Abstract
Autophagy, a lysosome-mediated intracellular degradation and recycling pathway, plays multiple context-dependent roles in tumorigenesis and treatment resistance. Encouraging results from various preclinical studies have led to the initiation of numerous clinical trials with the intention of targeting autophagy in various cancers. Accumulating knowledge of the particular mechanisms and players involved in different steps of autophagy regulation led to the ongoing discovery of small molecule inhibitors designed to disrupt this highly orchestrated process. However, the development of validated autophagy-related biomarkers, essential for rational selection of patients entering clinical trials involving autophagy inhibitors, is lagging behind. One possible source of biomarkers for this purpose is the autophagy machinery itself. In this review, we address the recent trends, challenges and advances in the assessment of the biomarker potential of clinically relevant autophagy proteins in human cancers.
Keywords: autophagy, cancer, biomarkers, immunohistochemistry
1. Introduction
Macroautophagy (herein referred to as autophagy) is a lysosome-mediated degradation and recycling process that functions in both tumor suppression and tumor progression, depending on the stage of tumorigenesis. In advanced malignancies, autophagy promotes cancer cell survival and contributes to cancer progression and drug resistance [1], hence becoming a promising target for anticancer therapy.
Numerous clinical trials investigating the pharmacologic autophagy inhibitors chloroquine (CQ) and hydroxychloroquine (HCQ) in various cancers are underway [2]. These lysosome-targeting inhibitors were previously approved by the Food and Drug Administration (FDA) for the treatment of malaria and later were repurposed for autophagy inhibition [3]. The first published results [4,5,6] demonstrate only a moderate effectiveness of HCQ in combination with chemotherapy, indicating the need for the development of more potent and selective autophagy inhibitors as well as reliable criteria for better selection of patients entering clinical trials. While autophagy inhibitors that target specific proteins involved in different steps of the autophagy process are under development [7,8,9,10,11,12,13,14], it is crucial to determine cancer types suitable for each of these autophagy inhibition strategies. Therefore, the search for markers of autophagy, or response to autophagy modulation, in cancer patients is a research area of relevance to both clinicians and scientists.
Due to the dynamic nature of autophagy and complexity of its regulation, simultaneous assessment of several autophagy markers will likely be required to assess autophagy status. However, many autophagy proteins have been shown to function in other cellular processes, including cell survival and apoptosis, modulation of cellular trafficking, protein secretion, cell signaling, transcription, translation and membrane reorganization [15]. Each protein known to be involved in the autophagy machinery, when evaluated separately from other autophagy proteins, could have an independent biomarker potential in cancer patients, which is not necessarily related to autophagy. Caution has to be taken, therefore, when interpreting the results from studies that report association of tumoral expression of autophagy-related proteins with clinicopathological characteristics and/or patient outcomes. Two distinct goals—evaluation of autophagy status versus assessment of biomarker potential—need to be clearly separated.
Biomarkers are typically classified as “prognostic” or “predictive”. A prognostic biomarker provides information on the likely outcome of the disease in an untreated individual and is helpful in identifying patients for adjuvant systemic therapies. A predictive biomarker helps select patients who will likely benefit from a given treatment [16,17,18]. In practice, the distinction between these categories is not straightforward, and many biomarkers have both prognostic and predictive values, and also serve as therapeutic targets [17,19]. Following the definitions above, all the markers reviewed here (Table 1) fall currently into the category of “prognostic” markers, and future studies are required to establish whether they may also have predictive values for specific treatment strategies.
Table 1.
Prognostic values of autophagy-related proteins in cancers.
| Candidate Marker * | Prognostic Value ** | |
|---|---|---|
| Poor | Favorable | |
| LC3B | BC [22,48,49], GC [50,51,52], CRC [53,54,55,56], melanoma [22], astrocytoma [57], esophageal cancer [58], oral SCC [59], and HCC [60] | NSCLC [61], BC [62] |
| LC3A | CRC [63], BC [64], GC [65], NSCLCs [66], HCC [67] and clear cell OC [68] (stone-like structures) | CRC [63] and BC [64] (juxtanuclear accumulation) |
| GABARAP | CRC patients [69] | |
| p62 | endometrial cancer, [70], oral SCC [59], epithelial OC [71], and NSCLC [72,73], BC [74,75] | |
| ULK1 | CRC [76], esophageal SCC [77], HCC [78], and nasopharygeal carcinoma [79] | BC [80], GC [51] |
| Beclin1 | CRC [48,81] ***; [56,82,83] | GC, BC, NSCLC, CRC, lymphoma [48] *** |
| ATG3 | GC [51] | |
| ATG10 | CRC [84] | GC [51] |
| FIP200 | BC [85] | |
| Autophagy “signature”: ULK1, Beclin 1, ATG3, and ATG10 | GC [51] | |
| Autophagy “signature”: LC3B, ATG5, Beclin 1, Ambra1 and Bif-1 | PDAC [86] | |
* Protein expression was evaluated by immunohistochemistry; ** Prognostic value was based on the association of high protein expression with patient outcomes. BC, breast cancer; GC, gastric cancer; CRC, colorectal cancer; HCC, hepatocellular carcinoma; NSCLC, non-small cell lung cancer; PDAC, pancreatic ductal adenocarcinoma; OC, ovarian cancer; SCC, squamous cell carcinoma; *** based on meta-analysis of multiple publications.
Numerous technologies are used to analyze biomarker values of different types of biomolecules such as DNA, RNA, proteins, and peptides [20,21]. Here, we will focus on the autophagy-related protein biomarkers, although validated autophagy-related protein biomarkers and protocols have not yet been approved for use in a clinical setting. Indeed, scoring systems for the evaluation of autophagy-related protein markers in tissues can differ dramatically between researchers. While manual scoring appears to be prevalent in the literature, some authors [22] take an automated approach, especially for large-scale studies. While acknowledging the variability of staining techniques and antibodies used, a lack of a uniform scoring system, as well as context-dependent roles of autophagy in cancers, below we summarize the recent trends in evaluating the biomarker potential of key autophagy-related proteins in various human cancers. We first provide a brief overview of the autophagy machinery itself to place the various protein biomarkers described into the context of the autophagy pathway.
2. Autophagy Machinery
Autophagy is a tightly-regulated multi-step process that involves more than 30 core autophagy-related (ATG) proteins [23,24]. The ATG proteins act to initiate, promote and complete the formation of double-membrane autophagosomes that fuse with lysosomes to form autolysosomes where the contents are degraded and recycled by the cell. A detailed overview of autophagic machinery has been described in several excellent reviews [1,25,26], and we include only a brief summary here.
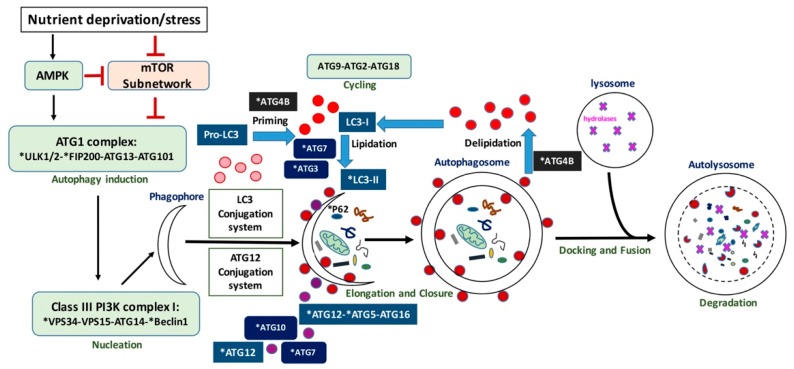
One of the main autophagy regulators is the mechanistic target of rapamycin (mTOR) protein kinase [27]; however, mTOR-independent autophagy [28] has also been described. As shown in Figure 1, autophagy is initiated by the activation of the serine/threonine Unc-51-like kinases 1 and 2 (ULK1 and ULK2), that receive signals from the master nutrient sensors mTOR and AMP-activated protein kinase (AMPK) [29]. ULK1/2 then forms a complex with ATG13, ATG101, and FIP200 [30]. This complex regulates the induction of autophagosome formation. The activity of the ULK1 kinase is required for the recruitment of the phosphatidylinositol 3-kinase catalytic subunit type 3 (PIK3C3/VPS34) to the phagophore—the cup-shaped double-membrane precursor of the autophagosome. Along with VPS15 (the PI3K regulatory subunit 4), ATG14, and the scaffold protein Beclin 1, VPS34 forms the class III PI3K complex I, which produces phosphatidylinositol 3-phosphate (PI(3)P) at the sites of phagophore nucleation. When clustered, PI(3)Ps create a cytosol-facing platform for the binding of proteins (such as WIPI/II) required for the recruitment of machineries, involved in the so-called “elongation reaction” [30,31]. This next stage in autophagosome formation requires two ubiquitin-like conjugation systems: the conjugation of ATG12 to ATG5 and ATG16L1 and, downstream to it, conjugation of Atg8 (in yeast)/microtubule-associated protein 1 light chain 3 (MAP1LC3, or LC3, in mammals) to phosphatidylethanolamine (PE). The E1-like enzyme ATG7 is involved in both conjugation processes; E2-like enzymes ATG10 and ATG3 function in ATG12-ATG5-ATG16L1 and LC3-PE conjugation systems, respectively [25,30,32,33]. In addition, the ATG12-ATG5 conjugate may act as an E3-like enzyme for the conjugation of LC3 to PE and, together with ATG16L1, is responsible for the recruitment of LC3-PE to the phagophore [34,35,36]. Prior to conjugation, LC3 is processed by the autophagy related 4B cysteine peptidase (ATG4B). ATG4B is responsible for the cleavage of the carboxyl terminus of newly synthesized pro-LC3 to provide LC3-I [37], a reaction essential for further LC3-I conjugation to PE to form a membrane-bound LC3-II during autophagosome formation. That is required for elongation of the autophagosome. ATG4B also functions in de-conjugation of LC3 from the autophagosome membrane to ensure recycling of LC3 in the cell [38]. ATG9 and its cycling system (ATG2, ATG9 and ATG18) play a role in lipid delivery to the expanding autophagosome membrane [25,30,32]. LC3 further interacts with various adaptor proteins such as p62/Sequestosome 1 (SQSTM1) that function to recruit cargo from the cytoplasm.
Figure 1.
Schematic representation of autophagy. Autophagy proteins indicated with an asterisk have been investigated as potential biomarkers.
The process of phagophore closure is poorly understood and possibly regulated by ATG2A and ATG2B which are recruited to early autophagosomal membranes enriched in PI(3)P, where they associate with WIPI1 (WD-repeat protein interacting with phosphoinositides 1) [39]. GABA Type A Receptor-Associated Protein Like 2 (GABARAPL2/GATE-16), one of the LC3/GABARAP family members, has also been shown to mediate closure of the vesicle [35]. After closure, autophagosomes fuse with lysosomes to form autolysosomes, where the contents are degraded by hydrolases. The degradation products (e.g., amino acids) are released to the cytosol in a process involving soluble NSF-attachment protein receptors (SNAREs) and recycled by the cell [2,40].
Some mammalian orthologs of yeast ATG genes are represented by families of paralogous genes. For instance, mentioned above ATG4B belongs to the ATG4 family of cysteine proteases, which in mammals also includes ATG4A, ATG4C, and ATG4D proteins [41]. MAP1LC3 is a member of the ATG8 family, represented by three subfamilies in mammals: LC3 (LC3A, LC3B, LC3B2, LC3C), GABARAP (GABARAP, GABARAPL1), and GATE-16/GABARAPL2 [42]. Each family member, while demonstrating some functional redundancy with other members, plays distinct roles in the autophagy process [35,43]. For example, LC3s are required for the elongation step [35,44], whereas the proteins from GABARAP/GATE-16 subfamilies are thought to play important roles in autophagy initiation [44], as well as autophagosome closure [35]. A recent study by Nguyen et al. [42] provided deeper insights into the functions of different LC3/GABARAP family proteins during PINK1/Parkin mitophagy and starvation. They showed that, while these proteins are crucial for autophagosome–lysosome fusion and are likely to be important for regulating autophagosome size, they are dispensable for autophagosome formation. In spite of these new findings, LC3 remains a prominent autophagy protein and one of the main autophagosomal markers, used in a number of standard autophagy assays [45].
3. Candidate Autophagy-Related Biomarkers
In this review, we focus on the potential targets for autophagy modulation in cancer patients and summarize the recent reports on biomarker potential of these targets in different cancers (Table 1). Currently, the development of drugs for autophagy modulation follows two major directions: (1) lysosomal inhibitors and their derivatives; and (2) small molecule inhibitors targeting autophagy proteins. Examples of the first approach include CQ and HCQ, the main players in today’s clinical trials, as well a novel dimeric derivative of chloroquine Lys05 [46,47] which is currently being optimized for clinical trials [2]. Common autophagy markers, such as LC3B and p62, utilized in a variety of standard assays to measure autophagy turnover (also known as flux) in vitro and in vivo, may serve as useful readouts for the effects of lysosomal inhibitors on autophagy status in tumors. The potential targets from the second group include Beclin-1, ULK1, ATG4, ATG7, and VPS34.
3.1. LC3B
One of the best studied autophagy-related proteins, the microtubule-associated protein 1 light chain 3B (MAP1LC3B, or LC3B), has long served as an autophagy marker in multiple in vitro assays. The expression levels of LC3B protein have also been examined by immunohistochemistry (IHC) in many cancers. However, only a few recent studies (see below) differentiated among the various forms of LC3B, particularly the cytosolic (LC3B-I) and membrane-bound (LC3B-II, or LC3B “puncta”) forms [87,88].
Taking into consideration that treatment with lysosomal inhibitors results in accumulation of LC3B-II due to blockade of autophagosome-lysosome fusion, which leads to inhibition of LC3B degradation, establishing the biomarker potential of LC3B in different cancers might be useful for the selection of patients for treatment with CQ and its derivatives.
Recent systematic review and analysis by He et al. [48] showed that high protein expression of LC3B predicted adverse overall survival in breast cancer (HR = 1.98, 95% CI = 1.25–3.13), however, none of the studies included in this meta-analysis specified the pattern of LC3B staining in tumor samples. Lazova et al. [22] specifically addressed detection of LC3B puncta, rather than diffuse staining, in specimens from a relatively large cohort of breast cancer patients (n = 640). In this study, increased LC3B puncta expression also significantly correlated with poor prognosis. As breast cancer is a highly heterogeneous group of diseases, several authors attempted to look at LC3B expression depending on breast cancer subtype. Choi et al. [89] reported the highest LC3B expression in triple-negative breast cancers, and Zhao et al. [49] observed the association of high LC3B expression with poor overall survival and disease progression in patients with this cancer subtype. In these two studies the staining pattern (puncta vs. diffuse) was not specified. In the study by Lazova et al. [22], LC3B puncta expression was highest in the HER2/neu-positive subtype, followed by the triple-negative, luminal B, and luminal A subtypes. Interestingly, association of LC3B expression with poor outcomes reached significance only in luminal A tumors; the prognostic associations in the other 3 subtypes (luminal B, HER2/neu positive, and triple negative) did not reach statistical significance, possibly due to the limited number of patient samples [22]. Another study by Cha et al. [90] compared expression of LC3B along with other autophagy-related proteins, including Beclin-1 and p62, in invasive lobular carcinoma (ILC) versus invasive ductal carcinoma (IDC), and found that the expression of all these proteins was significantly higher in IDC. These results indicate a clear need for additional studies in large patient cohorts to determine LC3B prognostic values in different breast cancer histological and molecular subtypes.
The association of high LC3B expression with aggressive disease and poor outcomes was repeatedly reported in other cancer types, including gastric adenocarcinoma [50,51,52], colorectal cancers [53,54,55,56], melanoma [22], astrocytoma [57], esophageal cancer [58], oral squamous cell carcinoma [59] and hepatocellular carcinoma [60]. Interestingly, high expression of LC3B was associated with decreased overall survival in the KRAS-mutated subgroup of colorectal cancers, but not in the KRAS-wildtype [53]. This finding together with the previous functional studies that showed “addiction” of KRAS-mutated cancers to autophagy [91,92,93] suggests that further patient stratification based on molecular alterations is required to fully evaluate the biomarker potential of LC3B in different cancers. For instance, a recent study in non-small cell lung cancer [61] evaluated the punctated pattern of LC3B together with p62 expression levels and pointed to the possibility of improved prognosis in the high-LC3B puncta expression group. It may be informative to further subgroup the patients based on mutation status, including alterations in KRAS.
3.2. Other LC3/GABARAP Family Members
Although less studied than LC3B, microtubule-associated protein 1 light chain 3A (LC3A) has also been investigated as an autophagy marker. Earlier studies defined three distinct patterns of IHC staining of LC3A in solid tumors: diffuse cytoplasmic staining, juxtanuclear staining, and staining of “stone-like” structures (SLS) [64]. Each of these patterns appears to bear different prognostic value. For instance, juxtanuclear accumulation of LC3A in tumor cells correlated with good prognosis in colorectal [63] and breast cancers [64], whereas increased numbers of SLS were linked to poor prognosis in colorectal [63], breast [64], gastric [65] and non-small cell lung cancers [66], as well as hepatocellular carcinoma [67] and clear cell ovarian carcinoma [68].
To the best of our knowledge, only one study explored the prognostic significance of another mammalian homolog of yeast Atg8, gamma-aminobutyric acid type A (GABAA) receptor-associated protein (GABARAP), in human cancer. High expression of GABARAP was associated with poor differentiation and shortened overall survival in colorectal cancer patients [69].
3.3. p62/SQSTM1
Initially identified as a mediator of NFκB signaling, p62/SQSTM1 is now known as a “signaling hub” for diverse cellular events including amino acid sensing and the oxidative stress response [94]. p62 also functions as a molecular adaptor between the autophagic machinery and its substrates [95]. Due to its degradation during the autophagic process, p62 was proposed to serve as a marker of autophagic flux. For instance, accumulation of p62 protein by Western blot is usually considered indicative of autophagy inhibition [45]. However, caution should be taken when interpreting the results of p62-related assays, as they might be affected by complex regulation of p62 at both the transcriptional and post-translational levels [95]. Despite these known caveats, many authors still use p62 as an indicator of autophagic flux both in vitro and in vivo, and there are numerous publications related to p62 as an autophagy biomarker in human cancer specimens.
With or without a connection to autophagy, the vast majority of reports show an association of high p62 expression with poor prognosis. The examples include, but are not limited to, endometrial cancer, [70], oral squamous cell carcinoma [59], epithelial ovarian cancer [71], and non-small cell lung cancer [72,73], where high expression of cytoplasmic p62 but low expression of nuclear p62 significantly correlated with aggressive tumors and adverse prognosis. In breast cancer, an earlier publication by Rolland et al. [74] showed that p62 cytoplasmic expression correlated with grade, distant metastasis, and reduced five-year survival; in addition, there was a significant association with EGF receptor (EGFR), HER2, HER3, and HER4 expression. High expression of p62 in triple-negative breast cancers was also shown to be prognostic of poor outcome [75]. It would be useful to know whether p62 (both cytoplasmic and nuclear-localized) is differentially expressed and/or has different prognostic values across various molecular subtypes in breast cancer and other cancers. Overall, published data to date indicate that p62 expression is a poor prognostic marker in various cancer types.
3.4. ULK-1/2
Being the only serine/threonine kinases in the core autophagy machinery and possibly the most upstream components of the canonical autophagy pathway [7,96,97], ULK-1 and ULK-2 became attractive drug targets [7,8]. While the development of ULK-1/2 small molecule inhibitors is underway [7,8,98], the data on the prognostic value of ULK-1/2 in different cancers is scarce and contradictory.
Tang et al. [80] reported an association of low expression of ULK-1 with adverse patient prognosis in breast cancer. Similarly, ULK-1 was shown to be a favorable prognostic marker in gastric cancer [51]. On the contrary, in colorectal cancer patients, high ULK-1 expression was found to be a predictor of poor prognosis [76]. Another study in colorectal cancer patients [53] did not show any correlation with survival, even after stratification according to KRAS status, but linked ULK-1 high expression to the presence of lymph node metastasis. Poor prognostic value of high ULK-1 expression was also shown in esophageal squamous cell carcinoma [77], hepatocellular carcinoma [78], and nasopharygeal carcinoma [79]. The expression level of ULK-2 was reported to be significantly higher in prostate cancer tissue than in the adjacent normal prostate tissue [99], however no association with prognosis was available from this or other studies. Additional studies in larger patient cohorts are required to better define the prognostic values of ULK1 and ULK2 in different cancer types.
3.5. Beclin-1 and VPS34
The Beclin 1-VPS34 complex is one of the central coordinators of autophagy downstream of ULK1 [96]. Several groups reported development of potent VPS34 inhibitors [11,12,13,14,100], but it remains unclear which cancer patients will most likely benefit from these inhibitors.
As Beclin 1 interacts with members of the anti-apoptotic Bcl-2 protein family [101], loss of Beclin 1 expression (allelic loss or suppression by microRNAs) defines poor prognosis presumably by enhancing anti-apoptotic pathways. Overexpression of Beclin 1, linked with tumor hypoxia and acidity, also defines subgroups of tumors with aggressive clinical behavior [82], presumably by promoting autophagy.
Systematic review and meta-analysis by He et al. [48] identified Beclin-1 as a favorable prognostic marker in gastric cancer, breast cancer, lung cancer, and lymphoma, whereas in colorectal cancer the results were split between favorable and poor prognostic values. Another meta-analysis by Han et al. [81] showed that high Beclin-1 expression in patients with colorectal cancer was associated with poor prognosis in terms of tumor distant metastasis and overall survival. Additional studies in colorectal cancer further supported the poor prognostic value of Beclin1 in colorectal cancers [56,82,83]. To address seemingly contradictory reports in colorectal cancer, Han et al. [81] stratified patients according to treatment status and found that high Beclin-1 expression was associated with reduced survival in the patients who received chemotherapy, while among the patients without chemotherapy, high Beclin-1 levels were associated with longer overall survival. Knowing that the majority of chemotherapeutic drugs induce autophagy [40,102], which often plays stress-adaptive roles, we can hypothesize that high Beclin-1 expression in chemotherapy-treated patients indicated increased autophagy levels which promoted resistance to the chemotherapy treatment.
As with other biomarkers in breast cancer, it will be important to examine the expression of Beclin-1 in different histologic and molecular subtypes as was done in a study by Cha et al. [90], who showed that ILC had lower expression of Beclin-1 compared to IDC; in ILC, Beclin-1 expression correlated significantly with ER negativity and was variably expressed according to molecular subtypes, with the highest expression in triple-negative breast cancer.
3.6. ATG4B
Due to its central enzymatic roles in the autophagy process, the cysteine protease ATG4B became one of the autophagy proteins being pursued as a potential therapeutic target [9,10], but little is known about its prognostic value in different cancers. Previous reports showed elevated ATG4B expression in colorectal tumor cells [103], chronic myeloid leukemia [104], and lung cancer cells [105]. However, there are no reports in the literature on the prognostic value of ATG4B, or any of the other ATG4 family members, in cancers.
3.7. Additional Autophagy-Related Biomarkers
Although not widely pursued or reported in the literature as potential targets, other autophagy-related proteins have been studied in terms of their biomarker potential. The results of these studies are inconsistent and suggest context-dependency. In gastric cancer, low expression of ATG10 and ATG3 were associated with lymph node metastasis and advanced TNM stage; both ATG10 and ATG3 were found to be favorable independent prognostic factors for overall survival [51]. In contrast, the opposite trend was reported in colorectal cancer patients: high ATG10 expression correlated with tumor lymph node metastasis, invasion, and adverse prognosis [84].
Although examined in multiple functional studies involving various in vivo models for autophagy modulation, ATG7 expression did not correlate with survival in lung [106] and gastric [51] cancers. Additional studies are required before eliminating ATG7 as a potential prognostic biomarker.
Similar uncertainty applies to another autophagy-related protein, ATG5. While several studies explored ATG5 expression in different cancers [107,108] only a few reported prognostic values. In breast cancer patients, ATG5, along with FIP200, was shown to be a favorable prognostic marker [85]. In contrast, in oral squamous cell carcinoma, ATG5 expression was associated with high tumor grade, advanced clinical stage, large tumor size, and lymph node metastasis; however, there was no statistically significant correlation with prognosis [109]. Additional studies are also needed to provide a better understanding of biomarker potential of ATG5.
3.8. Evaluation of an “Autophagy Signature” in Cancer
Several groups [51,53] have recently attempted to evaluate the expression of multiple autophagy-related proteins simultaneously to determine the prognostic value of a so-called “autophagy signature” in cancer. The idea behind this strategy was that the specific combination of autophagy-related markers evaluated concurrently might reflect the dynamic nature of the autophagy process and its role in tumorigenesis.
Cao et al. [51] assessed prognostic values of 10 markers (ULK1, Beclin 1, ATG3, ATG5, ATG7, ATG9, ATG10, ATG12, LC3B and p62/SQSTM1) in a relatively large cohort of 352 gastric cancer patients. Out of 10 markers, only four (ULK1, Beclin 1, ATG3, and ATG10) demonstrated correlation with prognosis, each being an independent favorable prognostic factor. In the combination analysis, patients with positive expression of all four markers had superior survival compared with those having less than four positive markers [51]. However, no correlation between the markers was reported, which makes the interpretation of these findings difficult. Another study by Ko et al. [86] presented an evaluation of the expression of five autophagy-related proteins—LC3B, ATG5, Beclin 1 and its two cofactors, AMBRA1 (activating molecule in beclin-1-regulated autophagy) and Bif-1 (Bax-interacting factor 1)—in the resected pancreatic ductal adenocarcinoma tissues from a relatively small cohort of 73 patients. The correlation between the expression of autophagy-related proteins was significant for all protein pairs. Multivariate analysis revealed that high beclin-1 expression and high expression of all autophagy-related proteins were independently associated with poor prognosis [86]. The whole cohort was split into “high” and “low” autophagy types according to the number of highly expressed markers (4–5 vs. 0–3, respectively). Although not statistically significant (probably due to the small cohort size), there was a trend towards decreased overall survival in the “high autophagy type” group [86]. In most other studies, though, the so-called “autophagy signature” included 2–4 markers only [50,53,56,85,89,109], often making the results difficult to interpret.
3.9. Secreted Factors as Potential Predictive Biomarkers
In addition to lysosomal degradation of autophagosomal contents, a novel role for autophagy that involves unconventional protein secretion was described [110]. Another recent study by Kraya et al. [111], which utilized quantitative proteomics to identify secreted factors characteristic of tumor cells with high autophagy levels compared to low-autophagy tumor cells, opened up an exciting possibility of indirect measurement of tumoral autophagy dynamics in plasma. Unique secreted factors may serve as predictive biomarkers and aid in patient selection for treatment involving autophagy inhibition, and might also be useful for patient follow-up to evaluate the subsequent response to a given treatment. These factors included LIF (leukemia inhibitory factor), IL1B (interleukin 1, β), CXCL8 (chemokine (C-X-C motif) ligand 8), FAM3C (family with sequence similarity 3, member C), and DKK3 (dickkopf WNT signaling pathway inhibitor 3)—factors implicated in immunity and inflammation [111]. The authors chose melanoma as a model, and although this particular set of markers validated well in both in vitro and in vivo (patient specimens), further studies are needed to show whether these candidate biomarkers are relevant for other cancer subtypes or whether the autophagy-dependent secretome signatures are context (i.e., cancer type)-specific. Nonetheless, with growing evidence for the role of autophagy in immunity, inflammation, and the tumor microenvironment [112,113,114], as well as the promises of “liquid biopsies”, great attention should be given to the development in this direction.
4. Discussion and Future Directions
In our previous review in 2012 [32] we summarized the literature regarding expression levels of autophagy proteins in various cancer tissues. At that time, no clear picture could be drawn from the published data, and there existed many examples of inconsistent and conflicting reports on autophagy protein expression patterns. The reasons for these discrepancies included small patient cohorts, absence of standard techniques to process the tissues and analyze the results, lack of independent validation cohorts, and a limited number of autophagy-related markers (LC3, p62, and Beclin-1) available for assessment in patient samples.
In this review, we highlight the more recent advances in the evaluation of the biomarker potential of autophagy proteins in various cancers. Over the past five years, multiple groups accumulated data regarding IHC assessment of additional autophagy-related biomarkers in human cancer specimens (Table 1). While patterns have emerged (Table 1), these studies also showed that prognostic values of specific autophagy-related proteins, some of which are currently pursued as therapeutic targets, vary from one tumor type to another, from one disease stage to another, and between untreated and treated patients. These findings further support the context-dependent roles of autophagy in cancer, and also emphasize the need to interpret biomarker data in a corresponding context-dependent manner. Appreciation of the complexity and dynamic nature of the autophagy process has translated to increased interest in the evaluation of so-called “autophagy signatures” in different malignancies. This approach, focused on simultaneous assessment of multiple proteins in the autophagy pathway, deserves further attention along with the investigation of autophagy associated secreted factors.
Across the large and growing number of autophagy-related biomarker studies, the main challenges encountered previously still remain for the most part unresolved. These challenges include differences in staining techniques, scoring systems and cut-off points, limited sample sizes, and absence of independent validation processes. In addition, it is important to emphasize that knowing the prognostic relevance of autophagy-related proteins does not necessarily translate into their predictive value for the associated targeted therapeutics. New information regarding autophagy-related predictive biomarkers from the ongoing or future clinical trials is highly anticipated.
The question of how to track response to autophagy inhibitors in cancer patients remains one of the most outstanding in the field. An increase or decrease in the expression level of autophagosomal markers (such as LC3B) may not directly correlate with changes in autophagic activity, and is difficult to interpret, as well as monitor over time, especially in tissues. A recent attempt by Mahalingam et al. [115] to analyze peripheral blood mononuclear cells (PBMCs) from patients treated with HCQ and vorinostat suggested that tumor samples, although not readily accessible, are far more informative for the assessment of autophagy inhibition in vivo compared to PBMCs. In this study, in addition to LC3B and p62 levels, the authors evaluated the levels of the lysosomal protease cathepsin D (CTSD), which they previously showed to be a key mediator of CQ/HCQ and HCQ plus vorinostat-induced apoptosis. In this context, novel biomarkers indirectly related to autophagic activity and its modulation, such as autophagy products rather than autophagy machinery, should also be explored.
Autophagy is a highly regulated process, and autophagy proteins are subject to regulatory post-translational modifications, including phosphorylation, ubiquitination, and acetylation [116]. It is possible, although technically challenging, that the evaluation of the expression of modified (e.g., phosphorylated) forms of autophagy-related proteins in tumor tissues, which might be feasible via mass spectrometry-based approaches [117], will provide useful information regarding the biomarker potential of these proteins in cancers, and this is an important area for future development.
Although this review focuses on IHC evaluation of autophagy-related protein levels in cancer tissues, it should be noted that additional techniques have been considered for the discovery and monitoring of autophagy-related alterations and their biomarker potential in various cancers. For instance, Rothe et al. [104] examined transcript levels of several key autophagy and autophagy-related genes in chronic myeloid leukemia (CML) stem cells, and showed that ATG4B and ATG5 were differentially expressed in imatinib-nonresponders vs. responders. The authors of this study suggested that the unique autophagy gene expression signature may serve as a novel, clinically useful biomarker for predicting response to tyrosine kinase inhibitor therapy. Eissa et al. [118] identified and validated a novel autophagy transcript signature for the diagnosis of human bladder cancer. In this study, the expression levels of a number of autophagy genes, including ATG12 and ULK1, in paired bladder tissue and urine samples were significantly lower in bladder cancer than in the control group. A large-scale analysis of the mutational status of the genes encoding the entire core autophagy machinery, published by Lebovitz et al. [119], indicated that the core autophagy machinery largely escapes genomic alterations in human cancers. However, those tumors in which mutations were found, should be given further attention [93].
Overall, significant progress has been made over the last several years, and the development of new strategies and standardized approaches for the comprehensive evaluation of the multiplayer and multistep autophagy process in human tissues is underway.
Acknowledgments
The authors thank Kevin C. Yang, Morgana Xu and Robert Camfield for helpful discussions and comments on the manuscript. S. Bortnik is supported by a CIHR Frederick Banting and Charles Best Canada Graduate Scholarship Doctoral Award, as well as the University of British Columbia Four Year Doctoral Fellowship (4YF) Award. The authors are grateful for support from CIHR in partnership with Avon Foundation for Women—Canada grant OBC127216.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Galluzzi L., Pietrocola F., Bravo-San Pedro J.M., Amaravadi R.K., Baehrecke E.H., Cecconi F., Codogno P., Debnath J., Gewirtz D.A., Karantza V., et al. Autophagy in malignant transformation and cancer progression. EMBO J. 2015;34:856–880. doi: 10.15252/embj.201490784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rebecca V.W., Amaravadi R.K. Emerging strategies to effectively target autophagy in cancer. Oncogene. 2016;35:1–11. doi: 10.1038/onc.2015.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solitro A.R., MacKeigan J.P. Leaving the lysosome behind: Novel developments in autophagy inhibition. Future Med. Chem. 2016;8:73–86. doi: 10.4155/fmc.15.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rangwala R., Leone R., Chang Y.C., Fecher L.A., Schuchter L.M., Kramer A., Tan K.-S., Heitjan D.F., Rodgers G., Gallagher M., et al. Phase I trial of hydroxychloroquine with dose-intense temozolomide in patients with advanced solid tumors and melanoma. Autophagy. 2014;10:1369–1379. doi: 10.4161/auto.29118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poklepovic A., Gewirtz D.A. Outcome of early clinical trials of the combination of hydroxychloroquine with chemotherapy in cancer. Autophagy. 2014;10:1478–1480. doi: 10.4161/auto.29428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenfeld M.R., Ye X., Supko J.G., Desideri S., Grossman S.A., Brem S., Mikkelson T., Wang D., Chang Y.C., Hu J., et al. A phase I/II trial of hydroxychloroquine in conjunction with radiation therapy and concurrent and adjuvant temozolomide in patients with newly diagnosed glioblastoma multiforme. Autophagy. 2014;10:1359–1368. doi: 10.4161/auto.28984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egan D.F., Chun M.G.H., Vamos M., Zou H., Rong J., Miller C.J., Lou H.J., Raveendra-Panickar D., Yang C.-C., Sheffler D.J., et al. Small molecule inhibition of the autophagy kinase ULK1 and Identification of ULK1 substrates. Mol. Cell. 2015;59:285–297. doi: 10.1016/j.molcel.2015.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petherick K.J., Conway O.J.L., Mpamhanga C., Osborne S.A., Kamal A., Saxty B., Ganley I.G. Pharmacological inhibition of ULK1 kinase blocks mammalian Target of Rapamycin (mTOR)-dependent autophagy. J. Biol. Chem. 2015;290:11376–11383. doi: 10.1074/jbc.C114.627778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen T.G., Honson N.S., Arns S., Davis T.L., Dhe-Paganon S., Kovacic S., Kumar N.S., Pfeifer T.A., Young R.N. Development of fluorescent substrates and assays for the key autophagy-related cysteine protease enzyme, ATG4B. Assay Drug Dev. Technol. 2014;12:176–189. doi: 10.1089/adt.2013.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akin D., Wang S.K., Habibzadegah-Tari P., Law B., Ostrov D., Li M., Yin X.-M., Kim J.-S., Horenstein N., Dunn W.A. A novel ATG4B antagonist inhibits autophagy and has a negative impact on osteosarcoma tumors. Autophagy. 2014;10:2021–2035. doi: 10.4161/auto.32229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bago R., Malik N., Munson M.J., Prescott A.R., Davies P., Sommer E., Shpiro N., Ward R., Cross D., Ganley I.G., et al. Characterization of VPS34-IN1, a selective inhibitor of Vps34, reveals that the phosphatidylinositol 3-phosphate-binding SGK3 protein kinase is a downstream target of class III phosphoinositide 3-kinase. Biochem. J. 2014;463:413–427. doi: 10.1042/BJ20140889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasquier B., El-Ahmad Y., Filoche-Rommé B., Dureuil C., Fassy F., Abecassis P.-Y., Mathieu M., Bertrand T., Benard T., Barrière C., et al. Discovery of (2S)-8-[(3R)-3-methylmorpholin-4-yl]-1-(3-methyl-2-oxobutyl)-2-(trifluoromethyl)-3,4-dihydro-2H-pyrimido[1,2-a]pyrimidin-6-one: A novel potent and selective inhibitor of Vps34 for the treatment of solid tumors. J. Med. Chem. 2015;58:376–400. doi: 10.1021/jm5013352. [DOI] [PubMed] [Google Scholar]
- 13.Dowdle W.E., Nyfeler B., Nagel J., Elling R.A., Liu S., Triantafellow E., Menon S., Wang Z., Honda A., Pardee G., et al. Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo. Nat. Cell Biol. 2014;16:1069–1079. doi: 10.1038/ncb3053. [DOI] [PubMed] [Google Scholar]
- 14.Ronan B., Flamand O., Vescovi L., Dureuil C., Durand L., Fassy F., Bachelot M.-F., Lamberton A., Mathieu M., Bertrand T., et al. A highly potent and selective Vps34 inhibitor alters vesicle trafficking and autophagy. Nat. Chem. Biol. 2014;10:1013–1019. doi: 10.1038/nchembio.1681. [DOI] [PubMed] [Google Scholar]
- 15.Subramani S., Malhotra V. Non-autophagic roles of autophagy-related proteins. EMBO Rep. 2013;14:143–151. doi: 10.1038/embor.2012.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Italiano A. Prognostic or predictive? It’s time to get back to definitions! J. Clin. Oncol. 2011;29:4718–4719. doi: 10.1200/JCO.2011.38.3729. [DOI] [PubMed] [Google Scholar]
- 17.Mehta S., Shelling A., Muthukaruppan A., Lasham A., Blenkiron C., Laking G., Print C. Predictive and prognostic molecular markers for cancer medicine. Ther. Adv. Med. Oncol. 2010;2:125–148. doi: 10.1177/1758834009360519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oldenhuis C.N.A.M., Oosting S.F., Gietema J.A., de Vries E.G.E. Prognostic versus predictive value of biomarkers in oncology. Eur. J. Cancer. 2008;44:946–953. doi: 10.1016/j.ejca.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Luo H.-Y., Xu R.-H. Predictive and prognostic biomarkers with therapeutic targets in advanced colorectal cancer. World J. Gastroenterol. WJG. 2014;20:3858–3874. doi: 10.3748/wjg.v20.i14.3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carvajal-Hausdorf D., Schalper K.A., Neumeister V., Rimm D.L. Quantitative measurement of cancer tissue biomarkers in the lab and in the clinic. Lab. Investig. 2015;95:385–396. doi: 10.1038/labinvest.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goossens N., Nakagawa S., Sun X., Hoshida Y. Cancer biomarker discovery and validation. Transl. Cancer Res. 2015;4:256–269. doi: 10.3978/j.issn.2218-676X.2015.06.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lazova R., Camp R.L., Klump V., Siddiqui S.F., Amaravadi R.K., Pawelek J.M. Punctate LC3B expression is a common feature of solid tumors and associated with proliferation, metastasis, and poor outcome. Clin. Cancer Res. J. Am. Assoc. Cancer Res. 2012;18:370–379. doi: 10.1158/1078-0432.CCR-11-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ravikumar B., Sarkar S., Davies J.E., Futter M., Garcia-Arencibia M., Green-Thompson Z.W., Jimenez-Sanchez M., Korolchuk V.I., Lichtenberg M., Luo S., et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol. Rev. 2010;90:1383–1435. doi: 10.1152/physrev.00030.2009. [DOI] [PubMed] [Google Scholar]
- 24.Yang Z., Klionsky D.J. Mammalian autophagy: Core molecular machinery and signaling regulation. Curr. Opin. Cell Biol. 2010;22:124–131. doi: 10.1016/j.ceb.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng Y., He D., Yao Z., Klionsky D.J. The machinery of macroautophagy. Cell Res. 2014;24:24–41. doi: 10.1038/cr.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mizushima N., Yoshimori T., Ohsumi Y. The role of Atg proteins in autophagosome formation. Annu. Rev. Cell Dev. Biol. 2011;27:107–132. doi: 10.1146/annurev-cellbio-092910-154005. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy B.K., Lamming D.W. The mechanistic target of rapamycin: The grand conducTOR of metabolism and aging. Cell Metab. 2016;23:990–1003. doi: 10.1016/j.cmet.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarkar S. Regulation of autophagy by mTOR-dependent and mTOR-independent pathways: Autophagy dysfunction in neurodegenerative diseases and therapeutic application of autophagy enhancers. Biochem. Soc. Trans. 2013;41:1103–1130. doi: 10.1042/BST20130134. [DOI] [PubMed] [Google Scholar]
- 29.McAlpine F., Williamson L.E., Tooze S.A., Chan E.Y.W. Regulation of nutrient-sensitive autophagy by uncoordinated 51-like kinases 1 and 2. Autophagy. 2013;9:361–373. doi: 10.4161/auto.23066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bento C.F., Renna M., Ghislat G., Puri C., Ashkenazi A., Vicinanza M., Menzies F.M., Rubinsztein D.C. Mammalian autophagy: How does it work? Annu. Rev. Biochem. 2016;85:685–713. doi: 10.1146/annurev-biochem-060815-014556. [DOI] [PubMed] [Google Scholar]
- 31.Green D.R., Levine B. To be or not to be?: How selective autophagy and cell death govern cell fate. Cell. 2014;157:65–75. doi: 10.1016/j.cell.2014.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lebovitz C.B., Bortnik S.B., Gorski S.M. Here, there be dragons: Charting autophagy-related alterations in human tumors. Clin. Cancer Res. J. Am. Assoc. Cancer Res. 2012;18:1214–1226. doi: 10.1158/1078-0432.CCR-11-2465. [DOI] [PubMed] [Google Scholar]
- 33.Geng J., Klionsky D.J. The Atg8 and Atg12 ubiquitin-like conjugation systems in macroautophagy. “Protein modifications: Beyond the usual suspects” review series. EMBO Rep. 2008;9:859–864. doi: 10.1038/embor.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanada T., Noda N.N., Satomi Y., Ichimura Y., Fujioka Y., Takao T., Inagaki F., Ohsumi Y. The Atg12-Atg5 conjugate has a novel E3-like activity for protein lipidation in autophagy. J. Biol. Chem. 2007;282:37298–37302. doi: 10.1074/jbc.C700195200. [DOI] [PubMed] [Google Scholar]
- 35.Weidberg H., Shvets E., Shpilka T., Shimron F., Shinder V., Elazar Z. LC3 and GATE-16/GABARAP subfamilies are both essential yet act differently in autophagosome biogenesis. EMBO J. 2010;29:1792–1802. doi: 10.1038/emboj.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujita N., Itoh T., Omori H., Fukuda M., Noda T., Yoshimori T. The Atg16L complex specifies the site of LC3 lipidation for membrane biogenesis in autophagy. Mol. Biol. Cell. 2008;19:2092–2100. doi: 10.1091/mbc.E07-12-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanida I., Sou Y., Ezaki J., Minematsu-Ikeguchi N., Ueno T., Kominami E. HsAtg4B/HsApg4B/autophagin-1 cleaves the carboxyl termini of three human Atg8 homologues and delipidates microtubule-associated protein light chain 3-and GABAA receptor-associated protein-phospholipid conjugates. J. Biol. Chem. 2004;279:36268–36276. doi: 10.1074/jbc.M401461200. [DOI] [PubMed] [Google Scholar]
- 38.Satoo K., Noda N.N., Kumeta H., Fujioka Y., Mizushima N., Ohsumi Y., Inagaki F. The structure of Atg4B–LC3 complex reveals the mechanism of LC3 processing and delipidation during autophagy. EMBO J. 2009;28:1341–1350. doi: 10.1038/emboj.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfisterer S.G., Bakula D., Frickey T., Cezanne A., Brigger D., Tschan M.P., Robenek H., Proikas-Cezanne T. Lipid droplet and early autophagosomal membrane targeting of Atg2A and Atg14L in human tumor cells. J. Lipid Res. 2014;55:1267–1278. doi: 10.1194/jlr.M046359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thorburn A., Thamm D.H., Gustafson D.L. Autophagy and cancer therapy. Mol. Pharmacol. 2014;85:830–838. doi: 10.1124/mol.114.091850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang L., Li J., Ouyang L., Liu B., Cheng Y. Unraveling the roles of Atg4 proteases from autophagy modulation to targeted cancer therapy. Cancer Lett. 2016;373:19–26. doi: 10.1016/j.canlet.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen T.N., Padman B.S., Usher J., Oorschot V., Ramm G., Lazarou M. Atg8 family LC3/GABARAP proteins are crucial for autophagosome–lysosome fusion but not autophagosome formation during PINK1/Parkin mitophagy and starvation. J. Cell Biol. 2016;215:857–874. doi: 10.1083/jcb.201607039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shpilka T., Weidberg H., Pietrokovski S., Elazar Z. Atg8: An autophagy-related ubiquitin-like protein family. Genome Biol. 2011;12:226. doi: 10.1186/gb-2011-12-7-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schaaf M.B.E., Keulers T.G., Vooijs M.A., Rouschop K.M.A. LC3/GABARAP family proteins: Autophagy-(un)related functions. FASEB J. 2016;30:3961–3978. doi: 10.1096/fj.201600698R. [DOI] [PubMed] [Google Scholar]
- 45.Klionsky D.J., Abdelmohsen K., Abe A., Abedin M.J., Abeliovich H., Acevedo Arozena A., Adachi H., Adams C.M., Adams P.D., Adeli K., et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) Autophagy. 2016;12:1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McAfee Q., Zhang Z., Samanta A., Levi S.M., Ma X.-H., Piao S., Lynch J.P., Uehara T., Sepulveda A.R., Davis L.E., et al. Autophagy inhibitor Lys05 has single-agent antitumor activity and reproduces the phenotype of a genetic autophagy deficiency. Proc. Natl. Acad. Sci. USA. 2012;109:8253–8258. doi: 10.1073/pnas.1118193109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma X.-H., Piao S.-F., Dey S., Mcafee Q., Karakousis G., Villanueva J., Hart L.S., Levi S., Hu J., Zhang G., et al. Targeting ER stress–induced autophagy overcomes BRAF inhibitor resistance in melanoma. J. Clin. Investig. 2014;124:1406–1417. doi: 10.1172/JCI70454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He Y., Zhao X., Subahan N.R., Fan L., Gao J., Chen H. The prognostic value of autophagy-related markers beclin-1 and microtubule-associated protein light chain 3B in cancers: A systematic review and meta-analysis. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2014;35:7317–7326. doi: 10.1007/s13277-014-2060-4. [DOI] [PubMed] [Google Scholar]
- 49.Zhao H., Yang M., Zhao J., Wang J., Zhang Y., Zhang Q. High expression of LC3B is associated with progression and poor outcome in triple-negative breast cancer. Med. Oncol. Northwood Lond. Engl. 2013;30:475. doi: 10.1007/s12032-013-0475-1. [DOI] [PubMed] [Google Scholar]
- 50.Masuda G., Yashiro M., Kitayama K., Miki Y., Kasashima H., Kinoshita H., Morisaki T., Fukuoka T., Hasegawa T., Sakurai K., et al. Clinicopathological correlations of autophagy-related proteins LC3, Beclin 1 and p62 in gastric cancer. Anticancer Res. 2016;36:129–136. [PubMed] [Google Scholar]
- 51.Cao Q.-H., Liu F., Yang Z.-L., Fu X.-H., Yang Z.-H., Liu Q., Wang L., Wan X.-B., Fan X.-J. Prognostic value of autophagy related proteins ULK1, Beclin 1, ATG3, ATG5, ATG7, ATG9, ATG10, ATG12, LC3B and p62/SQSTM1 in gastric cancer. Am. J. Transl. Res. 2016;8:3831–3847. [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou H., Yuan M., Yu Q., Zhou X., Min W., Gao D. Autophagy regulation and its role in gastric cancer and colorectal cancer. Cancer Biomark. Sect. Dis. Markers. 2016;17:1–10. doi: 10.3233/CBM-160613. [DOI] [PubMed] [Google Scholar]
- 53.Schmitz K.J., Ademi C., Bertram S., Schmid K.W., Baba H.A. Prognostic relevance of autophagy-related markers LC3, p62/sequestosome 1, Beclin-1 and ULK1 in colorectal cancer patients with respect to KRAS mutational status. World J. Surg. Oncol. 2016;14:189. doi: 10.1186/s12957-016-0946-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang M., Zhao H., Guo L., Zhang Q., Zhao L., Bai S., Zhang M., Xu S., Wang F., Wang X., et al. Autophagy-based survival prognosis in human colorectal carcinoma. Oncotarget. 2015;6:7084–7103. doi: 10.18632/oncotarget.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guo G.-F., Jiang W.-Q., Zhang B., Cai Y.-C., Xu R.-H., Chen X.-X., Wang F., Xia L.-P. Autophagy-related proteins Beclin-1 and LC3 predict cetuximab efficacy in advanced colorectal cancer. World J. Gastroenterol. 2011;17:4779–4786. doi: 10.3748/wjg.v17.i43.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu S., Sun C., Tian D., Li Y., Gao X., He S., Li T. Expression and clinical significances of Beclin1, LC3 and mTOR in colorectal cancer. Int. J. Clin. Exp. Pathol. 2015;8:3882–3891. [PMC free article] [PubMed] [Google Scholar]
- 57.Winardi D., Tsai H.-P., Chai C.-Y., Chung C.-L., Loh J.-K., Chen Y.-H., Hsieh C.-L. Correlation of altered expression of the autophagy marker lc3b with poor prognosis in astrocytoma. Bio. Med. Res. Int. 2014;2014 doi: 10.1155/2014/723176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.El-Mashed S., O’Donovan T.R., Kay E.W., Abdallah A.R., Cathcart M.-C., O’Sullivan J., O’Grady A., Reynolds J., O’Reilly S., O’Sullivan G.C., et al. LC3B globular structures correlate with survival in esophageal adenocarcinoma. BMC Cancer. 2015;15:582. doi: 10.1186/s12885-015-1574-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu J.-L., Chen F.-F., Lung J., Lo C.-H., Lee F.-H., Lu Y.-C., Hung C.-H. Prognostic significance of p62/SQSTM1 subcellular localization and LC3B in oral squamous cell carcinoma. Br. J. Cancer. 2014;111:944–954. doi: 10.1038/bjc.2014.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu D.-H., Jia C.-C., Chen J., Lin Z.-X., Ruan D.-Y., Li X., Lin Q., Dong M., Ma X.-K., Wan X.-B., et al. Autophagic LC3B overexpression correlates with malignant progression and predicts a poor prognosis in hepatocellular carcinoma. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2014;35:12225–12233. doi: 10.1007/s13277-014-2531-7. [DOI] [PubMed] [Google Scholar]
- 61.Schläfli A.M., Adams O., Galván J.A., Gugger M., Savic S., Bubendorf L., Schmid R.A., Becker K.-F., Tschan M.P., Langer R., et al. Prognostic value of the autophagy markers LC3 and p62/SQSTM1 in early-stage non-small cell lung cancer. Oncotarget. 2016;7:39544–39555. doi: 10.18632/oncotarget.9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ladoire S., Penault-Llorca F., Senovilla L., Dalban C., Enot D., Locher C., Prada N., Poirier-Colame V., Chaba K., Arnould L., et al. Combined evaluation of LC3B puncta and HMGB1 expression predicts residual risk of relapse after adjuvant chemotherapy in breast cancer. Autophagy. 2015;11:1878–1890. doi: 10.1080/15548627.2015.1082022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Giatromanolaki A., Koukourakis M.I., Harris A.L., Polychronidis A., Gatter K.C., Sivridis E. Prognostic relevance of light chain 3 (LC3A) autophagy patterns in colorectal adenocarcinomas. J. Clin. Pathol. 2010;63:867–872. doi: 10.1136/jcp.2010.079525. [DOI] [PubMed] [Google Scholar]
- 64.Sivridis E., Koukourakis M.I., Zois C.E., Ledaki I., Ferguson D.J.P., Harris A.L., Gatter K.C., Giatromanolaki A. LC3A-positive light microscopy detected patterns of autophagy and prognosis in operable breast carcinomas. Am. J. Pathol. 2010;176:2477–2489. doi: 10.2353/ajpath.2010.090049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liao W., Sun L., Wang C., Huang H., Liu J., Liao W., Shi M. LC3A-positive “stonelike” structures predict an adverse prognosis of gastric cancer. Anat. Rec. 2014;297:653–662. doi: 10.1002/ar.22895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Karpathiou G., Sivridis E., Koukourakis M.I., Mikroulis D., Bouros D., Froudarakis M.E., Giatromanolaki A. Light-chain 3A autophagic activity and prognostic significance in non-small cell lung carcinomas. Chest. 2011;140:127–134. doi: 10.1378/chest.10-1831. [DOI] [PubMed] [Google Scholar]
- 67.Xi S.-Y., Lu J.-B., Chen J.-W., Cao Y., Luo R.-Z., Wu Q.-L., Cai M.-Y. The “stone-like” pattern of LC3A expression and its clinicopathologic significance in hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2013;431:760–766. doi: 10.1016/j.bbrc.2012.12.151. [DOI] [PubMed] [Google Scholar]
- 68.Spowart J.E., Townsend K.N., Huwait H., Eshragh S., West N.R., Ries J.N., Kalloger S., Anglesio M., Gorski S.M., Watson P.H., et al. The autophagy protein LC3A correlates with hypoxia and is a prognostic marker of patient survival in clear cell ovarian cancer. J. Pathol. 2012;228:437–447. doi: 10.1002/path.4090. [DOI] [PubMed] [Google Scholar]
- 69.Miao Y., Zhang Y., Chen Y., Chen L., Wang F. GABARAP is overexpressed in colorectal carcinoma and correlates with shortened patient survival. Hepato-Gastroenterology. 2010;57:257–261. [PubMed] [Google Scholar]
- 70.Iwadate R., Inoue J., Tsuda H., Takano M., Furuya K., Hirasawa A., Aoki D., Inazawa J. High expression of p62 protein is associated with poor prognosis and aggressive phenotypes in endometrial cancer. Am. J. Pathol. 2015;185:2523–2533. doi: 10.1016/j.ajpath.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 71.Iwadate R., Inoue J., Tsuda H., Takano M., Furuya K., Hirasawa A., Aoki D., Inazawa J. High expression of SQSTM1/p62 protein is associated with poor prognosis in epithelial ovarian cancer. Acta. Histochem. Cytochem. 2014;47:295–301. doi: 10.1267/ahc.14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang X., Du Z., Li L., Shi M., Yu Y. Beclin 1 and p62 expression in non-small cell lung cancer: Relation with malignant behaviors and clinical outcome. Int. J. Clin. Exp. Pathol. 2015;8:10644–10652. [PMC free article] [PubMed] [Google Scholar]
- 73.Inoue D., Suzuki T., Mitsuishi Y., Miki Y., Suzuki S., Sugawara S., Watanabe M., Sakurada A., Endo C., Uruno A., et al. Accumulation of p62/SQSTM1 is associated with poor prognosis in patients with lung adenocarcinoma. Cancer Sci. 2012;103:760–766. doi: 10.1111/j.1349-7006.2012.02216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rolland P., Madjd Z., Durrant L., Ellis I.O., Layfield R., Spendlove I. The ubiquitin-binding protein p62 is expressed in breast cancers showing features of aggressive disease. Endocr. Relat. Cancer. 2007;14:73–80. doi: 10.1677/erc.1.01312. [DOI] [PubMed] [Google Scholar]
- 75.Luo R.-Z., Yuan Z.-Y., Li M., Xi S.-Y., Fu J., He J. Accumulation of p62 is associated with poor prognosis in patients with triple-negative breast cancer. OncoTargets Ther. 2013;6:883–888. doi: 10.2147/OTT.S46222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zou Y., Chen Z., He X., He X., Wu X., Chen Y., Wu X., Wang J., Lan P. High expression levels of unc-51-like kinase 1 as a predictor of poor prognosis in colorectal cancer. Oncol. Lett. 2015;10:1583–1588. doi: 10.3892/ol.2015.3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jiang L., Duan B.-S., Huang J.-X., Jiao X., Zhu X.-W., Sheng H.-H., Gao H.-J., Yu H. Association of the expression of unc-51-Like kinase 1 with lymph node metastasis and survival in patients with esophageal squamous cell carcinoma. Int. J. Clin. Exp. Med. 2014;7:1349–1354. [PMC free article] [PubMed] [Google Scholar]
- 78.Xu H., Yu H., Zhang X., Shen X., Zhang K., Sheng H., Dai S., Gao H. UNC51-like kinase 1 as a potential prognostic biomarker for hepatocellular carcinoma. Int. J. Clin. Exp. Pathol. 2013;6:711–717. [PMC free article] [PubMed] [Google Scholar]
- 79.Yun M., Bai H.-Y., Zhang J.-X., Rong J., Weng H.-W., Zheng Z.-S., Xu Y., Tong Z.-T., Huang X.-X., Liao Y.-J., et al. ULK1: A promising biomarker in predicting poor prognosis and therapeutic response in human nasopharygeal carcinoma. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0117375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tang J., Deng R., Luo R.-Z., Shen G.-P., Cai M.-Y., Du Z.-M., Jiang S., Yang M.-T., Fu J.-H., Zhu X.-F. Low expression of ULK1 is associated with operable breast cancer progression and is an adverse prognostic marker of survival for patients. Breast Cancer Res Treat. 2012;134:549–560. doi: 10.1007/s10549-012-2080-y. [DOI] [PubMed] [Google Scholar]
- 81.Han Y., Xue X.-F., Shen H.-G., Guo X.-B., Wang X., Yuan B., Guo X.-P., Kuang Y.-T., Zhi Q.-M., Zhao H. Prognostic significance of Beclin-1 expression in colorectal cancer: A meta-analysis. Asian Pac. J. Cancer Prev. 2014;15:4583–4587. doi: 10.7314/APJCP.2014.15.11.4583. [DOI] [PubMed] [Google Scholar]
- 82.Koukourakis M.I., Giatromanolaki A., Sivridis E., Pitiakoudis M., Gatter K.C., Harris A.L. Beclin 1 over-and underexpression in colorectal cancer: Distinct patterns relate to prognosis and tumour hypoxia. Br. J. Cancer. 2010;103:1209–1214. doi: 10.1038/sj.bjc.6605904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang M.-Y., Gou W.-F., Zhao S., Mao X.-Y., Zheng Z.-H., Takano Y., Zheng H.-C. Beclin 1 Expression is Closely Linked to Colorectal Carcinogenesis and Distant Metastasis of Colorectal Carcinoma. Int. J. Mol. Sci. 2014;15:14372–14385. doi: 10.3390/ijms150814372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jo Y.K., Kim S.C., Park I.J., Park S.J., Jin D.-H., Hong S.-W., Cho D.-H., Kim J.C. Increased expression of ATG10 in colorectal cancer is associated with lymphovascular invasion and lymph node metastasis. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0052705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang L., Yao L., Zheng Y.-Z., Xu Q., Liu X.-P., Hu X., Wang P., Shao Z.-M. Expression of autophagy-related proteins ATG5 and FIP200 predicts favorable disease-free survival in patients with breast cancer. Biochem. Biophys. Res. Commun. 2015;458:816–822. doi: 10.1016/j.bbrc.2015.02.037. [DOI] [PubMed] [Google Scholar]
- 86.Ko Y.H., Cho Y.-S., Won H.S., Jeon E.K., An H.J., Hong S.U., Park J.H., Lee M.A. Prognostic significance of autophagy-related protein expression in resected pancreatic ductal adenocarcinoma. Pancreas. 2013;42:829–835. doi: 10.1097/MPA.0b013e318279d0dc. [DOI] [PubMed] [Google Scholar]
- 87.Mizushima N., Yamamoto A., Matsui M., Yoshimori T., Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol. Biol. Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kabeya Y., Mizushima N., Ueno T., Yamamoto A., Kirisako T., Noda T., Kominami E., Ohsumi Y., Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Choi J., Jung W., Koo J.S. Expression of autophagy-related markers beclin-1, light chain 3A, light chain 3B and p62 according to the molecular subtype of breast cancer. Histopathology. 2013;62:275–286. doi: 10.1111/his.12002. [DOI] [PubMed] [Google Scholar]
- 90.Cha Y.J., Kim Y.H., Cho N.H., Koo J.S. Expression of autophagy related proteins in invasive lobular carcinoma: Comparison to invasive ductal carcinoma. Int. J. Clin. Exp. Pathol. 2014;7:3389–3398. [PMC free article] [PubMed] [Google Scholar]
- 91.Guo J.Y., Chen H.-Y., Mathew R., Fan J., Strohecker A.M., Karsli-Uzunbas G., Kamphorst J.J., Chen G., Lemons J.M.S., Karantza V., et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes. Dev. 2011;25:460–470. doi: 10.1101/gad.2016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guo J.Y., Xia B., White E. Autophagy-mediated tumor promotion. Cell. 2013;155:1216–1219. doi: 10.1016/j.cell.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Amaravadi R., Kimmelman A.C., White E. Recent insights into the function of autophagy in cancer. Genes. Dev. 2016;30:1913–1930. doi: 10.1101/gad.287524.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Katsuragi Y., Ichimura Y., Komatsu M. p62/SQSTM1 functions as a signaling hub and an autophagy adaptor. FEBS J. 2015;282:4672–4678. doi: 10.1111/febs.13540. [DOI] [PubMed] [Google Scholar]
- 95.Puissant A., Fenouille N., Auberger P. When autophagy meets cancer through p62/SQSTM1. Am. J. Cancer Res. 2012;2:397–413. [PMC free article] [PubMed] [Google Scholar]
- 96.Jiang X., Overholtzer M., Thompson C.B. Autophagy in cellular metabolism and cancer. J. Clin. Investig. 2015;125:47–54. doi: 10.1172/JCI73942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lin M.G., Hurley J.H. Structure and function of the ULK1 complex in autophagy. Curr. Opin. Cell Biol. 2016;39:61–68. doi: 10.1016/j.ceb.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lazarus M.B., Shokat K.M. Discovery and structure of a new inhibitor scaffold of the autophagy initiating kinase ULK1. Bioorg. Med. Chem. 2015;23:5483–5488. doi: 10.1016/j.bmc.2015.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.John Clotaire D.Z., Zhang B., Wei N., Gao R., Zhao F., Wang Y., Lei M., Huang W. miR-26b inhibits autophagy by targeting ULK2 in prostate cancer cells. Biochem. Biophys. Res. Commun. 2016;472:194–200. doi: 10.1016/j.bbrc.2016.02.093. [DOI] [PubMed] [Google Scholar]
- 100.Liu J., Xia H., Kim M., Xu L., Li Y., Zhang L., Cai Y., Norberg H.V., Zhang T., Furuya T., et al. Beclin1 controls the levels of p53 by regulating the deubiquitination activity of USP10 and USP13. Cell. 2011;147:223–234. doi: 10.1016/j.cell.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cao Y., Klionsky D.J. Physiological functions of Atg6/Beclin 1: A unique autophagy-related protein. Cell Res. 2007;17:839–849. doi: 10.1038/cr.2007.78. [DOI] [PubMed] [Google Scholar]
- 102.Shen S., Kepp O., Michaud M., Martins I., Minoux H., Métivier D., Maiuri M.C., Kroemer R.T., Kroemer G. Association and dissociation of autophagy, apoptosis and necrosis by systematic chemical study. Oncogene. 2011;30:4544–4556. doi: 10.1038/onc.2011.168. [DOI] [PubMed] [Google Scholar]
- 103.Liu P.-F., Leung C.-M., Chang Y.-H., Cheng J.-S., Chen J.-J., Weng C.-J., Tsai K.-W., Hsu C.-J., Liu Y.-C., Hsu P.-C., et al. ATG4B promotes colorectal cancer growth independent of autophagic flux. Autophagy. 2014;10:1454–1465. doi: 10.4161/auto.29556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rothe K., Lin H., Lin K.B.L., Leung A., Wang H.M., Malekesmaeili M., Brinkman R.R., Forrest D.L., Gorski S.M., Jiang X. The core autophagy protein ATG4B is a potential biomarker and therapeutic target in CML stem/progenitor cells. Blood. 2014;123:3622–3634. doi: 10.1182/blood-2013-07-516807. [DOI] [PubMed] [Google Scholar]
- 105.Wu S., Su J., Qian H., Guo T. SLC27A4 regulate ATG4B activity and control reactions to chemotherapeutics-induced autophagy in human lung cancer cells. Tumour. Biol. 2016;37:6943–6952. doi: 10.1007/s13277-015-4587-4. [DOI] [PubMed] [Google Scholar]
- 106.Sun S., Wang Z., Tang F., Hu P., Yang Z., Xue C., Gong J., Shi L., Xie C. ATG7 promotes the tumorigenesis of lung cancer but might be dispensable for prognosis predication: A clinicopathologic study. OncoTargets Ther. 2016;9:4975–4981. doi: 10.2147/OTT.S107876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim M.S., Song S.Y., Lee J.Y., Yoo N.J., Lee S.H. Expressional and mutational analyses of ATG5 gene in prostate cancers. APMIS Acta Pathol. Microbiol. Immunol. Scand. 2011;119:802–807. doi: 10.1111/j.1600-0463.2011.02812.x. [DOI] [PubMed] [Google Scholar]
- 108.An C.H., Kim M.S., Yoo N.J., Park S.W., Lee S.H. Mutational and expressional analyses of ATG5, an autophagy-related gene, in gastrointestinal cancers. Pathol. Res. Pract. 2011;207:433–437. doi: 10.1016/j.prp.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 109.Tang J.-Y., Fang Y.-Y., His E., Huang Y.-C., Hsu N.C.-H., Yang W.-C., Chang H.-W., Chai C.-Y., Chu P.-Y. Immunopositivity of Beclin-1 and ATG5 as indicators of survival and disease recurrence in oral squamous cell carcinoma. Anticancer Res. 2013;33:5611–5616. [PubMed] [Google Scholar]
- 110.Manjithaya R., Subramani S. Autophagy: A broad role in unconventional protein secretion? Trends Cell Biol. 2011;21:67–73. doi: 10.1016/j.tcb.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kraya A.A., Piao S., Xu X., Zhang G., Herlyn M., Gimotty P., Levine B., Amaravadi R.K., Speicher D.W. Identification of secreted proteins that reflect autophagy dynamics within tumor cells. Autophagy. 2015;11:60–74. doi: 10.4161/15548627.2014.984273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Deretic V., Saitoh T., Akira S. Autophagy in infection, inflammation and immunity. Nat. Rev. Immunol. 2013;13:722–737. doi: 10.1038/nri3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Levine B., Mizushima N., Virgin H.W. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhong Z., Sanchez-Lopez E., Karin M. Autophagy, inflammation, and immunity: A troika governing cancer and its treatment. Cell. 2016;166:288–298. doi: 10.1016/j.cell.2016.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mahalingam D., Mita M., Sarantopoulos J., Wood L., Amaravadi R.K., Davis L.E., Mita A.C., Curiel T.J., Espitia C.M., Nawrocki S.T., et al. Combined autophagy and HDAC inhibition. Autophagy. 2014;10:1403–1414. doi: 10.4161/auto.29231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wani W.Y., Boyer-Guittaut M., Dodson M., Chatham J., Darley-Usmar V., Zhang J. Regulation of autophagy by protein post-translational modification. Lab. Investig. 2015;95:14–25. doi: 10.1038/labinvest.2014.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Crutchfield C.A., Thomas S.N., Sokoll L.J., Chan D.W. Advances in mass spectrometry-based clinical biomarker discovery. Clin. Proteom. 2016;13 doi: 10.1186/s12014-015-9102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Eissa S., Matboli M., Awad N., Kotb Y. Identification and validation of a novel autophagy gene expression signature for human bladder cancer patients. Tumour. Biol. J. Int. Soc. Oncodev. Biol. Med. 2017;39 doi: 10.1177/1010428317698360. [DOI] [PubMed] [Google Scholar]
- 119.Lebovitz C.B., Robertson A.G., Goya R., Jones S.J., Morin R.D., Marra M.A., Gorski S.M. Cross-cancer profiling of molecular alterations within the human autophagy interaction network. Autophagy. 2015;11:1668–1687. doi: 10.1080/15548627.2015.1067362. [DOI] [PMC free article] [PubMed] [Google Scholar]