Abstract
Pre-mRNA splicing is the process by which introns are removed and the protein coding elements assembled into mature mRNAs. Alternative pre-mRNA splicing provides an important source of transcriptome and proteome complexity through selectively joining different coding elements to form mRNAs, which encode proteins with similar or distinct functions. In mammals, previous studies have shown the role of alternative splicing in regulating the function of the immune system, especially in the regulation of T-cell activation and function. As lower vertebrates, teleost fish mainly rely on a large family of pattern recognition receptors (PRRs) to recognize pathogen-associated molecular patterns (PAMPs) from various invading pathogens. In this review, we summarize recent advances in our understanding of alternative splicing of piscine PRRs including peptidoglycan recognition proteins (PGRPs), nucleotide binding and oligomerization domain (NOD)-like receptors (NLRs), retinoic acid-inducible gene-I (RIG-I)-like receptors (RLRs) and their downstream signaling molecules, compared to splicing in mammals. We also discuss what is known and unknown about the function of splicing isoforms in the innate immune responses against pathogens infection in mammals and teleost fish. Finally, we highlight the consequences of alternative splicing in the innate immune system and give our view of important directions for future studies.
Keywords: transcriptional regulation, alternative splicing, pattern recognition receptors, signaling molecules, pathogens infection, teleost fish
1. Introduction
In the initiation of innate immune responses against pathogens, pattern-recognition receptors (PRRs) have an essential role in recognizing the conserved pathogen-associated molecular patterns (PAMPs) and triggering immune responses to eliminate the invading microorganisms. In vertebrate, the most characteristic PRRs include Toll-like receptors (TLRs), peptidoglycan recognition proteins (PGRPs), Nucleotide binding and oligomerization domain (NOD)-like receptors (NLRs) and RIG-I-like receptors (RLRs). The activation of these PRRs could initiate transcriptional and nontranscriptional innate immune responses, which tightly controlled signal transduction pathways and even directed the appropriate adaptive response [1,2,3]. These PRRs-triggered responses are also regulated through themselves and through the involvement of intracellular regulators or amplifiers [4].
Alternative splicing is a versatile regulatory mechanism that allows individual genes to generate more than one mRNA isoform, which in many cases encode functionally distinct proteins [5]. In mammals, more than 90% of human genes undergo alternative splicing [6], and alternative splicing is especially prevalent in the nervous and immune systems [7,8,9]. The importance of alternative splicing is underscored by the fact that misregulated alternative splicing can lead to human disease [10], e.g., the generation of CD44 splice variants can be linked closely with gastric carcinoma tumorigenesis and differentiation, breast cancer development and progression [11,12,13]. Although numerous immunologically relevant genes, such as pro-inflammatory cytokines and chemokines, have been found to undergo alternative splicing [14,15,16], there has been little effort to develop a coherent picture of how alternative splicing might be used as a general mechanism to regulate the function of PRRs and PRRs-mediated innate immune signaling. In recent years, the alternative splicing and immune function of piscine PRRs and their downstream signaling molecules were investigated in our laboratory. In this review, we summarized what is known and unknown about the alternative splicing and the function of splicing isoforms from PGRPs, NLRs, RLRs and their downstream signaling molecules in response to pathogens infection in mammals and teleost fish.
2. Alternative Splicing and Immune Function of Peptidoglycan Recognition Proteins
Peptidoglycan recognition proteins (PGRPs) are evolutionarily conserved pattern recognition receptors from insects to mammals, which recognize bacterial PGN and function in antibacterial innate immunity. Insects PGRP genes are classified into short (S) and long (L) transcripts. The short PGRPs include PGRP-SA, SB1, SB2, SC1A, SC1B, SC2 and SD, with short transcripts and 5′-untranslated regions. The long PGRPs include PGRP-LA, LB, LC, LD and LE, with long transcripts and 5′-untranslated regions. Most PGRPs have one PGRP domain, which is homologous to bacteriophage and bacterial type 2 amidases [17]. Multiple alternative splicing patterns for the PGRP-LA, LB, LC and LD genes have been identified in the fruit fly Drosophila melanogaster [18]. The functions of PGRP-LC isoforms have been well studied. Alternative splicing of variable extracellular domain-encoding exons generates three membrane-bound receptor isoforms, namely PGRP-LCa, PGRP-LCx and PGRP-LCy. Among them, PGRP-LCx isoform is required to mediate signals from gram-positive bacteria and purified bacterial peptidoglycan. PGRP-LCa and LCx are required for the recognition of gram-negative bacteria and bacterial lipopolysaccharide. PGRP-LCy may have a minor role in antagonizing the immune response [19,20].
Mammals have a family of four secreted PGRPs named PGLYRP-1, PGLYRP-2, PGLYRP-3 and PGLYRP-4, respectively. PGLYRP-2 is an N-acetylmuramoyl-l-alanine amidase that hydrolyzes the lactyl bond between the MurNAc and L-alanine in bacterial peptidoglycan [21]. PGLYRP-1, PGLYRP-3 and PGLYRP-4 are a new class of bactericidal proteins different from currently known antimicrobial peptides in structure, mechanism of action and expression [22,23,24]. A splicing pattern of tagL (PGRPL) gene was described in the mouse (Mus musculus) [25]. The transcription of TagL-α′, TagL-β′ and TagL-ε′ splice variants starts from the exon I, TagL-α, TagL-γ and TagL-δ from the exon II. The N-terminal portion of all identified proteins is identical. Among them, TagL-α, TagL-α′ and TagL-β′ contain T phage lysozyme homology domain (also known as PGRP domain) on the C terminus. Frame shift occurring in TagL-γ, TagL-δ and TagL-μ results in the lack of PGRP domain. All these splice variants bound gram-positive, gram-negative bacteria and peptidoglycan, which suggest that the binding does not depend on the presence of PGRP domain.
Three members of the PGRP family were cloned in teleost fish. Unlike human PGRPs, PGLYRP-2 (or zfPGRP2), PGLYRP-5 (or zfPGRP-SC) and PGLYRP-6 (or zfPGRP6) from the zebrafish Danio rerio have both amidase and bactericidal activities [26]. zfPGRP6 and zfPGRP-SC also function as pattern recognition receptors to mediate signal transduction [27,28]. RNAi-mediated suppression of zfPGRP6 significantly down-regulated the expression of those genes involved in a Toll-like receptor signaling pathway [27]. zfPGRP-SC could mediate multiple intracellular signaling pathways which may connect with each other to form a complex network to regulate not just immune responses but also other processes such as development and apoptosis [28]. The alternative transcripts also exist in fish PGRP homologs. The long PGRPs in teleost fish have multiple alternatively spliced variants [29,30]. In comparison to genomic sequences, the splicing patterns of tnPGRP-L, zfPGRP2 and gcPGRP6 were determined in the spotted green pufferfish (Tetraodon nigroviridis), zebrafish (D. rerio) and grass carp (Ctenopharyngodon idella) [29,30]. These spliced variants were generated from the deletion of the partial exon 2 (tnPGRP-L2, gcPGRP6a and gcPGRP6c), the whole exon 2 (gcPGRP6d), partial exon 3 (tnPGRP-3 and zfPGRP-L), the whole exon 3 (tnPGRP-L4), or partial exon 2 and the whole exon 3 (gcPGRP6b). The functions of most spliced variants were unclear in teleost fish, except that a report showed that gcPGRP6 splice variants are able to bind microbial PAMPs and inhibit earlier stage growth of intracellular bacteria [30]. Interestingly, although all gcPGRP6 splice variants have an N-terminal signal peptide, immunofluorescence microscopy and Western blotting showed that the splice variants are intracellular proteins, which are different from the gcPGRP6 normal form [30,31].
3. Alternative Splicing and Immune Function of Nucleotide Binding and Oligomerization Domain-Like Receptors
Nucleotide binding and oligomerization domain (NOD)-like receptors (NLRs) were cytosolic sensors of microbial molecules, which have been shown to have many different and important roles in inflammatory responses and host defense against microbial pathogens [32,33,34,35], in maintaining immune homeostasis [36], in the control of autophagy [37] and in regulating early embryogenesis and reproduction [38]. Among four subfamilies that were subdivided according to their amino terminal effector domain [39], NLRA and NLRC subfamilies are conserved in mammals and teleost fish. The other two NLRB and NLRP subfamilies were not identified in teleost fish, and NLRP may represent a mammalian expansion of NLR proteins [40].
3.1. NLRA Subfamily
The NLRA subfamily includes only one member, the major histocompatibility complex (MHC-II) transactivator (CIITA). CIITA functions as a master control factor for MHC class II genes expression. CIITA contains an N-terminal acidic domain (AD), followed by a region rich in proline, serine and threonine (P/S/T region), a central GTP-binding domain (GBD) and a C-terminal leucine rich repeat domain [41]. Multiple variants and differential splicing patterns were found in mammalian CIITA.
Alternative promoter usage: Four isoforms of CIITA (CIITA type I, II, III and IV) were generated by alternative promoter usage. These CIITA isoforms are differed only in their N-terminal ends [42]. Of the four different CIITA isoforms, human CIITA type III corresponds to the previously described form of CIITA cDNA [41]. The CIITA type II and IV use the same ATG which is located 21 bp downstream of the 5′ end of the common nucleotide sequence, and encode the same protein. CIITA type I and III use the ATG located upstream of the common nucleotide sequence, and generate CIITA proteins with an additional 101 or 24 N-terminal amino acids respectively. The pattern of CIITA promoter usage was analyzed by RNase protection assays on the specific transcripts of the endogenous CIITA gene, which revealed a strong bias in the selective use of different CIITA promoters in the control of both constitutive and inducible expression of CIITA [42].
A variety of insertions and/or deletions were seen in the coding region and additional sequences were found at their 3′ ends: In MHC class II-positive B cells, CIITA cDNA clones showed alternative RNA splicing [43]. CIITA-8 was considered to produce a wild type (wt) protein. CIITA-2.11 contained an insertion of 479 bp within the coding sequence beginning at the base pair position 596 in wt CIITA, and also contained an additional 30 bp at the 3′ end. CIITA-1.23 contained the 3′ 248 bp of the inserted DNA found in the coding region of CIITA-2.11 at base pair position 596. DNA sequence analysis indicated that both CIITA-2.11 and CIITA-1.23 contained stop codons in all reading frames. CIITA-10 contained a 1 aa insertion at base pair 473, a 49 aa in-frame deletion between base pairs 596 and 744, and a stop codon resulting in a truncated protein of 884 aa instead of 1130 aa. Among these variants, only CIITA-8 was able to restore class II MHC gene expression.
Alternative splice donor site: Defective MHC class II expression in an MHC class II deficiency patient is caused by ATU CIITA, a novel deletion of a splice donor site in the CIITA gene [44]. ATU CIITA with the lack of 84 nucleotides failed to transactivate MHC class II genes and did not display a dominant negative effect on CIITA-mediated transactivation of various MHC class II promoters.
Exon skipping: In primary cells, two novel splice variants of human CIITA were identified [45]. One variant CIITAΔE7 is devoid of the entire exon 7, which results in the loss of aa 160–209 in the N-terminal part of P/S/T domain of the CIITA protein. CIITAΔE7 exhibits altered functions toward those chaperons involved in regulating HLA class II assembly and transport.
Intron retention: In K-562 cells, an alternatively spliced transcript of CIITA was identified [46]. This variant contains an insertion of 870 bp genomic sequence, which introduces a stop codon at nt 2796 and results in a truncated protein of 932 amino acids rather than 1130. The alternative transcript was not present in Raji cells. Although the alternative CIITA protein is able to associate with the MHC class II promoter and the RFX complex, the transactivation ability of CIITA variant is abolished, compared with wt CIITA.
Different from mammalian CIITA, the study on fish CIITA was rather limited. Only two reports showed the phylogeny and expression analysis of CIITA in channel catfish (Ictalurus punctatus) [47,48]. CIITA has been referred to as the "master control factor" for the expression of MHC class II genes [49]. Interestingly, the deficiency of zebrafish nucleotide-binding oligomerization domain-containing protein 1 (NOD1) significantly attenuated the expression of MHC-II β and mhc2dab [50], which suggested that NOD1 functioned as a new regulator to drive the expression of MHC genes. Further studies are needed to clarify if piscine CIITA plays redundant or exclusive roles with NOD1 in the regulation of the expression of MHC genes.
3.2. NLRC Subfamily
The NLRC subfamily consists of five members: NLRC1 (NOD1), NLRC2 (NOD2), NLRC3, NLRC4 and NLRC5. These NLRCs can function as either positive or negative regulators of inflammatory signaling cascades. Among these members, the homologues of mammalian NLRC4 were not identified in teleost fish. Here, the alternative splicing and immune function of other four members except NLRC4 are summarized in this review.
3.2.1. Nucleotide-Binding Oligomerization Domain-Containing Protein 1 and 2
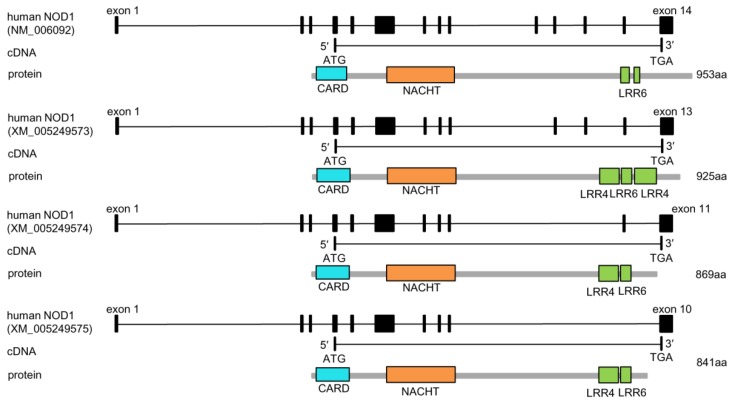
NOD1 and NOD2 are the best-characterized members of NLRC subfamily. Significant advances have been achieved regarding the function of NOD1 and NOD2 in innate immune responses to bacterial, parasite and viral infections [51,52,53,54]. Although alternative pre-mRNA splicing was only reported for NOD2 but not NOD1 in mammals and teleost fish, analysis of human databases revealed the existence of multiple splicing variants of NOD1 (Figure 1). Different from mammalian NOD1, only one form of NOD1 exists in zebrafish (D. rerio) since Western blotting exhibits the single band in the lysates from ZF4 cells and wild type zebrafish using monoclonal anti-NOD1 antibody [50].
Figure 1.
The alternative splicing of human NOD1. Exons are indicated as square boxes, and the introns as straight lines. CARD: caspase activation and recruitment domain; NACHT: nucleotide-binding oligomerization domain; LRR: Leucine-rich repeats.
The mammalian NOD2 gene has 12 exons and encodes a protein of 1040 amino acids [55]. At least 8 NOD2 splicing variants were identified. An abundant NOD2 splice variant lacking exon 3 leads to a predicted 21-kDa short NOD2 protein variant (NOD2-S) with a complete CARD1, a truncated CARD2 domain (54 amino acids) and 10 previously undescribed C-terminal amino acids. Besides NOD2-S, another N-terminally spliced variant NOD2-35 was generated by retention of part of intron 1 and a frameshift. NOD2-35 encoded only the first 25 N-terminal amino acid residues of NOD2, followed by a novel sequence of 10 amino acid residues [56]. A novel alternative promoter and novel first exon of NOD2 are responsible for producing a protein of 1023 amino acids, which is likely to be translated from the first ATG in exon 2 (known as Met28) [57,58]. In addition, five NOD2 variants are generated by alternatively spliced transcripts of the LRR domains [56]. Among these identified variants, NOD2-S interacts with NOD2 and RIP2 and abolishes MDP induced NOD2 self-association, and also functions as a negative regulator of NOD2/RIP2-induced NF-κB activation [59]. Except for NOD2-S, the functional significance of most NOD2 splicing variants is unknown.
Similar to mammalian NOD2, the piscine NOD2 gene undergoes splice variation. In zebrafish (D. rerio), a cryptic splice site in exon 1 resulted in a predicted NOD2 molecule with a single CARD but intact NOD and LRR domain [60]. In rainbow trout (Oncorhynchus mykiss), two NOD2 transcripts were confirmed by RT-PCR [61]. The shorter transcript of rainbow trout NOD2 (trNOD2a) encodes the normal form. Another transcript named trNOD2b had a longer 5′ untranslated region (UTR) and a 65 bp deletion including the normal start codon ATG, resulting in the predicted translation starting from the next downstream ATG. The first CARD domain is incomplete in trNOD2b. The 38 aa deletion in the first CARD domain of trout NOD2 has no significant effect on the induced expression of proinflammatory cytokines including IL-1β, tumor necrosis factor-α (TNF-α), IL-6 and IL-8, the antibacterial peptide cathelicidin-2, a variety of caspases including caspase-6, -7, -8, -9, and type I and type II IFN [61].
3.2.2. NLR Family CARD Domain Containing 3
NLR Family CARD Domain Containing 3 (NLRC3) was shown to be a negative regulator, which negatively regulates diverse aspects of host antiviral immunity including STING, type I IFN and TLR-induced NF-κB signaling to attenuate overzealous inflammation following virus infection [62,63]. In addition, NLRC3 negatively regulates T cell function [64], and also functions as an inhibitor of the mTOR pathways [65,66]. The splicing variants of NLRC3 were not found either in mammals or fish species. In teleost fish, several studies showed the cloning and expression pattern of NLRC3 in turbot (Scophthalmus maximus L.) [67], rainbow trout (O. mykiss) [68], Asian seabass (Lates calcarifer) [69], Japanese flounder (Paralichthys olivaceus) [70], miiuy croaker (miichthys miiuy) [71] and channel catfish (I. punctatus) [72,73]. The functions of piscine NLRC3 were quite unclear at present, although a study showed that zebrafish NLRC3-like, which contains the canonical pyrin (PYD) and NACHT domains but lacks the common LRRs, prevents inappropriate macrophage activation, thereby allowing normal microglia development [74].
3.2.3. NLR Family CARD Domain Containing 5
The role of mammalian NLRC5 (also known as NOD27 and CLR16.1) in regulating innate and adaptive immune responses has been controversial. The study by Cui et al. showed the negative regulation of NLRC5 in antiviral signaling and type I IFN production [75], but little or no role in regulating IFN levels or virus replication from the report of Kumar et al. [76]. The positive regulation of NLRC5 in IFN-dependent or RIG-I-mediated antiviral responses was reported in three other groups [77,78,79]. Besides this discrepancy, NLRC5 has been linked to the NLRP3 inflamasome and MHC class I transactivation [80,81,82,83]. NLRC5 interacts with NLRP3 to cooperatively activate the inflammasome [80,81]. NLRC5 exclusively transactivates MHC class I and related genes through a distinctive SXY module [84].
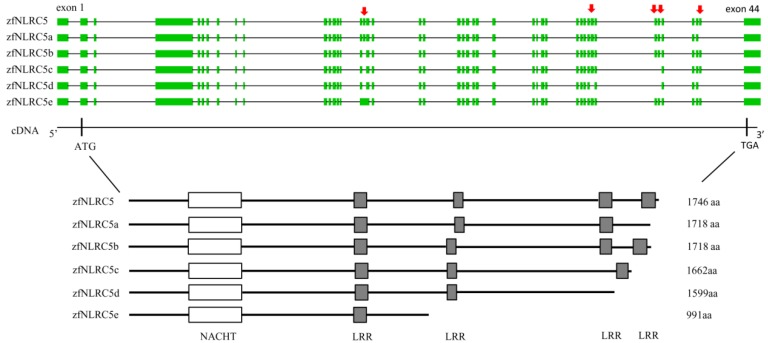
In human Homo sapiens [78] and zebrafish D. rerio (Figure 2), 5 different splice variants were obtained. All shared a conserved 5′ region but differed in the length of the LRRs. In mammals, the LRR domains of NLR proteins are essential for sensing of their PAMPs and DAMPs. The unusual structure of the NLRC5 LRR domain might thus be indicative for NLRC5 to respond to quite different stimuli than other NLRs [78]. Although the exact biological function of these NLRC5 isoforms has yet to be investigated, our unpublished studies in vivo and in vitro showed the functional difference of zebrafish NLRC5 isoforms in viral infection. Interestingly, our research also showed that zebrafish NLRC5 normal form is involved in an IFN-independent antiviral response and also functions as a transcriptional regulator of MHC class II genes [85], which is different from mammalian NLRC5. Further studies are needed to understand the function and the mechanism of NLRC5 isoforms in response to different pathogens infection.
Figure 2.
The alternative splicing of zebrafish NLRC5. Exons are indicated as square boxes, and the introns as straight lines. GenBank accession numbers for zebrafish NLRC5 isoforms are: zfNLRC5, AFN73230; zfNLRC5a, AFN73231; zfNLRC5b, AFN73232; zfNLRC5c, AFN73233; zfNLRC5d, AFN73234; zfNLRC5e, AFN73235. NACHT: nucleotide-binding oligomerization domain; LRR: Leucine-rich repeats. The alternatively spliced exons were indicated in the red arrows.
4. Alternative Splicing and Immune Function of Retinoic Acid-Inducible Gene-I-Like Receptors
RLRs are well conserved intracellular PRRs among vertebrates. The RLR family consists of retinoic acid-inducible gene-I (RIG-I), melanoma differentiation-associated factor 5 (MDA5) and laboratory of genetics and physiology 2 (LGP2). RIG-I and MDA5 share similar domain structures, including two N-terminal caspase activation and recruitment domains (CARDs), a distinct DEX/DH box RNA helicase domain and a C-terminal regulatory domain (CTD or RD) [86]. The N-terminal CARD domains facilitate RIG-I and MDA5 interacting with other CARD containing molecules. The central DExD/H-box region with ATP hydrolysis activity is homologous to RNA helicase domain, and involved in dsRNA interactions. The RD domain is crucial for the specific recognition of RNA substrate [86,87]. In mammals, RIG-I and MDA5 function as positive regulators in antiviral innate immunity [88]. The third RLR family member LGP2, also known as Dhx58, harbors a DExD/H-box helicase domain and a C-terminal RD but lacks any CARDs which functions as a positive [89] or negative regulator [90,91] in RIG-I- and MDA5-mediated antiviral responses.
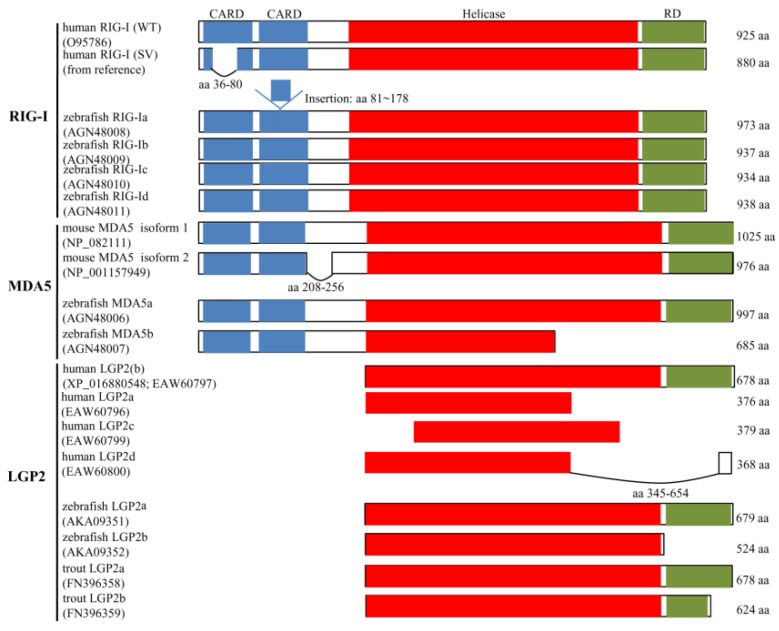
In teleost fish, RLRs were first found in 2008 using bioinformatic analysis [92]. RIG-I, MDA5 and LGP2 genes have been cloned in crucian carp (Carassius auratus) [93], common carp (Cyprinus carpio) [94], black carp (Mylopharyngodon piceus) [95,96], grass carp (C. idella) [97,98,99], zebrafish (D. rerio) [100,101,102,103], channel catfish (I. punctatus) [104], orange spotted grouper (Epinephelus coioides) [105,106], Atlantic salmon (Salmo salar) [107], rainbow trout (O. mykiss) [108], large yellow croaker (Larimichthys crocea) [109], green chromide (Etroplus suratensis) [110], sea perch (Lateolabrax japonicas) [111,112] and Japanese flounder (P. olivaceus) [113]. Similar to those orthologs in mammals, piscine RLRs could be spliced at RNA levels, which lead to sequence deletion or insertion in the open reading frame (ORF) (Figure 3).
Figure 3.
The alternative splicing of mammalian and piscine RLRs. CARD: caspase activation and recruitment domain; Helicase: helicase_insert_domain superfamily; RD: regulatory domain.
RIG-I gene in zebrafish had four different transcripts. Compared with RIG-I from mammalian and other fish species, zebrafish RIG-Ib encodes the normal form. The residues RKPFEIKISFTRVTWPQARRQEVKTEGALQIHRGALDL in RIG-Ia are inserted in the second CARD domain of RIG-I, which shows no sequence homology with any reported RIG-I in other fish species or in mammals [101]. Zebrafish RIG-Ic encodes a protein that lacks the first 189–192 amino acid region just behind the second CARD of RIG-I. Zebrafish RIG-Id encodes a protein that lacks 2 aa just behind the second CARD, however inserts 3 aa within the Helicase domain. Zebrafish RIG-I genomic DNA sequence has not yet been completely assembled in the latest version GRCz10 (Genome Reference Consortium Zebrafish Build 10). Different from RIG-I, the genomic DNA sequence of MDA5 is clear in zebrafish. The MDA5a gene consists of 16 exons, whereas MDA5b lacks partial exon 11, the entire exon 12 and partial exon 13. The C-terminal RD domain is absent for zebrafish MDA5b [100]. Two LGP2 splicing variants were identified both in rainbow trout (O. mykiss) and zebrafish (D. rerio) [103,108]. The identified trout LGP2 cDNA (named LGP2a) encodes a protein of 678 aa. Trout LGP2b is 54 aa shorter than LGP2a due to an intron of 1,040 bp retained at the 3′-end region of the ORF, which results in the early termination of translation [108]. The zebrafish LGP2b (DrLGP2b) is a truncated isoform of LGP2a (DrLGP2a). Compared with DrLGP2a, the DrLGP2b lack a regulatory domin (RD) (551–672 aa) at the C-terminal (Figure 3). All sequences of fish RLRs including RIG-I, MDA5 and LGP2 isoforms refer to transcripts.
In mammals, the function of RIG-I splicing variant was reported. The RIG-I SV, lacking a critical part of the first CARD, loses TRIM25 binding, CARD ubiquitination, and downstream signaling ability. Furthermore, RIG-I SV suppresses the RIG-I-mediated IFN-β production through inhibiting the formation of virus-induced RIG-I multimerization and RIG-I-mitochondrial antiviral signaling protein (MAVS) signaling complex [114]. In zebrafish, although the RIG-Ia variant, with 38 amino acids inserted in the second CARD, loses the activity to induce the activation of IFN promoter and protect cells against spring viraemia of carp rhabdovirus (SVCV) infection, RIG-Ia functions as an enhancer in the RIG-Ib/MAVS-mediated signaling pathway [101]. The functions of other two RIG-I variants (RIG-Ic and RIG-Id) are unclear at present. Similar to zebrafish MDA5a, the truncated MDA5b variant can also induce an antiviral response due to the presence of the intact tandem CARDs [100], which is consistent with the finding in RIG-I that the over-expression of the N-terminal CARDs was able to protect cells against virus infection [115]. In addition, MDA5b can augment the IFN production induced by MDA5a and MAVS [100]. In teleost fish, most studies showed piscine LGP2 functions as a positive regulator in antiviral responses [95,96,113,116], except for negative regulation of the antiviral response by LGP2 from orange-spotted grouper (E. coioides) and grass carp (C. idella) [105,117]. LGP2 splicing variants were only identified in zebrafish (D. rerio) [103] and rainbow trout (O. mykiss) [108]. Trout LGP2a acted as a positive regulator in antiviral responses, whereas LGP2b with a deletion of 54 amino acids at the C terminus RD domain acts as a negative regulator for LGP2a-elicited antiviral signaling by competing for the viral RNA PAMPs [108]. The exact roles of the two zebrafish LGP2 isoforms involved in viral infection are still unclear [103].
5. Alternative Splicing and Immune Function of Downstream Signaling Molecules
5.1. Mitochondrial Antiviral Signaling Protein
The mitochondrial antiviral signaling protein (MAVS), also known as CARDIF, IPS-1, KIAA1271 and VISA, is an innate immunity protein that functions downstream of RIG-I-like receptors (RLRs) to link RNA virus invasion to the type I interferon (IFN) pathway. Mammalian MAVS gene encodes a number of splice variants that have been proposed to negatively regulate MAVS signaling. Mammalian MAVS gene is encoded by a single gene composed of 6 exons. MAVS 1a (deletion of exon 2), containing a putative CARD domain and a TRAF2-binding motif, interacts with RIP1 and TRAF proteins and functions as an inhibitor against MAVS activation on IFN-β and NF-κB promoters through disrupting RIG-I/MAVS signaling complex formation. MAVS1b (deletion of exon 3) shares the first 97 residues with wt MAVS and 27 aa residues of unknown protein. Different from wt MAVS, which activates both NF-κB and IRF3 pathways, MAVS1b promotes signaling complex formation involving FADD and RIP1 for IFN-β activation. MAVS1c (deletion of exon 6), which encodes 386 aa residues and is a truncated form of MAVS, has no activity on either NF-κB or IRF3 pathway [118]. In addition, translation of mammalian MAVS can also be initiated by two different translation start sites. This alternative internal translation of MAVS results in the production of a shorter variant of 398 amino acids that lacks the CARD domain, and is referred to as miniMAVS which essentially serves as an inhibitor of wt MAVS signaling [119].
Piscine MAVS contains similar protein domains as in mammals, with an N-terminal CARD domain, a central proline-rich region and a C-terminal TM domain [115,120,121,122,123,124]. Except for wt MAVS, MAVS variant is only cloned in zebrafish (D. rerio). This shorter variant named MAVS_tv2, lacking a C-terminal TM domain, is generated from a frame shift due to intron insertion, whose C-terminal 41 aa residues share no sequence similarity to any known proteins in the database [125]. Interestingly, different expression constructs of MAVS_tv2 exhibited the functional differences [125,126]. The EPC cells transfected with ptGFP1-MAVS_tv2 were more resistant to SVCV infection than the control cells transfected with ptGFP1 empty plasmid. In addition, overexpression of MAVS_tv2-FLAG in EPC cells induced the activation of IFN1 and IFN3 promoters. Furthermore, overexpression of MAVS_tv2-FLAG in zebrafish embryos can significantly increase the expression of many antiviral genes such as IFN1, IFN2, IFN3, mxc and rsad2 [125]. All these data suggested the positive regulation of MAVS_tv2 in the antiviral response. Different from ptGFP1-MAVS_tv2 and MAVS_tv2-FLAG, overexpression of pcDNA-MAVS_tv2 could not affect the IFN1 activity. On the other hand, overexpression of pcDNA-MAVS_tv2 decreased the activation of IFN1 promoter and the transcriptional levels of several IFN-stimulated genes induced by IRF7, which suggested that MAVS_tv2 is a negative regulator of IFN1 by targeting IRF7 [126]. More studies are needed to make sure the exact function and mechanisms of MAVS_tv2 targeting in the different signaling molecule of RLRs signaling pathway in response to viral infection.
5.2. Stimulator of Interferon Genes
Stimulator of interferon genes (STING) (also known as MITA or ERIS) has been found to be another adaptor protein that links upstream pathogen sensing to downstream IFN induction [127,128]. MITA, comprising 5 putative transmembrane (TM) regions, predominantly resides in the endoplasmic reticulum and is able to activate both NF-κB and IRF3 transcription pathways to induce type I IFN [127]. Intensive studies have established the essential role of STING in sensing nucleic acids such as the cytosolic double-stranded DNAs and c-di-GMP or c-di-AMP [129,130,131,132,133,134,135,136]. The MITA/TBK1/IRF3 axis has been found to be important in RLRs-mediated and some DNA sensor-mediated antiviral signaling pathways [129,135,137,138]. MITA is also reported to be a target molecule for microbial pathogens such as yellow fever virus, dengue virus and hepatitis C virus to escape the innate immune response [139,140,141].
A splice variant of MITA (designated as MRP) lacking exon 7 was identified in human (H. sapiens). The absence of exon 7 resulted in a frame shift, whose putative protein is identical to aa 1-253 of wt MITA at the N-terminal but possesses a unique 30-aa sequence at the carboxyl terminal [142]. Interestingly, MRP plays a role as a negative regulator in MITA-induced activation of the IFN signaling pathway by sendai virus infection and cyclic diguanylate treatment, but enhanced the herpes simplex virus type 1 (HSV-1) induced IFN response [142]. In addition, a recent study showed that MRP, despite its inability to trigger IRF3 activation, could restrict hepatitis B virus (HBV) replication in vitro and in vivo via the activation of NF-κB pathway [143].
In teleost fish, MITA was only reported in crucian carp (C. auratus) [93], zebrafish (D. rerio), fathead minnow (Pimephales promelas) [144], orange spotted grouper (E. coioides) [145] and grass carp (C. idella) [146]. Similar to mammalian MITA, piscine MITA activates IFN response via MITA-TBK1-IRF3 signaling pathway [93,145], and is also the target of virus to escape the innate immune response [146]. Our unpublished data showed that zebrafish MITA variant is generated by Exon skipping. Zebrafish MITA variant is identical to aa 1–244 of wt MITA at the N-terminal but possesses a unique 17-aa sequence at the carboxyl terminal. The function of piscine MITA variant is unclear at present, and need to be further investigated.
5.3. TRAF Family Member-Associated NF-kappaB Activator (TANK) Binding Kinase 1
TANK binding kinase 1 (TBK1) is a serine/threonine-protein kinase, and acts as a critical player in the regulation of the immune response to bacterial and viral challenges, inflammatory responses, the insulin signaling pathway and autophagy [147,148,149,150,151]. As the pivotal role of TBK1 in various immunobiological and immunopathological events, its activity must be tightly regulated to effectively control pathogen infection and maintain immune homeostasis. TBK1 activity is regulated in a variety of ways including phosphorylation, ubiquitination, kinase activity modulation and prevention of functional TBK1-containing complexes formation [152]. The splice variant of TBK1 was only reported in human (H. sapiens) and mouse (M. musculus), and named as TBK1s. Excision of exons 3–6 of TBK1s results in translation from the second ATG and leads to an in-frame deletion of the kinase domain (amino acids 1–234). Different from TBK1, TBK1s can bind to RIG-I through its coiled-coil domain, and negatively regulates virus-triggered IFN-β signaling pathway by disrupting the interaction of RIG-I and MAVS [153].
The function of TBK1 in regulating IFN-I pathway was studied in teleost fish. The TBK1 (CiTBK1) from grass carp (C. idella) participates in the antibacterial and antiviral immune responses in different manners. After LPS stimulation, CiTBK1 triggered IFN-I activation which was independent of IRF3/IRF7. Post GCRV challenge, CiTBK1 mediated IFN-I response mainly by IRF7 not IRF3. In addition, CiTBK1 negatively regulated PGN-induced IRF3, IRF7, IFN-I and Mx1 immune response [154]. Similar to piscine MAVS and MITA, piscine TBK1 is also targeted by viruses as a major negative regulatory target to decrease the IFN response and facilitate viral replication. Spring viremia of carp virus (SVCV) P protein functions as a decoy substrate for cellular TBK1, leading to the reduction of IRF3 phosphorylation and suppression of IFN expression [155]. In zebrafish (D. rerio), a TBK1-like transcript (TBK1L), containing an incomplete S_TKc domain and lacking UBL_TBK1_like domain, was cloned. Overexpression of zebrafish TBK1L negatively regulated the production of IFN and IFN-stimulated genes through RLRs-MAVS-TBK1 pathway [156]. In addition, a study showed that the TBK1 from large yellow croaker (L. crocea) can be regulated by Nrdp1, an E3 ubiquitin ligase, and was involved in the immune defense against the pathogen infection [157].
5.4. Interferon Regulatory Factor 3
The transcription factor IRF3 plays a critical role in the regulation of IFN production following virus infection. The TBK1 and the inhibitor of NF-κB kinase-ε (IKKε) can phosphorylate IRF3. Phosphorylated IRF3 subsequently dissociates from the adaptor protein, and then forms a homo- or heterodimer with other transcriptional factors before translocating into the nucleus to induce transcription of IFNs [158,159]. In mammals, multiple IRF3 isoforms have been characterized. Different from the normal form of IRF3, an additional exon located between exon 2 and 3 was designated 3a, which encoded a distinct 20-amino-acid N terminus of IRF-3 [160]. Due to lack half of the DNA binding domain found in IRF-3 normal form, human IRF-3a spliced isoform failed to bind with ISRE sequences, and negatively regulated the transcriptional activity of IRF3 [161]. The second spliced isoform IRF3-nirs3, which lacked 127 amino acids in the regulatory domain (RD) of IRF3 normal form, was found in human hepatocellular carcinoma (HCC) cells. IRF3-nirs3 overexpression impaired significantly the expression of IFN-β, and was benefiting for viral replication [162]. The third spliced isoform IRF3-CL was ubiquitously expressed in various cell lines including liver and tumor cell lines. Compared with IRF3 normal form, the additional 16 nucleotides upstream of exon 7 in IRF3-CL generated a frame shift, which gave rise to a distinct carboxyl-terminus without the autoinhibition element (AIE) domain. IRF3-CL functioned as a competitive inhibitor of IRF3 [163]. Two novel IRF3 spliced isoforms, Int2V1 and Int2V2, were cloned from pheochromocytoma tissue. The expression of Int2V2 was higher than Int2V1 in a variety of tissues and cell lines examined, except for in HepG2 cell line [164]. The functions of Int2V1 and Int2V2 are unclear at present. In addition, five other splicing isoforms of IRF3, namely IRF-3b, -3c, -3d, -3e and -3f, were identified in human cells. These IRF3 isoforms were generated by exon deletions, and attenuated the transactivation activity of IRF3 [165].
Although IRF3 has been reported in multiple fish species such as miiuy croaker (m. miiuy) [166], grass carp (C. idella) [167], tilapia (Oreochromis niloticus) [168], half-smooth tongue sole (Cynoglossus semilaevis) [169], European eel (Anguilla anguilla) [170], large yellow croaker (L. crocea) [171], Japanese flounder (P. olivaceus) [172] and crucian carp (C. auratus) [93], splicing variants of IRF3 have still not been well studied. Analysis the zebrafish database and the results from our sequencing of IRF3, at least 3 splicing variants are found in zebrafish (Supplement Figure S1). Interestingly, IRF3c cloned by us may generate two ORFs, which both encode IRF3. The first ORF encodes 125 aa, which are corresponding to 1~125 aa of IRF3a (Supplement Figure S2). The second ORF encodes 337 aa, which is 87.24 and 88.08% identities with IRF3a and IRF3b, respectively (Supplement Figure S3). It is interesting to know the exact function of these piscine IRF3 variants.
5.5. Interferons and Their Receptors
Interferons (IFNs) play a major role in the defense against virus infection in vertebrates. There are three types of IFNs in mammals. Type I IFNs consist of seven classes: IFN-α, IFN-β, IFN-ε, IFN-κ, IFN-ω, IFN-δ, and IFN-τ. Type II IFN consists of IFN-γ only. The type III IFNs or IFN-λs, which are comprised of three intron-containing members and are known as interleukin (IL)-29, IL-28A and IL-28B [173,174]. Type I IFNs transduce intracellular signals through the common receptor IFNAR1/2 [175], Type II IFN by recognizing cell surface receptor IFNGR1/2 [176], type III IFNs by IL-28R1/IL-10R2 receptor complex [177]. Mammalian IFNAR genes encode multiple isoforms that contribute to the complexity of the functional receptor. The major ligand-binding subunit IFNAR-2 exists in 3 mRNA splice variants including 2 transmembrane isoforms (IFNAR-2b and IFNAR-2c) and a soluble isoform (IFNAR-2a) [178]. Among the 3 IFNAR-2 isoforms, IFNAR-2c is recognized as the functional one, whereas IFNAR-2b is unable to perform signal transduction [179] and may act as a dominant negative regulator of IFN responses [180]. Although lacking the transmembrane and intracytoplasmic domain, the third isoform IFNAR-2a is still capable of binding IFNs, and functions as an important regulatory factor of type I IFNs activity [181]. Different from IFNAR-2, only one IFNAR-1 isoform with transcriptional capacity was identified in normal cells [182,183].
Among the three IFN families, only type I and II IFN genes were identified and well documented in teleost fish [184]. In contrast with the single exon IFN genes in reptiles, birds and mammals, fish type I IFN genes are consisting of five exons and four introns [185,186]. The alternative splicing of piscine type I IFN genes has been reported in rainbow trout (O. mykiss) [187,188]. The splicing isoforms of trout IFN1 are generated by the absence or retention of the first and/or second introns and the usage of different ATG transcription start site. In rainbow trout, there are three ATG transcription start sites at the 5′ end of the transcript. Secretory sIFN1 with the retention of the first and second introns uses the second ATG for transcription initiation. Intracellular iIFN1a with the retention of the first intron uses the third ATG for transcription initiation. Intracellular iIFN1b without the first and second introns uses the first ATG for transcription initiation. Similar to secretory IFN1, the intracellular IFN1 isoforms possess antiviral activity and are able to activate cellular antiviral pathways [188]. Furthermore, membrane bound and intracellular IFN receptors, named as mIFNAR2 and iIFNAR2, are generated by alternative splicing. mIFNAR2 conserves the first intron in the coding region and uses the first ATG for transcription initiation. iIFNAR2 without intron retention uses the second ATG for transcription initiation. The intracellular IFNs induce the expression of antiviral genes and STAT phosphorylation through intracellular IFN receptors [188].
In addition to IFN-γ, the IFN-γrel molecule, which has duplicated from the IFN-γ gene, is generally accepted as a second member of the type II IFN family in teleost fish [184]. Two IFNγ spliced isoforms are found in catfish (I. punctatus) and medaka (Oryzias latipes) [189,190]. IFNγ2a and 2b were cloned from the gonadal cDNA of medaka. IFNγ2a exhibited ubiquitous expression, while IFNγ2b was only expressed predominantly in female germ cells than males. The alternative splicing of IFNγ in medaka is steroid-dependent [190]. Two IFNGR1 isoforms were also cloned in zebrafish (D. rerio) and goldfish (Carassius auratus L.) [191,192]. In vitro binding studies indicated that goldfish IFNGR1-1 bound to IFNγ1 but not IFNγ2, while the IFNGR1-2 bound to IFNγ2 [191].
6. Conclusions and Prospects
Those studies on PRRs and PRRs-mediated innate immune signaling highlight the pivotal role of innate immune in controlling pathogen infection. The importance of alternative splicing in finely regulating the immune homeostasis is now beginning to be appreciated. Indeed, those PRRs (PGRPs, NLRs and RLRs) and their downstream signaling molecules (MAVS, MITA, TBK1, IRF3, IFNs and their receptors) are alternatively spliced. In addition, those splicing isoforms are cross-modulated for PRRs-triggered responses in either a cooperative or an antagonistic manner. In mammals, plenty of work has been done to identify the sequences, proteins and mechanisms by which splicing are regulated in the PRRs-mediated signaling pathways, or splicing variants regulate the function of PRRs-triggered innate immune responses. Several intriguing and important aspects of the splicing and immune function for those members from NLRC and NLRP subfamilies are still unclear and therefore remain to be done in future research. In teleost fish, the alternative splicing of IFN and IFN receptors can lead to a functional intracellular IFN system, which acts as a novel defense to combat viral infection. Further studies will be needed to determine whether the intracellular IFN system function as a widespread mechanism in vertebrate evolution.
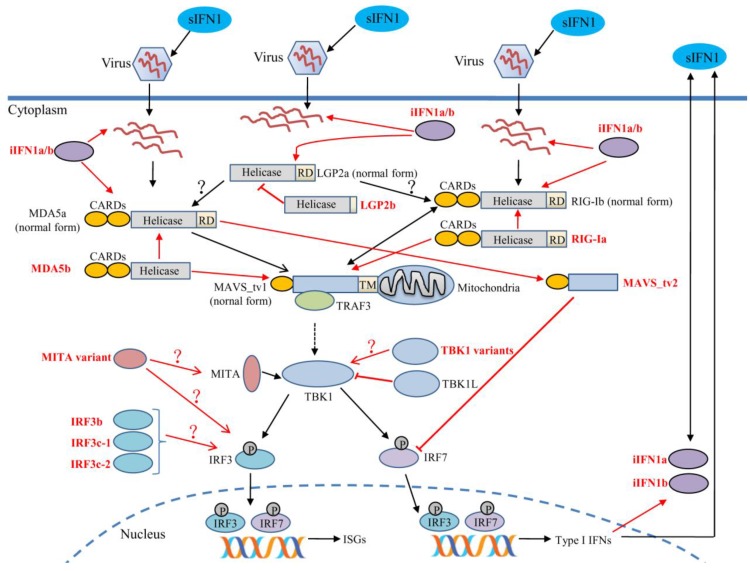
Although many splicing isoforms of most PRRs as well as the downstream signaling molecules have currently been identified in zebrafish, the exact mechanisms of splicing isoforms and the specific ligand(s) recognized by different PRR variants remain poorly understood in teleost fish. Our previous studies show clearly that splicing isoforms RIG-Ia, MDA5b, MAVS_tv2, iIFN1a and iIFN1b function as positive regulators for RLRs-triggered responses, whereas LGP2b and TBK1L as negative regulators for RLRs-triggered responses in teleost fish (Figure 4). Much work is needed to understand how different variants of NLRs family affect distinct signaling molecules, and also to understand the physiological and pathological significance of alternative splicing. In addition, the cross-regulation among different PRR variants may further endow them with the ability to properly respond to a large variety of invading pathogens. The interplay effect between PRR variants and/or other immune pathways on the host immune defense responses also requires further investigation.
Figure 4.
The alternative splicing and immune function of RLRs-mediated signaling pathways in response to viral infection in teleost fish. RIG-I, MDA5, LGP2, MAVS, MITA, TBK1, IRF3 and IFN1 undergo alternative splicing. RIG-Ia functions as an enhancer in the RIG-Ib/MAVS-mediated signaling pathway, MDA5b as an enhancer in the MDA5a/MAVS-mediated signaling pathway, LGP2b as a negative regulator for LGP2a-elicited antiviral signaling. MAVS_tv1 cooperates with RIG-Ib in a positive feedback loop and enhances RIG-Ib-mediated antiviral signaling, whereas MAVS_tv2 synergizes with MDA5a and enhances MAVS_tv2-mediated antiviral signaling. MAVS_tv2 may also function as a negative regulator of IFN1 by targeting IRF7. The function of those splicing isoforms of MITA, TBK1 and IRF3 is still unclear at present, and need to be further investigated, which were indicated by the arrows with “?”. Importantly, fish possess a functional intracellular IFN system. The cross-regulation among excellular and intracellular IFN system function as a positive feedback loop in RLRs-MAVS signaling pathways. In the signaling schematics, the signaling pathways mediated by splicing isoforms are marked with red arrows, black arrows for normal form or wild type of PRRs signaling molecules, bidirectional arrows for the interaction between different PRRs signaling molecules. The broken arrow indicates that the direct interaction need to be confirmed.
Given the fact that aberrant splicing is known to contribute to defects in immune function and that the expressions of splicing isoforms have been linked to the ability to induce a protective immune response to pathogens or the ability of the pathogen to evade host immune response, there is no doubt that alternative splicing has strong effects on the immune system. The knowledge of alternative splicing of PRRs and PRRs-mediated innate immune signaling will shed new light on the pathogenesis of inflammatory diseases and provide important clues for the control of pathogens infection.
Acknowledgments
This work was supported by National Natural Science Foundation of China Grants 31372531 and 31672687 (Ming Xian Chang).
Supplementary Materials
Supplementary materials can be found at www.mdpi.com/1422-0067/18/7/1530/s1.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Palm N.W., Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunol. Rev. 2009;227:221–233. doi: 10.1111/j.1600-065X.2008.00731.x. [DOI] [PubMed] [Google Scholar]
- 2.Brennan K., Bowie A.G. Activation of host pattern recognition receptors by viruses. Curr. Opin. Microbiol. 2010;13:503–507. doi: 10.1016/j.mib.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Deretic V., Saitoh T., Akira S. Autophagy in infection, inflammation and immunity. Nat. Rev. Immunol. 2013;13:722–737. doi: 10.1038/nri3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao X. Self-regulation and cross-regulation of pattern-recognition receptor signalling in health and disease. Nat. Rev. Immunol. 2016;16:35–50. doi: 10.1038/nri.2015.8. [DOI] [PubMed] [Google Scholar]
- 5.Nilsen T.W., Graveley B.R. Expansion of the eukaryotic proteome by alternative splicing. Nature. 2010;463:457–463. doi: 10.1038/nature08909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang E.T., Sandberg R., Luo S., Khrebtukova I., Zhang L., Mayr C., Kingsmore S.F., Schroth G.P., Burge C.B. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456:470–476. doi: 10.1038/nature07509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grabowski P. Alternative splicing takes shape during neuronal development. Curr. Opin. Genet. Dev. 2011;21:388–394. doi: 10.1016/j.gde.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Vuong C.K., Black D.L., Zheng S. The neurogenetics of alternative splicing. Nat. Rev. Neurosci. 2016;17:265–281. doi: 10.1038/nrn.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez N.M., Lynch K.W. Control of alternative splicing in immune responses: Many regulators, many predictions, much still to learn. Immunol. Rev. 2013;253:216–236. doi: 10.1111/imr.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faustino N.A., Cooper T.A. Pre-mRNA splicing and human disease. Genes Dev. 2003;17:419–437. doi: 10.1101/gad.1048803. [DOI] [PubMed] [Google Scholar]
- 11.Hsieh H.F., Yu J.C., Ho L.I., Chiu S.C., Harn H.J. Molecular studies into the role of CD44 variants in metastasis in gastric cancer. Mol. Pathol. 1999;52:25–28. doi: 10.1136/mp.52.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olsson E., Honeth G., Bendahl P.O., Saal L.H., Gruvberger-Saal S., Ringnér M., Vallon-Christersson J., Jönsson G., Holm K., Lövgren K., et al. CD44 isoforms are heterogeneously expressed in breast cancer and correlate with tumor subtypes and cancer stem cell markers. BMC Cancer. 2011;11:418. doi: 10.1186/1471-2407-11-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prochazka L., Tesarik R., Turanek J. Regulation of alternative splicing of CD44 in cancer. Cell Signal. 2014;26:2234–2239. doi: 10.1016/j.cellsig.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Moors M., Vudattu N.K., Abel J., Krämer U., Rane L., Ulfig N., Ceccatelli S., Seyfert-Margolies V., Fritsche E., Maeurer M.J. Interleukin-7 (IL-7) and IL-7 splice variants affect differentiation of human neural progenitor cells. Genes Immun. 2010;11:11–20. doi: 10.1038/gene.2009.77. [DOI] [PubMed] [Google Scholar]
- 15.Sahoo A., Im S.H. Interleukin and interleukin receptor diversity: Role of alternative splicing. Int. Rev. Immunol. 2010;29:77–109. doi: 10.3109/08830180903349651. [DOI] [PubMed] [Google Scholar]
- 16.Shakola F., Suri P., Ruggiu M. Splicing Regulation of pro-inflammatory cytokines and chemokines: At the interface of the neuroendocrine and immune systems. Biomolecules. 2015;5:2073–2100. doi: 10.3390/biom5032073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurata S. Peptidoglycan recognition proteins in Drosophila immunity. Dev. Comp. Immunol. 2014;42:36–41. doi: 10.1016/j.dci.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Werner T., Liu G., Kang D., Ekengren S., Steiner H., Hultmark D. A family of peptidoglycan recognition proteins in the fruit fly Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 2000;97:13772–13777. doi: 10.1073/pnas.97.25.13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Werner T., Borge-Renberg K., Mellroth P., Steiner H., Hultmark D. Functional diversity of the Drosophila PGRP-LC gene cluster in the response to lipopolysaccharide and peptidoglycan. J. Biol. Chem. 2003;278:26319–26322. doi: 10.1074/jbc.C300184200. [DOI] [PubMed] [Google Scholar]
- 20.Neyen C., Poidevin M., Roussel A., Lemaitre B. Tissue- and ligand-specific sensing of gram-negative infection in drosophila by PGRP-LC isoforms and PGRP-LE. J. Immunol. 2012;189:1886–1897. doi: 10.4049/jimmunol.1201022. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z.M., Li X., Cocklin R.R., Wang M., Wang M., Fukase K., Inamura S., Kusumoto S., Gupta D., Dziarski R. Human peptidoglycan recognition protein-L is an N-acetylmuramoyl-l-alanine amidase. J. Biol. Chem. 2003;278:49044–49052. doi: 10.1074/jbc.M307758200. [DOI] [PubMed] [Google Scholar]
- 22.Dziarski R., Gupta D. Mammalian PGRPs: Novel antibacterial proteins. Cell Microbiol. 2006;8:1059–1069. doi: 10.1111/j.1462-5822.2006.00726.x. [DOI] [PubMed] [Google Scholar]
- 23.Dziarski R., Gupta D. The peptidoglycan recognition proteins (PGRPs) Genome Biol. 2006;7:232. doi: 10.1186/gb-2006-7-8-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu X., Wang M., Qi J., Wang H., Li X., Gupta D., Dziarski R. Peptidoglycan recognition proteins are a new class of human bactericidal proteins. J. Biol. Chem. 2006;281:5895–5907. doi: 10.1074/jbc.M511631200. [DOI] [PubMed] [Google Scholar]
- 25.Kibardin A.V., Mirkina I.I., Baranova E.V., Zakeyeva I.R., Georgiev G.P., Kiselev S.L. The differentially spliced mouse tagL gene, homolog of tag7/PGRP gene family in mammals and Drosophila, can recognize gram-positive and gram-negative bacterial cell wall independently of T phage lysozyme homology domain. J. Mol. Biol. 2003;326:467–474. doi: 10.1016/S0022-2836(02)01401-8. [DOI] [PubMed] [Google Scholar]
- 26.Li X., Wang S., Qi J., Echtenkamp S.F., Chatterjee R., Wang M., Boons G.J., Dziarski R., Gupta D. Zebrafish peptidoglycan recognition proteins are bactericidal amidases essential for defense against bacterial infections. Immunity. 2007;27:518–529. doi: 10.1016/j.immuni.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang M.X., Nie P. RNAi suppression of zebrafish peptidoglycan recognition protein 6 (zfPGRP6) mediated differentially expressed genes involved in Toll-like receptor signaling pathway and caused increased susceptibility to Flavobacterium columnare. Vet. Immunol. Immunopathol. 2008;124:295–301. doi: 10.1016/j.vetimm.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Chang M.X., Wang Y.P., Nie P. Zebrafish peptidoglycan recognition protein SC (zfPGRP-SC) mediates multiple intracellular signaling pathways. Fish Shellfish Immunol. 2009;26:264–274. doi: 10.1016/j.fsi.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Chang M.X., Nie P., Wei L.L. Short and long peptidoglycan recognition proteins (PGRPs) in zebrafish, with findings of multiple PGRP homologs in teleost fish. Mol. Immunol. 2007;44:3005–3023. doi: 10.1016/j.molimm.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 30.Yu Z.L., Li J.H., Xue N.N., Nie P., Chang M.X. Expression and functional characterization of PGRP6 splice variants in grass carp Ctenopharyngodon idella. Dev. Comp. Immunol. 2014;47:264–274. doi: 10.1016/j.dci.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Li J.H., Yu Z.L., Xue N.N., Zou P.F., Hu J.Y., Nie P., Chang M.X. Molecular cloning and functional characterization of peptidoglycan recognition protein 6 in grass carp Ctenopharyngodon idella. Dev. Comp. Immunol. 2014;42:244–255. doi: 10.1016/j.dci.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 32.Koizumi Y., Toma C., Higa N., Nohara T., Nakasone N., Suzuki T. Inflammasome activation via intracellular NLRs triggered by bacterial infection. Cell Microbiol. 2012;14:149–154. doi: 10.1111/j.1462-5822.2011.01707.x. [DOI] [PubMed] [Google Scholar]
- 33.Jacobs S.R., Damania B. NLRs, inflammasomes, and viral infection. J. Leukoc. Biol. 2012;92:469–477. doi: 10.1189/jlb.0312132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wen H., Miao E.A., Ting J.P. Mechanisms of NOD-like receptor-associated inflammasome activation. Immunity. 2013;39:432–441. doi: 10.1016/j.immuni.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lupfer C., Kanneganti T.D. The expanding role of NLRs in antiviral immunity. Immunol. Rev. 2013;255:13–24. doi: 10.1111/imr.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parlato M., Yeretssian G. NOD-like receptors in intestinal homeostasis and epithelial tissue repair. Int. J. Mol. Sci. 2014;15:9594–9627. doi: 10.3390/ijms15069594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carneiro L.A., Travassos L.H. The interplay between NLRs and autophagy in immunity and inflammation. Front. Immunol. 2013;4:361. doi: 10.3389/fimmu.2013.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Gorp H., Kuchmiy A., Van Hauwermeiren F., Lamkanfi M. NOD-like receptors interfacing the immune and reproductive systems. FEBS. J. 2014;281:4568–4582. doi: 10.1111/febs.13014. [DOI] [PubMed] [Google Scholar]
- 39.Ting J.P., Lovering R.C., Alnemri E.S., Bertin J., Boss J.M., Davis B.K., Flavell R.A., Girardin S.E., Godzik A., Harton J.A., et al. The NLR gene family: A standard nomenclature. Immunity. 2008;28:285–287. doi: 10.1016/j.immuni.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stein C., Caccamo M., Laird G., Leptin M. Conservation and divergence of gene families encoding components of innate immune response systems in zebrafish. Genome Biol. 2007;8:R251. doi: 10.1186/gb-2007-8-11-r251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steimle V., Otten L.A., Zufferey M., Mach B. Complementation cloning of an MHC class II transactivator mutated in hereditary MHC class II deficiency (or bare lymphocyte syndrome) Cell. 1993;75:135–146. doi: 10.1016/S0092-8674(05)80090-X. [DOI] [PubMed] [Google Scholar]
- 42.Muhlethaler-Mottet A., Otten L.A., Steimle V., Mach B. Expression of MHC class II molecules in different cellular and functional compartments is controlled by differential usage of multiple promoters of the transactivator CIITA. EMBO. J. 1997;16:2851–2860. doi: 10.1093/emboj/16.10.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riley J.L., Westerheide S.D., Price J.A., Brown J.A., Boss J.M. Activation of class II MHC genes requires both the X box region and the class II transactivator (CIITA) Immunity. 1995;2:533–543. doi: 10.1016/1074-7613(95)90033-0. [DOI] [PubMed] [Google Scholar]
- 44.Peijnenburg A., Van den Berg R., Van Eggermond M.J., Sanal O., Vossen J.M., Lennon A.M., Alcaïde-Loridan C., Van den Elsen P.J. Defective MHC class II expression in an MHC class II deficiency patient is caused by a novel deletion of a splice donor site in the MHC class II transactivator gene. Immunogenetics. 2000;51:42–49. doi: 10.1007/s002510050007. [DOI] [PubMed] [Google Scholar]
- 45.Chiu B.L., Li C.H., Chang C.C. Selective modulation of MHC class II chaperons by a novel IFN-γ-inducible class II transactivator variant in lung adenocarcinoma A549 cells. Biochem. Biophys. Res. Commun. 2013;440:190–195. doi: 10.1016/j.bbrc.2013.09.066. [DOI] [PubMed] [Google Scholar]
- 46.Day N.E., Ugai H., Yokoyama K.K., Ichiki A.T. K-562 cells lack MHC class II expression due to an alternatively spliced CIITA transcript with a truncated coding region. Leuk. Res. 2003;27:1027–1038. doi: 10.1016/S0145-2126(03)00072-9. [DOI] [PubMed] [Google Scholar]
- 47.Rajendran K.V., Zhang J., Liu S., Kucuktas H., Wang X., Liu H., Sha Z., Terhune J., Peatman E., Liu Z. Pathogen recognition receptors in channel catfish: I Identification, phylogeny and expression of NOD-like receptors. Dev. Comp. Immunol. 2012;37:77–86. doi: 10.1016/j.dci.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 48.Liu Y., Meng Y., Wang Q., Sha Z. Class II, major histocompatibility complex, transactivator (CIITA) in channel catfish: Identification and expression patterns responding to different pathogens. Mol. Biol. Rep. 2012;39:11041–11050. doi: 10.1007/s11033-012-2007-z. [DOI] [PubMed] [Google Scholar]
- 49.LeibundGut-Landmann S., Waldburger J.M., Krawczyk M., Otten L.A., Suter T., Fontana A., Acha-Orbea H., Reith W. Mini-review: Specificity and expression of CIITA, the master regulator of MHC class II genes. Eur. J. Immunol. 2004;34:1513–1525. doi: 10.1002/eji.200424964. [DOI] [PubMed] [Google Scholar]
- 50.Hu Y.W., Wu X.M., Ren S.S., Cao L., Nie P., Chang M.X. NOD1 deficiency impairs CD44a/LCK as well as PI3K/Akt pathway. Sci. Rep. 2017;7:2979. doi: 10.1038/s41598-017-03258-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tattoli I., Travassos L.H., Carneiro L.A., Magalhaes J.G., Girardin S.E. The Nodosome: NOD1 and NOD2 control bacterial infections and inflammation. Semin. Immunopathol. 2007;29:289–301. doi: 10.1007/s00281-007-0083-2. [DOI] [PubMed] [Google Scholar]
- 52.Caruso R., Warner N., Inohara N., Núñez G. NOD1 and NOD2: Signaling, host defense, and inflammatory disease. Immunity. 2014;41:898–908. doi: 10.1016/j.immuni.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clay G.M., Sutterwala F.S., Wilson M.E. NLR proteins and parasitic disease. Immunol. Res. 2014;59:142–152. doi: 10.1007/s12026-014-8544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coutermarsh-Ott S., Eden K., Allen I.C. Beyond the inflammasome: Regulatory NOD-like receptor modulation of the host immune response following virus exposure. J. Gen. Virol. 2016;97:825–838. doi: 10.1099/jgv.0.000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ogura Y., Inohara N., Benito A., Chen F.F., Yamaoka S., Nunez G. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-κB. J. Biol. Chem. 2001;276:4812–4818. doi: 10.1074/jbc.M008072200. [DOI] [PubMed] [Google Scholar]
- 56.Leung E., Hong J., Fraser A., Krissansen G.W. Splicing of NOD2 (CARD15) RNA transcripts. Mol. Immunol. 2007;44:284–294. doi: 10.1016/j.molimm.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 57.King K., Bagnall R., Fisher S.A., Sheikh F., Cuthbert A., Tan S., Mundy N.I., Rosenstiel P., Schreiber S., Mathew C.G., et al. Identification, evolution, and association study of a novel promoter and first exon of the human NOD2 (CARD15) gene. Genomics. 2007;90:493–501. doi: 10.1016/j.ygeno.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 58.Rosenstiel P., Huse K., Franke A., Hampe J., Reichwald K., Platzer C., Roberts R.G., Mathew C.G., Platzer M., Schreiber S. Functional characterization of two novel 5′ untranslated exons reveals a complex regulation of NOD2 protein expression. BMC Genomics. 2007;8:472. doi: 10.1186/1471-2164-8-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosenstiel P., Huse K., Till A., Hampe J., Hellmig S., Sina C., Billmann S., von Kampen O., Waetzig G.H., Platzer M., et al. A short isoform of NOD2/CARD15, NOD2-S, is an endogenous inhibitor of NOD2/receptor-interacting protein kinase 2-induced signaling pathways. Proc. Natl. Acad. Sci. USA. 2006;103:3280–3285. doi: 10.1073/pnas.0505423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oehlers S.H., Flores M.V., Hall C.J., Swift S., Crosier K.E., Crosier P.S. The inflammatory bowel disease (IBD) susceptibility genes NOD1 and NOD2 have conserved anti-bacterial roles in zebrafish. Dis. Model. Mech. 2011;4:832–841. doi: 10.1242/dmm.006122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chang M., Wang T., Nie P., Zou J., Secombes C.J. Cloning of two rainbow trout nucleotide-binding oligomerization domain containing 2 (NOD2) splice variants and functional characterization of the NOD2 effector domains. Fish Shellfish Immunol. 2011;30:118–127. doi: 10.1016/j.fsi.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 62.Schneider M., Zimmermann A.G., Roberts R.A., Zhang L., Swanson K.V., Wen H., Davis B.K., Allen I.C., Holl E.K., Ye Z., et al. The innate immune sensor NLRC3 attenuates Toll-like receptor signaling via modification of the signaling adaptor TRAF6 and transcription factor NF-κB. Nat. Immunol. 2012;13:823–831. doi: 10.1038/ni.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang L., Mo J., Swanson K.V., Wen H., Petrucelli A., Gregory S.M., Zhang Z., Schneider M., Jiang Y., Fitzgerald K.A., et al. NLRC3, a member of the NLR family of proteins, is a negative regulator of innate immune signaling induced by the DNA sensor sting. Immunity. 2014;40:329–341. doi: 10.1016/j.immuni.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Conti B.J., Davis B.K., Zhang J., O′connor W., Williams K.L., Ting J.P. CATERPILLER 16.2 (CLR16.2), a novel NBD/LRR family member that negatively regulates T cell function. J. Biol. Chem. 2005;280:18375–18385. doi: 10.1074/jbc.M413169200. [DOI] [PubMed] [Google Scholar]
- 65.Karki R., Man S.M., Malireddi R.K., Kesavardhana S., Zhu Q., Burton A.R., Sharma B.R., Qi X., Pelletier S., Vogel P., et al. NLRC3 is an inhibitory sensor of PI3K-mTOR pathways in cancer. Nature. 2016 doi: 10.1038/nature20597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leavy O. Tumour immunology: NLRC3 inhibits mTOR in colorectal cancer. Nat. Rev. Immunol. 2017;17:79. doi: 10.1038/nri.2016.152. [DOI] [PubMed] [Google Scholar]
- 67.Hou Z., Ye Z., Zhang D., Gao C., Su B., Song L., Tan F., Song H., Wang Y., Li C. Characterization and expression profiling of NOD-like receptor C3 (NLRC3) in mucosal tissues of turbot (Scophthalmus maximus L.) following bacterial challenge. Fish Shellfish Immunol. 2017;66:231–239. doi: 10.1016/j.fsi.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 68.Álvarez C.A., Ramírez-Cepeda F., Santana P., Torres E., Cortés J., Guzmán F., Schmitt P., Mercado L. Insights into the diversity of NOD-like receptors: Identification and expression analysis of NLRC3, NLRC5 and NLRX1 in rainbow trout. Mol. Immunol. 2017;87:102–113. doi: 10.1016/j.molimm.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 69.Paria A., Deepika A., Sreedharan K., Makesh M., Chaudhari A., Purushothaman C.S., Thirunavukkarasu A.R., Rajendran K.V. Identification of Nod like receptor C3 (NLRC3) in Asian seabass, Lates calcarifer: Characterisation, ontogeny and expression analysis after experimental infection and ligand stimulation. Fish Shellfish Immunol. 2016;55:602–612. doi: 10.1016/j.fsi.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 70.Li S., Chen X., Hao G., Geng X., Zhan W., Sun J. Identification and characterization of a novel NOD-like receptor family CARD domain containing 3 gene in response to extracellular ATP stimulation and its role in regulating LPS-induced innate immune response in Japanese flounder (Paralichthys olivaceus) head kidney macrophages. Fish Shellfish Immunol. 2016;50:79–90. doi: 10.1016/j.fsi.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 71.Li J., Kong L., Gao Y., Wu C., Xu T. Characterization of NLR-A subfamily members in miiuy croaker and comparative genomics revealed NLRX1 underwent duplication and lose in actinopterygii. Fish Shellfish Immunol. 2015;47:397–406. doi: 10.1016/j.fsi.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 72.Sha Z., Abernathy J.W., Wang S., Li P., Kucuktas H., Liu H., Peatman E., Liu Z. NOD-like subfamily of the nucleotide-binding domain and leucine-rich repeat containing family receptors and their expression in channel catfish. Dev. Comp. Immunol. 2009;33:991–999. doi: 10.1016/j.dci.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 73.Li M., Wang Q.L., Lu Y., Chen S.L., Li Q., Sha Z.X. Expression profiles of NODs in channel catfish (Ictalurus punctatus) after infection with Edwardsiella tarda, Aeromonas hydrophila, Streptococcus iniae and channel catfish hemorrhage reovirus. Fish Shellfish Immunol. 2012;33:1033–1041. doi: 10.1016/j.fsi.2012.06.033. [DOI] [PubMed] [Google Scholar]
- 74.Shiau C.E., Monk K.R., Joo W., Talbot W.S. An anti-inflammatory NOD-like receptor is required for microglia development. Cell Rep. 2013;5:1342–1352. doi: 10.1016/j.celrep.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cui J., Zhu L., Xia X., Wang H.Y., Legras X., Hong J., Ji J., Shen P., Zheng S., Chen Z.J., et al. NLRC5 negatively regulates the NF-kappaB and type I interferon signaling pathways. Cell. 2010;141:483–496. doi: 10.1016/j.cell.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kumar H., Pandey S., Zou J., Kumagai Y., Takahashi K., Akira S., Kawai T. NLRC5 deficiency does not influence cytokine induction by virus and bacteria infections. J. Immunol. 2011;186:994–1000. doi: 10.4049/jimmunol.1002094. [DOI] [PubMed] [Google Scholar]
- 77.Kuenzel S., Till A., Winkler M., Häsler R., Lipinski S., Jung S., Grötzinger J., Fickenscher H., Schreiber S., Rosenstiel P. The nucleotide-binding oligomerization domain-like receptor NLRC5 is involved in IFN-dependent antiviral immune responses. J. Immunol. 2010;184:1990–2000. doi: 10.4049/jimmunol.0900557. [DOI] [PubMed] [Google Scholar]
- 78.Neerincx A., Lautz K., Menning M., Kremmer E., Zigrino P., Hösel M., Büning H., Schwarzenbacher R., Kufer T.A. A role for the human nucleotide-binding domain, leucine-rich repeat-containing family member NLRC5 in antiviral responses. J. Biol. Chem. 2010;285:26223–26232. doi: 10.1074/jbc.M110.109736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ranjan P., Singh N., Kumar A., Neerincx A., Kremmer E., Cao W., Davis W.G., Katz J.M., Gangappa S., Lin R., et al. NLRC5 interacts with RIG-I to induce a robust antiviral response against influenza virus infection. Eur. J. Immunol. 2015;45:758–772. doi: 10.1002/eji.201344412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Davis B.K., Roberts R.A., Huang M.T., Willingham S.B., Conti B.J., Brickey W.J., Barker B.R., Kwan M., Taxman D.J., Accavitti-Loper M.A., et al. Cutting edge: NLRC5-dependent activation of the inflammasome. J. Immunol. 2011;186:1333–1337. doi: 10.4049/jimmunol.1003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Triantafilou K., Kar S., van Kuppeveld F.J., Triantafilou M. Rhinovirus-induced calcium flux triggers NLRP3 and NLRC5 activation in bronchial cells. Am. J. Respir. Cell Mol. Biol. 2013;49:923–934. doi: 10.1165/rcmb.2013-0032OC. [DOI] [PubMed] [Google Scholar]
- 82.Meissner T.B., Li A., Kobayashi K.S. NLRC5: A newly discovered MHC class I transactivator (CITA) Microbes Infect. 2012;14:477–484. doi: 10.1016/j.micinf.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kobayashi K.S., van den Elsen P.J. NLRC5: A key regulator of MHC class I-dependent immune responses. Nat. Rev. Immunol. 2012;12:813–820. doi: 10.1038/nri3339. [DOI] [PubMed] [Google Scholar]
- 84.Ludigs K., Seguín-Estévez Q., Lemeille S., Ferrero I., Rota G., Chelbi S., Mattmann C., MacDonald H.R., Reith W., Guarda G. NLRC5 exclusively transactivates MHC class I and related genes through a distinctive SXY module. PLoS Genet. 2015;11:e1005088. doi: 10.1371/journal.pgen.1005088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu X.M., Hu Y.W., Xue N.N., Ren S.S., Chen S.N., Nie P., Chang M.X. Role of zebrafish NLRC5 in antiviral response and transcriptional regulation of MHC related genes. Dev. Comp. Immunol. 2017;68:58–68. doi: 10.1016/j.dci.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 86.Bruns A.M., Horvath C.M. Activation of RIG-I-like receptor signal transduction. Crit. Rev. Biochem. Mol. Biol. 2012;47:194–206. doi: 10.3109/10409238.2011.630974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ramos H.J., Gale M., Jr. RIG-I like receptors and their signaling crosstalk in the regulation of antiviral immunity. Curr. Opin. Virol. 2011;1:167–176. doi: 10.1016/j.coviro.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yoneyama M., Kikuchi M., Matsumoto K., Imaizumi T., Miyagishi M., Taira K., Foy E., Loo Y.M., Gale M., Jr., Akira S., et al. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J. Immunol. 2005;175:2851–2858. doi: 10.4049/jimmunol.175.5.2851. [DOI] [PubMed] [Google Scholar]
- 89.Satoh T., Kato H., Kumagai Y., Yoneyama M., Sato S., Matsushita K., Tsujimura T., Fujita T., Akira S., Takeuchi O. LGP2 is a positive regulator of RIG-I- and MDA5-mediated antiviral responses. Proc. Natl. Acad. Sci. USA. 2010;107:1512–1517. doi: 10.1073/pnas.0912986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rothenfusser S., Goutagny N., DiPerna G., Gong M., Monks B.G., Schoenemeyer A., Yamamoto M., Akira S., Fitzgerald K.A. The RNA helicase LGP2 inhibits TLR-independent sensing of viral replication by retinoic acid-inducible gene-I. J. Immunol. 2005;175:5260–5268. doi: 10.4049/jimmunol.175.8.5260. [DOI] [PubMed] [Google Scholar]
- 91.Saito T., Hirai R., Loo Y.M., Owen D., Johnson C.L., Sinha S.C., Akira S., Fujita T., Gale M., Jr. Regulation of innate antiviral defenses through a shared repressor domain in RIG-I and LGP2. Proc. Natl. Acad. Sci. USA. 2007;104:582–587. doi: 10.1073/pnas.0606699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sarkar D., Desalle R., Fisher P.B. Evolution of MDA-5/RIG-I-dependent innate immunity: Independent evolution by domain grafting. Proc. Natl. Acad. Sci. USA. 2008;105:17040–17045. doi: 10.1073/pnas.0804956105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun F., Zhang Y.B., Liu T.K., Shi J., Wang B., Gui J.F. Fish MITA serves as a mediator for distinct fish IFN gene activation dependent on IRF3 or IRF7. J. Immunol. 2011;187:2531–2539. doi: 10.4049/jimmunol.1100642. [DOI] [PubMed] [Google Scholar]
- 94.Feng H., Liu H., Kong R., Wang L., Wang Y., Hu W., Guo Q. Expression profiles of carp IRF-3/-7 correlate with the up-regulation of RIG-I/MAVS/TRAF3/TBK1, four pivotal molecules in RIG-I signaling pathway. Fish Shellfish Immunol. 2011;30:1159–1169. doi: 10.1016/j.fsi.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 95.Xiao J., Yan J., Chen H., Li J., Tian Y., Feng H. LGP2 of black carp plays an important role in the innate immune response against SVCV and GCRV. Fish Shellfish Immunol. 2016;57:127–135. doi: 10.1016/j.fsi.2016.08.031. [DOI] [PubMed] [Google Scholar]
- 96.Liu J., Li J., Xiao J., Chen H., Lu L., Wang X., Tian Y., Feng H. The antiviral signaling mediated by black carp MDA5 is positively regulated by LGP2. Fish Shellfish Immunol. 2017;66:360–371. doi: 10.1016/j.fsi.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 97.Su J., Huang T., Dong J., Heng J., Zhang R., Peng L. Molecular cloning and immune responsive expression of MDA5 gene, a pivotal member of the RLR gene family from grass carp Ctenopharyngodon idella. Fish Shellfish Immunol. 2010;28:712–718. doi: 10.1016/j.fsi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 98.Huang T., Su J., Heng J., Dong J., Zhang R., Zhu H. Identification and expression profiling analysis of grass carp Ctenopharyngodon idella LGP2 cDNA. Fish Shellfish Immunol. 2010;29:349–355. doi: 10.1016/j.fsi.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 99.Yang C., Su J., Huang T., Zhang R., Peng L. Identification of a retinoic acid-inducible gene I from grass carp (Ctenopharyngodon idella) and expression analysis in vivo and in vitro. Fish Shellfish Immunol. 2011;30:936–943. doi: 10.1016/j.fsi.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 100.Zou P.F., Chang M.X., Xue N.N., Liu X.Q., Li J.H., Fu J.P., Chen S.N., Nie P. Melanoma differentiation-associated gene 5 in zebrafish provoking higher interferon-promoter activity through signalling enhancing of its shorter splicing variant. Immunology. 2014;141:192–202. doi: 10.1111/imm.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zou P.F., Chang M.X., Li Y., Zhang S.H., Fu J.P., Chen S.N., Nie P. Higher antiviral response of RIG-I through enhancing RIG-I/MAVS-mediated signaling by its long insertion variant in zebrafish. Fish Shellfish Immunol. 2015;43:13–24. doi: 10.1016/j.fsi.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 102.Nie L., Zhang Y.S., Dong W.R., Xiang L.X., Shao J.Z. Involvement of zebrafish RIG-I in NF-κB and IFN signaling pathways: Insights into functional conservation of RIG-I in antiviral innate immunity. Dev. Comp. Immunol. 2015;48:95–101. doi: 10.1016/j.dci.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 103.Wang W., Asim M., Yi L., Hegazy A.M., Hu X., Zhou Y., Ai T., Lin L. Abortive infection of snakehead fish vesiculovirus in ZF4 cells was associated with the RLRs pathway activation by viral replicative intermediates. Int. J. Mol. Sci. 2015;16:6235–6250. doi: 10.3390/ijms16036235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rajendran K.V., Zhang J., Liu S., Peatman E., Kucuktas H., Wang X., Liu H., Wood T., Terhune J., Liu Z. Pathogen recognition receptors in channel catfish: II Identification, phylogeny and expression of retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs) Dev. Comp. Immunol. 2012;37:381–389. doi: 10.1016/j.dci.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 105.Yu Y., Huang Y., Yang Y., Wang S., Yang M., Huang X., Qin Q. Negative regulation of the antiviral response by grouper LGP2 against fish viruses. Fish Shellfish Immunol. 2016;56:358–366. doi: 10.1016/j.fsi.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 106.Huang Y., Yu Y., Yang Y., Yang M., Zhou L., Huang X., Qin Q. Antiviral function of grouper MDA5 against iridovirus and nodavirus. Fish Shellfish Immunol. 2016;54:188–196. doi: 10.1016/j.fsi.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 107.Nerbøvik I.G., Solheim M.A., Eggestøl H.Ø., Rønneseth A., Jakobsen R.A., Wergeland H.I., Haugland G.T. Molecular cloning of MDA5, phylogenetic analysis of RIG-I-like receptors (RLRs) and differential gene expression of RLRs, interferons and proinflammatory cytokines after in vitro challenge with IPNV, ISAV and SAV in the salmonid cell line TO. J. Fish. Dis. 2017 doi: 10.1111/jfd.12622. [DOI] [PubMed] [Google Scholar]
- 108.Chang M., Collet B., Nie P., Lester K., Campbell S., Secombes C.J., Zou J. Expression and functional characterization of the RIG-I-like receptors MDA5 and LGP2 in Rainbow trout (Oncorhynchus mykiss) J. Virol. 2011;85:8403–8412. doi: 10.1128/JVI.00445-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shen B., Hu Y., Zhang S., Zheng J., Zeng L., Zhang J., Zhu A., Wu C. Molecular characterization and expression analyses of three RIG-I-like receptor signaling pathway genes (MDA5, LGP2 and MAVS) in Larimichthys crocea. Fish Shellfish Immunol. 2016;55:535–549. doi: 10.1016/j.fsi.2016.06.032. [DOI] [PubMed] [Google Scholar]
- 110.Bhat A., Paria A., Deepika A., Sreedharan K., Makesh M., Bedekar M.K., Purushothaman C.S., Rajendran K.V. Molecular cloning, characterisation and expression analysis of melanoma differentiation associated gene 5 (MDA5) of green chromide, Etroplus suratensis. Gene. 2015;557:172–181. doi: 10.1016/j.gene.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 111.Jia P., Zhang J., Jin Y., Zeng L., Jia K., Yi M. Characterization and expression analysis of laboratory of genetics and physiology 2 gene in sea perch, Lateolabrax japonicus. Fish Shellfish Immunol. 2015;47:214–220. doi: 10.1016/j.fsi.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 112.Jia P., Jia K., Chen L., Le Y., Jin Y., Zhang J., Zhu L., Zhang L., Yi M. Identification and characterization of the melanoma differentiation-associated gene 5 in sea perch, Lateolabrax japonicus. Dev. Comp. Immunol. 2016;61:161–168. doi: 10.1016/j.dci.2016.03.029. [DOI] [PubMed] [Google Scholar]
- 113.Ohtani M., Hikima J., Kondo H., Hirono I., Jung T.S., Aoki T. Evolutional conservation of molecular structure and antiviral function of a viral RNA receptor, LGP2, in Japanese flounder, Paralichthys olivaceus. J. Immunol. 2010;185:7507–7517. doi: 10.4049/jimmunol.1001850. [DOI] [PubMed] [Google Scholar]
- 114.Gack M.U., Kirchhofer A., Shin Y.C., Inn K.S., Liang C., Cui S., Myong S., Ha T., Hopfner K.P., Jung J.U. Roles of RIG-I N-terminal tandem CARD and splice variant in TRIM25-mediated antiviral signal transduction. Proc. Natl. Acad. Sci. USA. 2008;105:16743–16748. doi: 10.1073/pnas.0804947105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Biacchesi S., LeBerre M., Lamoureux A., Louise Y., Lauret E., Boudinot P., Brémont M. Mitochondrial antiviral signaling protein plays a major role in induction of the fish innate immune response against RNA and DNA viruses. J. Virol. 2009;83:7815–7827. doi: 10.1128/JVI.00404-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Han J., Wang Y., Chu Q., Xu T. The evolution and functional characterization of Miiuy croaker cytosolic gene LGP2 involved in immune response. Fish Shellfish Immunol. 2016;58:193–202. doi: 10.1016/j.fsi.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 117.Rao Y., Wan Q., Yang C., Su J. Grass carp laboratory of genetics and physiology 2 serves as a negative regulator in retinoic acid-inducible gene I- and melanoma differentiation-associated gene 5-mediated antiviral signaling in resting state and early stage of grass carp reovirus infection. Front Immunol. 2017;8:352. doi: 10.3389/fimmu.2017.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lad S.P., Yang G., Scott D.A., Chao T.H., Correia J.d.S., de la Torre J.C., Li E. Identification of MAVS splicing variants that interfere with RIGI/MAVS pathway signaling. Mol. Immunol. 2008;45:2277–2287. doi: 10.1016/j.molimm.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 119.Brubaker S.W., Gauthier A.E., Mills E.W., Ingolia N.T., Kagan J.C. A bicistronic MAVS transcript highlights a class of truncated variants in antiviral immunity. Cell. 2014;156:800–811. doi: 10.1016/j.cell.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jia P., Jin Y., Chen L., Zhang J., Jia K., Yi M. Molecular characterization and expression analysis of mitochondrial antiviral signaling protein gene in sea perch, Lateolabrax japonicus. Dev. Comp. Immunol. 2016;55:188–193. doi: 10.1016/j.dci.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 121.Zhou W., Zhou J., Lv Y., Qu Y., Chi M., Li J., Feng H. Identification and characterization of MAVS from black carp Mylopharyngodon piceus. Fish Shellfish Immunol. 2015;43:460–468. doi: 10.1016/j.fsi.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 122.Xiang Z., Qi L., Chen W., Dong C., Liu Z., Liu D., Huang M., Li W., Yang G., Weng S., et al. Characterization of a TnMAVS protein from Tetraodon nigroviridis. Dev. Comp. Immunol. 2011;35:1103–1115. doi: 10.1016/j.dci.2011.03.029. [DOI] [PubMed] [Google Scholar]
- 123.Simora R.M., Ohtani M., Hikima J., Kondo H., Hirono I., Jung T.S., Aoki T. Molecular cloning and antiviral activity of IFN-β promoter stimulator-1 (IPS-1) gene in Japanese flounder, Paralichthys olivaceus. Fish Shellfish Immunol. 2010;29:979–986. doi: 10.1016/j.fsi.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 124.Su J., Huang T., Yang C., Zhang R. Molecular cloning, characterization and expression analysis of interferon-β promoter stimulator 1 (IPS-1) gene from grass carp Ctenopharyngodon idella. Fish Shellfish Immunol. 2011;30:317–323. doi: 10.1016/j.fsi.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 125.Chen W.Q., Hu Y.W., Zou P.F., Ren S.S., Nie P., Chang M.X. MAVS splicing variants contribute to the induction of interferon and interferon-stimulated genes mediated by RIG-I-like receptors. Dev Comp Immunol. 2015;49:19–30. doi: 10.1016/j.dci.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 126.Lu L.F., Li S., Lu X.B., Zhang Y.A. Functions of the two zebrafish MAVS variants are opposite in the induction of IFN1 by targeting IRF7. Fish Shellfish Immunol. 2015;45:574–582. doi: 10.1016/j.fsi.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 127.Ishikawa H., Barber G.N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sun W., Li Y., Chen L., Chen H., You F., Zhou X., Zhou Y., Zhai Z., Chen D., Jiang Z. ERIS, an endoplasmic reticulum IFN stimulator, activates innate immune signaling through dimerization. Proc. Natl. Acad. Sci. USA. 2009;106:8653–8658. doi: 10.1073/pnas.0900850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhong B., Yang Y., Li S., Wang Y.Y., Li Y., Diao F., Lei C., He X., Zhang L., Tien P., et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity. 2008;29:538–550. doi: 10.1016/j.immuni.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 130.Ishikawa H., Ma Z., Barber G.N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Burdette D.L., Monroe K.M., Sotelo-Troha K., Iwig J.S., Eckert B., Hyodo M., Hayakawa Y., Vance R.E. STING is a direct innate immune sensor of cyclic di-GMP. Nature. 2011;478:515–518. doi: 10.1038/nature10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shu C., Yi G., Watts T., Kao C.C., Li P. Structure of STING bound to cyclic di-GMP reveals the mechanism of cyclic dinucleotide recognition by the immune system. Nat. Struct. Mol. Biol. 2012;19:722–724. doi: 10.1038/nsmb.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shang G., Zhu D., Li N., Zhang J., Zhu C., Lu D., Liu C., Yu Q., Zhao Y., Xu S., et al. Crystal structures of STING protein reveal basis for recognition of cyclic di-GMP. Nat. Struct. Mol. Biol. 2012;19:725–727. doi: 10.1038/nsmb.2332. [DOI] [PubMed] [Google Scholar]
- 134.Ouyang S., Song X., Wang Y., Ru H., Shaw N., Jiang Y., Niu F., Zhu Y., Qiu W., Parvatiyar K., et al. Structural analysis of the STING adaptor protein reveals a hydrophobic dimer interface and mode of cyclic di-GMP binding. Immunity. 2012;36:1073–1086. doi: 10.1016/j.immuni.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yin Q., Tian Y., Kabaleeswaran V., Jiang X., Tu D., Eck M.J., Chen Z.J., Wu H. Cyclic di-GMP sensing via the innate immune signaling protein STING. Mol. Cell. 2012;46:735–745. doi: 10.1016/j.molcel.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Härtlova A., Erttmann S.F., Raffi F.A., Schmalz A.M., Resch U., Anugula S., Lienenklaus S., Nilsson L.M., Kröger A., Nilsson J.A., et al. DNA damage primes the type I interferon system via the cytosolic DNA sensor STING to promote anti-microbial innate immunity. Immunity. 2015;42:332–343. doi: 10.1016/j.immuni.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 137.Unterholzner L., Keating S.E., Baran M., Horan K.A., Jensen S.B., Sharma S., Sirois C.M., Jin T., Latz E., Xiao T.S., et al. IFI16 is an innate immune sensor for intracellular DNA. Nat. Immunol. 2010;11:997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang Z., Yuan B., Bao M., Lu N., Kim T., Liu Y.J. The helicase DDX41 senses intracellular DNA mediated by the adaptor STING in dendritic cells. Nat. Immunol. 2011;12:959–965. doi: 10.1038/ni.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Aguirre S., Maestre A.M., Pagni S., Patel J.R., Savage T., Gutman D., Maringer K., Bernal-Rubio D., Shabman R.S., Simon V., et al. DENV inhibits type I IFN production in infected cells by cleaving human STING. PLoS Pathog. 2012;8:e1002934. doi: 10.1371/journal.ppat.1002934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yu C.Y., Chang T.H., Liang J.J., Chiang R.L., Lee Y.L., Liao C.L., Lin Y.L. Dengue virus targets the adaptor protein MITA to subvert host innate immunity. PLoS Pathog. 2012;8:e1002780. doi: 10.1371/journal.ppat.1002780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nitta S., Sakamoto N., Nakagawa M., Kakinuma S., Mishima K., Kusano-Kitazume A., Kiyohashi K., Murakawa M., Nishimura-Sakurai Y., Azuma S., et al. Hepatitis C virus NS4B protein targets STING and abrogates RIG-I-mediated type I interferon-dependent innate immunity. Hepatology. 2013;57:46–58. doi: 10.1002/hep.26017. [DOI] [PubMed] [Google Scholar]
- 142.Chen H., Pei R., Zhu W., Zeng R., Wang Y., Wang Y., Lu M., Chen X. An alternative splicing isoform of MITA antagonizes MITA-mediated induction of type I IFNs. J. Immunol. 2014;192:1162–1170. doi: 10.4049/jimmunol.1300798. [DOI] [PubMed] [Google Scholar]
- 143.Liu S., Zhao K., Su X., Lu L., Zhao H., Zhang X., Wang Y., Wu C., Chen J., Zhou Y., et al. MITA/STING and its alternative splicing isoform MRP restrict hepatitis B virus replication. PLoS ONE. 2017;12:e0169701. doi: 10.1371/journal.pone.0169701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Biacchesi S., Mérour E., Lamoureux A., Bernard J., Brémont M. Both STING and MAVS fish orthologs contribute to the induction of interferon mediated by RIG-I. PLoS ONE. 2012;7:e47737. doi: 10.1371/journal.pone.0047737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Huang Y., Ouyang Z., Wang W., Yu Y., Li P., Zhou S., Wei S., Wei J., Huang X., Qin Q. Antiviral role of grouper STING against iridovirus infection. Fish Shellfish Immunol. 2015;47:157–167. doi: 10.1016/j.fsi.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 146.Lu L.F., Li S., Wang Z.X., Du S.Q., Chen D.D., Nie P., Zhang Y.A. Grass carp reovirus VP41 targets fish MITA to abrogate the IFN response. J. Virol. 2017:JVI-00390. doi: 10.1128/JVI.00390-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Perry A.K., Chow E.K., Goodnough J.B., Yeh W.C., Cheng G. Differential requirement for TANK-binding kinase-1 in type I interferon responses to Toll-like receptor activation and viral infection. J. Exp. Med. 2004;199:1651–1658. doi: 10.1084/jem.20040528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Muñoz M.C., Giani J.F., Mayer M.A., Toblli J.E., Turyn D., Dominici F.P. TANK-binding kinase 1 mediates phosphorylation of insulin receptor at serine residue 994: A potential link between inflammation and insulin resistance. J. Endocrinol. 2009;201:185–197. doi: 10.1677/JOE-08-0276. [DOI] [PubMed] [Google Scholar]
- 149.Miyahira A.K., Shahangian A., Hwang S., Sun R., Cheng G. TANK-binding kinase-1 plays an important role during in vitro and in vivo type I IFN responses to DNA virus infections. J. Immunol. 2009;182:2248–2257. doi: 10.4049/jimmunol.0802466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Weidberg H., Elazar Z. TBK1 mediates crosstalk between the innate immune response and autophagy. Sci. Signal. 2011;4:pe39. doi: 10.1126/scisignal.2002355. [DOI] [PubMed] [Google Scholar]
- 151.Yu T., Yi Y.S., Yang Y., Oh J., Jeong D., Cho J.Y. The pivotal role of TBK1 in inflammatory responses mediated by macrophages. Mediators Inflamm. 2012;2012:979105. doi: 10.1155/2012/979105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhao W. Negative regulation of TBK1-mediated antiviral immunity. FEBS Lett. 2013;587:542–548. doi: 10.1016/j.febslet.2013.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Deng W., Shi M., Han M., Zhong J., Li Z., Li W., Hu Y., Yan L., Wang J., He Y., et al. Negative regulation of virus-triggered IFN-β signaling pathway by alternative splicing of TBK1. J. Biol. Chem. 2008;283:35590–35597. doi: 10.1074/jbc.M805775200. [DOI] [PubMed] [Google Scholar]
- 154.Feng X., Su J., Yang C., Yan N., Rao Y., Chen X. Molecular characterizations of grass carp (Ctenopharyngodon idella) TBK1 gene and its roles in regulating IFN-I pathway. Dev. Comp. Immunol. 2014;45:278–290. doi: 10.1016/j.dci.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 155.Li S., Lu L.F., Wang Z.X., Lu X.B., Chen D.D., Nie P., Zhang Y.A. The P protein of spring viremia of carp virus negatively regulates the fish interferon response by inhibiting the kinase activity of TANK-binding kinase 1. J. Virol. 2016;90:10728–10737. doi: 10.1128/JVI.01381-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang L., Chen W.Q., Hu Y.W., Wu X.M., Nie P., Chang M.X. TBK1-like transcript negatively regulates the production of IFN and IFN-stimulated genes through RLRs-MAVS-TBK1 pathway. Fish Shellfish Immunol. 2016;54:135–143. doi: 10.1016/j.fsi.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 157.Zhang D.L., Yu D.H., Chen J., Fan S., Wang Z.Y. Expression profiles and interaction suggest TBK1 can be regulated by Nrdp1 in response to immune stimulation in large yellow croaker Larimichthys crocea. Fish Shellfish Immunol. 2015;46:745–752. doi: 10.1016/j.fsi.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 158.Fitzgerald K.A., McWhirter S.M., Faia K.L., Rowe D.C., Latz E., Golenbock D.T., Coyle A.J., Liao S.M., Maniatis T. IKKε and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 2003;4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 159.Hiscott J. Triggering the innate antiviral response through IRF-3 activation. J. Biol. Chem. 2007;282:15325–15329. doi: 10.1074/jbc.R700002200. [DOI] [PubMed] [Google Scholar]
- 160.Karpova A.Y., Howley P.M., Ronco L.V. Dual utilization of an acceptor/donor splice site governs the alternative splicing of the IRF-3 gene. Genes Dev. 2000;14:2813–2818. doi: 10.1101/gad.813800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Karpova A.Y., Ronco L.V., Howley P.M. Functional characterization of interferon regulatory factor 3a (IRF-3a), an alternative splice isoform of IRF-3. Mol. Cell Biol. 2001;21:4169–4176. doi: 10.1128/MCB.21.13.4169-4176.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Marozin S., Altomonte J., Stadler F., Thasler W.E., Schmid R.M., Ebert O. Inhibition of the IFN-β response in hepatocellular carcinoma by alternative spliced isoform of IFN regulatory factor-3. Mol. Ther. 2008;16:1789–1797. doi: 10.1038/mt.2008.201. [DOI] [PubMed] [Google Scholar]
- 163.Li C., Ma L., Chen X. Interferon regulatory factor 3-CL, an isoform of IRF3, antagonizes activity of IRF3. Cell. Mol. Immunol. 2011;8:67–74. doi: 10.1038/cmi.2010.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ren W., Xu H.G., Lu C., Jin R., Zou L., Wang Y., Zhou G.P. The characterization of two novel IRF-3 transcripts starting from intron 2 of the wild type of IRF-3. Mol. Biol. Rep. 2011;38:4415–4421. doi: 10.1007/s11033-010-0569-1. [DOI] [PubMed] [Google Scholar]
- 165.Li Y., Hu X., Song Y., Lu Z., Ning T., Cai H., Ke Y. Identification of novel alternative splicing variants of interferon regulatory factor 3. Biochim. Biophys. Acta. 2011;1809:166–175. doi: 10.1016/j.bbagrm.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 166.Shu C., Chu Q., Bi D., Wang Y., Xu T. Identification and functional characterization of miiuy croaker IRF3 as an inducible protein involved regulation of IFN response. Fish Shellfish Immunol. 2016;54:499–506. doi: 10.1016/j.fsi.2016.04.136. [DOI] [PubMed] [Google Scholar]
- 167.Xu X., Lai Q., Gu M., Liu D., Hou Q., Liu X., Mi Y., Sun Z., Wang H., Lin G., et al. Fish IRF3 up-regulates the transcriptional level of IRF1, IRF2, IRF3 and IRF7 in CIK cells. Fish Shellfish Immunol. 2015;47:978–985. doi: 10.1016/j.fsi.2015.10.039. [DOI] [PubMed] [Google Scholar]
- 168.Gu Y.F., Wei Q., Tang S.J., Chen X.W., Zhao J.L. Molecular characterization and functional analysis of IRF3 in tilapia (Oreochromis niloticus) Dev. Comp. Immunol. 2016;55:130–137. doi: 10.1016/j.dci.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 169.Zhang J., Li Y.X., Hu Y.H. Molecular characterization and expression analysis of eleven interferon regulatory factors in half-smooth tongue sole, Cynoglossus semilaevis. Fish Shellfish Immunol. 2015;44:272–282. doi: 10.1016/j.fsi.2015.02.033. [DOI] [PubMed] [Google Scholar]
- 170.Huang B., Huang W.S., Nie P. Cloning and expression analyses of interferon regulatory factor (IRF) 3 and 7 genes in European eel, Anguilla anguilla with the identification of genes involved in IFN production. Fish Shellfish Immunol. 2014;37:239–247. doi: 10.1016/j.fsi.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 171.Yao C.L., Huang X.N., Fan Z., Kong P., Wang Z.Y. Cloning and expression analysis of interferon regulatory factor (IRF) 3 and 7 in large yellow croaker, Larimichthys crocea. Fish Shellfish Immunol. 2012;32:869–878. doi: 10.1016/j.fsi.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 172.Ohtani M., Hikima J., Hwang S.D., Morita T., Suzuki Y., Kato G., Kondo H., Hirono I., Jung T.S., Aoki T. Transcriptional regulation of type I interferon gene expression by interferon regulatory factor-3 in Japanese flounder, Paralichthys olivaceus. Dev. Comp. Immunol. 2012;36:697–706. doi: 10.1016/j.dci.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 173.Pestka S., Krause C.D., Walter M.R. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004;202:8–32. doi: 10.1111/j.0105-2896.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- 174.Bonjardim C.A., Ferreira P.C., Kroon E.G. Interferons: Signaling, antiviral and viral evasion. Immunol. Lett. 2009;122:1–11. doi: 10.1016/j.imlet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Novick D., Cohen B., Rubinstein M. The human interferon α/β receptor: Characterization and molecular cloning. Cell. 1994;77:391–400. doi: 10.1016/0092-8674(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 176.Farrar M.A., Schreiber R.D. The molecular cell biology of interferon-γ and its receptor. Annu. Rev. Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- 177.Witte K., Witte E., Sabat R., Wolk K. IL-28A, IL-28B, and IL-29: Promising cytokines with type I interferon-like properties. Cytokine Growth Factor Rev. 2010;21:237–251. doi: 10.1016/j.cytogfr.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 178.Lutfalla G., Holland S.J., Cinato E., Monneron D., Reboul J., Rogers N.C., Smith J.M., Stark G.R., Gardiner K., Mogensen K.E., et al. Mutant U5A cells are complemented by an interferon-α β receptor subunit generated by alternative processing of a new member of a cytokine receptor gene cluster. EMBO J. 1995;14:5100–5108. doi: 10.1002/j.1460-2075.1995.tb00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Domanski P., Colamonici O.R. The type-I interferon receptor. The long and short of it. Cytokine Growth Factor Rev. 1996;7:143–151. doi: 10.1016/1359-6101(96)00017-2. [DOI] [PubMed] [Google Scholar]
- 180.Gazziola C., Cordani N., Carta S., De Lorenzo E., Colombatti A., Perris R. The relative endogenous expression levels of the IFNAR2 isoforms influence the cytostatic and pro-apoptotic effect of IFNα on pleomorphic sarcoma cells. IntJ. Oncol. 2005;26:129–140. doi: 10.3892/ijo.26.1.129. [DOI] [PubMed] [Google Scholar]
- 181.McKenna S.D., Vergilis K., Arulanandam A.R., Weiser W.Y., Nabioullin R., Tepper M.A. Formation of human IFN-β complex with the soluble type I interferon receptor IFNAR-2 leads to enhanced IFN stability, pharmacokinetics, and antitumor activity in xenografted SCID mice. J. Interferon Cytokine Res. 2004;24:119–129. doi: 10.1089/107999004322813363. [DOI] [PubMed] [Google Scholar]
- 182.Cook J.R., Cleary C.M., Mariano T.M., Izotova L., Pestka S. Differential responsiveness of a splice variant of the human type I interferon receptor to interferons. J. Biol. Chem. 1996;271:13448–13453. doi: 10.1074/jbc.271.23.13448. [DOI] [PubMed] [Google Scholar]
- 183.Gilli F. Role of differential expression of interferon receptor isoforms on the response of multiple sclerosis patients to therapy with interferon α. J Interferon Cytokine Res. 2010;30:733–741. doi: 10.1089/jir.2010.0098. [DOI] [PubMed] [Google Scholar]
- 184.Zou J., Secombes C.J. Teleost fish interferons and their role in immunity. Dev. Comp. Immunol. 2011;35:1376–1387. doi: 10.1016/j.dci.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 185.Robertsen B. The interferon system of teleost fish. Fish Shellfish Immunol. 2006;20:172–191. doi: 10.1016/j.fsi.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 186.Sun B., Robertsen B., Wang Z., Liu B. Identification of an Atlantic salmon IFN multigene cluster encoding three IFN subtypes with very different expression properties. Dev. Comp. Immunol. 2009;33:547–558. doi: 10.1016/j.dci.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 187.Purcell M.K., Laing K.J., Woodson J.C., Thorgaard G.H., Hansen J.D. Characterization of the interferon genes in homozygous rainbow trout reveals two novel genes, alternate splicing and differential regulation of duplicated genes. Fish Shellfish Immunol. 2009;26:293–304. doi: 10.1016/j.fsi.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 188.Chang M.X., Zou J., Nie P., Huang B., Yu Z., Collet B., Secombes C.J. Intracellular interferons in fish: A unique means to combat viral infection. PLoS Pathog. 2013;9:e1003736. doi: 10.1371/journal.ppat.1003736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Milev-Milovanovic I., Long S., Wilson M., Bengten E., Miller N.W., Chinchar V.G. Identification and expression analysis of interferon γ genes in channel catfish. Immunogenetics. 2006;58:70–80. doi: 10.1007/s00251-006-0081-x. [DOI] [PubMed] [Google Scholar]
- 190.Mohapatra S., Chakraborty T., Miyagawa S., Zhou L., Ohta K., Iguchi T., Nagahama Y. Steroid responsive regulation of IFNγ2 alternative splicing and its possible role in germ cell proliferation in medaka. Mol. Cell. Endocrinol. 2015;400:61–70. doi: 10.1016/j.mce.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 191.Grayfer L., Belosevic M. Molecular characterization of novel interferon γ receptor 1 isoforms in zebrafish (Danio rerio) and goldfish (Carassius auratus L.) Mol. Immunol. 2009;46:3050–3059. doi: 10.1016/j.molimm.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 192.Yabu T., Toda H., Shibasaki Y., Araki K., Yamashita M., Anzai H., Mano N., Masuhiro Y., Hanazawa S., Shiba H., et al. Antiviral protection mechanisms mediated by ginbuna crucian carp interferon γ isoforms 1 and 2 through two distinct interferon γ-receptors. J. Biochem. 2011;150:635–648. doi: 10.1093/jb/mvr108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.