Abstract
Phosphorus (P) limits the production of maize, one of the major food crops in China. Phosphate-solubilizing bacteria (PSB) have the capacity to solubilize phosphate complexes into plant absorbable and utilizable forms by the process of acidification, chelation, and exchange reactions. In this study, six bacteria, including one Paenibacillus sp. B1 strain, four Pseudomonas sp. strains (B10, B14, SX1, and SX2) and one Sphingobium sp. SX14 strain, were those isolated from the maize rhizosphere and identified based on their 16S rRNA sequences. All strains could solubilize inorganic P (Ca3(PO4)2, FePO4 and AlPO4), and only B1 and B10 organic P (lecithin). All strains, except of SX1, produced IAA, and SX14 and B1 showed the highest level. B1 incited the highest increase in root length and the second increase in shoot and total dry weight, shoot length, and total P and nitrogen (N), along with increased root length. In addition, by confocal laser scanning microscopy (CLSM), we found that green fluorescent protein (GFP)-labeled B1 mainly colonized root surfaces and in epidermal and cortical tissue. Importantly, B1 can survive through forming spores under adverse conditions and prolong quality guarantee period of bio-fertilizer. Therefore, it can act as a good substitute for bio-fertilizer to promote agricultural sustainability.
Keywords: maize growth promotion, phosphate solubilizing bacteria, Paenibacillus, green fluorescent protein
1. Introduction
Maize (Zea mays L.), a major food and energy crop, is the third most important food crop in China after wheat and rice [1]. Phosphorus (P) functions as the second largest nutrient after nitrogen, is essential for plant growth and development [2], and takes part in some major metabolic processes, such as signal transduction, macromolecular biosynthesis, energy transfer, photosynthesis, respiration and macromolecular biosynthesis [3]. However, P deficiency constrains biomass accumulation in the agroecosystem [4]. Although there are large amounts of inorganic and organic forms in soils, only 0.1% total P is available for assimilation by plants [5]. This leads to strong demand for the application of P fertilizer. However, frequent application of P fertilizer is not only expensive but also eco-unfriendly [6,7]. It may lead to algal blooms by causing the eutrophication of lakes [8] and the loss of soil fertility [9]. In fact, the P fertilizer utilization is less than 30% [10], because soluble P is quickly fixed by reacting with free Al3+, Ca2+, Mg2+, and Fe3+ upon its application in the soil [10,11]. Leaching and run-off also result in a loss of P fertilizer [12]. P ore, a finite resource, is the primary original material of P fertilizer. Cordell et al. [13] estimated these high-quality P ores may run out within 50–100 years. Khan et al. [14] indicated mobilization of all insoluble P accumulated in agricultural soils would be enough to be utilized by crops for about 100 years. Therefore, applying bio-fertilizers possessing P-solubilizing traits shows promise as an alternative to chemical fertilizer [10].
Most plant-growth-promoting rhizobacteria (PGPR) can promote plant growth by multiple mechanisms, such as nitrogen fixation, phosphate solubilization, indole-3-acetic acid (IAA) production and siderophore secretion, which may contribute to differential growth responses of inoculated plants [15,16]. Phosphate solubilizing bacteria (PSB) play a critical role in biogeochemical cycling models of soluble and insoluble P cycling forms in agricultural ecosystems. They can convert insoluble P (inorganic and organic forms) to soluble P (ionic phosphate and low molecular-weight organic phosphate) accessible to plants [17,18,19]. The main mechanisms contain: (a) dissolving directly mineral complexes to release soluble P [20] and (b) chelating Ca2+, Fe3+, and Al3+ to form stable complexes available for plants [21,22]. Insoluble organic P can be converted into available forms for plant uptake through mineralization by PSB [23], and mineralization of most insoluble organic P is performed mainly by phosphatase enzymes such as acid phosphatases [20]. PSB can secrete low molecular weight organic acids such as gluconic, acetic, fumaric, and citric acid to solubilize inorganic P complexes [24,25].
PSB of Pseudomonas, Acinetobacter, Sinorhizobium, and Arthrobacter could promote growth of pea, cucumber, medicago truncatula, and rice, respectively [26,27,28,29,30]. For instance, Yazdani et al. [30] demonstrated application of PSB could reduce the application rate of P fertilizer by 50% without reducing maize yield in field trials. The endophytic strains Pseudomonas fluorescens L321 isolated from Miscanthus giganteus improved pea growth promotion [26]. Moreover, PSB might also enhance drought tolerance in plant [31] and promote phytoremediation of contaminated soil by heavy metal [32,33].
The green fluorescent protein (GFP) from the jellyfish Aequorea victoria is a small protein [34]. It has been widely applied in protein localization and gene expression in various organisms. Valdivia et al. [35] explored the potential GFP applications of host-parasite interactions, indicating GFP expression in the pathogens did not reduce bacteria survival, their infective ability to mammalian cells or their survival within macrophages. Now, GFP has been successfully employed to observe plant-PGPR interaction [36,37,38]. However, until now, no data have been available with respect to the colonization of PSB.
We have isolated bacteria from main maize growing areas of North China. These areas share about a third of total maize planting area of China [39]. It was hypothesized the plant rhizosphere of these areas have some potential fine PSB that can greatly promote plant P-nutrition and growth, especially for maize. With this purpose, six PSB strains were obtained and a comparative research was carried out for phosphate solubilization, IAA production and maize growth promotion. In addition, the colonization pattern and ability of Paenibacillus sp. B1 in plant tissues were addressed using GFP-label combined with confocal laser scanning microscopy (CLSM). To the best of our knowledge, this is the first report of Paenibacillus isolated from maize rhizosphere that presents a phosphate solubilization ability.
2. Results
2.1. Characterization and Identification of Isolated Phosphate-Solubilizing Bacteria
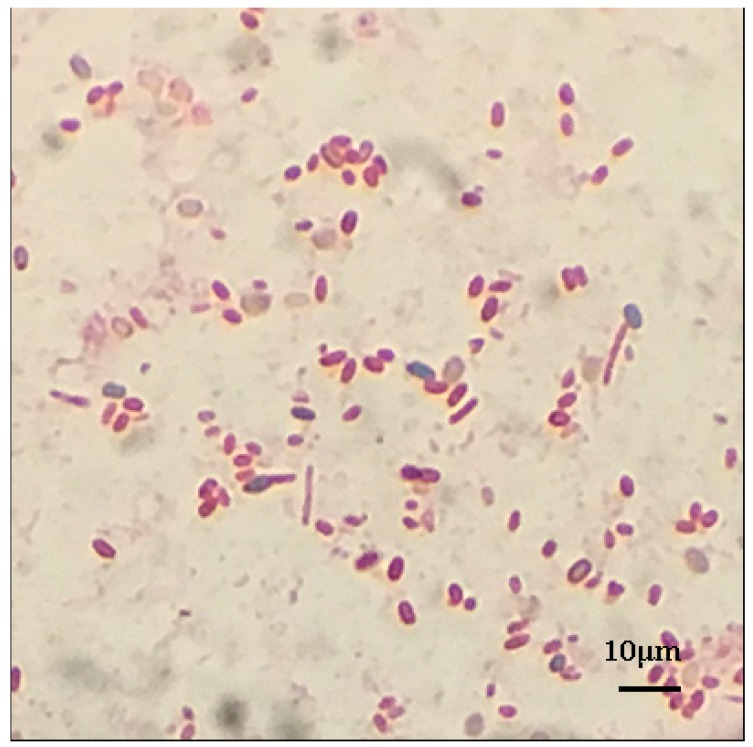
Six bacteria strains producing obvious transparent zone in Pikovskaya (PVK) medium were purified from rhizosphere soil (Table 1). Strains B1, B10, and B14, which were obtained from Beijing, China, showed white colony with rods. Strains SX1, SX2, and SX14, which were obtained from Shanxi Province, China, showed milky white colony with short rods, white colony with short rods and vitelline colony with rods, respectively. All strains except B1 were Gram-negative, and B1 was the only endospore-forming bacteria (Figure 1).
Table 1.
Characterization and identification of all strains.
| Physiological Characteristics | Analysis of 16s rRNA Gene Sequence | |||||
|---|---|---|---|---|---|---|
| Strain Code | Location | Cell Morphology | Colony Morphology | Gram Stain | Maximum Similarity of 16S rRNA with (%) | Accession Number |
| B1 | BJ | Rods | White, regular | + | Paenibacillus illinoisensis | KY111475 |
| NR_113828 (99%) | ||||||
| B10 | BJ | Short rods | White, irregular | – | Pseudomonas thivervalensis | KY111476 |
| KJ420530 (99%) | ||||||
| B14 | BJ | Short rods | white | – | Pseudomonas fluorescens | KY111477 |
| KU977136 (99%) | ||||||
| SX1 | SX | Short rods | milky white | – | Pseudomonas koreensis | KY122023 |
| GQ368179 (99%) | ||||||
| SX2 | SX | Short rods | white | – | Pseudomonas syringae | KY122024 |
| KU977139 (99%) | ||||||
| SX14 | SX | rods | vitelline | – | Sphingobium mellinum | KY122025 |
| NR_133859 (98%) | ||||||
BJ, Beijing; SX, Shanxi; +, positive; –, negative; Accession number: the accession number of the strains obtained from the GenBank (NCBI).
Figure 1.
Spore stain of Paenibacillus sp. B1. Red represents the cells, and blue the spores.
Based on the analysis of the 16S rRNA gene sequence, B1 was presumably identified as Paenibacillus sp., SX14 as Sphingobium sp. and others as Pseudomonas sp., all except SX14 showing 99% homologies with the respective sequences in the GenBank database (Table 1). The 16S rRNA gene sequences were submitted to the GenBank nucleotide database under the accession numbers KY111475, KY111476, KY111477, KY122023, KY122024, and KY122025.
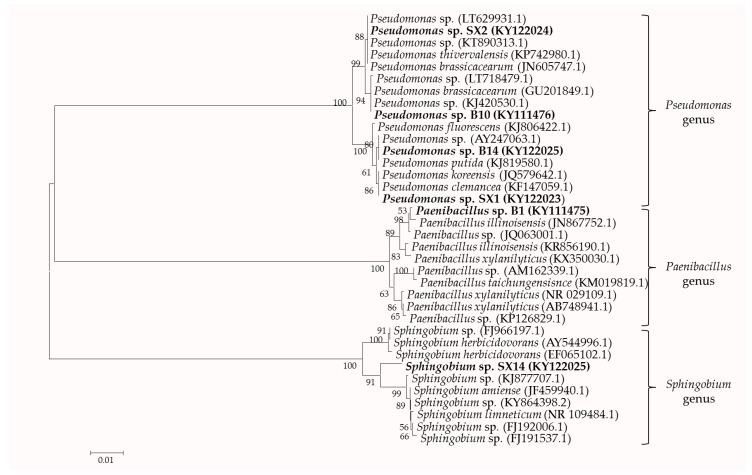
Phylogenetic analysis (Figure 2) based on the neighbor joining method revealed three different genera involved Pseudomonas, Paenibacillus, and Sphingomonas, with high bootstrap values. Strains B10, B14, SX1, and SX2 were affiliated Pseudomonas cluster, while B1 and SX14 belong to Paenibacillus and Sphingomonas, respectively.
Figure 2.
Phylogenetic tree based on 16S rRNA sequence shows the position of isolated strains with other closely related strains. These 16S rRNA sequences of related strains were downloaded from NCBI GenBank database. The tree was structured using neighbor joining method, with the bootstrap analyses of 1000 cycles. Only bootstrap values greater than 50% is shown at the branching points. Bar represents sequence divergence of 0.01 nucleotides. Strains isolated in this study are underlined with the bold letters.
2.2. Qualitative and Quantitative Analysis of Phosphate Solubilization Ability of Isolated Phosphate-Solubilizing Bacteria
After inoculation of these strains on modified PVK medium plates for 7 days at 30 °C, the strains generated visible halo zones, and showed considerable variation in phosphate solubilization index (PSI) ranged from 2.03 to 7.64 (Table 2), and B14 showed the largest PSI, reaching up to 7.64.
Table 2.
Qualitative analysis of phosphate solubilization ability and IAA production by isolated phosphate-solubilizing bacteria (PSB).
| Strain | Qualitative Analysis | IAA Production (mg L−1) | ||
|---|---|---|---|---|
| Diameter of Halo/cm | Diameter of Colony/cm | PSI | ||
| B1 | 0.56 ± 0.06 b | 0.40 ± 0.07 a | 2.46 ± 0.24 c | 20.30 ± 2.69 a |
| B10 | 0.59 ± 0.02 b | 0.12 ± 0.01 c | 5.78 ± 0.26 b | 8.97 ± 1.18 b,c |
| B14 | 0.95 ± 0.07 a | 0.15 ± 0.04 c | 7.64 ± 0.62 a | 5.84 ± 1.64 c |
| SX1 | 0.65 ± 0.08 b | 0.14 ± 0.04 c | 5.87 ± 0.49 b | ND |
| SX2 | 0.61 ± 0.02 b | 0.10 ± 0.01 c | 7.32 ± 0.35 a | 13.20 ± 2.32 b |
| SX14 | 0.31 ± 0.02 c | 0.30 ± 0.02 b | 2.03 ± 0.02c | 22.70 ± 3.37 a |
Data are means ± SE of three independent biological replicates. Bearing different letters in the same row are significantly different from each other according to the least significant difference (LSD) test (p < 0.05). ND: not detected.
All strains solubilized Ca3(PO4)2, AlPO4, and FePO4 accompanied with a decrease of the pH of supernatants in the National Botanical Research Institute’s Phosphate (NBRIP). Only B1 and B10 solubilized lecithin (Table 3). The concentration of soluble P released from Ca3(PO4)2 ranged from 71.7 to 530 mg·L−1 and the strains SX2 secreted the highest amounts of soluble P (530 mg·L−1). The values of soluble P released from AlPO4 ranged from 9.53 to 95.18 mg·L−1 and the strains B1 secreted the highest amounts of soluble P into the medium (95.2 mg·L−1) and showed the lowest value of pH in their supernatants (3.13). The values of soluble P released from FePO4 ranged from 4.59 to 47.2 mg·L−1 and the strains B1 and SX2 secreted the highest amounts of soluble P in to the medium (23.0 and 47.2 mg·L−1, respectively). Only both strains B10 and B1 could solubilize organic lecithin in the NBRIP broth medium (2.04 and 0.43 mg·L−1, respectively).
Table 3.
Quantitative analysis of phosphate solubilization ability in National Botanical Research Institute’s Phosphate (NBRIP) broth medium with Ca3(PO4)2, AlPO4, FePO4, and lecithin by isolated PSB.
| Strain | NBRIP Medium with Ca3(PO4)2 | NBRIP Medium with AlPO4 | NBRIP Medium with FePO4 | NBRIP Medium with Lecithin | ||||
|---|---|---|---|---|---|---|---|---|
| Soluble P (mg·L−1) | PH | Soluble P (mg·L−1) | PH | Soluble P (mg·L−1) | PH | Soluble P (mg·L−1) | PH | |
| B1 | 378.0 ± 35.7 b | 5.17 ± 0.03 c | 95.2 ± 8.2 a | 3.13 ± 0.06 d | 23.0 ± 1.9 b | 3.52 ± 0.08 d | 0.4 ± 0.1 b | 4.28 ± 0.02 c |
| B10 | 208.9 ± 27.3 c | 5.06 ± 0.22 c | 9.5 ± 1.3 d | 3.51 ± 0.05 c | 16.0 ± 2.7 b,c | 3.07 ± 0.10 e | 2.0 ± 0.2 a | 3.58 ± 0.04 d |
| B14 | 307.8 ± 13.7 b | 5.07 ± 0.41 c | 23.4 ± 2.9 d | 3.28 ± 0.04 d | 4.6 ± 0.4 c | 4.12 ± 0.07 b | ND | 3.28 ± 0.02 e |
| SX1 | 493.1 ± 21.2 a | 4.03 ± 0.08 d | 56.0 ± 6.4 c | 3.59 ± 0.07 c | 17.1 ± 2.3 b | 3.91 ± 0.04 c | ND | 2.93 ± 0.04 f |
| SX2 | 529.7 ± 45.9 a | 5.49 ± 0.10 b,c | 73.0 ± 5.4 b | 4.11 ± 0.07 b | 47.2 ± 8.4 a | 4.09 ± 0.07 b | ND | 3.66 ± 0.03 d |
| SX14 | 71.7 ± 12.9 d | 5.82 ± 0.03 b | 16.4 ± 1.4 d | 3.62 ± 0.11 c | 16.2 ± 2.4b,c | 3.19 ± 0.08 e | ND | 4.75 ± 0.03 b |
| JM109 | ND | 6.43 ± 0.03 a | ND | 6.48 ± 0.06 a | ND | 6.63 ± 0.07 a | ND | 6.52 ± 0.18 a |
Data are means ± SE of three independent biological replicates. Bearing different letters in the same row are significantly different from each other according to the LSD test (p < 0.05). ND: not detected.
Correlation analysis indicated that the values of PSI and the concentration of soluble P in liquid medium NBRIP medium containing Ca3(PO4)2 had a low positive relationship (r = 0.526). Between soluble P released in liquid medium NBRIP with Ca3(PO4)2 and pH of supernatants having a low negative relationship (r = −0.524), the values of soluble P released from AlPO4 and FePO4 and supernatants pH was not directly correlated (r = 0.001 and r = 0.235, respectively). With the exception of control, the pH of all the bacterial supernatants was below 6.0, because PSB can excrete organic acids into the medium, i.e., gluconic acid and citric acid, which can reduce the pH of medium and solubilize mineral phosphates.
All strains, with the exception of SX1, produced IAA. SX14 and B1 produced the highest amount of IAA (22.7 and 20.3 mg·L−1, respectively).
2.3. Growth Promotion Potential of Isolated Phosphate-Solubilizing Bacteria
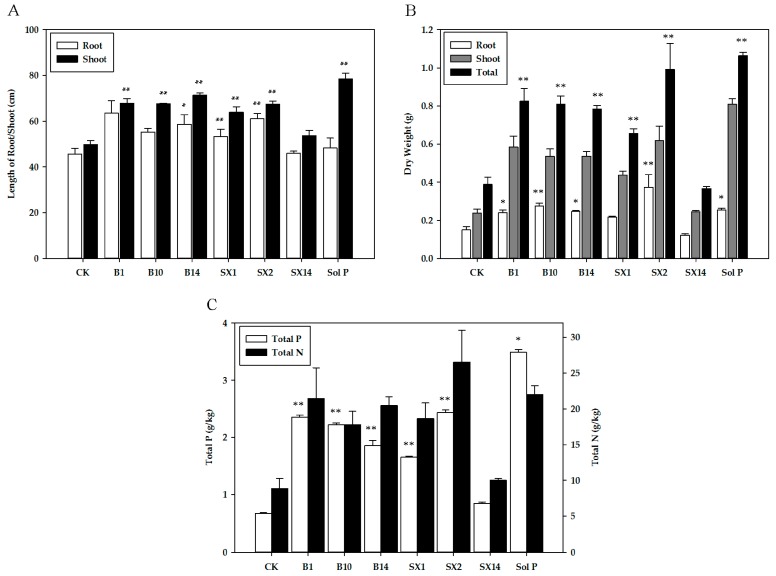
Overall, inoculation with SX2 and B1 exerted substantial increase in plant growth parameters. Most isolated PSB strains greatly increased the length of root and shoot (Figure 3A), and plants inoculated with B1 showed maximum increase in root length (39.2%), followed by those inoculated with SX2 (33.5%) and B14 (28.4%). The ones inoculated with B14 showed a maximum increase in the shoot length (43.1%), followed by B1 (35.9%) and B10 (35.5%). In Figure 3B, inoculation with SX2 significantly outperformed all six of the other PSB inoculated treatments for root dry weight, shoot dry weight, and total dry weight, and inoculation with B1 had the second highest increase of shoot dry weight and total dry weight. Inoculation with PSB strains significantly enhanced P and N uptake (Figure 3C). The seeding inoculated with SX2 had the highest total N (26.5 mg·L−1) and total P (2.44 mg·L−1) among six treatments. And the seedling with B1 ranked second. Especially total N (21.5 mg·L−1), which was even equal to the one in Sol P (22 mg·L−1). In addition, B10, B14, and SX1 also had significantly positive effects on the P and N uptake. Compared with CK, inoculation with SX14 had no obvious effect on N uptake, but also promote P uptake. As expected, un-inoculated plants treated with the soluble P (positive control) yielded the maximum quantity of biomass (shoot length, shoot dry weight, total weight dry, and total P).
Figure 3.
Effects of phosphate solubilizing bacteria inoculation on shoot and root length (A), dry weight (B), and total P and N of plant (C). The values are means from three biological replicates. The bars represent the standard errors of the means. CK: un-inoculated seedlings. Sol P: un-inoculated seedlings watered with nutrient solution containing 0.25 mM KH2PO4. Single asterisks or double asterisks (* or **) indicate significant differences between negative control and other treatment determined by LSD at p < 0.05 or p < 0.01.
2.4. Fluorescent Microscopy of GFP-Labelled Paenibacillus sp. B1 in Maize Root
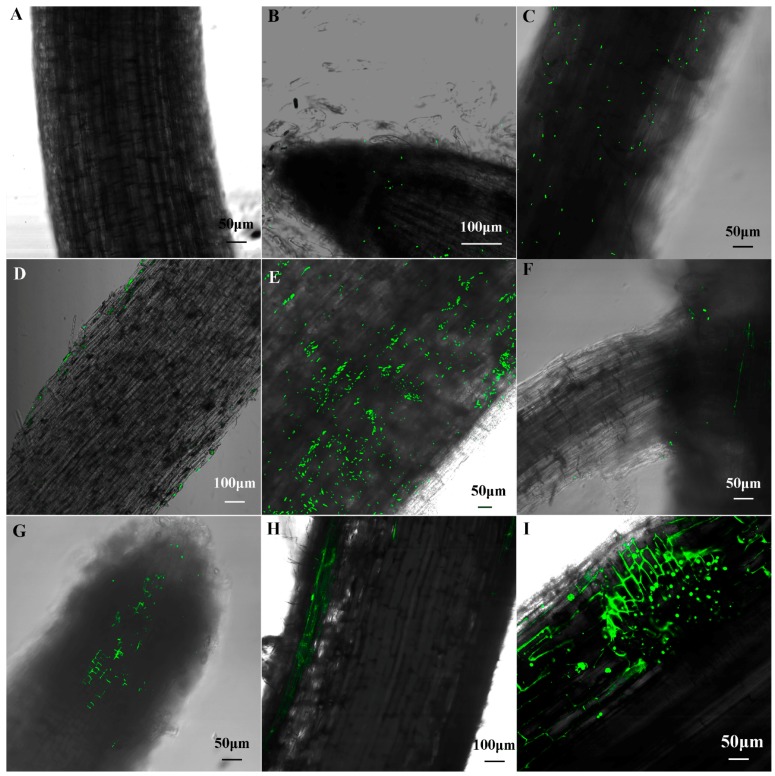
No bacterial cells were observed in the un-inoculated control maize seedlings (Figure 4A). One day after inoculation, a small number of cells with green fluorescence were found to colonize on the surface of root tip and primary root (Figure 4B,C). Longitudinal sections of primary root showed these cells only colonized on the surface of root (Figure 4D). Three days after inoculation, more cells were observed on the surface of root (Figure 4E), and, some bacteria were observed in the junction of the primary and lateral root (Figure 4F) and longitudinal sections of root showed some cells had distributed to the cell gap of the meristem region (Figure 4G), indicating that B1 strains invaded into inside the meristem region of maize root firstly. After seedlings were infected five days, the bacteria cells were found within epidermal cells and the lateral root primordial (Figure 4H,I). Seven and nine days after inoculation, B1 was not further observed within the cortex and vascular bundle (figure not present).
Figure 4.
Colonization of the GFP-labeled cells in maize root. (A) Non-inoculated control plant; (B–D) Colonization patterns of the GFP-labeled Paenibacillus sp. B1 in maize root after one day of inoculation; (E–G) Colonization patterns after three days of inoculation; (H,I) Colonization patterns after five days of inoculation. Green dot represents the GFP-labeled Paenibacillus sp. B1.
3. Discussion
The narrow zone, which is particularly affected by the root activity, is designated as the rhizosphere [40], which is a habitat of a unique population of plant-associated microorganisms-beneficial, harmful, or neutral. Those beneficial rhizobacteria could successfully colonize plant roots and positively enhance plant growth through various properties, e.g., nitrogen fixation, IAA production, phosphate solubilization, siderophore secretion, or induced systemic resistance (ISR) [41,42]. Lower effective P in soil and large available P demanded for plant growth is contradictory, so the ability of phosphate solubilization is an important parameter for assessing PGPR [43]. North China is the prime area for maize production in China and main crop rotations is winter wheat-summer maize [44]. In these areas, the farmers apply P fertilizer about 75 kg·hm−2 for maize production [45]. To overcome dependency on P fertilizer and reduce the cost of production in agriculture, we isolated six strains from the maize rhizosphere. Phylogenetic analysis showed three clusters including Pseudomonas, Paenibacillus, and Sphingomonas. The cluster of Pseudomonas encompasses two subgroups. In fact, Pseudomonas sp. SX2 clustered with the Pseudomonas sp. B10 and Pseudomonas sp. B14 with Pseudomonas sp. SX1. In addition, Paenibacillus sp. B1 grouped in a separate subcluster with P. illinoisensis JN867752.1. Though Sphingomonas sp. SX14 grouped in the cluster of Sphingomonas with high bootstrap values, it did not group in a separate subcluster with others. Some members included in the phylogenetic tree have been reported because of their plant growth promoting or biocontrol functions. Pseudomonas brassicacearum Zy-2-1 (GU201849.1) was isolated from root nodules of the wild legume Sphaerophysa salsula, and inoculation with Zy-2-1 had impact on growth-promoting activity, nodule number and average nodule weight of S. salsula plants [46]. Pseudomonas sp. S50 (KT890313.1), isolated from potato, presented the anti-Phytophthora potential through the emissions of volatile organic compounds [47]. Pseudomonas brassicacearum J12 (JN605747), isolated from tomato, could serve as an antagonist against Ralstonia solanacearum [48].
In this study, six PSB were isolated on PVK agar plate containing insoluble tricalcium phosphate. Nautiyal [49] demonstrated the NBRIP medium presented the same efficiency as PVK medium in a plate assay, however, NBRIP medium could reach three-fold higher than PVK medium in a broth assay. The NBRIP broth assay was considered more reliable than the plate assay in measuring a strain as PSB [50]. In our qualitative and quantitative assays, contradictory results were observed. Of the six strains, B14 produced the largest value of PSI, but the fourth highest soluble P in the liquid NBRIP medium containing Ca3(PO4)2. Similar results were found when measuring P solubilization ability [51]. The value of PSI is not adequate for estimating the P solubilization ability of PSB, although it is simple and fast. Therefore, further quantitative determination this trait in NBRIP broth medium was of great significance. There is no metal-P complex that can act as the general selection factor for PSB because of the variability of soils in pH and some chemical properties [52]. Hence, it is imperative to measure the P solubilization ability using a combination of two or more P complexes together. So insoluble inorganic P (Ca3(PO4)2, AlPO4, and FePO4) and organic P (lecithin) were elected to quantify this ability of PSB. All strains solubilized Ca3(PO4)2 to a greater extent than other insoluble P. Similar results were reported when quantitative determination of the P solubilization ability [53,54] because tricalcium phosphate was considerably more soluble than iron/aluminum phosphate [10]. Our results indicated there was no obvious correlation between the supernatant pH and soluble P released from mineral P by PSB. Acidification may not be the only mechanism of P solubilization, and chelation is also an important one. Only B1 and B10 presented the ability to solubilize organic P (lecithin). The mechanism of organic P solubilization is mineralization by enzymolysis.
PSB can excrete organic acids into the soil, i.e., gluconic acid and citric acid, which can reduce the pH of soil and solubilize mineral phosphates for the plant growth and development [26]. PSB can also adsorb micronutrients, e.g., iron, zinc, and copper, around the root of crops by secreting organic acids, and then stimulate the rapid growth of plants and enhance plant cold, drought, and salinity tolerance [55]. The P solubilization ability appears frequently associated with PGPR, however, it is not necessarily related with the ability to promote plant growth [54,56,57]. The “real” PSB must be directly dedicated to P plant nutrition [58]. So, IAA production was also analyzed in this study and SX14 and B1 secreted the highest amount of IAA. The pot experiment showed maize seedlings inoculated with PSB, except Sphingobium sp. SX14, grew faster than the negative control. Inoculation with B1 and SX2 showed maximum beneficial effects on biomass accumulation, P uptake, and N uptake, which we tentatively link to the abilities to produce high amounts of IAA, as well as soluble P. IAA can positively promote plant growth, such as lateral root development and root elongation [15,16]. It is interesting that inoculation had a significantly positive effect on N uptake. Maize seedlings inoculated with SX2 and B1 accumulated the highest amount total N among all those inoculated. Yang et al. [59] proved the fungal Phomopsis liquidambari colonization could induce several genes related to N uptake and N metabolism to express differentially to enhance N utilization efficiency in rice. Therefore, we hypothesize SX2 and B1 colonization can also effectively improve N uptake in maize just as described above. SX14 did not induce the plant-promoting growth, though, owing the abilities of P solubilization and IAA production. Whether SX14 is a kind of beneficial plant-associated microorganism needs to be investigated further.
Of the six PSB strains, Paenibacillus sp. B1 and Pseudomonas sp. SX2 presented a positive plant growth response and the finest PGPR traits (P solubilization and IAA production). The PGPR can function only if they successfully colonize in the plant roots [60]. GFP has been widely used for studying plant-microbe interactions [37,38,61,62]. Pseudomonas, a non-endospore-forming bacterium, could colonize velamen and core parenchyma of Dendrobium nobile [63]. P. polymyxa WLY78 (azotobacter) could colonize wheat, maize, and cucumber [38], though some PGPR such as Pseudomonas used in commercial bio-fertilizers, the utilization of bio-fertilizers are still a small fraction in the agriculture industry. In the first instance, PGPR is hindered by environmental variables, such as pH, salinity, and climatic conditions in the field application [10]. Second, these existing bio-fertilizers have many disadvantages, such as a short retention period and unstable activity, posing great difficulties to practical application. However, the ability to form endospores can allow PGPR to survive in a wide range of environmental variables [64], thus facilitating to produce a high-quality bio-fertilizer having long retention period. Taken together, Paenibacillus sp. B1 is considered as a better candidate to produce bio-fertilizer than Pseudomonas sp. SX2. Hence, only B1 was chosen to employ a colonization assay in the gnotobiotic model system, and it has been demonstrated the gnotobiotic model system could reflect the natural colonization pattern in soil [65]. GFP-labelled B1 could successfully colonize on the surface and within the epidermis cells of maize roots.
4. Materials and Methods
4.1. Soil Sampling and Phosphate-Solubilizing Bacteria Isolation
Rhizosphere soil samples were derived from two different sites, including Wenshui County, Shanxi Province (112.03° E and 37.43° N) and Beijing City (116.10° E and 40.08° N), China. The plant was uprooted by scoop and loosely adhering soil was removed by shaking gently, then the tightly-adhering soil around the root, which is regarded as rhizosphere soil, was placed in a sterilized valve bag and transported to the laboratory for further isolation of PSB strains as soon as possible.
PSB strains were isolated by the standard dilution plating technique on modified PVK medium, which was made as follows: C6H12O6, 10.0 g; Ca3(PO4)2, 5.0 g; (NH4)2SO4, 0.5 g; NaCl, 0.3 g; MgSO4·7H2O, 0.3 g; KCl, 0.3 g; MnSO4·4H2O, 0.03 g; FeSO4·7H2O, 0.03 g; agar, 18.0 g, H2O 1 L; pH (at 25 °C) 7.2 ± 0.2 [66]. The plates were incubated for seven days at 30 °C, and then colonies with transparent zones were picked and purified three times on Luria Bertani (LB) agar plates and then stored at 4 °C. For long-term storage, these purified PSB strains were stored in the medium with 30% glycerol (v/v) at −80 °C.
4.2. Morphological Characterization of Isolated Phosphate-Solubilizing Bacteria
The bacterial strains were streaked on LB agar plates, followed by incubation of these plates for 48 h at 30 °C, and then colony properties of strains were observed. Cell morphology was described by light microscopy (Olympus CX22LED, Olympus Corporation, Tokyo, Japan). All strains were stained with Gram stain as described by Vincent [67]. Spore staining was performed by Schaeffer-Fulton’s method [68].
4.3. Amplication and Sequencing of 16S rRNA Gene and Phylogenetic Analysis
Strains were individually grown on LB broth medium until the OD600nm = 0.8 and then cells were collected by centrifugation. Genomic DNA was extracted and purified using the TIANamp Bacteria DNA Kit (Tiangen Biotech (Beijing) Co., Ltd., Beijing, China) according to the manufacturer’s instructions. The amplication of 16S rRNA gene sequence was performed with the universal primers 27F (5′ AGAGTTTGATC (AC) TGGCTCAG 3′) and 1492R (5′ CGG (CT) TACCTTGTTACGACTT 3′) as described by Khan et al. [69]. The PCR process was performed according to Shahid et al. [70]. Then 16S rRNA was sequenced commercially by Shanghai Majorbio Bio-Pharm Technology Co., Ltd., Shanghai, China. The sequenced products were conducted by BLAST software from NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi) against the GenBank database.
The phylogenetic tree, which contains our sequencing 16S rRNA gene and sequences with high similarity scores from the GenBank database, was constructed with MEGA software package(MEGA 6.0, Kumar, Stecher, and Tamura 2015) [71] using the neighbor-joining method. Bootstrap analysis were performed with 1000 cycles, and only bootstrap values greater than 50% were shown at the branch points.
4.4. Qualitative and Quantitative Determination of Phosphate Solubilization Ability of Isolated Phosphate Solubilizing Bacteria
A preliminary determination of phosphate-dissolving ability was evaluated by modified PVK agar plate assay. Each strain was prick inoculated in the center of the modified PVK medium containing tricalcium phosphate (Ca3(PO4)2) as insoluble phosphate source with sterile toothpick. All of these plates were incubated at 30 °C for seven days. Experiments were carried in triplicate. The diameter of the colony (d) and halo zone (D) around the colony were measured and PSI was calculated using Equation (1) [72]:
| PSI = (D + d)/d | (1) |
In order to quantify the ability of PSB to solubilize inorganic and organic mineralize P complexes, we chose modified NBRIP [49] broth medium, which was made as follows: C6H12O6, 10 g; MgCl2, 5 g; MgSO4·7H2O, 0.25 g; (NH4)2SO4, 0.1 g; KCl, 0.2 g; Ca3(PO4)2, 5 g; H2O, 1 L; pH (at 25 °C) 6.75 ± 0.25. FePO4 (1 g·L−1), AlPO4 (2 g·L−1), or lecithin (2 g·L−1) was used in replacement of Ca3(PO4)2. To replace the calcium source CaCl2 (1 g·L−1) was added. The PSB strains were grown in LB broth medium at 30 °C for 36 h, respectively, and OD600nm adjusted to 1.0. Then strains were inoculated in 50 mL Erlenmeyer flasks containing 20 mL NBRIP broth medium and incubated at 180 rpm for seven days at 30 °C. The medium was inoculated with E. coli JM109 and non-inoculated as the control. Each treatment had three biological replicates. Clear supernatant was obtained by centrifuging at 12,000 rpm for 10 min. And then the concentration of soluble phosphate was analyzed based on the Fiske and Subbarow method [73]. Finally, a subsample was used to record the final pH by pH acidity meter.
4.5. Determination of Indole-3-Acetic Acid (IAA) Production
The IAA production was measured by colorimetric analysis. The PSB strains were grown in 50 mL Erlenmeyer flasks containing 20 mL King B broth medium with 0.1% (w/v) Trp for three days at 30 °C. This medium comprised: peptone, 20 g; K2HPO4, 1.15 g; MgSO4·7H2O, 1.5 g; glycerol, 10 g; H2O, 1 L; pH (at 25 °C) 7.2 ± 0.2. Non-inoculated King B broth medium was considered as the negative control. Each treatment had three biological replicates. Clear supernatant was obtained by centrifuging at 12,000 rpm for 10 min. The IAA concentration was measured as described by Glickmann et al. [74]. Briefly, 2 mL (S2/1 method) of Salkowski reagent (1 mL 0.5 M FeCl3, 30 mL concentrated H2SO4, and 50 mL distilled H2O) was added to 1 mL of above supernatant. Then, the mixtures were reacted in the dark at room temperature for 30 min. Thereafter, the optical density of reaction solutions at 530 nm was recorded using a spectrophotometer (Shimadzu UVmini-1240, Shimadzu Instruments Suzhou Limited, Soochow, China), and the IAA concentration was estimated against the prepared IAA standard curve.
4.6. Growth Promotion Potential of Isolated Phosphate Solubilizing Bacteria
A pot experiment was employed in the greenhouse of the China Agricultural University, Beijing, China to evaluate the effects of these strains on maize growth and development.
The above strains were cultured in 100 mL of LB broth medium for 36 h at 30 °C, respectively, and then cells were harvested by centrifugation at 6000 rpm for 5 min at 4 °C and adjusted to 108 cells mL−1 with sterile deionized water. Vermiculite was used as substrate for plant growth in this study, washed three times with deionized water to remove the dissoluble phosphorus, and air-dried at room temperature. 700 g of the air-dried vermiculite was put into each pot (25 cm diameter, 35 cm high). At the same time, Ca3(PO4)2 (3.5 g per pot) was completely blended with the vermiculite as an insoluble phosphate source.
Plump maize seeds were sterilized with 10% sodium hypochlorite for 10 min followed by washing with sterilized deionized water three times and then germinated on sterile Petri dishes containing moist filters in darkness at room temperature for 3–5 days. Homogenous seedlings were selected, some of which were soaked with above bacterial strains suspensions (108 cells·mL−1) for 30 min. Then, three seedlings were sown in each plastic pot, and grown in the greenhouse (16 h day/8 h night and 20/8 °C day/night temperature). Every treatment had three pots. The experimental treatments contained: (1) inoculated maize seedlings; (2) un-inoculated maize seedlings as a negative control; and (3) un-inoculated seedlings which were watered regularly with nutrient solution as a positive control containing soluble phosphate (0.25 mM KH2PO4). Plants were irrigated with nutrient solution (0.65 mM MgSO4, 2 mM NH4NO3, 2 mM CaCl2, 0.75 mM K2SO4, 0.1 mM KCl, 0.25 mM KH2PO4, 0.2 mM Fe-EDTA, 1 × 10−3 mM MnSO4, 1 × 10−3 mM ZnSO4, 1 × 10−4 mM CuSO4, and 5 × 10−6 mM (NH4)6Mo7O24, 1 × 10−3 mM H3BO3) in the presence or absence of KH2PO4, where applicable (30 mL per pot).
When maize seedlings were grown in pots for three weeks, whole plants were harvested and root samples were carefully washed with deionized water to remove the adhering soil. Then three plants were picked out from each treatment (one pot with one plant) to immediately measure the length of the shoot and root. Subsequently, the above plant samples were oven-dried at 105 °C for 30 min to de-enzyme, and then dried at 65 °C until a constant weight for dry weight analysis.
The oven-dried samples were weighed and ground into fine powders. Appropriate amounts of ground tissue were digested by an H2SO4-H2O2 mixture at 370 °C and then N concentration was analyzed using a modified Kjeldahl method [75] and P concentration was determined using the standard vanadomolybdate method [76].
4.7. Colonization of Maize by GFP-Labelled B1
The transformation vector plasmid pGFP300 [38], which was a shuttle vector in E. coli and, expresses GFP from its promoter. This plasmid also contains the tetracycline resistance gene tet, which is used as a selectable marker when transformed into E. coli and B. subtilis cells. A electrotransformation method for constructing GFP-labelled Panebacillus sp. B1 was used according to Zhang et al. [77].
The maize seedlings inoculated with GFP-labelled Panebacillus sp. B1 was obtained as described above. Then one seedling was sown in each sterile flask (6 cm diameter, 10 cm high) containing 100 mL 1/2 × Murashige and Skoog (MS) semisolid agar medium [78] under gnotobiotic condition and grown in the light growth chamber (27 °C, 70% humidity, and 16 h day/8 h night conditions).
The maize seedling tissues were sampled after sowing for certain times and processed for microscopy (Olympus FluoView™ FV1000 confocal microscope, Olympus Corporation, Tokyo, Japan). These images were collected using FV10-ASW software (Version 03.01.02.02, Olympus Europa Holding GmbH, Hamburg Germany) and assembled using Adobe Photoshop CC 2015 and Adobe Illustrator CS6 (Adobe, San Jose, CA, USA).
4.8. Statistical Analysis
Each experimental treatment had three replicates. The results of the measurements were analyzed by analysis of one-way analysis of variance (ANOVA) using SPSS software version 20 (SPSS Inc., Chicago, IL, USA). Means of different treatments were compared using the least significant difference (LSD) at a 0.05 or 0.01 level of probability. The Pearson correlation coefficient was analyzed using double-variable analysis.
5. Conclusions
Among all the isolated PSB associated with maize rhizosphere, Paenibacillus sp. B1 can produce higher levels of IAA to promote plant growth and development. Secondly, B1 can solubilize various insoluble inorganic P and organic P (lecithin). It can also colonize on maize root surfaces and in epidermal and cortical tissues. More importantly, it can generate spores under unfavorable conditions. Therefore, this strain may be an ideal candidate for producing bio-fertilizer having a long quality guarantee period.
Acknowledgments
This work is supported by Beijing municipal commission of rural affairs (no. 20170152) and the National High Technology Program (“863” Program) of China (no. 2013AA102802-04).
Abbreviations
| P | Phosphorus |
| N | Nitrogen |
| PSB | Phosphate solubilizing bacteria |
| CLSM | Confocal laser scanning microscopy |
| PGPR | Plant-growth-promoting rhizobacteria |
| PVK | Pikovskaya |
| PSI | Phosphate solubilization index |
| NBRIP | National Botanical Research Institute Phosphate |
| ISR | Induced systemic resistance |
| LSD | Least significant difference |
Author Contributions
Yongbin Li and Sanfeng Chen conceived and designed the experiments; Yongbin Li and Tianyi Hao performed the experiments; Yongbin Li and Xiaomeng Liu analyzed the data; and Yongbin Li wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Fan X., Tan J., Yang J. Utilization of exotic tropical and subtropical maize germplasm. Southwest China J. Agric. Sci. 2000;13:107–111. doi: 10.3969/j.issn.1001-4829.2000.01.022. [DOI] [Google Scholar]
- 2.Vance C.P. Symbiotic nitrogen fixation and phosphorus acquisition. Plant nutrition in a world of declining renewable resources. Plant Physiol. 2001;127:390–397. doi: 10.1104/pp.010331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan M.S., Zaidi A., Ahemad M., Oves M., Wani P.A. Plant growth promotion by phosphate solubilizing fungi-current perspective. Arch. Agron. Soil Sci. 2010;56:73–98. doi: 10.1080/03650340902806469. [DOI] [Google Scholar]
- 4.Lyu Y., Tang H.L., Li H.G., Zhang F.S., Rengel Z., Whalley W.R., Shen J.B. Major crop species show differential balance between root morphological and physiological responses to variable phosphorus supply. Front. Plant Sci. 2016:7. doi: 10.3389/fpls.2016.01939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zou X.M., Binkley D., Doxtader K.G. A new method for estimating gross phosphorus mineralization and immobilization rates in soils. Plant Soil. 1992;147:243–250. doi: 10.1007/BF00029076. [DOI] [Google Scholar]
- 6.Singh H., Reddy M.S. Effect of inoculation with phosphate solubilizing fungus on growth and nutrient uptake of wheat and maize plants fertilized with rock phosphate in alkaline soils. Eur. J. Soil Biol. 2011;47:30–34. doi: 10.1016/j.ejsobi.2010.10.005. [DOI] [Google Scholar]
- 7.Tilman D., Cassman K.G., Matson P.A., Naylor R., Polasky S. Agricultural sustainability and intensive production practices. Nature. 2002;418:671–677. doi: 10.1038/nature01014. [DOI] [PubMed] [Google Scholar]
- 8.Schindler D.W., Hecky R.E., Findlay D.L., Stainton M.P., Parker B.R., Paterson M.J., Beaty K.G., Lyng M., Kasian S.E. Eutrophication of lakes cannot be controlled by reducing nitrogen input: Results of a 37-year whole-ecosystem experiment. Proc. Natl. Acad. Sci. USA. 2008;105:11254–11258. doi: 10.1073/pnas.0805108105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gyaneshwar P., Kumar G.N., Parekh L.J., Poole P.S. Role of soil microorganisms in improving P nutrition of plants. Plant Soil. 2002;245:83–93. doi: 10.1023/A:1020663916259. [DOI] [Google Scholar]
- 10.Sharma S.B., Sayyed R.Z., Trivedi M.H., Gobi T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus. 2013;2:587. doi: 10.1186/2193-1801-2-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagin J., Hadas A. Solubility of calcium phosphate in calcareous soils. Nature. 1962;193:1211–1212. doi: 10.1038/1931211a0. [DOI] [Google Scholar]
- 12.Sashidhar B., Podile A.R. Transgenic expression of glucose dehydrogenase in Azotobacter vinelandii enhances mineral phosphate solubilization and growth of sorghum seedlings. Microb. Biotechnol. 2009;2:521–529. doi: 10.1111/j.1751-7915.2009.00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cordell D., Drangert J.O., White S. The story of phosphorus: Global food security and food for thought. Global. Environ. Chang. 2009;19:292–305. doi: 10.1016/j.gloenvcha.2008.10.009. [DOI] [Google Scholar]
- 14.Khan M.S., Zaidi A., Wani P.A. Role of phosphate-solubilizing microorganisms in sustainable agriculture—A review. Agron. Sustain. Dev. 2007;27:29–43. doi: 10.1051/agro:2006011. [DOI] [Google Scholar]
- 15.Ghyselinck J., Velivelli S.L., Heylen K., O’Herlihy E., Franco J., Rojas M., De Vos P., Prestwich B.D. Bioprospecting in potato fields in the Central Andean Highlands: Screening of rhizobacteria for plant growth-promoting properties. Syst. Appl. Microbiol. 2013;36:116–127. doi: 10.1016/j.syapm.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Naqqash T., Hameed S., Imran A., Hanif M.K., Majeed A., van Elsas J.D. Differential response of potato toward inoculation with taxonomically fiverse plant growth promoting rhizobacteria. Front. Plant Sci. 2016;7:144. doi: 10.3389/fpls.2016.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Acevedo E., Galindo-Castañeda T., Prada F., Navia M., Romero H.M. Phosphate-solubilizing microorganisms associated with the rhizosphere of oil palm (Elaeis guineensis Jacq.) in Colombia. Appl. Soil Ecol. 2014;80:26–33. doi: 10.1016/j.apsoil.2014.03.011. [DOI] [Google Scholar]
- 18.Rodriguez H., Gonzalez T., Goire I., Bashan Y. Gluconic acid production and phosphate solubilization by the plant growth-promoting bacterium Azospirillum spp. Naturwissenschaften. 2004;91:552–555. doi: 10.1007/s00114-004-0566-0. [DOI] [PubMed] [Google Scholar]
- 19.Chung H., Park M., Madhaiyan M., Seshadri S., Song J., Cho H., Sa T. Isolation and characterization of phosphate solubilizing bacteria from the rhizosphere of crop plants of Korea. Soil Biol. Biochem. 2005;37:1970–1974. doi: 10.1016/j.soilbio.2005.02.025. [DOI] [Google Scholar]
- 20.Rodriguez H., Fraga R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999;17:319–339. doi: 10.1016/S0734-9750(99)00014-2. [DOI] [PubMed] [Google Scholar]
- 21.Montenegro A., Zapata F. Rape genotypic differences in P uptake and utilization from phosphate rocks in an Andisol of Chile. Nutr. Cycl. Agroecosyst. 2002;63:27–33. doi: 10.1023/A:1020523625712. [DOI] [Google Scholar]
- 22.Omar S.A. The role of rock-phosphate-solubilizing fungi and vesicular-arbuscular-mycorrhiza (VAM) in growth of wheat plants fertilized with rock phosphate. World J. Microbiol. Biotechnol. 1997;14:211–218. doi: 10.1023/A:1008830129262. [DOI] [Google Scholar]
- 23.Khan A.A., Jilani G., Akhtar M.S., Naqvi S.M.S., Rasheed M. Phosphorus solubilizing bacteria: Occurrence, mechanisms and their role in crop production. J. Agric. Biol. Sci. 2009;1:48–58. [Google Scholar]
- 24.Chen Y.P., Rekha P.D., Arun A.B., Shen F.T., Lai W.A., Young C.C. Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl. Soil Ecol. 2006;34:33–41. doi: 10.1016/j.apsoil.2005.12.002. [DOI] [Google Scholar]
- 25.Zeng Q., Wu X., Wang J., Ding X. Phosphate solubilization and gene expression of phosphate-solubilizing bacterium Burkholderia multivorans WS-FJ9 under different levels of soluble phosphate. J. Microbiol. Biotechnol. 2017;27:844–855. doi: 10.4014/jmb.1611.11057. [DOI] [PubMed] [Google Scholar]
- 26.Oteino N., Lally R.D., Kiwanuka S., Lloyd A., Ryan D., Germaine K.J., Dowling D.N. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front. Microbiol. 2015;6:745. doi: 10.3389/fmicb.2015.00745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang S.M., Joo G.J., Hamayun M., Na C.I., Shin D.H., Kim H.Y., Hong J.K., Lee I.J. Gibberellin production and phosphate solubilization by newly isolated strain of Acinetobacter calcoaceticus and its effect on plant growth. Biotechnol. Lett. 2009;31:277–281. doi: 10.1007/s10529-008-9867-2. [DOI] [PubMed] [Google Scholar]
- 28.Bianco C., Defez R. Improvement of phosphate solubilization and Medicago plant yield by an indole-3-acetic acid-overproducing strain of Sinorhizobium meliloti. Appl. Environ. Microbiol. 2010;76:4626–4632. doi: 10.1128/AEM.02756-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gusain Y.S., Kamal R., Mehta C.M., Singh U.S., Sharma A.K. Phosphate solubilizing and indole-3-acetic acid producing bacteria from the soil of Garhwal Himalaya aimed to improve the growth of rice. J. Environ. Biol. 2015;36:301–307. [PubMed] [Google Scholar]
- 30.Jilani G., Akram A., Ali R.M., Hafeez F.Y., Shamsi I.H., Chaudhry A.N., Chaudhry A.G. Enhancing crop growth, nutrients availability, economics and beneficial rhizosphere microflora through organic and biofertilizers. Ann. Microbiol. 2007;57:177–184. doi: 10.1007/BF03175204. [DOI] [Google Scholar]
- 31.Rahimzadeh S., Pirzad A. Arbuscular mycorrhizal fungi and Pseudomonas in reduce drought stress damage in flax (Linum usitatissimum L.): A field study. Mycorrhiza. 2017 doi: 10.1007/s00572-017-0775-y. [DOI] [PubMed] [Google Scholar]
- 32.Monica S., Harshada J. Study of phosphate solubilizing activity of lead tolerant Pseudomonas aeruginosa HMT 51 isolated from Zawar mines, Udaipur, India. Res. J. Recent Sci. 2015;5:280–282. [Google Scholar]
- 33.Gupta P., Kumar V. Value added phytoremediation of metal stressed soils using phosphate solubilizing microbial consortium. World J. Microbiol. Biotechnol. 2017;33 doi: 10.1007/s11274-016-2176-3. [DOI] [PubMed] [Google Scholar]
- 34.Prasher D.C., Eckenrode V.K., Ward W.W., Prendergast F.G., Cormier M.J. Primary structure of the Aequorea victoria green-fluorescent protein. Gene. 1992;111:229–233. doi: 10.1016/0378-1119(92)90691-H. [DOI] [PubMed] [Google Scholar]
- 35.Valdivia R.H., Hromockyj A.E., Monack D., Ramakrishnan L., Falkow S. Applications for green fluorescent protein (GFP) in the study of hostpathogen interactions. Gene. 1996;173:47–52. doi: 10.1016/0378-1119(95)00706-7. [DOI] [PubMed] [Google Scholar]
- 36.Reinhold-Hurek B., Hurek T. Life in grasses: Diazotrophic endophytes. Trends Microbiol. 1998;6:139–144. doi: 10.1016/S0966-842X(98)01229-3. [DOI] [PubMed] [Google Scholar]
- 37.Liu X., Zhao H., Chen S. Colonization of maize and rice plants by strain Bacillus megaterium C4. Curr. Microbiol. 2006;52:186–190. doi: 10.1007/s00284-005-0162-3. [DOI] [PubMed] [Google Scholar]
- 38.Hao T., Chen S. Colonization of wheat, maize and cucumber by Paenibacillus polymyxa WLY78. PLoS ONE. 2017;12:e0169980. doi: 10.1371/journal.pone.0169980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang T. Analysis of the temperature of com production in North China. Hans J. Agric. Sci. 2013;1:28–29. [Google Scholar]
- 40.Dobbelaere S., Vanderleyden J., Okon Y. Plant growth-promoting effects of diazotrophs in the rhizosphere. Crit. Rev. Plant Sci. 2003;22:107–149. doi: 10.1080/713610853. [DOI] [Google Scholar]
- 41.Glick B.R. The enhancement of plant growth by free-living bacteria. Can. J. Microbiol. 1995;41:109–117. doi: 10.1139/m95-015. [DOI] [Google Scholar]
- 42.Senthilkumar M., Govindasamy V., Annapurna K. Role of antibiosis in suppression of charcoal rot disease by soybean endophyte Paenibacillus sp. HKA-15. Curr. Microbiol. 2007;55:25–29. doi: 10.1007/s00284-006-0500-0. [DOI] [PubMed] [Google Scholar]
- 43.Yazdani M., Bahmanyar M.A., Pirdashti H., Esmaili M.A. Effect of phosphate solubilization microorganisms (PSM) and plant growth promoting rhizobacteria (PGPR) on yield and yield components of corn (Zea mays L.) World Acad. Sci. Eng. Technol. 2009;49:90–92. [Google Scholar]
- 44.Peng Z., Liu Y., Li Y., Abawi Y., Wang Y., Men M., An-Vo D.A. Responses of nitrogen utilization and apparent nitrogen loss to different control measures in the wheat and maize rotation system. Front. Plant Sci. 2017;8:160. doi: 10.3389/fpls.2017.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shao-Yun M.A., Liu Y.N., Wang Y.Q., Qin Y.N., Liu H.L., Peng Z.P. Study on the characters of living condition and nutrient balance of high-yield wheat and maize rotation system in Hebei province. J. Agric. Univ. Hebei. 2015;38:8–13. doi: 10.13320/j.cnki.jaun.2015.0002. [DOI] [Google Scholar]
- 46.Deng Z.S., Zhao L.F., Kong Z.Y., Yang W.Q., Lindström K., Wang E.T., Wei G.H. Diversity of endophytic bacteria within nodules of the Sphaerophysa salsula in different regions of Loess Plateau in China. FEMS Microbiol. Ecol. 2011;76:463–475. doi: 10.1111/j.1574-6941.2011.01063.x. [DOI] [PubMed] [Google Scholar]
- 47.Vrieze M.D., Pandey P., Bucheli T.D., Varadarajan A.R., Ahrens C.H., Weisskopf L., Bailly A. Volatile organic compounds from native potato-associated Pseudomonasas potential anti-oomycete agents. Front. Microbiol. 2015;6:1295. doi: 10.3389/fmicb.2015.01295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou T., Chen D., Li C., Sun Q., Li L., Liu F., Shen Q., Shen B. Isolation and characterization of Pseudomonas brassicacearum J12 as an antagonist against Ralstonia solanacearum and identification of its antimicrobial components. Microbiol. Res. 2012;167:388–394. doi: 10.1016/j.micres.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 49.Nautiyal C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999;170:265–270. doi: 10.1111/j.1574-6968.1999.tb13383.x. [DOI] [PubMed] [Google Scholar]
- 50.Johri J.K., Surange S., Nautiyal C.S. Occurrence of salt, pH, and temperature-tolerant, phosphate-solubilizing bacteria in alkaline soils. Curr. Microbiol. 1999;39:89–93. doi: 10.1007/s002849900424. [DOI] [PubMed] [Google Scholar]
- 51.Louw H., Webley D. A study of soil bacteria dissolving certain mineral phosphate fertilizers and related compounds. J. Appl. Microbiol. 1959;22:227–233. doi: 10.1111/j.1365-2672.1959.tb00155.x. [DOI] [Google Scholar]
- 52.Bashan Y., Kamnev A.A., Debashan L.E. Tricalcium phosphate is inappropriate as a universal selection factor for isolating and testing phosphate-solubilizing bacteria that enhance plant growth: A proposal for an alternative procedure. Biol. Fertil. Soils. 2013;49:465–479. doi: 10.1007/s00374-012-0737-7. [DOI] [Google Scholar]
- 53.Gadagi R.S., Sa T. New isolation method for microorganisms solulbilizing iron and aluminum phosphates using dyes. Soil Sci. Plant Nutr. 2002;48:615–618. doi: 10.1080/00380768.2002.10409246. [DOI] [Google Scholar]
- 54.Collavino M.M., Sansberro P.A., Mroginski L.A., Aguilar O.M. Comparison of in vitro solubilization activity of diverse phosphate-solubilizing bacteria native to acid soil and their ability to promote Phaseolus vulgaris growth. Biol. Fertil. Soils. 2010;46:727–738. doi: 10.1007/s00374-010-0480-x. [DOI] [Google Scholar]
- 55.Chaiharn M., Lumyong S. Phosphate solubilization potential and stress tolerance of rhizobacteria from rice soil in Northern Thailand. World J. Microboil. Biotechnol. 2008;25:305–314. doi: 10.1007/s11274-008-9892-2. [DOI] [Google Scholar]
- 56.Ahmad F., Ahmad I., Khan M.S. Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol. Res. 2008;163:173–181. doi: 10.1016/j.micres.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 57.Baig K.S., Arshad M., Shaharoona B., Khalid A., Ahmed I. Comparative effectiveness of Bacillus spp. possessing either dual or single growth-promoting traits for improving phosphorus uptake, growth and yield of wheat (Triticum aestivum L.) Ann. Microbiol. 2012;62:1109–1119. doi: 10.1007/s13213-011-0352-0. [DOI] [Google Scholar]
- 58.Bashan Y., Kamnev A.A., de-Bashan L.E. A proposal for isolating and testing phosphate-solubilizing bacteria that enhance plant growth. Biol. Fertil. Soils. 2013;49:1–2. doi: 10.1007/s00374-012-0756-4. [DOI] [Google Scholar]
- 59.Yang B., Wang X.-M., Ma H.-Y., Jia Y., Li X., Dai C.-C. Effects of the fungal endophyte Phomopsis liquidambari on nitrogen uptake and metabolism in rice. Plant Growth Regul. 2013;73:165–179. doi: 10.1007/s10725-013-9878-4. [DOI] [Google Scholar]
- 60.Hallmann J., Quadt-Hallmann A., Mahaffee W.F., Kloepper J.W. Bacterial endophytes in agricultural crops. Can. J. Microbiol. 1997;43:895–914. doi: 10.1139/m97-131. [DOI] [Google Scholar]
- 61.Errampalli D., Leung K., Cassidy M.B., Kostrzynska M., Blears M., Lee H., Trevors J.T. Applications of the green fluorescent protein as a molecular marker in environmental microorganisms. J. Microbilol. Methods. 1999;35:187–199. doi: 10.1016/S0167-7012(99)00024-X. [DOI] [PubMed] [Google Scholar]
- 62.Zhen Y. Bacillus megatherium WY4 labeled by GFP and its colonization in Chinese Cabbage (Brassica chinensis) J. Agric. Biotechnol. 2016;24:1925–1934. [Google Scholar]
- 63.Pavlova A.S., Leontieva M.R., Smirnova T.A., Kolomeitseva G.L., Netrusov A.I., Tsavkelova E.A. Colonization strategy of the endophytic plant growth promoting strains of Pseudomonas fluorescens and Klebsiella oxytoca on the seeds, seedlings and roots of the epiphytic orchid, Dendrobium nobile Lindl. J. Appl. Microbiol. 2017 doi: 10.1111/jam.13481. [DOI] [PubMed] [Google Scholar]
- 64.Pérez-García A., Romero D., de Vicente A. Plant protection and growth stimulation by microorganisms: Biotechnological applications of Bacilli in agriculture. Curr. Opin. Biotechnol. 2011;22:187–193. doi: 10.1016/j.copbio.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 65.Timmusk S., Grantcharova N., Wagner E.G. Paenibacillus polymyxa invades plant roots and forms biofilms. Appl. Environ. Microbiol. 2005;71:7292–7300. doi: 10.1128/AEM.71.11.7292-7300.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alagawadi A.R. Associative effect of Rhizobium and phosphate-solubilizing bacteria on the yield and nutrient uptake of chickpea. Plant Soil. 1988;105:241–246. doi: 10.1007/BF02376788. [DOI] [Google Scholar]
- 67.Vincent J. A Manual for the Practical Study of the Root-Nodule Bacteria. Blackwell Scientific Publications; Oxford, UK: 1970. Int. Biol. Prog. Handbook No. 15. [Google Scholar]
- 68.RM S., NR K. Phenotypic characterization. In: Ghardt P., Murray R.G.E., Wood W.A., Krieg N.R., editors. Methods for General and Molecular Bacteriology. ASM Press; Washington, DC, USA: 1994. pp. 607–657. [Google Scholar]
- 69.Khan A.L., Waqas M., Kang S.M., Al-Harrasi A., Hussain J., Al-Rawahi A., Al-Khiziri S., Ullah I., Ali L., Jung H.Y., et al. Bacterial endophyte Sphingomonas sp. LK11 produces gibberellins and IAA and promotes tomato plant growth. J. Microbiol. 2014;52:689–695. doi: 10.1007/s12275-014-4002-7. [DOI] [PubMed] [Google Scholar]
- 70.Shahid M., Hameed S., Tariq M., Zafar M., Ali A., Ahmad N. Characterization of mineral phosphate-solubilizing bacteria for enhanced sunflower growth and yield-attributing traits. Ann. Microbiol. 2014;65:1525–1536. doi: 10.1007/s13213-014-0991-z. [DOI] [Google Scholar]
- 71.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Premono M.E., Moawad A.M., Vleck P.L. G. Effect of phosphate solubilizing Pseudmonas putida on the growth of maize and its survival in the rhizosphere. Indonasian J. Crop Sci. 1996;11:13–23. doi: 10.1016/j.biortech.2017.04.013. [DOI] [Google Scholar]
- 73.Fiske C.H., Subbarow Y. A colorimetric determination of phosphorus. J. Biol. Chem. 1925;66:375–400. [Google Scholar]
- 74.Glickmann E., Dessaux Y. A critical examination of the specificity of the Salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl. Environ. Microb. 1995;61:793–796. doi: 10.1128/aem.61.2.793-796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baker W.H., Thompson T.L. Determination of total nitrogen in plant samples by Kjeldahl. In: Plank C.O., editor. Plant Analysis Reference Procedures for the Southern Region of the United States. Southern Cooperative Series Bulletin; Washington, DC, USA: 1992. pp. 13–16. [Google Scholar]
- 76.Hanson W.C. The photometric determination of phosphorus in fertilizers using the phosphovanadomolybdate complex. J. Sci. Food Agric. 1950;1:172–173. doi: 10.1002/jsfa.2740010604. [DOI] [Google Scholar]
- 77.Zhang W., Ding Y., Yao L., Liu K., Du B. Construction of gene knock-out system for Paenibacillus polymyxa SC2. Acta Microbiol. Sin. 2013;53:1258–1266. [PubMed] [Google Scholar]
- 78.Murashige T., Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Phys. Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]