Abstract
Drug-induced liver injury (DILI), although rare, is a frequent cause of adverse drug reactions resulting in warnings and withdrawals of numerous medications. Despite the research community’s best efforts, current testing strategies aimed at identifying hepatotoxic drugs prior to human trials are not sufficiently powered to predict the complex mechanisms leading to DILI. In our previous studies, we demonstrated lipophilicity and dose to be associated with increased DILI risk and, and in our latest work, we factored reactive metabolites into the algorithm to predict DILI. Given the inconsistency in determining the potential for drugs to cause DILI, the present study comprehensively assesses the relationship between DILI risk and lipophilicity and the extent of metabolism using a large published dataset of 1036 Food and Drug Administration (FDA)-approved drugs by considering five independent DILI annotations. We found that lipophilicity and the extent of metabolism alone were associated with increased risk for DILI. Moreover, when analyzed in combination with high daily dose (≥100 mg), lipophilicity was statistically significantly associated with the risk of DILI across all datasets (p < 0.05). Similarly, the combination of extensive hepatic metabolism (≥50%) and high daily dose (≥100 mg) was also strongly associated with an increased risk of DILI among all datasets analyzed (p < 0.05). Our results suggest that both lipophilicity and the extent of hepatic metabolism can be considered important risk factors for DILI in humans, and that this relationship to DILI risk is much stronger when considered in combination with dose. The proposed paradigm allows the convergence of different published annotations to a more uniform assessment.
Keywords: hepatotoxicity, drug dose, drug lipophilicity, metabolism, risk factor, annotation
1. Introduction
Drug-induced liver injury (DILI) can result in severe clinical outcomes such as acute liver failure, and, although rare, DILI is encountered frequently during the drug development process and therefore presents a significant challenge to drug developers and regulators. Importantly, the complexity of the disease, the lack of predictive biomarkers as well as the difficulty to diagnose and determine causality are obstacles that need to be overcome [1,2,3]. Our current understanding of DILI pathogenesis is limited, and therefore identifying risk factors is critical for a better understanding and avoidance of DILI risks.
An increased risk of developing DILI is thought to be caused by interactions among the host, drug, and environmental factors [3,4]. Genetic studies have found single nucleotide polymorphisms in a number of genes, including human leukocyte antigen (HLA) regions that were associated with increased risk for DILI as shown for flucloxacillin, amoxicillin-clavulanate, and lapatinib [5,6,7,8,9]. Non-genetic host factors, such as age, gender, and underlying liver disease, were also found to be associated with increased DILI risk; however, many of these factors may be associated only with specific drugs [10]. Underlying liver diseases affect the activities of certain CYP enzymes such that drugs that are substrates of these enzymes should be given at lower doses [11]. Furthermore, there is evidence for serum acute phase reactants to hallmark healthy individuals at risk to develop DILI prior to drug treatment, therefore carrying the potential to identify individuals likely to develop DILI [12].
When considering drug properties that may have influence on DILI, lipophilicity is an important criterion that strongly contributes to the distribution of drugs throughout the body and is generally associated with a large volume of distribution. There is also increasing interest in investigating drug characteristics and their relationship to DILI risk. Lammert et al. found that exposure to oral medications given at doses greater than 50 mg/day was associated with a higher DILI risk [13]. This finding was later confirmed in the Spanish DILI registry, where 77% of the drugs that caused DILI were given at doses of 50 mg/day or more [14]. In addition to dose, extensive metabolism (>50%) is also associated with increased risk of hepatic adverse events from orally administered drugs [15]. Recently, we identified drugs with daily doses (DD) greater than 100 mg/day and high lipophilicity (measured by log value for octanol-water partition coefficient p, i.e., logP ≥ 3) to be associated with increased DILI risk [16]. These two drug characteristics (i.e., extent of metabolism and lipophilicity) look promising and have been widely cited by seminal reviews [3,10], but further validation to test the reliability of the findings in larger datasets is warranted.
In this study, we aim to comprehensively revisit the relationship between DILI risk and lipophilicity and the extent of metabolism using five independent annotations for drugs within the DILIrank dataset, a large published drug reference dataset with 1036 Food and Drug Administration FDA-approved drugs [17]. We found that lipophilicity and the extent of metabolism alone were associated with increased DILI risk, but that the strength of association varied considerably across different annotations. However, the combination of high daily dose with high lipophilicity or significant hepatic metabolism was consistently associated with increased DILI risk across all annotated datasets.
2. Results
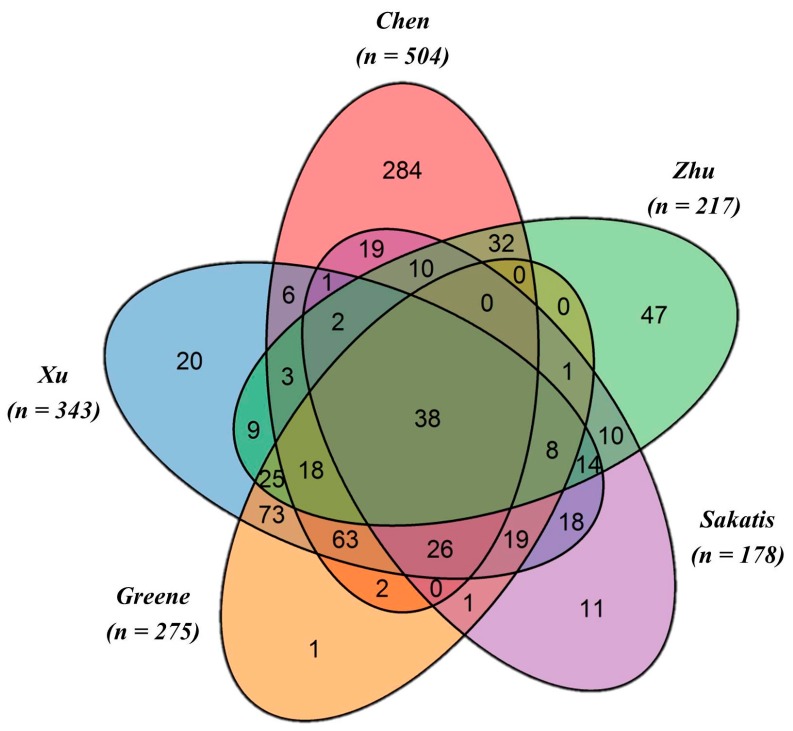
The Venn diagram (Figure 1) shows the commonality and the specificities of the DILI datasets retrieved from the five independent annotations for n = 1036 DILIrank drugs. A large portion of the drugs (n = 398) are annotated by at least two datasets, but only n = 38 drugs are in common among all five datasets. Chen et al. provides the largest annotation dataset of n = 504, with n = 192 vMost-DILI-concern and n = 312 vNo-DILI-concern drugs, followed by Xu et al. of n = 343, with n = 195 DILI positives and n = 148 negatives, and Greene et al. of n = 275, with n = 189 drugs having human evidence of toxicity and n = 86 drugs with no evidence of human toxicity. Additionally, the Sakatis dataset annotated n = 178 drugs, including n = 92 hepatoxicants and n = 86 as non-hepatotoxicants, and the Zhu et al. dataset labeled n = 217 drugs, of which n = 161 were hepatotoxic and n = 56 were non-hepatotoxic. Notably, a total of n = 275 DILIrank drugs were not evaluated in the analysis due to their ambiguous annotations.
Figure 1.
Venn diagram showing the commonality and specificities among the five individually annotated datasets. Among the different authors, a total of 38 drugs were commonly annotated. Highlighted in the Venn diagram are also specific data sets. For example, the Zhu et al. dataset contains n = 47 drugs uniquely annotated by these authors. Similarly, n = 11 unique drugs are considered in the Sakatis et al. data set. The R package VennDiagram [18] was used to generate this figure.
2.1. Lipophilicity and DILI Risk
We analyzed 763 medications retrieved from the DILIrank dataset with both daily dose and logP data available. To comprehensively assess the relationship between lipophilicity and hepatotoxicity risk, five independent DILI annotations were used, as detailed above. We used Fisher’s exact test to determine the statistical significance of the association between high daily dose, high lipophilicity (measured by logP), and DILI risk. The extent of the association was measured using odds ratio (OR) calculated by logistic regression.
As illustrated in Table 1, the combination of high lipophilicity (logP ≥ 3) and DD ≥ 100 mg, as defined by the Rule of Two (RO2) [16], is statistically significantly associated with the risk of DILI across all datasets (p < 0.05); however, the estimated OR ranges from 2.32 to 11.50, depending on the annotations used.
Table 1.
The assessment of the relationship between DILI risk, lipophilicity, and daily dose.
| Annotated Datasets | DILI Classification | Positives | Negatives | Odds Ratio (95% Confidence Interval) | p-Value |
|---|---|---|---|---|---|
| LogP ≥ 3 and Daily Dose ≥ 100 mg | |||||
| Chen [17] | vMost-concern (n = 172) | 71 | 101 | 11.50 (5.42–24.82) | <0.05 |
| vNo-concern (n = 173) | 10 | 163 | |||
| Greene [19] | Human hepatotoxicity (n = 174) | 51 | 123 | 4.08 (1.57–11.23) | <0.05 |
| No evidence (n = 65) | 6 | 59 | |||
| Zhu [20] | Hepatotoxic (n = 152) | 42 | 110 | 5.47 (1.52–23.42) | <0.05 |
| Non-hepatotoxic (n = 46) | 3 | 43 | |||
| Sakatis [21] | Hepatotoxic (n = 89) | 28 | 61 | 2.80 (1.20–6.57) | <0.05 |
| Non-hepatotoxic (n = 78) | 11 | 67 | |||
| Xu [22] | Positive (n = 179) | 56 | 123 | 2.32 (1.27–4.24) | <0.05 |
| Negative (n = 128) | 21 | 107 | |||
| Consensus | Positive (n = 313) | 99 | 214 | 4.77 (2.79–7.86) | <0.05 |
| Negative (n = 255) | 23 | 232 | |||
| LogP ≥ 3 | |||||
| Chen [17] | vMost-concern (n = 172) | 87 | 85 | 2.26 (1.42–3.58) | <0.05 |
| vNo-concern (n = 173) | 54 | 119 | |||
| Greene [19] | Human hepatotoxicity (n = 174) | 69 | 105 | 1.72 (0.88–3.36) | 0.098 |
| No evidence (n = 65) | 18 | 47 | |||
| Zhu [20] | Hepatotoxic (n = 152) | 64 | 88 | 2.31 (1.03–5.26) | <0.05 |
| Non-hepatotoxic (n = 46) | 11 | 35 | |||
| Sakatis [21] | Hepatotoxic (n = 89) | 35 | 54 | 1.03 (0.53–2.03) | 1.0 |
| Non-hepatotoxic (n = 78) | 30 | 48 | |||
| Xu [22] | Positive (n = 179) | 74 | 105 | 0.97 (0.59–1.57) | 0.91 |
| Negative (n = 128) | 54 | 74 | |||
| Consensus | Positive (n = 313) | 138 | 175 | 1.55 (1.09–2.22) | <0.05 |
| Negative (n = 255) | 86 | 169 | |||
| Daily Dose ≥ 100 mg | |||||
| Chen [17] | vMost-concern (n = 172) | 139 | 33 | 6.20 (3.71–10.39) | <0.05 |
| vNo-concern (n = 173) | 70 | 103 | |||
| Greene [19] | Human hepatotoxicity (n = 174) | 123 | 51 | 2.65 (1.41–4.96) | <0.05 |
| No evidence (n = 65) | 31 | 34 | |||
| Zhu [20] | Hepatotoxic (n = 152) | 110 | 42 | 2.86 (1.37–5.96) | <0.05 |
| Non-hepatotoxic (n = 46) | 22 | 24 | |||
| Sakatis [21] | Hepatotoxic (n = 89) | 73 | 16 | 7.3 (3.41–15.83) | <0.05 |
| Non-hepatotoxic (n = 78) | 30 | 48 | |||
| Xu [22] | Positive (n = 179) | 127 | 52 | 2.52 (1.52–4.16) | <0.05 |
| Negative (n = 128) | 63 | 65 | |||
| Consensus | Positive (n = 313) | 225 | 88 | 3.54 (2.46–5.10) | <0.05 |
| Negative (n = 255) | 107 | 148 | |||
The association between DILI risk and high lipophilicity is statistically significant only when considering the annotations generated by Chen, Greene, and Zhu; however, no significant relationship was seen when the Sakatis or Xu annotations were considered. Note, DD ≥ 100 mg was significantly associated with DILI risk in all datasets. Furthermore, when the datasets of Chen, Greene and Zhu were evaluated, the OR for logP alone is significantly lower than those calculated for the combination of DD ≥ 100 mg and logP ≥ 3, suggesting a weaker association between logP alone with DILI risk as compared to the combination of DD ≥ 100 mg and logP ≥ 3.
We also analyzed the RO2 results across the five annotated datasets. As shown in Figure 2, there were 13% of RO2 positives but <1% RO2 negatives which were shared by the five datasets. Similar trends were observed for the percentage of RO2 positives and negatives shared by three or four datasets. The higher consensus in RO2 positives than in RO2 negatives agrees with our previous report regarding the unique characteristics of the RO2 model, which has a high true positive rate but a high false negative rate [16].
Figure 2.
The overlap of Rule of Two (RO2) positives and negatives across the five investigated datasets. From the left to the right: Red bars depict drugs that are RO2 positives and the percentage of RO2 positives that were identified as hepatotoxic among all datasets (left) to only one dataset (right). Green bars depict drugs that are RO2 negatives and the percentage of RO2 negatives are in agreement with all five datasets (left) to only one dataset (right). To calculate the overlap of RO2 positives and negatives across the investigated datasets, a total of n = 763 drugs were considered, of which n = 139 were RO2 positives and n = 624 were RO2 negatives. The percentage of RO2 positives shared by all five datasets is much higher than the percentage of RO2 negatives. Importantly, the percent of RO2 negatives sharply decreases as the number of datasets increases, indicating a lack of consensus in the DILI classifications of RO2 negatives as compared to that of RO2 positives.
2.2. Metabolism and DILI Risk
Similarly, a total of 559 DILIrank drugs with daily dose and available metabolism data were used to assess the relationship between DILI risk and the extent of metabolism. As given in Table 2, the combination of hepatic metabolism ≥50% and DD ≥ 100 mg was significantly associated with an increased risk of DILI across all datasets (p < 0.05), and the extent of association, as measured by ORs, ranged from 3.79 to 11.09 depending on the annotations used. Significant hepatic metabolism (≥50%) alone was significantly associated with DILI risk in most annotations except for the Zhu et al. and Sakatis et al. datasets. Similar to logP, the calculated ORs for the combination of metabolism and daily dose were higher than those for metabolism alone. This was seen in any of the five annotated datasets, which demonstrates that the combination with daily dose enhances the association between the extent of metabolism and DILI risk. As in the preceding analysis, DD ≥ 100 mg alone was significantly associated with DILI risk across all annotated datasets.
Table 2.
The assessment of the relationship between DILI risk, the extent of metabolism, and daily dose.
| Annotated Datasets | DILI Classification | Positives | Negatives | Odds Ratio (95% Confidence Interval) | p-Value |
|---|---|---|---|---|---|
| Hepatic Metabolism ≥ 50% and Daily Dose ≥ 100 mg | |||||
| Chen [17] | vMost-concern (n = 107) | 76 | 31 | 11.09 (5.54–22.48) | <0.05 |
| vNo-concern (n = 105) | 19 | 86 | |||
| Greene [19] | Human hepatotoxicity (n = 139) | 81 | 58 | 4.32 (1.91–9.92) | <0.05 |
| No evidence (n = 45) | 11 | 34 | |||
| Zhu [20] | Hepatotoxic (n = 127) | 70 | 57 | 5.32 (1.90–15.57) | <0.05 |
| Non-hepatotoxic (n = 32) | 6 | 26 | |||
| Sakatis [21] | Hepatotoxic (n = 73) | 53 | 20 | 7.48 (3.30–17.20) | <0.05 |
| Non-hepatotoxic (n = 65) | 17 | 48 | |||
| Xu [22] | Positive (n = 141) | 84 | 57 | 3.79 (2.11–6.84) | <0.05 |
| Negative (n = 100) | 28 | 72 | |||
| Consensus | Positive (n = 221) | 127 | 94 | 5.48 (3.40–8.84) | <0.05 |
| Negative (n = 177) | 35 | 142 | |||
| Hepatic Metabolism ≥ 50% | |||||
| Chen [17] | vMost-concern (n = 107) | 91 | 16 | 2.67 (1.27–5.40) | <0.05 |
| vNo-concern (n = 105) | 72 | 33 | |||
| Greene [19] | Human hepatotoxicity (n = 139) | 109 | 30 | 2.42 (1.11–5.29) | <0.05 |
| No evidence (n = 45) | 27 | 18 | |||
| Zhu [20] | Hepatotoxic (n = 127) | 98 | 29 | 1.77 (0.70–4.42) | 0.18 |
| Non-hepatotoxic (n = 32) | 21 | 11 | |||
| Sakatis [21] | Hepatotoxic (n = 73) | 61 | 12 | 1.80 (0.73–4.48) | 0.21 |
| Non-hepatotoxic (n = 65) | 48 | 17 | |||
| Xu [22] | Positive (n = 141) | 112 | 29 | 1.92 (1.02–3.56) | <0.05 |
| Negative (n = 100) | 67 | 33 | |||
| Consensus | Positive (n = 221) | 174 | 47 | 1.90 (1.18–3.05) | <0.05 |
| Negative (n = 177) | 117 | 60 | |||
| Daily Dose ≥ 100 mg | |||||
| Chen [17] | vMost-concern (n = 107) | 87 | 20 | 7.67 (3.92–15.15) | <0.05 |
| vNo-concern (n = 105) | 38 | 67 | |||
| Greene [23] | Human hepatotoxicity (n = 139) | 99 | 40 | 2.37 (1.12–5.00) | <0.05 |
| No evidence (n = 45) | 23 | 22 | |||
| Zhu [20] | Hepatotoxic (n = 127) | 90 | 37 | 3.13 (1.32–7.49) | <0.05 |
| Non-hepatotoxic (n = 32) | 14 | 18 | |||
| Sakatis [21] | Hepatotoxic (n = 73) | 61 | 12 | 7.63 (3.23–18.33) | <0.05 |
| Non-hepatotoxic (n = 65) | 26 | 39 | |||
| Xu [22] | Positive (n = 141) | 101 | 40 | 2.43 (1.37–4.30) | <0.05 |
| Negative (n = 100) | 51 | 49 | |||
| Consensus | Positive (n = 221) | 160 | 61 | 4.01 (2.57–6.26) | <0.05 |
| Negative (n = 177) | 70 | 107 | |||
3. Discussion
The identification of DILI risk factors is of critical importance to protect individuals from adverse drug reactions. Recent seminal reviews include lipophilicity and the extent of metabolism as potential risk factors in the pathogenesis of DILI [3,10]. Given the inconsistencies among DILI annotations, it is important to further validate contributing factors to DILI onset and progression. In the present study, we utilized a large published DILIrank dataset with 1036 FDA-approved drugs and five independent DILI annotations to comprehensively assess the association between DILI risk and two drug properties, namely lipophilicity and the extent of metabolism. Based on a consensus approach, our results suggest that lipophilicity and the extent of metabolism alone had weak, but statistically significant, associations with an increase in DILI risk, and that factoring daily dose into the algorithm significantly strengthened these associations.
In our analysis, we did observe significant variation across different annotations regarding the strength of the association between DILI risk and lipophilicity as well as the extent of metabolism. Obviously, the accuracy of annotating DILI risks varied among the datasets and this could affect the outcome when assessing risk factors. No golden standard for DILI annotation has been established so far, and each annotation utilizes different sources and criteria to define the risk of liver injury. For example, in the Xu et al. dataset, drugs with more than 10 clinical reports of Hy’s law cases are positive, while in the Sakatis et al. dataset, having 50 case reports of clinically significant hepatotoxicity was set as a threshold for drugs to be considered as DILI positive. Furthermore, to establish causality, the data needs to be evaluated by accepted methods for causality assessment such as RUCAM [24], though expert opinion is of equal importance [25]. Unfortunately, different outcomes among various causality assessment scales have been observed [26]; nonetheless, for some drugs the reported evidence of hepatotoxicity is vague [27,28]. Although not all of the datasets compared in the present study considered the RUCAM score, determining dose and lipophilicity in combination (or RO2) greatly improved the coherence among the different datasets, as shown in Figure 2.
DILI negative annotation are even more problematic, as demonstrated by a recent report [17] which suggested that as many as 40% of drugs defined as negatives by some means were also reported as DILI positives in other registries with established causality. Such contradictions, together with the significant variation in our assessment of risk factors, highlight the importance of selecting appropriately annotated datasets for the identification of DILI risk factors.
We therefore established a consensus annotation by cataloging the majority vote of the five selected annotations. Based on this consensus annotation, both lipophilicity and the extent of metabolism were significantly associated with increased DILI risk, and the strength of the association increased when combined with daily dose. The association between DILI risk and lipophilicity or metabolism was also consistently significant when assessed by the Chen and Greene annotations. In addition to annotation accuracy, an appropriate data analysis is crucial to ascertain correct conclusions. In a recent article, Weng et al. [29] reported that the combination of daily dose and metabolism or logP did not improve the prediction of drug-induced liver injury (DILI). Unfortunately, the data analysis is flawed, particularly when considering the methods for defining DILI negatives. Here, the authors made the assumption that DILI negatives could be defined by simply subtracting the positives for a given condition from the total number of drugs. In our reanalysis, however, we removed inappropriately assigned DILI negative drugs (e.g., “all human Adverse Drug Reactions (hADRs)” or “severe hADRs” in the study by Weng et al.), and found that the odd ratios for the combined daily dose and metabolism or logP were statistically significant, a finding distorted by the inappropriate assignment of DILI negatives in the study by Weng et al. [29]. This highlights the importance of a careful assessment of DILI annotations and the necessity of the consensus approach as employed in the present study.
The mechanisms by which DILI occurs are complex and are frequently idiosyncratic in nature; nonetheless, lipophilicity and metabolism are contributing factors. Lipophilic drugs often display high volumes of distribution and are therefore distributed amongst many different tissues and organs and need to be converted into hydrophilic metabolites to be eliminated [23,30]. Furthermore, extensively metabolized drugs have a greater potential to form toxic reactive metabolites [15]. Toxic reactive metabolites may irreversibly form covalent bonds, inhibiting transport proteins or triggering an immune response. Reactive metabolites may also interfere with mitochondrial function or cause oxidative stress. Whether or not these interactions lead to liver injury appears to be dependent on the accumulation of the reactive metabolites beyond a critical level [2,31]. Hepatic exposure and the amount of parent drug or metabolite that accumulates depend on dose, which might explain why the combination of daily dose significantly improves the association between DILI risk and lipophilicity and metabolism.
Several limitations must be considered in this study. First, the lipophilicity and metabolism data used in this study were collected from different literature sources, yielding data that were likely not consistently measured or that may have been derived from different study protocols. Second, defining DILI risk associated with a drug is challenging and, without a “golden standard” annotation, relies on a variety of different sources and methods to collect data and define DILI risk, resulting in DILI annotations that differ for certain drugs. Third, our study is based on setting thresholds for daily dose and logP which could lead to some bias, especially for the drugs with values close to the thresholds. Fourth, not all of the datasets in the present study considered the causality assessment (e.g., RUCAM score), which is essential in future studies to characterize DILI, as reports suggest that some hepatotoxicity recorded in the literature is vague [27,28]. Finally, this study is a retrospective analysis and requires further validation based on a prospective study design.
Despite these limitations, our evaluation permitted the evaluation of five independently annotated datasets to overwhelmingly suggest that both lipophilicity and the extent of metabolism can be considered as risk factors of DILI. However, neither lipophilicity nor the extent of metabolism alone is a strong predictor for DILI risk, but together with daily dose, the association is clear.
4. Materials and Methods
4.1. Drug Datasets
To test the relationship between DILI risk, lipophilicity, and the extent of hepatic metabolism, we collected information for drugs included in the DILI rank dataset, which is a large dataset of 1036 drugs approved by the US FDA before 2010 [17].
The characterization of drugs according to their potential to cause liver injury was collected from the dataset by Chen et al., as shown in the Table S1 [17]. Chen et al. [17] systematically divided drugs into four categories (vMost-, vLess-, vNo-DILI-concern, and Ambiguous DILI concern) based on FDA labeling and causality evidence determined by the Roussel Uclaf Causality Assessment Method (RUCAM) [24] or expert evaluation [25]. As in our previous study [16], only vMost- and vNo-DILI-concern drugs were considered in detail. The vMost-DILI-concern group includes drugs that, due to their potential to cause DILI, were withdrawn or given a boxed warning or severe DILI indication in their Warnings and Precautions. Included in the vNo-DILI-concern group are drugs with neither DILI indications in their labeling nor causality evidence.
Additional information for the 1036 drugs in the DILIrank dataset were collected from four publically available datasets in order to comprehensively assess the relationship between DILI risk, lipophilicity, and the extent of hepatic metabolism. The annotation by Greene et al. [19] classifies drugs based on human and animal evidence of toxicity, and only drugs categorized as having human evidence of toxicity (HH) or no evidence of human toxicity (NE) were used for this analysis. The annotation by Xu et al. [22] defines DILI positive drugs as those that, due to reports of hepatotoxicity, were withdrawn from the market or given warnings in their labeling, or those that had more than 10 clinical reports of serious hepatotoxicity. Those that not met the criteria of positives were defined as DILI negatives. In the annotation by Sakatis et al. [21], drugs were categorized as hepatotoxic or non-hepatotoxic based on the availability of clinical hepatotoxicity reported in literature and drug labeling. The annotation by Zhu et al. [20] classifies drugs as known hepatotoxicants, according to the assignment from Suzuki et al. [32], the causality of which has been judged by the RUCAM method. Conversely, drugs marketed for at least five years without reports of hepatotoxicity in PubMed or MedWatch were assigned as non-hepatotoxicants. All data were collected from original publications without further judgment on causality.
Additionally, we created a consensus annotation from the annotations by Chen [17], Greene [19], Zhu [20], Sakatis [21], and Xu [22]. In the consensus annotation, a drug will be considered as DILI positive if the majority of available annotations are positive, otherwise it will be considered as negative. Drugs with an equal number of available positive and negative annotations will be considered as positive because, in the annotations, DILI positives were justified with clinic evidences whereas DILI negatives were instead defined by a lack of evidence.
4.2. Daily Dose, Lipophilicity, and Metabolism
Daily dose for a total of n = 763 DILIrank drugs could be retrieved from the World Health Organization (WHO) Anatomical Therapeutic Chemical (ATC) database (http://www.whocc.no/atc_ddd_index) and from FDA-approved drug labels (http://dailymed.nlm.nih.gov/dailymed/about.cfm) and literature sources as described in our previous publication [16]. LogP values for n = 944 drugs were also collected, including n = 734 drugs of experimental logP values from the Drugbank database (www.drugbank.ca) and n = 210 drugs with calculated logP values from AlogPS 2.1 (http://www.vcclab.org/lab/alogps/start.html).
A total of n = 640 DILIrank drugs were categorized as having significant or insignificant hepatic metabolism based on metabolism data collected from Micromedex® 2.0 (http://www.micromedexsolutions.com/micromedex2/librarian/), NIH LiverTox database (https://livertox.nih.gov/), Lammert et al. [15], Drugbank [33], Pharmapendium (https://www.pharmapendium.com/), and literature sources. Significant metabolism was defined as ≥50%, as suggested by Lammert et al. [15]. For a small number of compounds (i.e., n = 16), no detailed information on the extent of metabolism was available; however, the amount of unchanged drugs in urine was <20% to suggest extensive metabolism.
4.3. Statistical Analysis
The odds ratio with a 95% confidence interval derived from logistic regression was used to measure the strength of association between DILI risk and a specific risk factor (e.g., logP ≥ 3). A two-sided Fisher’s exact test was used to determine the statistical significance of the association. The logistic regression was computed using R software (version 3.2.2, R Development Core Team, Vienna, Austria) and the Bioconductor package (version 3.4, http://www.bioconductor.org/).
Acknowledgments
We would like to thank Wei Zhuang for the discussion on the statistical analysis.
Supplementary Materials
Supplementary materials can be found at www.mdpi.com/1422-0067/18/7/1335/s1.
Author Contributions
Minjun Chen conceived and designed the experiments; Kristin McEuen performed the experiments; Kristin McEuen, Jürgen Borlak, Weida Tong and Minjun Chen analyzed the data and wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest. The views expressed are those of the authors and do not necessarily represent the position of, nor imply endorsement from, the U.S. Food and Drug Administration or the U.S. Government.
References
- 1.Chalasani N., Björnsson E. Risk factors for idiosyncratic drug-induced liver injury. Gastroenterology. 2010;138:2246–2259. doi: 10.1053/j.gastro.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amacher D.E. The primary role of hepatic metabolism in idiosyncratic drug-induced liver injury. Expert Opin. Drug Metab. Toxicol. 2012;8:335–347. doi: 10.1517/17425255.2012.658041. [DOI] [PubMed] [Google Scholar]
- 3.Fontana R.J. Pathogenesis of idiosyncratic drug-induced liver injury and clinical perspectives. Gastroenterology. 2014;146:914–928. doi: 10.1053/j.gastro.2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen M., Suzuki A., Borlak J., Andrade R.J., Lucena M.I. Drug-induced liver injury: Interactions between drug properties and host factors. J. Hepatol. 2015;63:503–514. doi: 10.1016/j.jhep.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 5.Daly A.K., Donaldson P.T., Bhatnagar P., Shen Y., Pe’er I., Floratos A., Daly M.J., Goldstein D.B., John S., Nelson M.R., et al. HLA-b* 5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat. Genet. 2009;41:816–819. doi: 10.1038/ng.379. [DOI] [PubMed] [Google Scholar]
- 6.Lucena M.I., Molokhia M., Shen Y., Urban T.J., Aithal G.P., Andrade R.J., Day C.P., Ruiz-Cabello F., Donaldson P.T., Stephens C., et al. Susceptibility to amoxicillin-clavulanate-induced liver injury is influenced by multiple HLA class I and II alleles. Gastroenterology. 2011;141:338–347. doi: 10.1053/j.gastro.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monshi M.M., Faulkner L., Gibson A., Jenkins R.E., Farrell J., Earnshaw C.J., Alfirevic A., Cederbrant K., Daly A.K., French N., et al. Human leukocyte antigen (HLA)-B* 57: 01-Restricted activation of drug-specific T cells provides the immunological basis for flucloxacillin-induced liver injury. Hepatology. 2013;57:727–739. doi: 10.1002/hep.26077. [DOI] [PubMed] [Google Scholar]
- 8.Donaldson P.T., Daly A.K., Henderson J., Graham J., Pirmohamed M., Bernal W., Day C.P., Aithal G.P. Human leucocyte antigen class II genotype in susceptibility and resistance to co-amoxiclav-induced liver injury. J. Hepatol. 2010;53:1049–1053. doi: 10.1016/j.jhep.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 9.Spraggs C.F., Budde L.R., Briley L.P., Bing N., Cox C.J., King K.S., Whittaker J.C., Mooser V.E., Preston A.J., Stein S.H., et al. HLA-DQA1* 02:01 is a major risk factor for lapatinib-induced hepatotoxicity in women with advanced breast cancer. J. Clin. Oncol. 2011;29:667–673. doi: 10.1200/JCO.2010.31.3197. [DOI] [PubMed] [Google Scholar]
- 10.Leise M.D., Poterucha J.J., Talwalkar J.A. Mayo Clinic Proceedings. Elsevier; Amsterdam, the Netherlands: 2014. Drug-induced liver injury; pp. 95–106. [DOI] [PubMed] [Google Scholar]
- 11.Lauschke V.M., Ingelman-Sundberg M. The importance of patient-specific factors for hepatic drug response and toxicity. Int. J. Mol. Sci. 2016;17:1714. doi: 10.3390/ijms17101714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borlak J., Chatterji B., Londhe K.B., Watkins P.B. Serum acute phase reactants hallmark healthy individuals at risk for acetaminophen-induced liver injury. Genome Med. 2013;5:86. doi: 10.1186/gm493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lammert C., Einarsson S., Saha C., Niklasson A., Bjornsson E., Chalasani N. Relationship between daily dose of oral medications and idiosyncratic drug-induced liver injury: Search for signals. Hepatology. 2008;47:2003–2009. doi: 10.1002/hep.22272. [DOI] [PubMed] [Google Scholar]
- 14.Lucena M.I., Andrade R.J., Kaplowitz N., García-Cortes M., Fernández M.C., Romero-Gomez M., Bruguera M., Hallal H., Robles-Diaz M., Rodriguez-González J.F., et al. Phenotypic characterization of idiosyncratic drug-induced liver injury: The influence of age and sex. Hepatology. 2009;49:2001–2009. doi: 10.1002/hep.22895. [DOI] [PubMed] [Google Scholar]
- 15.Lammert C., Bjornsson E., Niklasson A., Chalasani N. Oral medications with significant hepatic metabolism at higher risk for hepatic adverse events. Hepatology. 2010;51:615–620. doi: 10.1002/hep.23317. [DOI] [PubMed] [Google Scholar]
- 16.Chen M., Borlak J., Tong W. High lipophilicity and high daily dose of oral medications are associated with significant risk for drug-induced liver injury. Hepatology. 2013;58:388–396. doi: 10.1002/hep.26208. [DOI] [PubMed] [Google Scholar]
- 17.Chen M., Suzuki A., Thakkar S., Yu K., Hu C., Tong W. Dilirank: The largest reference drug list ranked by the risk for developing drug-induced liver injury in humans. Drug Discov. Today. 2016;21:648–653. doi: 10.1016/j.drudis.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Chen H., Boutros P.C. Venndiagram: A package for the generation of highly-customizable Venn and Euler diagrams in r. BMC Bioinform. 2011;12:1. doi: 10.1186/1471-2105-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greene N., Fisk L., Naven R.T., Note R.R., Patel M.L., Pelletier D.J. Developing structure—Activity relationships for the prediction of hepatotoxicity. Chem. Res. Toxicol. 2010;23:1215–1222. doi: 10.1021/tx1000865. [DOI] [PubMed] [Google Scholar]
- 20.Zhu X., Kruhlak N.L. Construction and analysis of a human hepatotoxicity database suitable for QSAR modeling using post-market safety data. Toxicology. 2014;321:62–72. doi: 10.1016/j.tox.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Sakatis M.Z., Reese M.J., Harrell A.W., Taylor M.A., Baines I.A., Chen L., Bloomer J.C., Yang E.Y., Ellens H.M., Ambroso J.L., et al. Preclinical strategy to reduce clinical hepatotoxicity using in vitro bioactivation data for >200 compounds. Chem. Res. Toxicol. 2012;25:2067–2082. doi: 10.1021/tx300075j. [DOI] [PubMed] [Google Scholar]
- 22.Xu J.J., Henstock P.V., Dunn M.C., Smith A.R., Chabot J.R., de Graaf D. Cellular imaging predictions of clinical drug-induced liver injury. Toxicol. Sci. 2008;105:97–105. doi: 10.1093/toxsci/kfn109. [DOI] [PubMed] [Google Scholar]
- 23.Kaplowitz N. Avoiding idiosyncratic DILI: Two is better than one. Hepatology. 2013;58:15–17. doi: 10.1002/hep.26295. [DOI] [PubMed] [Google Scholar]
- 24.Danan G., Teschke R. RUCAM in drug and herb induced liver injury: The update. Int. J. Mol. Sci. 2015;17:14. doi: 10.3390/ijms17010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis J.H. Causality assessment: Which is best—Expert opinion or rucam? Clin. Liver Dis. 2014;4:4–8. doi: 10.1002/cld.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.García-Cortés M., Lucena M., Pachkoria K., Borraz Y., Hidalgo R., Andrade R. Evaluation of naranjo adverse drug reactions probability scale in causality assessment of drug-induced liver injury. Aliment. Pharmacol. Ther. 2008;27:780–789. doi: 10.1111/j.1365-2036.2008.03655.x. [DOI] [PubMed] [Google Scholar]
- 27.Björnsson E.S. Hepatotoxicity by drugs: The most common implicated agents. Int. J. Mol. Sci. 2016;17:224. doi: 10.3390/ijms17020224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teschke R., Frenzel C., Wolff A., Eickhoff A., Schulze J. Drug induced liver injury: Accuracy of diagnosis in published reports. Ann. Hepatol. 2014;13:248–255. [PubMed] [Google Scholar]
- 29.Weng Z., Wang K., Li H., Shi Q. A comprehensive study of the association between drug hepatotoxicity and daily dose, liver metabolism, and lipophilicity using 975 oral medications. Oncotarget. 2015;6:17031–17038. doi: 10.18632/oncotarget.4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ortega-Alonso A., Stephens C., Lucena M.I., Andrade R.J. Case characterization, clinical features and risk factors in drug-induced liver injury. Int. J. Mol. Sci. 2016;17:714. doi: 10.3390/ijms17050714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corsini A., Bortolini M. Drug-induced liver injury: The role of drug metabolism and transport. J. Clin. Pharmacol. 2013;53:463–474. doi: 10.1002/jcph.23. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki A., Andrade R.J., Bjornsson E., Lucena M.I., Lee W.M., Yuen N.A., Hunt C.M., Freston J.W. Drugs associated with hepatotoxicity and their reporting frequency of liver adverse events in vigibase™. Drug Saf. 2010;33:503–522. doi: 10.2165/11535340-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 33.Wishart D.S., Knox C., Guo A.C., Shrivastava S., Hassanali M., Stothard P., Chang Z., Woolsey J. Drugbank: A comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006;34:D668–D672. doi: 10.1093/nar/gkj067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.