Abstract
Genes involved in regulation of the nuclear factor-κB (NF-κB)—pathway are suggested to play a role in pathogenesis of rheumatoid arthritis (RA). In the present study, genetic polymorphisms of TLR2, TLR4, TLR9 and NF-κB1 genes were investigated to assess their associations with RA susceptibility, progression and response to anti-TNF-α therapy. A group of 110 RA patients and 126 healthy individuals were genotyped for TLR2 (rs111200466), TLR4 (rs4986790, rs4986791), TLR9 (rs5743836, rs187084) and NF-κB1 (rs28362491) alleles. The presence of the TLR9 −1486 T variant (p < 0.0001) and its homozygosity (p < 0.0001) were found to be associated with disease susceptibility. The TLR9 −1237 C allele was associated with predisposition to RA in females only (p = 0.005). Moreover, the TLR4 rs4986791 G (rs4986790 T) alleles were more frequently detected among patients with the stage IV disease (p = 0.045), and were associated with more effective response to anti-TNF-α therapy (p = 0.012). More efficient response to anti-TNF-α treatment was also observed in patients with del within the NF-κB1 gene (p = 0.047), while for the TLR9 −1486 T homozygotes, the treatment was ineffective (p = 0.018). TLR polymorphisms affect disease susceptibility and response to therapy with TNF-α inhibitors in RA patients of Caucasian origin.
Keywords: disease association, gene polymorphism, nuclear factor-κB pathway, rheumatoid arthritis, Toll-like receptors
1. Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disorder characterized by systemic inflammation and persistent synovitis that affects joints and promotes joint destruction [1]. The signal cascade mediated by Toll-Like Receptors (TLRs) and the nuclear factor κB (NF-κB) results in an immune response via rapidly increased production of cytokines, such as TNF-α, and chemokines, that may lead to inflammatory and joint destructive process.
TLRs, a family of phylogenetically conserved receptors that recognize self- and non-self-molecular patterns, which have a critical function in both innate and adaptive immune responses [2] and have been suggested to play a role in RA. High expression of TLRs 1–6 by synovial fibroblasts has been observed at an early stage of RA [3]. TLR3 and TLR4 constituted the most abundant TLRs in synovial fibroblasts and their expression levels were comparable to the levels of patients with longstanding disease. Moreover, one of the TLR4 gene polymorphisms was reported to impair response to single disease-modifying antirheumatic drug (DMARD) treatment in recent-onset RA [4].
The nuclear factor-κB (NF-κB) family of transcription factors was identified more than 20 years ago [5]. The NF-κB is an important regulator of innate and adaptive immune responses, and it affects expression of hundreds of genes involved in regulation of proliferation, survival, stress responses, angiogenesis, inflammation and even malignant transformation. The transcription factor NF-κB is a central regulator of inflammation and can be activated by TLRs. TLR2 and TLR9 activate inflammation through the canonical NF-κB pathway, while TLR4 is involved in activation of inflammation through the canonical or non-canonical NF-κB pathway. The TLRs initiate a kinase cascade that ultimately activates the IκB kinase (IKK) complex, which phosphorylates and degrades the NF-κB inhibitor IκBa. NF-κB is shuttled from the cytosol to the nucleus, where it initiates expression of pro- and anti-inflammatory cytokines [6].
Our and other recent studies have documented that polymorphisms located within genes encoding cytokines regulated by NF-κB, IL-17A and IL-17F [7,8,9], or by TNF-α and its receptor [10], may be associated with RA susceptibility and response to therapy with TNF-α inhibitors. Clinical factors only partly explain variation in response to anti-TNF therapy. It has been suggested that gender is probably not a predictor of response, but disease activity and poor functional ability at baseline could be [11].
The present study aimed to assess the effect of the polymorphisms in TLR2, TLR4, TLR9 and NF-κB1 genes, involved in regulation of the NF-κB pathway, on susceptibility to RA, progression of the disease and response to therapy with TNF-α blocking agents.
For this purpose, 110 patients with high disease activity (the 28-joint Disease Activity Score; DAS 28 > 5.1) at baseline and 126 healthy individuals were investigated and typed for the TLR2 (rs111200466, −196/−174 del/ins), TLR4 (rs4986790, Asp299Gly, 13,843 A > G; rs4986791, Thr399Ile, 14,143 C > T), TLR9 (rs5743836, −1237 C > T; rs187084, −1486 T > C) and NF-κB1 (rs28362491, −94 ins/del ATTG) alleles.
2. Results
2.1. Response to Treatment
Clinical data of 87 Caucasian patients with RA treated with TNF-α inhibitors were analyzed. Among them, 50% were treated with etanercept (ETA), 36% with adalimumab (ADA), 8% with infliximab (INF) and 6% with certolizumab pegol (CER) (Table 1). Mean DAS28 at the onset of biological treatment was 6.59 ± 0.73 (range 5.14–8.05). Among subgroups treated with different anti-TNF agents, DAS28 values at the beginning were as follows: ETA—6.64 ± 0.74, ADA—6.54 ± 0.81, INF—6.64 ± 0.62, CER—6.53 ± 0.43 (ns). Mean DAS28 after 24 weeks of treatment was 4.0 ± 1.12 (range 1.97–6.88) for the whole group of patients, while in the subgroups treated with TNF inhibitors DAS28 were: ETA—3.84 ± 1.13, ADA—4.11 ± 0.95, INF—4.87 ± 1.71, CER—3.34 ± 0.14 (ns).
Table 1.
Characteristics of RA patients for whom associations of single nucleotide polymorphisms with disease progression and response to therapy with TNF-α inhibitors were analyzed.
| RA Patients (N =) | 87 |
|---|---|
| Sex (female/male); n (%) | 71 (82%)/16 (18%) |
| Age (years) | 50.7 ± 12.3 (range: 17–77) |
| Females (%) | 71 (82%) |
| Disease duration (years) | 12.4 ± 8.3 (range 1–39) |
| Disease onset (years) | 38.8 ± 12.0 (range 15–65) |
| Current smokers (%) | 14 |
| RF+ Rheumatoid factor positive, n (%) | 72 |
| ACPA+/Anti-CCP present, n (%) | 47 |
| Stage, n (%) | |
| 1 | 2 (2.3%) |
| 2 | 20 (23%) |
| 3 | 51 (58.6%) |
| 4 | 14 (16.1%) |
| DAS28 at baseline | 6.59 ± 0.73 (range 5.14–8.05) |
| DAS28 at week 24 | 4.0 ± 1.12 (range 1.97–6.88) |
| anti-TNF drug | |
| etanercept (%) | 44 (50%) |
| adalimumab (%) | 32 (36%) |
| infliximab (%) | 7 (8%) |
| certolizumab pegol (%) | 5 (6%) |
| Glucocorticosteroids % | 79 (mean dose 9.3 mg prednisone daily) |
| Methotrexate % | 71 (mean dose 20.4 mg weekly) |
RA—rheumatoid arthritis, RF—rheumatoid factor, ACPA—anti-citrullinated protein antibodies, DAS 28—disease activity score 28, TNF—tumour necrosis factor.
2.2. Distribution of Alleles and Genotypes of TLRs and NF-κB Encoding Genes in RA Patients and Controls, Associations with Disease Susceptibility and Progression
All allelic variants were detected in both groups of individuals studied. Minor allele frequency (MAFs) values (Table 2) were similar in patients and controls, except for the TLR9 (rs187084; −1486 T > C) polymorphism.
Table 2.
Minor allele frequencies (MAFs) of the polymorphisms studied in Polish patients with rheumatoid arthritis and healthy individuals.
| Polymorphism | Minor Allele | MAF RA Patients | MAF Controls |
|---|---|---|---|
| NF-κB1 (rs28362491, −94 del/ins ATTG) | del | 0.423 | 0.385 |
| TLR2 (rs111200466, −196/−174 del/ins) | del | 0.168 | 0.179 |
| TLR4 (rs4986790, 13843 A > G) | G | 0.077 | 0.065 |
| TLR4 (rs4986791, 14143 C > T) | T | 0.077 | 0.074 |
| TLR9 (rs5743836; −1237 C > T) | C | 0.135 | 0.083 |
| TLR9 (rs187084; −1486 T > C) | T | 0.495 | 0.131 |
Of the six polymorphisms studied, 2 single nucleotide polymorphisms (SNP) within the TLR9 gene (rs5743836, −1237 C > T and rs187084, −1486 T > C) were found to be associated with predisposition to the disease. Distribution of alleles and genotypes of the TLR2, TLR4, TLR9 and NF-κB1 genes is given in Table 3.
The TLR9 −1237 C wild type allele was more frequently detected among female patients than controls. This allele was detected in 23 out of 87 (26.44%) female patients with RA and in 5 out of 63 (7.94%) healthy females (OR = 3.816, p = 0.005). Thus, the TLR9 gene (rs5743836, −1237 C > T) substitution seemed to be associated with predisposition to the disease in females only.
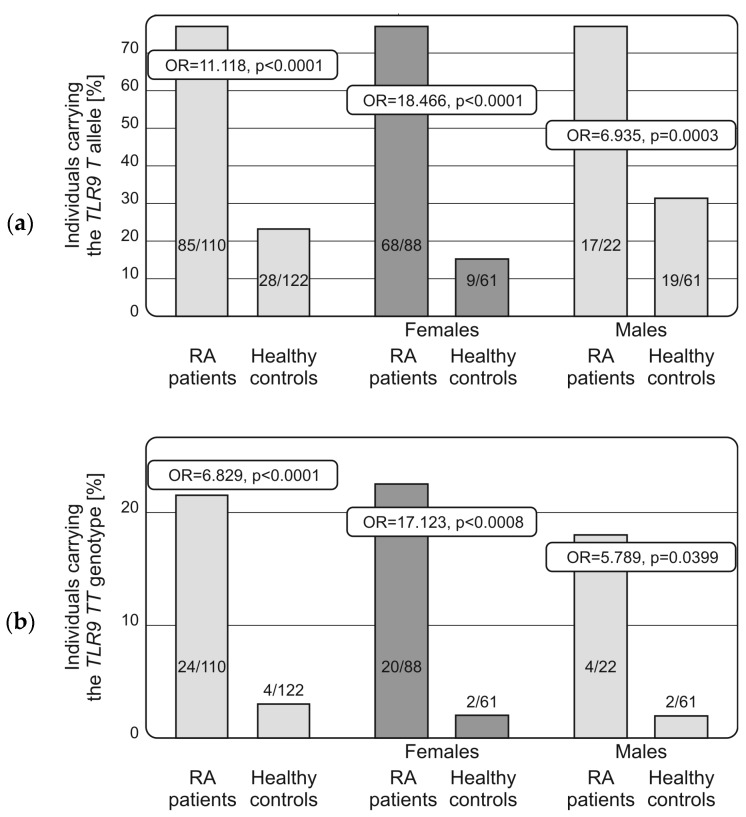
Interestingly, the TLR9 −1486 T allele was more frequently detected among patients with RA than healthy individuals, independently of the sex (OR = 11.118, p < 0.001, Table 3; OR = 18.466 and OR = 6.935 for females and males, respectively, with p < 0.0001 in both cases, Figure 1).
Table 3.
Distribution of the TLRs and NF-κB1 alleles and genotypes in Polish patients with rheumatoid arthritis and healthy individuals. The TLR2 and TLR9 polymorphisms were associated with predisposition to RA. HWE stands for Chi2 p-values calculated for Hardy-Weinberg Equilibrium assessment.
| Polymorphism | RA Patients | Controls | OR, p | ||
|---|---|---|---|---|---|
| n | (%) | n | (%) | ||
| NF-κB1 (rs28362491, −94 del/ins ATTG) | |||||
| del/del | 19 | 17.3 | 14 | 11.1 | |
| del/ins | 55 | 50.0 | 69 | 54.8 | |
| ins/ins | 36 | 32.7 | 43 | 34.1 | |
| del | 74 | 67.3 | 83 | 65.9 | |
| ins | 91 | 82.7 | 112 | 88.9 | |
| HWE | p = 0.798 | p = 0.079 | |||
| TLR2 (rs111200466, −196/−174 del/ins) | |||||
| del/del | 1 | 1.8 | 7 | 5.5 | |
| del/ins | 35 | 31.8 | 31 | 24.6 | |
| ins/ins | 74 | 67.3 | 88 | 69.8 | |
| del | 36 | 32.7 | 39 | 30.9 | |
| ins | 109 | 90.1 | 119 | 94.4 | |
| HWE | p = 0.150 | p = 0.070 | |||
| TLR4 (rs4986790, Asp299Gly, 13843 A > G) | |||||
| AA | 94 | 85.5 | 107 | 87.0 | |
| AG | 15 | 13.6 | 16 | 13.0 | |
| GG | 1 | 0.9 | 0 | 0.0 | |
| A | 109 | 99.1 | 123 | 100.0 | |
| G | 16 | 14.5 | 16 | 13.0 | |
| HWE | p = 0.646 | p = 0.440 | |||
| TLR4 (rs4986791, Thr399Ile, 14,143 C > T) | |||||
| CC | 94 | 85.5 | 104 | 85.2 | |
| CT | 15 | 13.6 | 18 | 14.8 | |
| TT | 1 | 0.9 | 0 | 0.0 | |
| C | 109 | 99.1 | 122 | 100.0 | |
| T | 16 | 14.5 | 0 | 0.0 | |
| HWE | p = 0.646 | p = 0.379 | |||
| TLR9 (rs5743836; −1237 C > T) | |||||
| CC | 1 | 0.9 | 0 | 0.0 | |
| CT | 28 | 25.2 | 21 | 16.7 | |
| TT | 81 | 73.9 | 105 | 83.3 | |
| C | 29 | 26.4 | 21 | 16.7 | (association in females only) # |
| T | 109 | 99.1 | 105 | 83.3 | |
| HWE | p = 0.397 | p = 0.308 | |||
| TLR9 (rs187084; −1486 T > C) | |||||
| TT | 24 | 21.8 | 4 | 3.3 | OR = 6.829, p < 0.0001 |
| TC | 61 | 55.5 | 24 | 19.7 | OR = 4.995, p < 0.0001 |
| CC | 25 | 22.7 | 94 | 77.0 | |
| T | 85 | 77.3 | 28 | 22.9 | OR = 11.118, p < 0.0001 |
| C | 86 | 78.2 | 118 | 96.7 | |
| HWE | p = 0.252 | p = 0.131 | |||
# Association of the C allele with RA risk: OR = 3.816, p = 0.005 observed in females only (23 out of 87 (26.44%) female patients with RA vs. 5 out 63 (7.94%) of healthy females).
Figure 1.
Distribution of the TLR9 (rs187084) T allele (a) and TT homozygous genotype (b) in female and male patients with rheumatoid arthritis and healthy individuals. The presence of the T allele and its homozygosity was found to be associated with predisposition to the disease in females and males.
Similarly, the TT homozygosity was found to be associated with RA susceptibility and detected in 24 out of 110 patients and 4 out of 112 controls (OR = 6.829, p < 0.001, Table 3), respectively. This relationship was independent of the sex (OR = 7.123, p = 0.0008 and OR = 5.789, p = 0.0399 for females and males, respectively, Figure 1).
There were no significant differences in the distribution of the NF-κB1 (rs28362491; −94 ins/del ATTG), TLR2 (rs201786064, −196/−174 del/ins) and TLR4 (rs4986790; Asp299Gly, 13,843 A > G; and rs4986791, Thr399Ile, 14,143 C > T) alleles and genotypes between the groups of RA patients and healthy controls (Table 2). The analyzed non-synonymous TLR4 polymorphisms were in strong linkage disequilibrium in both patients and healthy individuals. The TLR4 rs4986790 A allele was detected together with the C allele of the TLR4 rs4986791 and, in an analogous manner, the G allele—alongside the T allele. Only two individuals within the control group differed and were characterized by the rs498679 AA/rs4986791 CT haplotype.
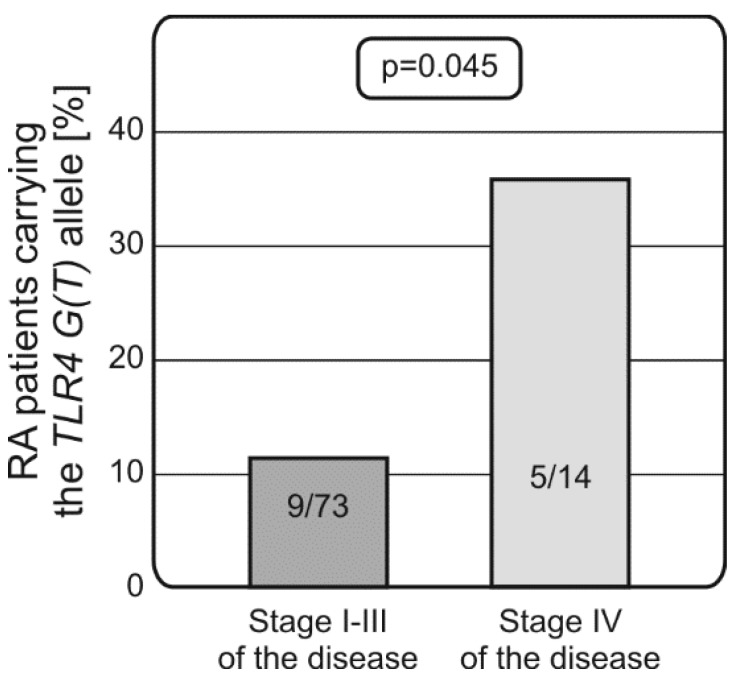
Interestingly, patients carrying the TLR4 rs4986790 G (rs4986791 T) alleles were more likely to display stage IV of the disease (0.36 vs. 0.12, p = 0.045, Figure 2).
Figure 2.
Both nonsynonymous TLR4 (rs4986790; Asp299Gly, 13,843 A > G; and rs4986791, Thr399Ile, 14,143 C > T) polymorphisms are associated with predisposition to stage IV of RA. The presence of the TLR4 rs4986791 G (rs4986790 T) allele was more frequently detected in patients presenting with stage IV of the disease.
However, no significant relationship was observed between any of the polymorphisms studied and RA diagnostic markers (RF, anti-CCP, CRP).
2.3. Genetic Variability within the TLRs and NF-κB1 Genes and Response to Therapy with TNF-α Blocking Agents
Analyses of the response to therapy with TNF inhibitors in relation to the studied genetic polymorphisms within the TLRs and NF-κB encoding genes showed some significant associations.
Worse (EULAR) response to the anti-TNF-α therapy after 6 months of treatment was observed in patients carrying the TLR9 −1486 TT homozygous genotype (11/60 patients with DAS28 <5.1 vs. 6/11 patients with DAS28 >5.1 after 6 months of treatment, p = 0.018). Of note, this homozygosity was found to be associated with RA manifestation in both females and males.
Moreover, patients carrying the TLR4 rs4986790 G (rs4986791 T) alleles (more frequently present among patients in stage IV disease) were characterized by less effective response to the anti-TNF-α treatment (p = 0.012), similarly to those carrying the ATTG insertion in the NF-κB1 gene (rs28362491; −94 ins/del). The ins/ins homozygosity within the NF-κB1 gene, associated with increased promoter activity (and thus a higher expression of proinflammatory cytokines), was more frequently detected among RA patients with inefficient response to anti-TNF treatment (39% vs. 17%, p = 0.047). The presence of the del allele increased this efficiency and the NF-κB1 heterozygosity showed the most promising effect (p = 0.005).
3. Discussion
In the present study, we aimed to find out whether polymorphic variants of the gene coding for TLR2, TLR4 and TLR9 innate receptors, as well as NF-κB1 transcription factor, could be associated with predisposition to rheumatoid arthritis, progression of the disease, and response to anti-TNF treatment. The previous studies have suggested an influence of these polymorphisms on the innate immune response and etiology of some autoimmune diseases, however, none of those studies concerned Polish populations.
Some potential associations of polymorphic variants of the genes coding for TLRs with RA susceptibility in various populations have been described [12,13,14]. A relationship between TLR3 rs3775291 genetic variants and sero-negative RA has been reported in Caucasian population from Denmark [12]. Also, a significant correlation of SNPs rs2072493 in TLR5 and rs3853839 in TLR7 with RA risk was observed in Dutch population, however replication cohorts of this study did not confirm these findings [13].
The TLR4 polymorphism resulting in the Asp299Gly amino acid substitution (rs4986790) did not appear to affect susceptibility to RA in UK patients [15], nor in 5 other European studies [16], which also concurs with our current observations. Additionally, a recently published meta-analysis did not show any significant association of this SNP with predisposition to RA [14].
In the current study, the presence of the TLR4 rs4986790 G (rs4986791 T) allele, resulting in 299Gly (399Ile), was more frequently detected among patients with stage IV disease, and was also associated with more effective response to anti-TNF-α therapy. In line with our observations, Kuuliala et al. [4] found that RA patients carrying the TLR4 G allele (299Gly) were characterized by significantly higher DAS28 and lower remission rate after single DMARD treatment.
Davis et al. [17] demonstrated other SNPs within the TLR4 gene-rs1927911 to affect RA progression. In their study, disease activity measures progressed less over time in the homozygous minor allele group rs1927911. In addition, Davis et al. [17] reported an influence of TLR4 rs1927911 polymorphism on disease progression in RA. Clinical measures of disease progression, including DAS28, correlated inversely with presence of the TLR4 rs1927911 TT genotype among patients.
The results of the present study also suggest that two SNPs located within the TLR9 gene might be involved in RA pathogenesis, supporting the hypothesis that microbial infection may play a role in RA and that the infection triggers inflammatory responses recognized by pattern recognition receptors (including TLRs). In our study, both TLR9 polymorphisms were found to be associated with predisposition to the disease (the TLR9 −1237 C allele and the TLR9 −1486 T allele and its homozygosity). These relationships have not been previously described in the literature, although these SNPs were studied in context of other autoimmune disorders.
The T allele of TLR9 gene polymorphism (rs5743836, −1237 C > T) was found to act as a risk factor of systemic lupus erythematosus (SLE) in South Indian Tamils [18]. The inconsistent results of this report and the present study stem from ethnic and sex from differences between studied populations.
The TLR9 −1486 T > C polymorphism (rs187084) has also been described to be significantly associated with increased susceptibility to ophthalmopathy in Taiwanese male patients with Graves’ disease [19]. In addition, relationships between two other TLR9 gene polymorphisms (rs352139, rs352140) and lupus nephritis development in a Chinese Han population were observed [20].
Interestingly, Bharti et al. [21] have recently found that the presence of the TLR9 −1486 C allele increases transcriptional activity of the TLR9 gene, which in turn induces high levels of Interferon gamma-induced protein 10 (IP-10) in patients with pulmonary tuberculosis; however, expression of proinflammatory cytokines such as IFNγ and TNFα was significantly lower in TLR9 C allele carriers as compared to those with the TLR9 −1486 T allele. These results suggest that individuals carrying the TLR9 −1486 T genetic variant (including the TLR9 −1486 TT homozygotes) produce more IFNγ and TNFα, whose increased levels have been observed in RA patients. Moreover, our preliminary results suggested a strong tendency between TLR9 −1486 C > T SNP and TNFα levels was observed in our study (187.787 vs. 119.608 pg/mL; T = 1.725, p = 0.0973; T-Test for 2 Independent Means; for TT vs. C carriers 3 months after anti-TNF treatment, respectively). Of note, both TLR9 −1486 T allele and its homozygosity correlated with predisposition to RA development while the TLR9 −1486 T homozygous genotype was associated with less favorable response to therapy with TNFα inhibitors.
According to our current knowledge, there has been only one published study to date suggesting the potential role of the TLRs genes in response to anti-TNFα treatment. Coenen et al. [13] reported the association of the SNP rs2072493 in TLR5 gene with anti-TNF treatment outcome in Dutch patients with RA however this relationship wasn’t observed in the Swedish population. There have been also no previous studies showing the effect of the NF-κB1 genetic variants on the anti-TNF therapy outcome, although our results suggest that the presence of the del allele within the NF-κB1 gene favors positive response to therapy with TNFα blocking agents.
Taken together, the present study contributes to the reports on genetic factors associated with RA and broaden our knowledge regarding RA pathogenesis. Our results suggest that 2 out of 6 polymorphisms studied, SNPs rs5743836 and rs187084 within the TLR9 gene, could be considered valuable prediction factors in estimation of the risk for RA development and prognosis. Moreover, anti-TNFα treatment outcome was found to be affected by both TLR9 SNPs (rs5743836 and rs187084), TLR4 non-synonymous substitutions and ins/del polymorphisms within the NF-κB1 gene.
Obviously, due to relatively small number of individuals investigated, our findings need to be confirmed by extended studies involving an independent cohort of patients before drawing the final conclusions. It would be also interesting to relate polymorphisms and expression/protein production of the investigated genes in RA patients with different manifestations of the disease and response to therapy with TNFα blocking agents. Further studies are warranted to elucidate the role of the TLR9 and TLR2 polymorphisms in pathogenesis of RA.
4. Materials and Methods
4.1. Patients and Controls
One hundred and ten patients (female/male: 87/23) diagnosed with RA were investigated. The following inclusion criteria were used: consent to participate in the study; confirmed RA based on the 2010 American College of Rheumatology/European League Against Rheumatism (EULAR) classification criteria; active form of the disease: DAS 28 > 5.1; age over 18; women and men with reproductive potential had to use reliable contraception; taking NSAIDs and glucocorticosteroids in stable doses was allowed.
The following exclusion criteria were used: pregnancy or breastfeeding; coexistence of other systemic diseases of connective tissue besides RA; clinically significant impairment of hepatic or renal function; alcohol abuse; infection with hepatotropic viruses; infections resistant to therapy; ongoing history of cancer if no cure was achieved; uncontrolled diabetes; patient unwilling or unable to cooperate.
Patients who had been treated with recommended doses of TNF-α inhibitors (adalimumab, etanercept, infliximab, certolizumab) for at least 3 months or had stopped therapy because of adverse events were investigated. To examine the response to anti-TNF therapy in RA, blood samples, laboratory data and clinical data were collected at baseline (prior to anti-TNF therapy), 3 and 6 months after treatment. Clinical evaluation was based on medical history, number of painful and swollen joints, pain intensity assessed by the patient on a 100 mm visual analogue scale (VAS) and laboratory tests (ESR, CRP). The parameters made it possible to determine improvement according to the criteria based on DAS 28, suggested by the EULAR.
One hundred and ten patients were analyzed for disease susceptibility (in comparison to healthy individuals). Eighty-seven cases were investigated for associations with disease progression and response to therapy with TNF-α inhibitors (Table 1). All patients provided written informed consent. The study was approved by the Wroclaw Medical University Ethics Committee (No. KB – 625/2016, 29 December 2016) and the procedures were in accordance with the ethical standards of the Helsinki Declaration, as revised in 2013.
Stages of RA were assessed according to Wheeless [22]. Following this classification, first stage RA is characterized by synovitis or an inflammation of the synovial membrane causing swelling of involved joints and pain upon motion. However, there is no x-ray evidence of joint destruction, with the exception of swelling of soft tissues or early stages of osteoporosis. In stage II, there is a spread of inflammation in synovial tissue affecting joint cavity space across joint cartilage. This inflammation leads to gradual destruction of cartilage accompanied by narrowing of the joint. Severe RA, stage III, is marked by formation of pannus in the synovium. Loss of joint cartilage exposes bone beneath the cartilage. These changes become evident in x-rays, together with erosions and signs of deformation. Stage IV is called terminal or end stage RA. The inflammatory process has subsided and formation of fibrous tissue and/or fusing of bone results in ceased joint function. Rheumatoid nodules may also be present in patients in stage IV of the disease.
In addition, 126 healthy individuals of both sexes (female/male: 63/63) served as controls.
4.2. DNA Isolation
DNA was extracted from samples of peripheral blood taken on EDTA using Maxwell 16 Blood DNA Purification Kit (Promega Corp., Madison, WI, USA) or silica membranes (Qiagen, Hilden, Germany) following the recommendations of the manufacturers.
4.3. NF-κB1 Genotyping
The NF-κB1 (rs28362491; −94 ins/del ATTG) polymorphism was analyzed using polymerase chain reaction combined with capillary gel electrophoresis performed in the 3500 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) instrument. Electrophoresis demonstrated the presence of a 154 bp fragment for the del allele and/or of a 158 bp fragment in length for the ins allele.
4.4. TLR4 Genotyping
Two SNPs within the exon 3 of the TLR4 gene (rs4986790, Asp299Gly, 13,843 A > G; and rs4986791, Thr399Ile, 14,143 C > T) were determined by real-time polymerase chain reaction (PCR) amplifications using LightSNiP assays designed for both polymorphisms by TIB MOLBIOL (Berlin, Germany). The reaction was performed following the recommendation of the manufacturer on the LightCycler 480 instrument (Roche Diagnostics GmbH, Basel, Switzerland).
4.5. TLR2 Genotyping
The PCR was employed for analysis of the 23 bp del/ins polymorphism within the TLR2 (rs111200466, −196/−174 del/ins) gene using the following oligonucleotides: forward primer: 5′-GCG GAC TTT CCC TTT TGC T-3′ and reverse primer: 5′-GTG CAG AGA GAC CAC ACG AG-3′. The PCR conditions were as follows: 95 °C for 5 min; 35 cycles of 95 °C for 30 s, 57 °C for 30 s and 72 °C for 30 s; and a final elongation step at 72 °C for 10 min. Amplified products of either 134 bp (deletion) or 157 bp in length (insertion) were then analyzed by electrophoresis in a 2.5% agarose gel stained with Simply Safe (EURx, Gdańsk, Poland) and visualized under UV light.
4.6. TLR9 Genotyping
For analysis of the rs5743836 (−1237 C > T) and rs187084 (−1486 T > C) polymorphisms in the TLR9 gene, polymerase chain reaction restriction fragment length polymorphism (PCR-RFLP) assay was employed [23]. Sequences of forward primers for the TLR9 rs5743836 and rs187084 genotypings were 5′-ATG GGA GCA GAG ACA TAA TGG A-3′ and 5′-TCC CAG CAG CAA CAA TTC ATT A-3′, respectively. The common reverse primer 5′-CTG CTT GCA GTT GAC TGT GT-3′ was used for both analyses. The PCR conditions were alike for both rs5743836 and rs187084 polymorphisms: 95 °C for 5 min; 35 cycles of 95 °C for 30 s, 60 °C for 30 s and 72 °C for 30 s; and finally, 72 °C for 10 min. Validations of the amplification products of 135 bp in length for the rs5743836 and 499 bp for the rs187084 polymorphism were performed via electrophoresis in a 2% agarose gel with Simply Safe (EURx, Gdańsk, Poland) and visualization in the UV light. The next step was digestion of the PCR products with either BstNI (for the rs5743836 polymorphism) or AflII restriction endonuclease (for the rs187084 polymorphism) (both from New England BioLabs Inc., Ipswich, MA, USA). Analysis of the final products was performed in a 3% agarose gel stained with Simply Safe (EURx, Poland) and visualized under UV light. The observed patterns were 60 bp, 48 bp and 27 bp for the C allele or 108 bp and 27 bp for the T allele of the TLR9 rs5743836 polymorphism. As for the rs187084 polymorphism in the TLR9 gene, final products were of 499 bp for the C allele or 172 bp and 327 bp in length for the T allele.
4.7. Statistical Analysis
Genotype and allele frequencies were compared between the study groups by the Chi2 test with Yates correction or Fisher’s exact test when necessary, using online tools (Available online: http://www.socscistatistics.com/tests/Default.aspx). The odds ratio (OR) was calculated by Haldane’s modification of Woolf’s method and the significance of its deviation from unity was estimated by Fisher’s exact test. Probability values <0.01 were considered statistically significant, and those between 0.05 and 0.1 as indicative of a trend.
Acknowledgments
Supported by the UMO-2016/21/B/NZ5/01901 scientific grant from the National Science Center and by Wroclaw Centre of Biotechnology program—The Leading National Research Centre (KNOW) for years 2014–2018 (519-1-4-3-174).
Abbreviations
| DAS 28 | 28-Joint Disease Activity Score |
| DMARD | Disease-Modifying Antirheumatic Drug |
| EULAR | European League Against Rheumatism |
| HWE | Hardy-Weinberg Equilibrium |
| IKK | IκB Kinase |
| MAF | Minor Allele Frequency |
| NF-κB | Nuclear Factor-κB |
| OR | Odds Ratio |
| p | Probability |
| RA | Rheumatoid Arthritis |
| RF | Rheumatoid Factor |
| SNP | Single Nucleotide Polymorphism |
| TLR | Toll-Like Receptor |
| TNF-α | Tumor Necrosis Factor α |
| VAS | Visual Analogue Scale |
Author Contributions
Katarzyna Bogunia-Kubik conceived and designed the experiments; Katarzyna Gębura and Barbara Wysoczańska performed the experiments; Katarzyna Gębura, Jerzy Świerkot, Barbara Wysoczańska and Beata Nowak analyzed the data; Jerzy Świerkot, Lucyna Korman, Piotr Wiland and Katarzyna Bogunia-Kubik contributed reagents/materials/analysis tools; Katarzyna Gębura and Katarzyna Bogunia-Kubik wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Lee D.M., Weinblatt M.E. Rheumatoid arthritis. Lancet. 2001;358:903–911. doi: 10.1016/S0140-6736(01)06075-5. [DOI] [PubMed] [Google Scholar]
- 2.Shin H.S., Jung Y.S., Rim H. Toll-like receptors. Br. J. Med. Med. Res. 2013;3:58–68. doi: 10.9734/BJMMR/2013/2071. [DOI] [Google Scholar]
- 3.Ospelt C., Brentano F., Rengel Y., Stanczyk J., Kolling C., Tak P.P., Gay R.E., Gay S., Kyburz D. Overexpression of toll-like receptors 3 and 4 in synovial tissue from patients with early rheumatoid arthritis: Toll-like receptor expression in early and longstanding arthritis. Arthritis Rheum. 2008;58:3684–3692. doi: 10.1002/art.24140. [DOI] [PubMed] [Google Scholar]
- 4.Kuuliala K., Orpana A., Leirisalo-Repo M., Kautiainen H., Hurme M., Hannonen P., Korpela M., Möttönen T., Paimela L., Puolakka K., et al. Polymorphism at position +896 of the toll-like receptor 4 gene interferes with rapid response to treatment in rheumatoid arthritis. Ann. Rheum. Dis. 2006;65:1241–1243. doi: 10.1136/ard.2006.055137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986;46:705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- 6.Verstrepen L., Bekaert T., Chau T.L., Tavernier J., Chariot A., Beyaert R. TLR-4, IL-1R and TNF-R signaling to NF-κB: Variations on a common theme. Cell. Mol. Life Sci. 2008;65:2964–2978. doi: 10.1007/s00018-008-8064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bogunia-Kubik K., Świerkot J., Malak A., Wysoczańska B., Nowak B., Białowąs K., Gębura K., Korman L., Wiland P. IL-17A, IL-17F and IL-23R Gene Polymorphisms in Polish Patients with Rheumatoid Arthritis. Arch. Immunol. Ther. Exp. 2015;63:215–221. doi: 10.1007/s00005-014-0319-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marwa O.S., Kalthoum T., Wajih K., Kamel H. Association of IL17A and IL17F genes with rheumatoid arthritis disease and the impact of genetic polymorphisms on response to treatment. Immunol. Lett. 2017;183:24–36. doi: 10.1016/j.imlet.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Paradowska-Gorycka A., Wojtecka-Lukasik E., Trefler J., Wojciechowska B., Lacki J.K., Maslinski S. Association between IL-17F gene polymorphisms and susceptibility to and severity of rheumatoid arthritis (RA) Scand. J. Immunol. 2010;72:134–141. doi: 10.1111/j.1365-3083.2010.02411.x. [DOI] [PubMed] [Google Scholar]
- 10.Świerkot J., Bogunia-Kubik K., Nowak B., Bialowas K., Korman L., Gebura K., Kolossa K., Jeka S., Wiland P. Analysis of associations between polymorphisms within genes coding for tumour necrosis factor (TNF)-α and TNF receptors and responsiveness to TNF-α blockers in patients with rheumatoid arthritis. Jt. Bone Spine. 2015;82:94–99. doi: 10.1016/j.jbspin.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Emery P., Dörner T. Optimising treatment in rheumatoid arthritis: A review of potential biological markers of response. Ann. Rheum. Dis. 2011;70:2063–2070. doi: 10.1136/ard.2010.148015. [DOI] [PubMed] [Google Scholar]
- 12.Laska M.J., Hansen B., Troldborg A., Lorenzen T., Stengaard-Pedersen K., Junker P., Nexø B.A., Lindegaard H.M. A non-synonymous single-nucleotide polymorphism in the gene encoding Toll-like Receptor 3 (TLR3) is associated with sero-negative Rheumatoid Arthritis (RA) in a Danish population. BMC Res. Notes. 2014;7:716. doi: 10.1186/1756-0500-7-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coenen M.J., Enevold C., Barrera P., Schijvenaars M.M., Toonen E.J., Scheffer H., Padyukov L., Kastbom A., Klareskog L., Barton A., et al. Genetic variants in toll-like receptors are not associated with rheumatoid arthritis susceptibility or anti-tumour necrosis factor treatment outcome. PLoS ONE. 2010;5:e14326. doi: 10.1371/journal.pone.0014326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tizaoui K., Naouali A., Kaabachi W., Hamzaoui A., Hamzaoui K. Association of Toll like receptor Asp299Gly with rheumatoid arthritis risk: A systematic review of case-control studies and meta-analysis. Pathol. Res. Pract. 2015;211:219–225. doi: 10.1016/j.prp.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Kilding R., Akil M., Till S., Amos R., Winfield J., Iles M.M., Wilson A.G. A biologically important single nucleotide polymorphism within the toll-like receptor-4 gene is not associated with rheumatoid arthritis. Clin. Exp. Rheumatol. 2003;21:340–342. [PubMed] [Google Scholar]
- 16.Lee Y.H., Bae S.C., Kim J.H., Song G.G. Toll-like receptor polymorphisms and rheumatoid arthritis: A systematic review. Rheumatol. Int. 2014;34:111–116. doi: 10.1007/s00296-013-2666-7. [DOI] [PubMed] [Google Scholar]
- 17.Davis M.L., LeVan T.D., Yu F., Sayles H., Sokolove J., Robinson W., Michaud K., Thiele G.M., Mikuls T.R. Associations of toll-like receptor (TLR)-4 single nucleotide polymorphisms and rheumatoid arthritis disease progression: An observational cohort study. Int. Immunopharmacol. 2015;24:346–352. doi: 10.1016/j.intimp.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 18.Devaraju P., Gulati R., Antony P.T., Mithun C.B., Negi V.S. Susceptibility to SLE in South Indian Tamils may be influenced by genetic selection pressure on TLR2 and TLR9 genes. Mol. Immunol. 2015;64:123–126. doi: 10.1016/j.molimm.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Liao W.L., Chen R.H., Lin H.J., Liu Y.H., Chen W.C., Tsai Y., Wan L., Tsai F.J. Toll-like receptor gene polymorphisms are associated with susceptibility to Graves’ ophthalmopathy in Taiwan males. BMC Med. Genet. 2010;11:154. doi: 10.1186/1471-2350-11-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou X.J., Lv J.C., Cheng W.R., Yu L., Zhao M.H., Zhang H. Association of TLR9 gene polymorphisms with lupus nephritis in a Chinese Han population. Clin. Exp. Rheumatol. 2010;28:397–400. [PubMed] [Google Scholar]
- 21.Bharti D., Kumar A., Mahla R.S., Kumar S., Ingle H., Shankar H., Joshi B., Raut A.A., Kumar H. The role of TLR9 polymorphism in susceptibility to pulmonary tuberculosis. Immunogenetics. 2014;66:675–681. doi: 10.1007/s00251-014-0806-1. [DOI] [PubMed] [Google Scholar]
- 22.Wheeless C.R. Rheumatoid arthritis. In: Wheeless C.R., Nunley J.A., Urbaniak J.R., editors. Wheeless’ Textbook of Orthopaedics. Data Trace Internet Publishing, LLC; Broklandville, MA, USA: 2012. [Google Scholar]
- 23.Liu F., Lu W., Qian Q., Qi W., Hu J., Feng B. Frequency of TLR 2, 4, and 9 gene polymorphisms in Chinese population and their susceptibility to type 2 diabetes and coronary artery disease. J. Biomed. Biotechnol. 2012;2012:373945. doi: 10.1155/2012/373945. [DOI] [PMC free article] [PubMed] [Google Scholar]