated. It is notable that many of the newly discovered PFASs in AFFF or AFFF-impacted drinking water could serve as precursors for PFHxS and/or PFHxA (Barzen-Hanson et al., 2017). For PFHxA, the high detection frequency of this PFAA compared to NHANES may be due to the fact that the laboratory where these samples were analyzed was recently established, resulting in very little blank contamination and low detection limits. Alternatively, a higher detection frequency may also be reflective of the phase out of longer-chain PFASs such as PFOA, resulting in greater uses of PFHxA-derived products more recently.
Several factors were determined to have a significant impact on the levels of PFAAs present in the blood of our study participants. Sex was a major predictor of serum levels in multiple regressions, and males had 1.15–2.13 times as much serum PFAAs as females in our cohort. This is similar to previously published data, whereby males consistently had higher blood PFAS levels than females (Calafat et al., 2007; Health Canada, 2013). Higher serum levels in males could be due to a variety of reasons, including fewer excretion pathways compared to females, longer half lives in males, and behavioral differences leading to increased exposure (Fromme et al., 2009). Females are hypothesized to have lower serum half-lives of PFAAs due to sex-specific elimination pathways, including placental transfer of precursors and metabolites during pregnancy and breastfeeding as well as depuration during menstruation (Wong et al., 2014; Yang et al., 2016). Support for this theory comes from research indicating that women with hysterectomies have higher PFAA serum levels than those that do not, suggesting that menstruation is an important route of elimination for females (Knox et al., 2011). This study also found that postmenopausal females tended to have higher serum PFAA levels than those still menstruating, which also supports this theory (Knox et al., 2011; Taylor et al., 2014). To our knowledge, the females in the Duke cohort were nulliparous, so offloading during pregnancy and breastfeeding are not expected depuration mechanisms. However, the elimination of PFAAs through menstruation could explain some of the sex differences in serum levels in this group.
Additionally, sex-related differences in personal behaviors may also be driving higher serum levels of PFAAs in males. The results from the self-administered questionnaire provide more insight on how different behaviors may contribute to exposure, which has not been well explored previously in the literature. To our knowledge, this is the first investigation that has studied the associations between personal cleaning behaviors and serum PFAA levels. Our results indicated that there were several behaviors potentially associated with either increases or decreases in serum concentrations, including consumption of microwave meals, water filtration use, and vacuuming frequency. Interestingly, serum levels of PFHxS appeared most strongly associated with personal behaviors. Over all, our results suggest that improved hygiene (more frequent vacuuming and dusting) could be associated with decreases in PFHxS exposure. Most behaviors were associated with unidirectional changes across all PFAAs. However, water filtration appeared to be associated with higher PFHxA and lower PFOA levels in the serum. The reasons for this difference are unclear. Certainly, one might anticipate that sorption of PFOA may occur on most filter cartridges, and thus use of water filtration may reduce exposure to PFOA. However, it is unclear why PFHxA levels would increase with use of water filtration. It is possible that retention of PFHxA precursors could occur on water filtration devices, and transformation to PFHxA in a water filter could result in greater exposure. If drinking water is a source of PFOA exposure in this population, we would expect that lower water consumption rates would mute or mask an association with water filter use, compared to individuals who consumed more water on a daily basis. However, in our population, the water filter users consumed more water on average than individuals that did not use water filters. Therefore, these differences cannot be explained by differences in water consumption rates. Differences in water consumption may also explain patterns observed for PFHxA.
Our results should be interpreted in the context of several important limitations. Our study population was relatively small and should not be considered representative of the general population; participants came from a single region of the U.S., were similar in age, and were generally not racially or ethnically diverse. Although this may limit the generalizability of our results to other populations, we would not expect impacts on the validity of observed associations. In addition, participants in our study population were surveyed and provided samples at one point in time. It is possible that participants could not recall certain pieces of information (e.g. whether or not their clothing was stain or water repellant). PFAAs have a relatively long half-life in the human body, suggesting that current serum levels represent both current and past exposures. Additional information on participants’ past behavior could provide additional insights in future studies.
5. Conclusion
While the size of the population in this study was rather small (n=37), our results indicate that exposure to PFAAs (or their precursors) is likely related to some personal behaviors. This information may be helpful in informing larger studies about information that should be collected to identify determinants of exposure. The higher concentrations of the PFHxS in this population compared to NHANES suggest that exposure could be increasing, and may be related to levels now being detected in water sources throughout the country (Hu et al., 2016; Barzen-Hanson et al., 2017). Further studies should investigate the relationships between PFAS levels in drinking water and serum levels, taking into account personal behaviors that may determine exposure (e.g. water filters).
Supplementary Material
Highlights.
PFASs were measured in serum samples collected from North Carolina adults.
PFHxS levels were higher than other U.S. cohorts and were related to behavior.
PFOA, PFHxS and PFHxA were associated with the use of water filtration devices.
Acknowledgments
Funding for this research was provided by grants from the Environmental Protection Agency (Grant 83564201) and NIEHS (R01 ES016099). We also gratefully acknowledge the samples provided by our research participants.
Abbreviations
- CI
confidence interval
- diPAPs
di-substituted polyfluoroalkyl phosphates
- FTOHs
fluorotelomer alcohols
- MDL
method detection limit
- monoPAPs
mono-substituted polyfluoroalkyl phosphates
- PFAAs
perfluoroalkyl acids
- PFASs
poly- and perfluoroalkyl substances
- PFHxS
perfluorohexane sulfonate
- PFHxA
perfluorohexanoic acid
- PFOA
perfluorooctanoic acid
- PFOS
perfluorooctane sulfonate
- PFPeA
perfluoropentanoic acid
- PFNA
perfluorononanoic acid
- PFHpA
perfluoroheptanoic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bach CC, Vested A, Jorgensen KT, Bonde JPE, Henriksen TB, Toft G. Perfluoroalkyl and polyfluoroalkyl substances and measures of human fertility: a systematic review. Crit. Rev. Toxicol. 2016;46:735–755. doi: 10.1080/10408444.2016.1182117. [DOI] [PubMed] [Google Scholar]
- Barzen-Hanson KA, Roberts SC, Choyke S, Oetjen K, McAlees A, Riddell N, McCrindle R, Ferguson PL, Higgins CP, Field JA. Discovery of 40 Classes of Per- and Polyfluoroalkyl Substances in Historical Aqueous Film-Forming Foams (AFFFs) and AFFF-Impacted Groundwater. Environ. Sci. Technol. 2017 doi: 10.1021/acs.est.6b05843. [DOI] [PubMed] [Google Scholar]
- Butt CM, Muir DCG, Mabury Sa. Biotransformation pathways of fluorotelomer-based polyfluoroalkyl substances: a review. Environ. Toxicol. Chem. 2014;33:243–67. doi: 10.1002/etc.2407. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL. Polyfluoroalkyl chemicals in the U.S. population: Data from the national health and nutrition examination survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ. Health Perspect. 2007;115:1596–1602. doi: 10.1289/ehp.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. NHANES 2011–2012 Laboratory Data [WWW Document] Natl. Heal. Nutr. Exam. Surv. 2014 URL https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Laboratory&CycleBeginYear=2011.
- D’eon JC, Mabury Sa. Production of perfluorinated carboxylic acids (PFCAs) from the biotransformation of polyfluoroalkyl phosphate surfactants (PAPS): Exploring routes of human contamination. Environ. Sci. Technol. 2007;41:4799–4805. doi: 10.1021/es070126x. [DOI] [PubMed] [Google Scholar]
- D’eon JC, Simpson AJ, Kumar R, Baer AJ, Mabury Sa. Determining the molecular interactions of perfluorinated carboxylic acids with human sera and isolated human serum albumin using nuclear magnetic resonance spectroscopy. Environ. Toxicol. Chem. 2010;29:1678–1688. doi: 10.1002/etc.204. [DOI] [PubMed] [Google Scholar]
- Fromme H, Tittlemier Sa, Völkel W, Wilhelm M, Twardella D. Perfluorinated compounds--exposure assessment for the general population in Western countries. Int. J. Hyg. Environ. Health. 2009;212:239–70. doi: 10.1016/j.ijheh.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Fujii Y, Harada KH, Koizumi A. Occurrence of perfluorinated carboxylic acids (PFCAs) in personal care products and compounding agents. Chemosphere. 2013;93:538–44. doi: 10.1016/j.chemosphere.2013.06.049. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Heilmann C, Weihe P, Nielsen F, Mogensen UB, Budtz-Jørgensen E. Serum Vaccine Antibody Concentrations in Adolescents Exposed to Perfluorinated Compounds. Environ. Health Perspect. 2016 doi: 10.1289/EHP275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel DR. Fabricating data: How substituting values for nondetects can ruin results, and what can be done about it. Chemosphere. 2006;65:2434–2439. doi: 10.1016/j.chemosphere.2006.04.051. [DOI] [PubMed] [Google Scholar]
- Haug LS, Huber S, Becher G, Thomsen C. Characterisation of human exposure pathways to perfluorinated compounds--comparing exposure estimates with biomarkers of exposure. Environ. Int. 2011;37:687–93. doi: 10.1016/j.envint.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Health Canada. Second Report on Human Biomonitoring of Environmental Chemicals in Canada: Results of the Canadian Health Measures Survey Cycle 2 (2009–2011) Ottawa: 2013. [Google Scholar]
- Hu XC, Andrews DQ, Lindstrom AB, Bruton TA, Schaider LA, Grandjean P, Lohmann R, Carignan CC, Blum A, Balan SA, Higgins CP, Sunderland EM. Detection of Poly- and Perfluoroalkyl Substances (PFASs) in U.S. Drinking Water Linked to Industrial Sites, Military Fire Training Areas, and Wastewater Treatment Plants. Environ. Sci. Technol. Lett. 2016;3:344–350. doi: 10.1021/acs.estlett.6b00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM. Trends in exposure to polyfluoroalkyl chemicals in the U.S. population: 1999–2008. Environ. Sci. Technol. 2011;45:8037–8045. doi: 10.1021/es1043613. [DOI] [PubMed] [Google Scholar]
- Keller JM, Calafat AM, Kato K, Ellefson ME, Reagen WK, Strynar M, O’Connell S, Butt CM, Mabury Sa, Small J, Muir DCG, Leigh SD, Schantz MM. Determination of perfluorinated alkyl acid concentrations in human serum and milk standard reference materials. Anal. Bioanal. Chem. 2010;397:439–451. doi: 10.1007/s00216-009-3222-x. [DOI] [PubMed] [Google Scholar]
- Kielsen K, Shamim Z, Ryder LP, Nielsen F, Grandjean P, Budtz-Jorgensen E, Heilmann C. Antibody response to booster vaccination with tetanus and diphtheria in adults exposed to perfluorinated alkylates. J. Immunotoxicol. 2016;13:270–273. doi: 10.3109/1547691X.2015.1067259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox SS, Jackson T, Javins B, Frisbee SJ, Shankar A, Ducatman AM. Implications of early menopause in women exposed to perfluorocarbons. J. Clin. Endocrinol. Metab. 2011;96:1747–1753. doi: 10.1210/jc.2010-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotthoff M, Müller J, Jürling H, Schlummer M, Fiedler D. Perfluoroalkyl and polyfluoroalkyl substances in consumer products. Environ. Sci. Pollut. Res. 2015;22:14546–14559. doi: 10.1007/s11356-015-4202-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krafft MP, Riess JG. Selected physicochemical aspects of poly- and perfluoroalkylated substances relevant to performance, environment and sustainability-Part one. Chemosphere. 2015;129:4–19. doi: 10.1016/j.chemosphere.2014.08.039. [DOI] [PubMed] [Google Scholar]
- Liu Y, Pereira AS, Beesoon S, Vestergren R, Berger U, Olsen GW, Glynn A, Martin JW. Temporal trends of perfluorooctanesulfonate isomer and enantiomer patterns in archived Swedish and American serum samples. Environ. Int. 2015;75:215–222. doi: 10.1016/j.envint.2014.11.014. [DOI] [PubMed] [Google Scholar]
- Mogensen UB, Grandjean P, Heilmann C, Nielsen F, Weihe P, Budtz-Jørgensen E. Structural equation modeling of immunotoxicity associated with exposure to perfluorinated alkylates. Environ. Health. 2015;14:47. doi: 10.1186/s12940-015-0032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Standards & Technology. Standard Reference Material 1957: Organic Contaminants in Non-Fortified Human Serum. Gaithersburg: 2016. [Google Scholar]
- Olsen GW, Lange CC, Ellefson ME, Mair DC, Church TR, Goldberg CL, Herron RM, Medhdizadehkashi Z, Nobiletti JB, Rios JA, Reagen WK, Zobel LR. Temporal trends of perfluoroalkyl concentrations in American Red Cross adult blood donors, 2000–2010. Environ. Sci. Technol. 2012;46:6330–6338. doi: 10.1021/es300604p. [DOI] [PubMed] [Google Scholar]
- Rosenmai aK, Nielsen FK, Pedersen M, Hadrup N, Trier X, Christensen JH, Vinggaard aM. Fluorochemicals used in food packaging inhibit male sex hormone synthesis. Toxicol. Appl. Pharmacol. 2013;266:132–42. doi: 10.1016/j.taap.2012.10.022. [DOI] [PubMed] [Google Scholar]
- Russell MH, Waterland RL, Wong F. Calculation of chemical elimination half-life from blood with an ongoing exposure source: The example of perfluorooctanoic acid (PFOA) Chemosphere. 2015;129:210–216. doi: 10.1016/j.chemosphere.2014.07.061. [DOI] [PubMed] [Google Scholar]
- Stahl T, Mattern D, Brunn H. Toxicology of perfluorinated compounds. Environ. Sci. Eur. 2011;23:1–52. doi: 10.1186/2190-4715-23-38. [DOI] [Google Scholar]
- Taylor KW, Hoffman K, Thayer KA, Daniels JL. Polyfluoroalkyl chemicals and menopause among women 20–65 years of age (NHANES) Environ. Health Perspect. 2014;122:145–150. doi: 10.1289/ehp.1306707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA. Drinking Water Health Advisories for PFOA and PFOS [WWW Document] 2016 doi: 10.1111/gwat.13303. URL https://www.epa.gov/ground-water-and-drinking-water/drinking-water-health-advisories-pfoa-and-pfos. [DOI] [PubMed]
- Wang Z, Cousins IT, Scheringer M, Hungerbühler K. Fluorinated alternatives to long-chain perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkane sulfonic acids (PFSAs) and their potential precursors. Environ. Int. 2013;60:242–248. doi: 10.1016/j.envint.2013.08.021. [DOI] [PubMed] [Google Scholar]
- Wong F, MacLeod M, Mueller JF, Cousins IT. Enhanced elimination of perfluorooctane sulfonic acid by menstruating women: Evidence from population-based pharmacokinetic modeling. Environ. Sci. Technol. 2014;48:8807–8814. doi: 10.1021/es500796y. [DOI] [PubMed] [Google Scholar]
- Wu XM, Bennett DH, Calafat AM, Kato K, Strynar M, Andersen E, Moran RE, Tancredi DJ, Tulve NS, Hertz-Picciotto I. Serum concentrations of perfluorinated compounds (PFC) among selected populations of children and Adults in California. Environ. Res. 2015;136:264–273. doi: 10.1016/j.envres.2014.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Wang Z, Shi Y, Li J, Wang Y, Zhao Y, Wu Y, Cai Z. Human placental transfer of perfluoroalkyl acid precursors: Levels and profiles in paired maternal and cord serum. Chemosphere. 2016;144:1631–1638. doi: 10.1016/j.chemosphere.2015.10.063. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Beezoon S, Zhu L, Martin JW. Biomonitoring of Perfluoroalkyl Acids in Human Urine and Estimates of Biological Half-Life Title. Environ. Sci. Technol. 2013;47:10619–10627. doi: 10.1021/es401905e. [DOI] [PubMed] [Google Scholar]
- Zheng X-M, Liu H-L, Shi W, Wei S, Giesy JP, Yu H-X. Effects of perfluorinated compounds on development of zebrafish embryos. Environ. Sci. Pollut. Res. Int. 2012;19:2498–2505. doi: 10.1007/s11356-012-0977-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.