Abstract
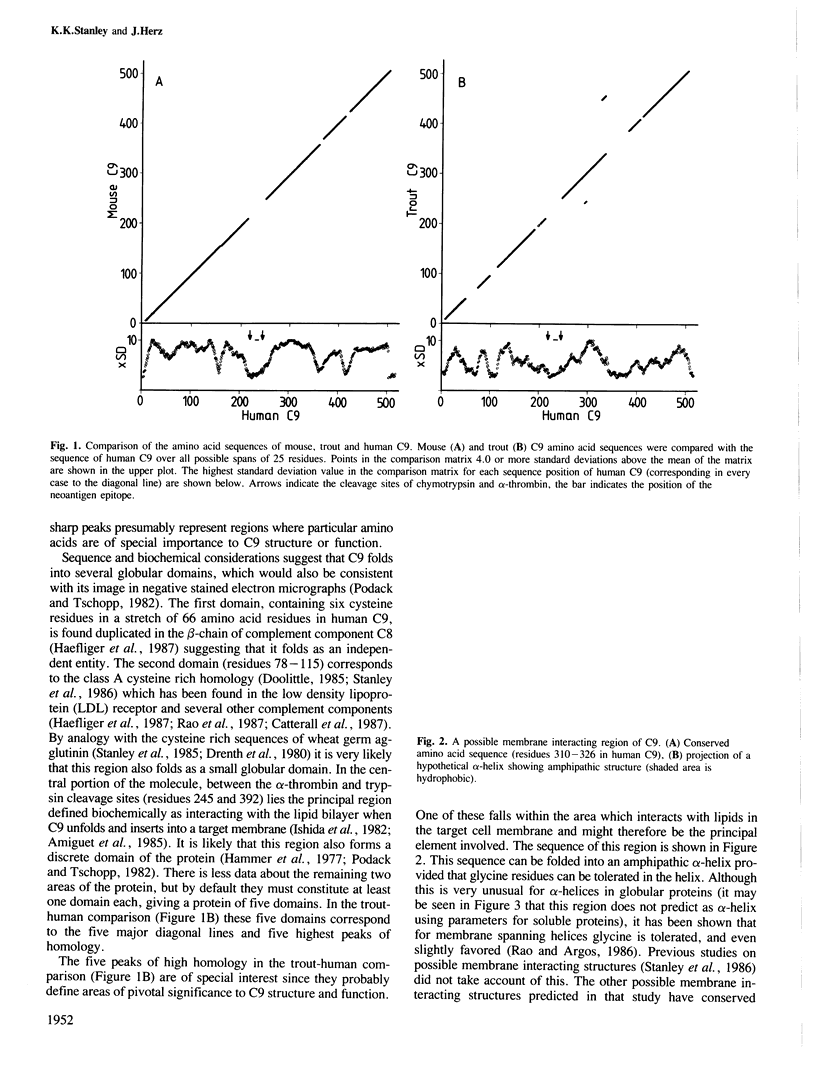
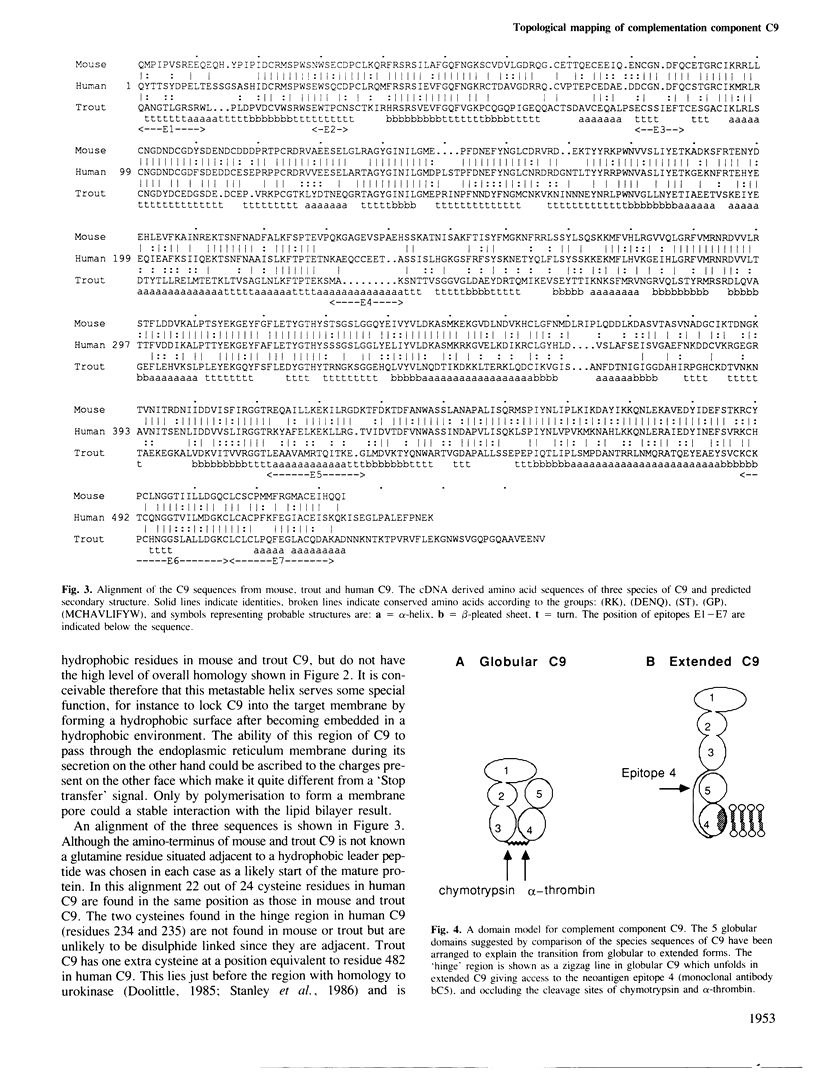
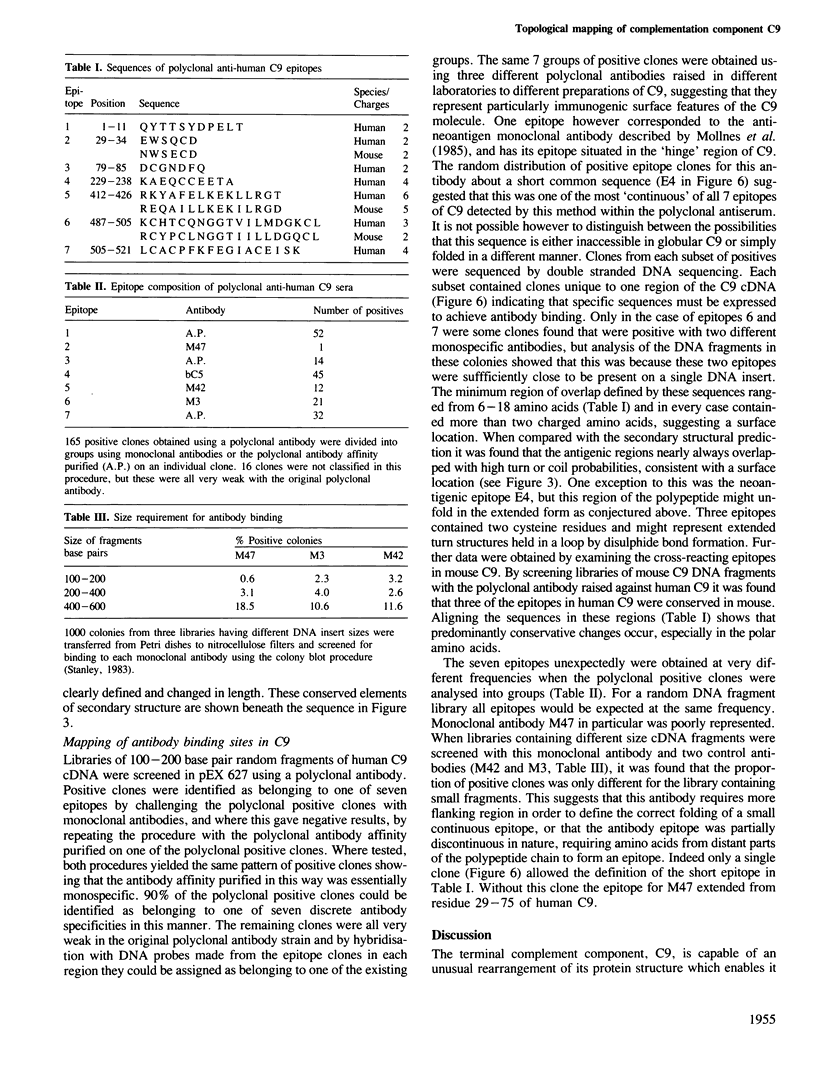
cDNA molecules coding for mouse and trout C9 have been isolated and the derived amino acid sequences compared with that of human C9. Regions of high homology between the closely related species (mouse and human) correlate with putative domains in the protein structure supporting a model of C9 having five globular domains. Comparison between the more distant species (trout and human) suggests regions of particular importance to C9 structure and function. In addition the three related sequences allow the secondary structure to be predicted with more confidence and we have tested the prediction by mapping surface features of the protein. Reported here is a recombinant DNA approach to fine mapping of antibody epitopes. Two of the putative domains of C9 are connected by a stretch of about 40 amino acid residues in which features characteristic of individual conformational forms of C9 are concentrated. We suggest that this region might act as a hinge allowing the rearrangement of globular domains necessary for membrane insertion. In the membrane inserting domain one highly conserved sequence has the potential to form an amphipathic alpha-helix once it is buried in the lipid bilayer. These features suggest a novel mechanism for the irreversible, post-translational insertion of C9 into target membranes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amiguet P., Brunner J., Tschopp J. The membrane attack complex of complement: lipid insertion of tubular and nontubular polymerized C9. Biochemistry. 1985 Dec 3;24(25):7328–7334. doi: 10.1021/bi00346a046. [DOI] [PubMed] [Google Scholar]
- Argos P. A sensitive procedure to compare amino acid sequences. J Mol Biol. 1987 Jan 20;193(2):385–396. doi: 10.1016/0022-2836(87)90226-9. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z. Antigenic structures of proteins. Their determination has revealed important aspects of immune recognition and generated strategies for synthetic mimicking of protein binding sites. Eur J Biochem. 1984 Nov 15;145(1):1–20. doi: 10.1111/j.1432-1033.1984.tb08516.x. [DOI] [PubMed] [Google Scholar]
- Boyle M. D., Langone J. J., Borsos T. Studies on the terminal stages of immune hemolysis. III. Distinction between the insertion of C9 and the formation of a transmembrane channel. J Immunol. 1978 May;120(5):1721–1725. [PubMed] [Google Scholar]
- Catterall C. F., Lyons A., Sim R. B., Day A. J., Harris T. J. Characterization of primary amino acid sequence of human complement control protein factor I from an analysis of cDNA clones. Biochem J. 1987 Mar 15;242(3):849–856. doi: 10.1042/bj2420849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Deeley R. G., Gordon J. I., Burns A. T., Mullinix K. P., Binastein M., Goldberg R. F. Primary activation of the vitellogenin gene in the rooster. J Biol Chem. 1977 Nov 25;252(22):8310–8319. [PubMed] [Google Scholar]
- Drenth J., Low B. W., Richardson J. S., Wright C. S. The toxin-agglutinin fold. A new group of small protein structures organized around a four-disulfide core. J Biol Chem. 1980 Apr 10;255(7):2652–2655. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Hadding U., Müller-Eberhard H. J. The ninth component of human complement: isolation, description and mode of action. Immunology. 1969 Jun;16(6):719–735. [PMC free article] [PubMed] [Google Scholar]
- Hammer C. H., Shin M. L., Abramovitz A. S., Mayer M. M. On the mechanism of cell membrane damage by complement: evidence on insertion of polypeptide chains from C8 and C9 into the lipid bilayer of erythrocytes. J Immunol. 1977 Jul;119(1):1–8. [PubMed] [Google Scholar]
- Haymerle H., Herz J., Bressan G. M., Frank R., Stanley K. K. Efficient construction of cDNA libraries in plasmid expression vectors using an adaptor strategy. Nucleic Acids Res. 1986 Nov 11;14(21):8615–8624. doi: 10.1093/nar/14.21.8615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innerarity T. L., Weisgraber K. H., Arnold K. S., Rall S. C., Jr, Mahley R. W. Normalization of receptor binding of apolipoprotein E2. Evidence for modulation of the binding site conformation. J Biol Chem. 1984 Jun 10;259(11):7261–7267. [PubMed] [Google Scholar]
- Ishida B., Wisnieski B. J., Lavine C. H., Esser A. F. Photolabeling of a hydrophobic domain of the ninth component of human complement. J Biol Chem. 1982 Sep 25;257(18):10551–10553. [PubMed] [Google Scholar]
- Knott T. J., Pease R. J., Powell L. M., Wallis S. C., Rall S. C., Jr, Innerarity T. L., Blackhart B., Taylor W. H., Marcel Y., Milne R. Complete protein sequence and identification of structural domains of human apolipoprotein B. Nature. 1986 Oct 23;323(6090):734–738. doi: 10.1038/323734a0. [DOI] [PubMed] [Google Scholar]
- Labeit S., Lehrach H., Goody R. S. DNA sequencing using alpha-thiodeoxynucleotides. Methods Enzymol. 1987;155:166–177. doi: 10.1016/0076-6879(87)55015-7. [DOI] [PubMed] [Google Scholar]
- Mehra V., Sweetser D., Young R. A. Efficient mapping of protein antigenic determinants. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7013–7017. doi: 10.1073/pnas.83.18.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohana Rao J. K., Argos P. A conformational preference parameter to predict helices in integral membrane proteins. Biochim Biophys Acta. 1986 Jan 30;869(2):197–214. doi: 10.1016/0167-4838(86)90295-5. [DOI] [PubMed] [Google Scholar]
- Mollnes T. E., Lea T., Harboe M., Tschopp J. Monoclonal antibodies recognizing a neoantigen of poly(C9) detect the human terminal complement complex in tissue and plasma. Scand J Immunol. 1985 Aug;22(2):183–195. doi: 10.1111/j.1365-3083.1985.tb01870.x. [DOI] [PubMed] [Google Scholar]
- Morgan B. P., Daw R. A., Siddle K., Luzio J. P., Campbell A. K. Immunoaffinity purification of human complement component C9 using monoclonal antibodies. J Immunol Methods. 1983 Nov 25;64(3):269–281. doi: 10.1016/0022-1759(83)90434-9. [DOI] [PubMed] [Google Scholar]
- Morgan B. P., Luzio J. P., Campbell A. K. Inhibition of complement-induced [14C]sucrose release by intracellular and extracellular monoclonal antibodies to C9: evidence that C9 is a transmembrane protein. Biochem Biophys Res Commun. 1984 Jan 30;118(2):616–622. doi: 10.1016/0006-291x(84)91347-0. [DOI] [PubMed] [Google Scholar]
- Nonaka M., Yamaguchi N., Natsuume-Sakai S., Takahashi M. The complement system of rainbow trout (Salmo gairdneri). I. Identification of the serum lytic system homologous to mammalian complement. J Immunol. 1981 Apr;126(4):1489–1494. [PubMed] [Google Scholar]
- Nunberg J. H., Rodgers G., Gilbert J. H., Snead R. M. Method to map antigenic determinants recognized by monoclonal antibodies: localization of a determinant of virus neutralization on the feline leukemia virus envelope protein gp70. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3675–3679. doi: 10.1073/pnas.81.12.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S., Ullrich A. Epidermal growth factor. Is the precursor a receptor? Nature. 1985 Jan 17;313(5999):184–184. doi: 10.1038/313184a0. [DOI] [PubMed] [Google Scholar]
- Podack E. R. Molecular composition of the tubular structure of the membrane attack complex of complement. J Biol Chem. 1984 Jul 10;259(13):8641–8647. [PubMed] [Google Scholar]
- Podack E. R., Tschoop J., Müller-Eberhard H. J. Molecular organization of C9 within the membrane attack complex of complement. Induction of circular C9 polymerization by the C5b-8 assembly. J Exp Med. 1982 Jul 1;156(1):268–282. doi: 10.1084/jem.156.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podack E. R., Tschopp J. Polymerization of the ninth component of complement (C9): formation of poly(C9) with a tubular ultrastructure resembling the membrane attack complex of complement. Proc Natl Acad Sci U S A. 1982 Jan;79(2):574–578. doi: 10.1073/pnas.79.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silversmith R. E., Nelsestuen G. L. Assembly of the membrane attack complex of complement on small unilamellar phospholipid vesicles. Biochemistry. 1986 Feb 25;25(4):852–860. doi: 10.1021/bi00352a017. [DOI] [PubMed] [Google Scholar]
- Stanley K. K., Kocher H. P., Luzio J. P., Jackson P., Tschopp J. The sequence and topology of human complement component C9. EMBO J. 1985 Feb;4(2):375–382. doi: 10.1002/j.1460-2075.1985.tb03639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley K. K., Luzio J. P. Construction of a new family of high efficiency bacterial expression vectors: identification of cDNA clones coding for human liver proteins. EMBO J. 1984 Jun;3(6):1429–1434. doi: 10.1002/j.1460-2075.1984.tb01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley K. K., Page M., Campbell A. K., Luzio J. P. A mechanism for the insertion of complement component C9 into target membranes. Mol Immunol. 1986 May;23(5):451–458. doi: 10.1016/0161-5890(86)90108-2. [DOI] [PubMed] [Google Scholar]
- Stanley K. K. Solubilization and immune-detection of beta-galactosidase hybrid proteins carrying foreign antigenic determinants. Nucleic Acids Res. 1983 Jun 25;11(12):4077–4092. doi: 10.1093/nar/11.12.4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze S. A., Stanley K. K. Identification of two epitopes in the carboxyterminal 15 amino acids of the E1 glycoprotein of mouse hepatitis virus A59 by using hybrid proteins. J Virol. 1986 Dec;60(3):928–934. doi: 10.1128/jvi.60.3.928-934.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp J., Masson D., Stanley K. K. Structural/functional similarity between proteins involved in complement- and cytotoxic T-lymphocyte-mediated cytolysis. 1986 Aug 28-Sep 3Nature. 322(6082):831–834. doi: 10.1038/322831a0. [DOI] [PubMed] [Google Scholar]
- Tschopp J., Müller-Eberhard H. J., Podack E. R. Formation of transmembrane tubules by spontaneous polymerization of the hydrophilic complement protein C9. Nature. 1982 Aug 5;298(5874):534–538. doi: 10.1038/298534a0. [DOI] [PubMed] [Google Scholar]
- Tschopp J., Podack E. R., Müller-Eberhard H. J. Ultrastructure of the membrane attack complex of complement: detection of the tetramolecular C9-polymerizing complex C5b-8. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7474–7478. doi: 10.1073/pnas.79.23.7474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner D., Chemla Y., Herzberg M. Isolation of poly(A)+ RNA by paper affinity chromatography. Anal Biochem. 1984 Sep;141(2):329–336. doi: 10.1016/0003-2697(84)90050-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Davis C. G., Brown M. S., Schneider W. J., Casey M. L., Goldstein J. L., Russell D. W. The human LDL receptor: a cysteine-rich protein with multiple Alu sequences in its mRNA. Cell. 1984 Nov;39(1):27–38. doi: 10.1016/0092-8674(84)90188-0. [DOI] [PubMed] [Google Scholar]




