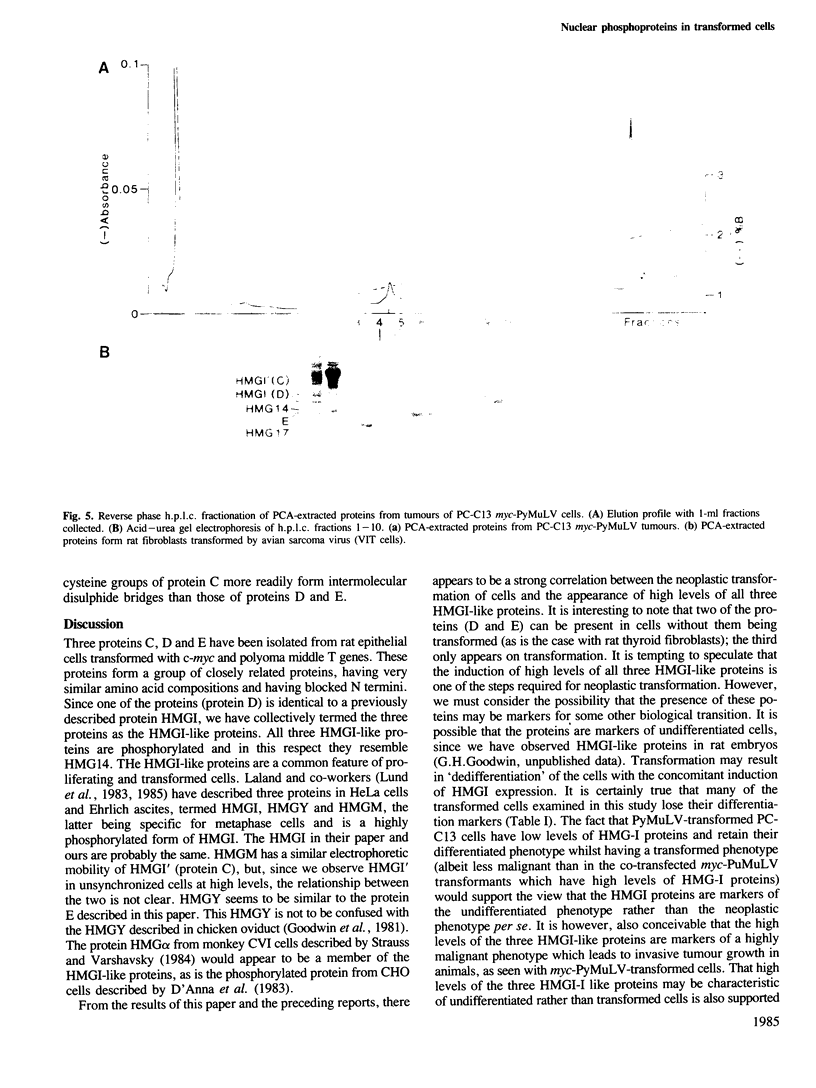
Abstract
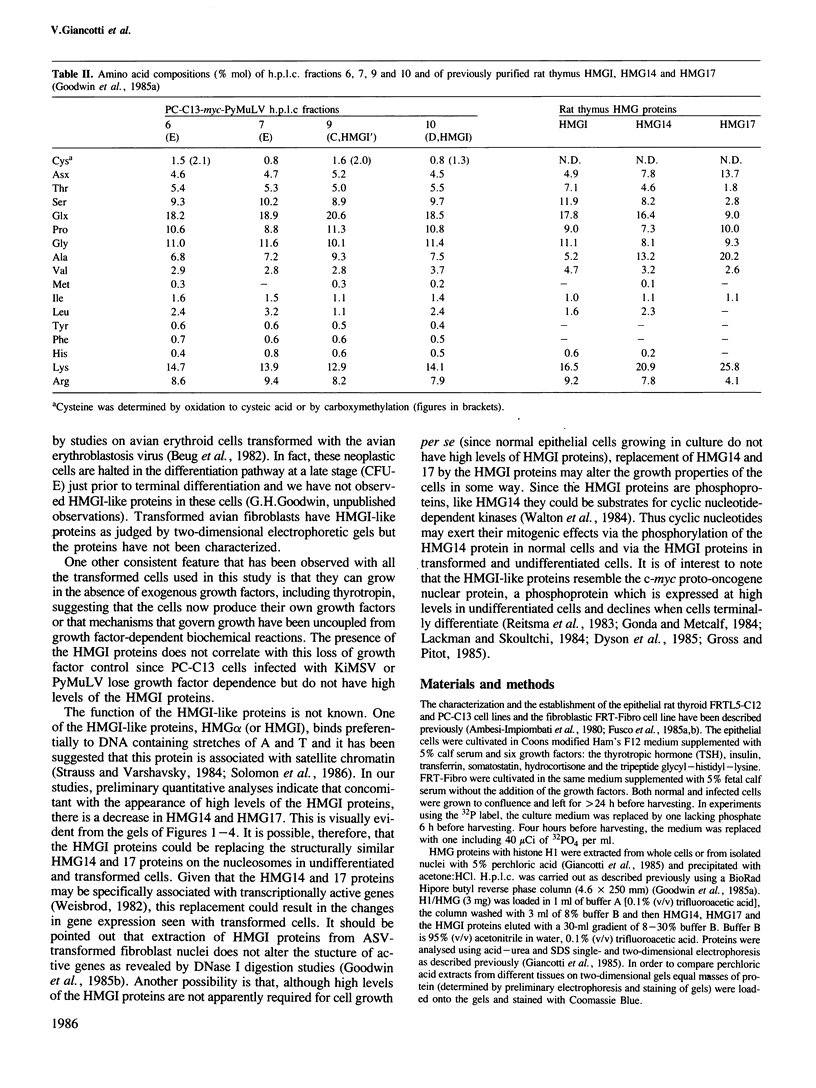
Transformation of a rat thyroid epithelial cell line (FRTL5-C12) with Kirsten and Harvey murine sarcoma viruses (carrying the ras oncogenes) results in elevated levels of three perchloric acid-soluble nuclear phosphoproteins. These three proteins are also induced to high levels in the PC-C13 thyroid epithelial cell line when transformed by the myeloproliferative sarcoma virus (carrying the v-mos oncogene) and when transformed by transfection with the c-myc proto-oncogene followed by infection with the polyoma leukaemia virus (PyMuLV) carry the polyoma middle T antigen gene. Neither c-myc or PyMuLV alone induced high levels of the three nuclear proteins. Untransformed thyroid fibroblasts have high levels of two of the three proteins and can be transformed by PyMuLV alone resulting in the appearance of the third protein. Transformation with Harvey sarcoma virus also results in the induction of the third protein. The three phosphoproteins have been purified by h.p.l.c. and shown to be related to the HeLa protein HMGI already described. The results of these studies indicate that elevated levels of these HMGI-like proteins are associated with neoplastic transformation and/or with an undifferentiated phenotype.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambesi-Impiombato F. S., Parks L. A., Coon H. G. Culture of hormone-dependent functional epithelial cells from rat thyroids. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3455–3459. doi: 10.1073/pnas.77.6.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beug H., Palmieri S., Freudenstein C., Zentgraf H., Graf T. Hormone-dependent terminal differentiation in vitro of chicken erythroleukemia cells transformed by ts mutants of avian erythroblastosis virus. Cell. 1982 Apr;28(4):907–919. doi: 10.1016/0092-8674(82)90070-8. [DOI] [PubMed] [Google Scholar]
- D'Anna J. A., Becker R. R., Tobey R. A., Gurley L. R. Composition and synthesis during G1 and S phase of a high mobility group-E/G component from Chinese hamster ovary cells. Biochim Biophys Acta. 1983 Mar 10;739(2):197–206. doi: 10.1016/0167-4781(83)90030-1. [DOI] [PubMed] [Google Scholar]
- Dyson P. J., Littlewood T. D., Forster A., Rabbitts T. H. Chromatin structure of transcriptionally active and inactive human c-myc alleles. EMBO J. 1985 Nov;4(11):2885–2891. doi: 10.1002/j.1460-2075.1985.tb04018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber E., Cameron R. The sequential analysis of cancer development. Adv Cancer Res. 1980;31:125–226. doi: 10.1016/s0065-230x(08)60658-2. [DOI] [PubMed] [Google Scholar]
- Fusco A., Pinto A., Ambesi-Impiombato F. S., Vecchio G., Tsuchida N. Transformation of rat thyroid epithelial cells by Kirsten murine sarcoma virus. Int J Cancer. 1981 Nov 15;28(5):655–662. doi: 10.1002/ijc.2910280519. [DOI] [PubMed] [Google Scholar]
- Fusco A., Pinto A., Tramontano D., Tajana G., Vecchio G., Tsuchida N. Block in the expression of differentiation markers of rat thyroid epithelial cells by transformation with Kirsten murine sarcoma virus. Cancer Res. 1982 Feb;42(2):618–626. [PubMed] [Google Scholar]
- Fusco A., Portella G., Di Fiore P. P., Berlingieri M. T., Di Lauro R., Schneider A. B., Vecchio G. A mos oncogene-containing retrovirus, myeloproliferative sarcoma virus, transforms rat thyroid epithelial cells and irreversibly blocks their differentiation pattern. J Virol. 1985 Oct;56(1):284–292. doi: 10.1128/JVI.56.1.284-292.1985. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Giancotti V., Berlingieri M. T., DiFiore P. P., Fusco A., Vecchio G., Crane-Robinson C. Changes in nuclear proteins on transformation of rat epithelial thyroid cells by a murine sarcoma retrovirus. Cancer Res. 1985 Dec;45(12 Pt 1):6051–6057. [PubMed] [Google Scholar]
- Gonda T. J., Metcalf D. Expression of myb, myc and fos proto-oncogenes during the differentiation of a murine myeloid leukaemia. Nature. 1984 Jul 19;310(5974):249–251. doi: 10.1038/310249a0. [DOI] [PubMed] [Google Scholar]
- Goodwin G. H., Cockerill P. N., Kellam S., Wright C. A. Fractionation by high-performance liquid chromatography of the low-molecular-mass high-mobility-group (HMG) chromosomal proteins present in proliferating rat cells and an investigation of the HMG proteins present in virus transformed cells. Eur J Biochem. 1985 May 15;149(1):47–51. doi: 10.1111/j.1432-1033.1985.tb08891.x. [DOI] [PubMed] [Google Scholar]
- Goodwin G. H., Nicolas R. H., Cockerill P. N., Zavou S., Wright C. A. The effect of salt extraction on the structure of transcriptionally active genes; evidence for a DNAseI-sensitive structure which could be dependent on chromatin structure at levels higher than the 30 nm fibre. Nucleic Acids Res. 1985 May 24;13(10):3561–3579. doi: 10.1093/nar/13.10.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin G. H., Wright C. A., Johns E. W. The characterisation of 1SF monomer nucleosomes from hen oviduct and the partial characterisation of a third HMG14/17-like in such nucleosomes. Nucleic Acids Res. 1981 Jun 25;9(12):2761–2775. doi: 10.1093/nar/9.12.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosso L. E., Pitot H. C. Transcriptional regulation of c-myc during chemically induced differentiation of HL-60 cultures. Cancer Res. 1985 Feb;45(2):847–850. [PubMed] [Google Scholar]
- Lachman H. M., Skoultchi A. I. Expression of c-myc changes during differentiation of mouse erythroleukaemia cells. Nature. 1984 Aug 16;310(5978):592–594. doi: 10.1038/310592a0. [DOI] [PubMed] [Google Scholar]
- Lund T., Holtlund J., Fredriksen M., Laland S. G. On the presence of two new high mobility group-like proteins in HeLa S3 cells. FEBS Lett. 1983 Feb 21;152(2):163–167. doi: 10.1016/0014-5793(83)80370-6. [DOI] [PubMed] [Google Scholar]
- Pragnell I. B., Fusco A., Arbuthnott C., Smadja-Joffe F., Klein B., Jasmin C., Ostertag W. Analysis of the myeloproliferative sarcoma virus genome: limited changes in the prototype lead to altered target cell specificity. J Virol. 1981 Jun;38(3):952–957. doi: 10.1128/jvi.38.3.952-957.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitsma P. H., Rothberg P. G., Astrin S. M., Trial J., Bar-Shavit Z., Hall A., Teitelbaum S. L., Kahn A. J. Regulation of myc gene expression in HL-60 leukaemia cells by a vitamin D metabolite. Nature. 1983 Dec 1;306(5942):492–494. doi: 10.1038/306492a0. [DOI] [PubMed] [Google Scholar]
- Scott M. R., Westphal K. H., Rigby P. W. Activation of mouse genes in transformed cells. Cell. 1983 Sep;34(2):557–567. doi: 10.1016/0092-8674(83)90388-4. [DOI] [PubMed] [Google Scholar]
- Solomon M. J., Strauss F., Varshavsky A. A mammalian high mobility group protein recognizes any stretch of six A.T base pairs in duplex DNA. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1276–1280. doi: 10.1073/pnas.83.5.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spandidos D. A. Mechanism of carcinogenesis: the role of oncogenes, transcriptional enhancers and growth factors. Anticancer Res. 1985 Sep-Oct;5(5):485–498. [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Autocrine growth factors and cancer. 1985 Feb 28-Mar 6Nature. 313(6005):745–747. doi: 10.1038/313745a0. [DOI] [PubMed] [Google Scholar]
- Strauss F., Varshavsky A. A protein binds to a satellite DNA repeat at three specific sites that would be brought into mutual proximity by DNA folding in the nucleosome. Cell. 1984 Jul;37(3):889–901. doi: 10.1016/0092-8674(84)90424-0. [DOI] [PubMed] [Google Scholar]
- Walker J. M., Hastings J. R., Johns E. W. The primary structure of a non-histone chromosomal protein. Eur J Biochem. 1977 Jun 15;76(2):461–468. doi: 10.1111/j.1432-1033.1977.tb11616.x. [DOI] [PubMed] [Google Scholar]
- Walton G. M., Gill G. N., Cooper E., Spaulding S. W. Thyrotropin-stimulated phosphorylation of high mobility group protein 14 in vivo at the site catalyzed by cyclic nucleotide-dependent protein kinases in vitro. J Biol Chem. 1984 Jan 10;259(1):601–607. [PubMed] [Google Scholar]
- Weisbrod S. Active chromatin. Nature. 1982 May 27;297(5864):289–295. doi: 10.1038/297289a0. [DOI] [PubMed] [Google Scholar]