Abstract
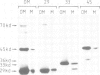
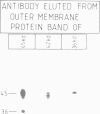
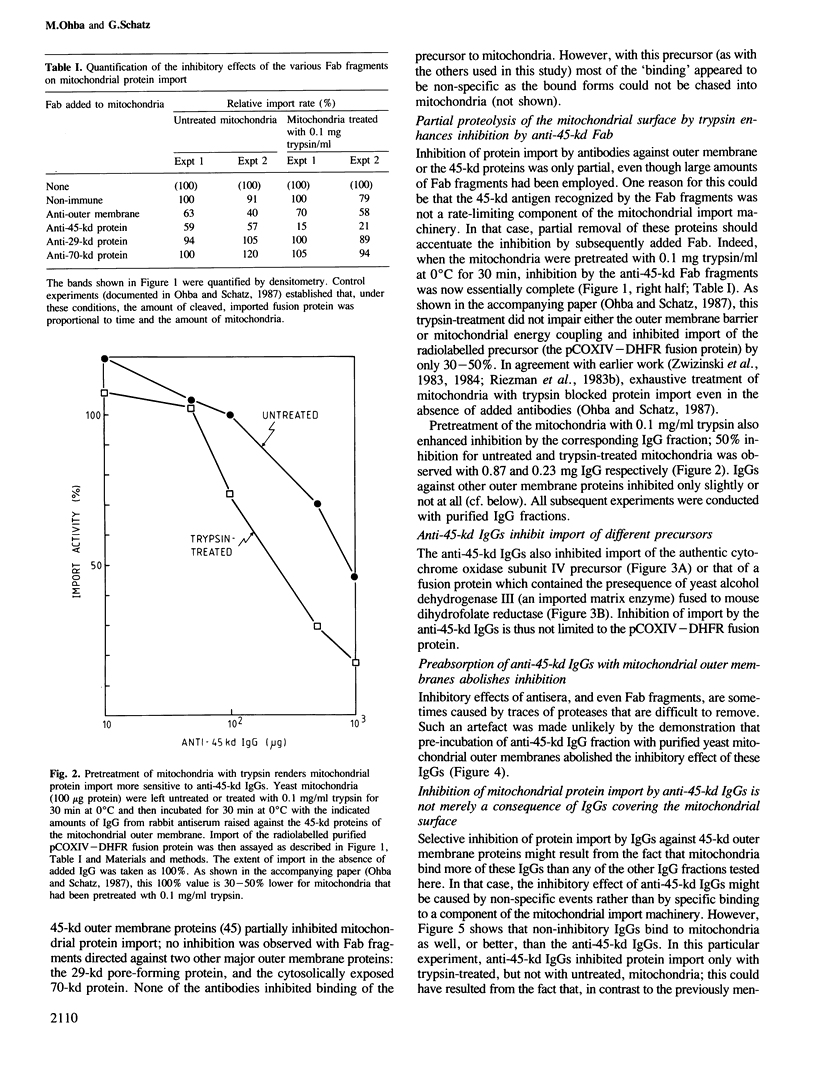
Import of several precursor proteins into isolated yeast mitochondria is inhibited by rabbit antiserum raised against the total mitochondrial outer membrane or against electrophoretically purified 45-kd outer membrane proteins. Antisera against other outer membrane proteins are only marginally active or inactive. Inhibition by the antiserum against 45-kd proteins is only weak with untreated mitochondria, but reaches 80-90% with mitochondria that had been pretreated with 0.1 mg/ml trypsin. This trypsin pretreatment by itself inhibits precursor import only slightly (30-50%). Selective inhibition of import does not correlate with binding of the various IgGs to the mitochondrial surface and is also observed with the corresponding Fab fragments. Inhibition by antibodies against 45-kd outer membrane proteins strongly suggests the existence of a mitochondrial surface protein mediating protein import and offers a means of isolating this protein.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison D. S., Schatz G. Artificial mitochondrial presequences. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9011–9015. doi: 10.1073/pnas.83.23.9011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argan C., Lusty C. J., Shore G. C. Membrane and cytosolic components affecting transport of the precursor for ornithine carbamyltransferase into mitochondria. J Biol Chem. 1983 Jun 10;258(11):6667–6670. [PubMed] [Google Scholar]
- Cox J. A., Comte M., Fitton J. E., DeGrado W. F. The interaction of calmodulin with amphiphilic peptides. J Biol Chem. 1985 Feb 25;260(4):2527–2534. [PubMed] [Google Scholar]
- Daum G., Böhni P. C., Schatz G. Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J Biol Chem. 1982 Nov 10;257(21):13028–13033. [PubMed] [Google Scholar]
- Eilers M., Schatz G. Binding of a specific ligand inhibits import of a purified precursor protein into mitochondria. Nature. 1986 Jul 17;322(6076):228–232. doi: 10.1038/322228a0. [DOI] [PubMed] [Google Scholar]
- Gilmore R., Walter P., Blobel G. Protein translocation across the endoplasmic reticulum. II. Isolation and characterization of the signal recognition particle receptor. J Cell Biol. 1982 Nov;95(2 Pt 1):470–477. doi: 10.1083/jcb.95.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haid A., Suissa M. Immunochemical identification of membrane proteins after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Methods Enzymol. 1983;96:192–205. doi: 10.1016/s0076-6879(83)96017-2. [DOI] [PubMed] [Google Scholar]
- Meyer D. I., Krause E., Dobberstein B. Secretory protein translocation across membranes-the role of the "docking protein'. Nature. 1982 Jun 24;297(5868):647–650. doi: 10.1038/297647a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ohba M., Schatz G. Disruption of the outer membrane restores protein import to trypsin-treated yeast mitochondria. EMBO J. 1987 Jul;6(7):2117–2122. doi: 10.1002/j.1460-2075.1987.tb02478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riezman H., Hay R., Gasser S., Daum G., Schneider G., Witte C., Schatz G. The outer membrane of yeast mitochondria: isolation of outside-out sealed vesicles. EMBO J. 1983;2(7):1105–1111. doi: 10.1002/j.1460-2075.1983.tb01553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riezman H., Hay R., Witte C., Nelson N., Schatz G. Yeast mitochondrial outer membrane specifically binds cytoplasmically-synthesized precursors of mitochondrial proteins. EMBO J. 1983;2(7):1113–1118. doi: 10.1002/j.1460-2075.1983.tb01554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roise D., Horvath S. J., Tomich J. M., Richards J. H., Schatz G. A chemically synthesized pre-sequence of an imported mitochondrial protein can form an amphiphilic helix and perturb natural and artificial phospholipid bilayers. EMBO J. 1986 Jun;5(6):1327–1334. doi: 10.1002/j.1460-2075.1986.tb04363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Gilmore R., Blobel G. Protein translocation across the endoplasmic reticulum. Cell. 1984 Aug;38(1):5–8. doi: 10.1016/0092-8674(84)90520-8. [DOI] [PubMed] [Google Scholar]
- Yaffe M. P., Schatz G. Two nuclear mutations that block mitochondrial protein import in yeast. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4819–4823. doi: 10.1073/pnas.81.15.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwizinski C., Schleyer M., Neupert W. Proteinaceous receptors for the import of mitochondrial precursor proteins. J Biol Chem. 1984 Jun 25;259(12):7850–7856. [PubMed] [Google Scholar]
- Zwizinski C., Schleyer M., Neupert W. Transfer of proteins into mitochondria. Precursor to the ADP/ATP carrier binds to receptor sites on isolated mitochondria. J Biol Chem. 1983 Apr 10;258(7):4071–4074. [PubMed] [Google Scholar]
- van Loon A. P., Brändli A. W., Schatz G. The presequences of two imported mitochondrial proteins contain information for intracellular and intramitochondrial sorting. Cell. 1986 Mar 14;44(5):801–812. doi: 10.1016/0092-8674(86)90846-9. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Mitochondrial targeting sequences may form amphiphilic helices. EMBO J. 1986 Jun;5(6):1335–1342. doi: 10.1002/j.1460-2075.1986.tb04364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]