Abstract
Thrombospondins (TSPs) represent extracellular matrix (ECM) proteins belonging to the TSP family that comprises five members. All TSPs have a complex multidomain structure that permits the interaction with various partners including other ECM proteins, cytokines, receptors, growth factors, etc. Among TSPs, TSP1, TSP2, and TSP4 are the most studied and functionally tested. TSP1 possesses anti-angiogenic activity and is able to activate transforming growth factor (TGF)-β, a potent profibrotic and anti-inflammatory factor. Both TSP2 and TSP4 are implicated in the control of ECM composition in hypertrophic hearts. TSP1, TSP2, and TSP4 also influence cardiac remodeling by affecting collagen production, activity of matrix metalloproteinases and TGF-β signaling, myofibroblast differentiation, cardiomyocyte apoptosis, and stretch-mediated enhancement of myocardial contraction. The development and evaluation of TSP-deficient animal models provided an option to assess the contribution of TSPs to cardiovascular pathology such as (myocardial infarction) MI, cardiac hypertrophy, heart failure, atherosclerosis, and aortic valve stenosis. Targeting of TSPs has a significant therapeutic value for treatment of cardiovascular disease. The activation of cardiac TSP signaling in stress and pressure overload may be therefore beneficial.
Keywords: thrombospondins, cardiac remodeling, cardiac hypertrophy, heart failure, atherosclerosis, myocardial infarction, cardiac fibrosis
1. Introduction
The function of the extracellular matrix (ECM) is not only limited by providing structural support and immobilization of cells. Functional significance of the ECM also relies in mediating cell-cell and cell-matrix contacts, signaling conduction, and triggering cell adhesion, motility, and differentiation. The composition and functional properties of the ECM vary depending on the cell type and tissue/organ specificity. For example, in the cardiovascular system, the ECM is involved in maintaining structural continuity of the heart and blood vessels, providing an essential scaffold for cell attachment and functioning, control of cell growth, viability and death, regulation of diastolic stiffness, and performing tissue repair/remodeling in a case of cardiovascular damage and inflammation [1]. In the ECM, structural changes induced by local microenvironment can lead to functional matrix alterations. Changes in the matrix may be then conducted to adjacent cells and affect their activity and behavior. For example, the cardiovascular ECM mediates blood flow-induced mechanotransduction [2,3] and adaptive responses of vascular cells and cardiomyocytes to various stress stimuli [4,5].
In cardiovascular pathology such as atherosclerosis, arterial restenosis or heart failure, ECM-associated responses are frequently maladaptive and may lead to adverse tissue remodeling and fibrosis [6,7]. Inflammatory, profibrotic, prooxidant, hypoxic, and other pathological stimuli may induce substantial modifications and impair matrix turnover, which in turn may cause qualitative and quantitative changes in matrix architecture and composition by increasing content of certain ECM proteins and decreasing amount of other matrix components [8,9]. For instance, in failing hearts of patients with dilated cardiomyopathy, significant changes in the content of some non-fibrillar matrix and matricellular proteins were found. Implementation of the mechanical unloading of the left ventricle by left ventricular assist device resulted in the restoration of the fibrillar ECM and basement membrane and improved clinical outcome [9].
Matricellular proteins comprise non-structural ECM proteins that modulate cell function and behavior. Matricellular proteins include thrombospondins (TSPs), tenascins, periostin, osteopontin, CCN proteins, and osteonectin/secreted protein acidic and rich in cysteine (SPARC) [10]. Tenascins contain three members (tenascin C, tenascin R, and tenascin X) that are especially abundant in developing embryonic tissues like cartilage, tendon, bone, and nervous system where these proteins promote migration, proliferation, and differentiation of stem and lineage-specific progenitor cells [11,12]. Periostin plays multiple physiological and pathogenic roles including regulation of mesenchymal differentiation in the developing heart, tissue repair, and involvement in cancer and valvular heart disease [13,14,15]. Osteopontin triggers biomineralization, bone remodeling, and immunity as well as pathological ectopic calcification [16,17,18]. Osteonectin is a Ca2+-binding protein whose primary function is to contribute to osteogenesis by conducting biomineralization of the bone and cartilage [19]. CCN proteins include at least six members (CCN1–6) with a complex multidomain structure that provides an option to bind numerous ligands and participate in a variety of biological processes such as angiogenesis, inflammation, tissue repair, fibrosis, and carcinogenesis [20]. Like CNN proteins, TSPs have several functional domains and indeed are able to interact with multiple partners. TSPs are abundantly distributed in various tissues and organs including the cardiovascular system. At steady state, TSP expression is low but can be up-regulated in response to wounding. TSPs are likely to contribute to post-injury tissue repair and remodeling [21]. In this review, we consider structural and functional aspects of the TSP protein family in relation to cardiovascular disease.
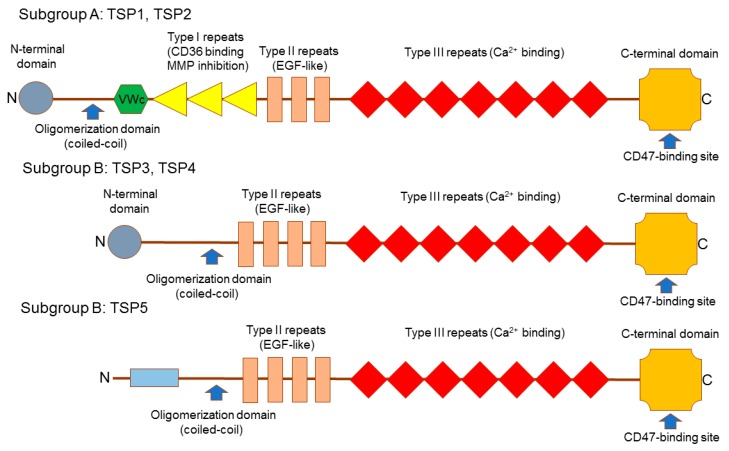
2. Thrombospondins Structure
The TSP family contains five members (TSP1–5) that represent multimeric glycoproteins, which bind Ca2+, interact with other ECM proteins, and contribute to the associations between cells and between cells and ECM. TSMs divided to two subgroups: trimeric subgroup A (TSP1 and TSP2) and pentameric subgroup B (TSP3, TSP4, and TSP5). TSPs have a complex multidomain structure (Figure 1). The C-terminal domain, type III repeats and epidermal growth factor (EGF)-like repeats are present in all TSPs and underline the TSP family. The oligomerization domain can be also found in all family members but it is more variable compared with other shared structures [22]. The subgroup A has three EGF-like repeats and type I repeats (also known as thrombospondin repeats; TSRs), von Willebrand factor type C (vWC) domains, and the N-terminal domain. The subgroup B contains four EGF-like repeats but vWC domains and TSRs are missing. While TSP3 and TSP4 have the N-terminal domain, TSP5 has not [23].
Figure 1.
Structure of thrombospondins (TPSs). TSP family includes five members: TSP1–5. Subgroup A comprises TSP1 and TSP2 that form pentamers while subgroup B contains trimeric TSP3–5. TSPs have a complex multidomain architecture that provides an option to bind various ligands. For example, C-terminal domain contains CD47-binding site. Type III repeats are involved in Ca2+ binding while Type I repeats are responsible for the interaction with CD36, a receptor for TSP1 and TSP2, and inhibition of matrix proteinases (MMPs). Type II EGF-like domains are involved in the regulation of various signaling pathways such Notch and others. C-terminal domain, Type III repeats and Type II epidermal growth factor (EGF)-like repeats share high homology in all TSPs and represent a signature of the TSP family. Von Willebrand factor type C (VWc) domain is cysteine-rich and is implicated in binding members of the transforming growth factor-β (TGF-β) superfamily. The oligomerization (coiled-coil) domain drives formation of TSH homooligomers. The N-terminal domain, which is present in TSP1–4 and absent in TSP5, is less conservative. This domain regulates structure and stability of the coiled-coil region and binds heparin.
The evolutionary analysis showed that the subgroup B is evolutionary younger than the subgroup A [24]. In fish, a TSP4-like sequence, an ortholog of the tetrapod TSP5 gene is found [25]. The TSP5-coding sequence evolved more quickly from the TSP4-like sequence as an innovation in the tetrapod lineage. Thus, all TSP genes show conservation of synteny between fish and tetrapods. In humans, the TSP1, TSP3, TSP4 and TSP5 genes reside within paralogous regions and are the result of gene duplications [24].
Due to the availability of various structural domains, TSPs can interact with different surface receptors and ECM proteins. In the TSP molecule, EGF-like domains are employed for binding of integrins and Ca2+, TSPs are needed to bind transforming growth factor (TGF)-β and CD36, while the N-terminal domain is required for binding heparin and integrins [26]. The C-terminal domain contains a binding site for CD47, an essential TSP receptor [27]. TSP-mediated effects indeed depend on the availability of the binding partner and local microenvironment that explains cell- and tissue-specific actions of TSPs. In addition, TSPs display different cellular distributions, different temporal expression profiles and have distinct functional responsibilities and modes of transcriptional regulation.
TSP1 that was identified first represents the most studied thrombospondin. TSP1 and TSP2 are expressed by several cell types in response to damage or during remodeling [28,29]. TSP1 is known due to the functional significance in the control of angiogenesis and thrombosis and capacity to increase bioavailability TGF-β by liberating this cytokine from its latent form [23]. In the heart, TSP2 contributes to maintaining of cardiac matrix integrity via its actions on matrix metalloproteinases (MMPs) [30].
The highest expression levels of TSP3 and TSP5 were detected in the vascular wall and tendon [31]. However, both these TSPs are the least studied among TSP family members. TSP4 has a role in vascular inflammation [31], regulation of myocyte contractility, angiogenesis, and ECM remodeling [32,33].
In our understanding of TSP functions, the most profound recent progress was made in studying the cardiovascular system. Genetic studies showed association between single nucleotide polymorphisms (SNPs) in TSP1, TSP2, and TSP4 genes with cardiovascular pathology [34,35,36,37,38,39]. All TSP disease-associated SNPs are functional. For example, the A387P polymorphism of TSP4 and N700S polymorphism of TSP1 alter Ca2+-binding sites [40]. Ca2+ binding is essential for TSP structure and function. The TSP1 S700 variant had significantly less capacity to bind Ca2+ compared to the N700 allele. In fact, the P387 variant of TSP4 represents a gain-of-function allele because it acquired an additional Ca2+-binding site absent in the A387 allele [40]. A recent meta-analysis showed that the N700S polymorphism of TSP1 is associated with coronary artery disease (CAD) especially in Asian populations (heterozygote model: odds ratio (OR) = 1.57 [95% confidence interval (CI): 1.01–2.44]; dominant model: OR = 1.56 [95% CI: 1.00–2.43]). The TSP4 A387P polymorphism is associated with increased CAD risk in American population (homozygote model: OR = 1.29 [95% CI: 1.04–1.61]; recessive model: OR = 1.27 [95% CI: 1.02–1.58]). No association was shown for the THS2 3′ (untranslated region) UTR polymorphism and higher CAD risk [41].
3. Cardiac Integrity
Expression of all TSP family members was found in the heart. In cardiac remodeling, TSP1, TSP2, and TSP4 are up-regulated [42,43,44,45]. In pressure overload, myocardial expression of TSP3 and TSP5 was shown to be also increased [46].
TSP1, TSP2, and TSP4 are involved in cardiac fibrosis but possess opposite effects. While TSP1 and TSP2 promote fibrosis [47,48], TSP4 inhibits profibrotic mechanisms as was shown in animal models [49,50] and human heart allografts [43,48]. In human allografts, increased levels of TSP1 and TSP2 suggested for induction of both the fibrotic response and allograft rejection [43,48]. In TSP1 or TSP2-deficient murine cardiac remodeling models (i.e., those affected with doxorubicin-induced cardiomyopathy [51], diabetic cardiomyopathy [52,53], dilated cardiomyopathy [54], or MI [44,55,56] the profibrotic role of both TSPs was confirmed. Mechanistically, profibrotic effects of TSP1 and TSP2 in the heart lead to the stimulation of TGF-β, a key inducer of cardiac fibrosis [48,57,58], suppression of MMPs [48,59], and inhibition of angiogenesis [43,47,55]. Profibrotic activity of TSP1 can be also mediated the calreticulin/low density lipoprotein receptor-related protein 1 (LRP1) complex whose stimulation results in the activation of prosurvival protein kinases such as PI3K and migration of fibroblasts [60,61]. The N-terminal domain of TSP1 has the calreticulin-binding site to stimulate association of calreticulin with LRP1 to the signal focal adhesion disassembly and providing signal into the cytoplasm [62].
TSP2 expression was observed only in biopsy specimens from the hypertrophic hearts of rats that rapidly developed heart failure suggesting for a possible value of TSP2 to serve as a marker of early onset of heart failure [59]. TSP2-deficient mice were extremely vulnerable to rapid progression from angiotensin II-induced cardiac hypertrophy to cardiac failure and fatal rupture since 70% of animals died from cardiac rupture whereas the rest of them progressed to heart failure [59]. These data therefore indicate a role of TSP2 as an essential regulator of cardiac integrity.
In the heart, lack of TSP4 leads to advanced fibrosis [49,50] suggesting for the anti-fibrotic role. TSP4 also triggers heart stress adaptation by increasing intracellular Ca2+ content in cardiac muscle cells and enhancing contractility [63]. TSP4-induced adaptive response against ER stress also protects cardiomyocytes from pressure overload [1]. In TSP4-deficient mice, tendon collagen fibrils were found to be significantly larger than in wild-type mice suggesting for the negative TSP4-dependent regulation of collagen synthesis in ligaments [64]. However, it is unknown whether this TSP inhibit cardiac collagen production.
The reason of profound differences in the actions of TSP4 and TSP1/TSP2 on myocardial fibrosis may rely on the structural differences between these TSPs. TSP4 lacks domains responsible for the control of MMP function, angiogenesis and TGF-β stimulation. These domains are present in TSP1 and TSP2 [23]. Thus, in order to recognize effects of every TSP in heart remodeling, it is necessary to monitor expression of each TSP in various steps of remodeling and adaptive reaction to heart damage.
Expression of TSP1–4 was found in human aortic valves [65]. In fibrosclerotic and stenotic valves, TSP2 production was increased. TSP2 was up-regulated in myofibroblasts and some endothelial cells (ECs) and was associated with myofibroblast proliferation and neovascularization. TSP2 activation was followed by suppression of Akt and NF-κB [65]. Cardiac-related expression of TSP4 was highest in the valves suggesting for a potential activity in these regions [32,50].
4. TSPs in Angiogenesis
Angiogenesis is an essential physiological process involved in the developmental vasculogenesis and tissue repair after injury. In pathology, formation of neovessels occurs in vascular proliferative diseases such as atherosclerosis and in tumors. The anti-angiogenic properties of TSP1 and TSP2 are established and confirmed in different models of angiogenesis [66,67]. The anti-angiogenic activity of TSP1 and TSP2 may be especially attractive in the context of anti-tumor therapy because both TSPs are able to inhibit tumor-associated angiogenesis and suppress tumor growth [68,69]. Studying of TSP1 effects on tumor neovessel formation provided new insights into a specific role and a power of this TSP in preventing angiogenesis. It became obvious that tissue expression of TSP1 is able to define fate of angiogenesis even without influencing by pro-angiogenic signals [70].
A phenomenon of dormant tumors that have a microscopic size and do not expand is associated with high expression and inhibitory effects of TSP1 and tissue inhibitor of matrix proteinases TIMP-1 [71]. Down-regulation of TSP1 and reduced tumor sensitivity to angiostatin leads to proangiogenic switch and induction of rapid growth and tumor expansion [72]. MicroRNA (miR)-467 was reported to function as a negative regulator of TSP1 expression [73]. This miRNA is induced by high glucose and leads to the sequestration of TSP1 mRNA in the non-polysomal fraction of tumor cells and induction of angiogenesis. Inhibition of miR-467 suppresses tumor growth and angiogenesis [74].
The anti-angiogenic activity of TSP1 is attributed to a structural domain known as the TSP type I repeat [75]. This domain serves as a single Ca2+-binding site for endothelial receptor CD36 that is essential for mediating anti-angiogenic effects of TSP1 and TSP2 [76,77]. Histidine-rich glycoprotein (HRGP), a circulating protein, contains a CD36 homology domain and blocks TSP-dependent anti-angiogenic effects by binding to either TSP1 or TSP2 [75,78]. Another TSP-1-dependent anti-angiogenic mechanism is consisted of the engagement of CD47 that disrupts CD47 interaction with vascular endothelial growth factor (VEGF) receptor 2 (VEGFR2) and blunts VEGFR2-mediated proangiogenic signaling associated with activation of endothelial NO synthase (eNOS) and soluble guanylate cyclase (sGC) [79]. In silico analysis showed that TSP1 binding to CD47 can also enhance VEGFR2 degradation [80]. By contrast, in TSP1-deficient mice, phosphorylation of endothelial VEGFR2 is up-regulated thereby providing a stimulatory signal to Akt or Src that in turn activate eNOS through phosphorylation [79]. Another suppressive mechanism, by which TSP1 can inhibit myristic acid-stimulated eNOS-dependent signaling that leads to the induction of increased adhesion properties of ECs and vascular smooth muscle cells (VSMCs), is dampening of the CD36-mediated uptake of free fatty acids or engagement of CD47 [81,82].
TSP1 can also diminish the sGC/3′, 5′-cyclic GMP (cGMP) signaling by limiting cGMP-dependent activation of the downstream cGMP-activated kinase (PKG) [83]. The inhibitory effect of TSP1 on eNOS/cGMP signaling in ECs are more potent than that of TSP2 suggesting for a role of TSP1 as a dominant regulator of NO/cGMP signaling pathway through CD47 [84].
NO is essential for activation sGC that contains a heme responsible for NO binding [85]. NO-dependent stimulation of sGC leads to intensive production of cGMP, a signaling messenger that is involved in the vascular tone regulation by relaxation of VSMC contractility [86], inhibiting platelet aggregation [87] and blood cell adhesion to the endothelium [88]. Except for limiting NO bioavailability, inhibitory effects of TSP1 on sGC activity and cGMP production can also involve suppression of the hydrogen sulfide (H2S)-mediated signaling through blocking activity of H2S-biosynthesiting enzymes, cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) [89]. Another inhibitory mechanism may involve TSP1-dependent stimulation of reactive oxygen (ROS) and nitrogen species production [90], which in turn inactivate sGC via covalent enzyme modification [91] or heme nitrosylation [92].
Compared to TSP1 and TSP2, TSP4 exerts proangiogenic properties [93]. TSP4 was detected in the lumen of neovessels. TSP4-deficient mice have diminished angiogenesis in comparison with wild-type mice. Mice transgenic for the CAD-associated human TSP4 P387 variant displayed more intensive angiogenesis compared with mice bearing the A387 allele of TSP4. Pulmonary ECs derived from TSP-lacking mice exhibited reduced adhesion and migratory properties in contrast to wild-type ECs. In addition, recombinant TSP4 was shown to stimulate vessel development and EC motility/proliferation through binding to integrin-α2 and gabapentin receptor α2δ-1 [93].
5. TSPs in Atherosclerotic Blood Vessels
Vascular expression was shown for all TSPs [31]. In blood vessels, TSP1, TSP2, and TSP4 are involved in the functional and structural regulation of the vascular wall and interactions with blood-borne cells. In diabetic rats, TSP1 levels were increased after vascular wounding [94]. In rats with balloon-induced injury, blockade of TSP1 in carotid artery with antibody resulted in enhanced reendothelization and decreased neointima formation [95]. In apolipoprotein E (ApoE)-deficient mice, an atherosclerotic animal model, inhibition of TSP1 and TSP4 caused delayed atherosclerosis progression and less inflammatory conditions. However, at late atherogenic stages, the plaques developed proinflammatory content and had the same size as lesions in control mice [31,96]. TSP-1 or TSP4 did not alter intraplaque lipid content but induced dramatic changes in macrophages counts within the plaque by influencing either function (TSP1) or infiltration (TSP4) of macrophages to the lesion. In advanced lesions, TSP2 plays an anti-atherogenic role by stimulating phagocytic function of macrophages needed to perform clearance of apoptotic and necrotic cells and cell debris [96].
In ApoE-deficient mice, TSP4 depletion does not affect lesional matrix deposition [31] while TSP1 deficiency leads to the formation of more fibrotic lesions of less size but also increases inflammation associated with enhanced activity of macrophages [96]. These macrophages are involved in intensive degradation of the fibrous cap associated with increased accumulation of MMP-9 in the cap but display altered phagocytosis that finally results in increase of the necrotic core and plaque destabilization. It appears that TSP1 promotes atherogenesis in early stages through induction of endothelial dysfunction, stimulation of VSMC proliferation and inhibiting collagen deposition. However, in late stages, TSP1 switches this role to the anti-atherogenic function by repressing lesional maturation via stimulation of the phagocytic activity of macrophages and reducing necrosis [97].
In ApoE-deficient mice fed on high-fat diet, strong plaque TSP1 expression was detected in fibrous cap-associated VSMCs and inflammatory cells in the shoulder of the plaque and foam cells. Weak TSP1 expression was found in the adventitia and media of the atherosclerotic wall [96]. Expression of TSP2 was found in human arterial VSMCs [98] but was not detectable in the endothelial plaque lining and intraplaque neovessels [99]. TSP3, TSP4, and TSP5 are all also linked to atherosclerotic plaques. Expression of TSP3 was detected in the tunica media and tunica adventitia, on the luminal endothelial surface, and in the plaques in ApoE-deficient mice [31]. TSP4 exerts proatherosclerotic and proinflammatory effects in vessels since TSP4-knockout mice developed less inflammatory lesions with lowered macrophage content, diminished activation of ECs, and reduced production of proinflammatory cytokines [31]. TSP4 stimulates adhesion and movement of macrophages and neutrophils in an integrin αvβ3-dependent manner [13,40]. The CAD risk-associated TSP4 P387 variant was shown to enhance leukocyte attachment to ECs and motility and promote proinflammatory signaling in vascular and blood-borne cells likely due the ability to bind more Ca2+. This ability leads to conformational changes in the mutant TSP4 molecule and provides better interaction with cell surface receptors [13,40].
TSP5 protein was histochemically detected in normal and affected (i.e., atherosclerotic and stenotic) human arteries where it is produced by VSMCs [100]. In ApoE-deficient mice, TSP5 expression is associated with tunica media and a few plaque cells [31]. TSP5 is involved in maintaining VSMC quiescence and contractile phenotype via interaction with integrin α7β1 [101]. A disintegrin and metalloproteinase with thrombospondin motifs 7 (ADAMTS7) that is also expressed in VSMCs can degrade TSP5 and promote VSMC migration and recruitment for neointima formation [102].
CD47 (also known as the integrin-associated receptor; IAP) is the receptor for TSP1 [103]. After stimulation with TSP1, CD47-mediated pathway is involved in the control of leukocyte functions, vascular resistance, and intracellular signaling in ECs and VSMCs [104]. TSP1 binding to CD47 has global functional consequences by inhibiting endothelial nitric oxide (NO) production, controlling vascular tone, and maintaining systemic hemodynamics and cardiac dynamics in stressful conditions [105,106,107,108]. The interaction between TSP1 and CD47 also regulates thrombosis/hemostasis, immune responses, and mitochondrial function [109]. CD47-binding capacity can be shared between all TSP family members since CD47-binding site is located in the C-terminal region, which is homologous in all TSHs.
TSP1-mediated overactivation of NADPH oxidase may exert important pathogenic effects in cardiovascular pathology. TSP1 is able to stimulate Nox1 and Nox4 through CD47-dependent signaling. In VSMCs, TSP1 binding to CD47 leads to phospholipase C (PLC)-catalyzed biosynthesis of diacyl glycerol, a stimulator of protein kinase C (PKC) that in turn phosphorylates the NADPH oxidase core subunit p47phox followed by activation of Nox1. Nox1 overactivity enhances ROS formation that further inhibits VSMC-dependent vasorelaxation and induces vascular dysfunction by promoting oxidative stress [90]. In ischemic VSMCs, TSP1 can also increase ROS generation via stimulation of the cell surface receptor signal-regulatory protein-α (SIRP-α) and subsequent recruitment of the p47phox subunit [110].
TSP1-induced Nox4 up-regulation stimulates ROS-dependent proliferation of VSMCs and neointimal formation, a hallmark of the proatherogenic arterial remodeling [111,112]. In addition, TSP1/CD47-dependent stimulation of Nox1 enhances micropinocytosis of non-modified low density lipoprotein (LDL) by macrophages and promotes their transformation to foam cells, another key characteristics of atherogenesis. In macrophages, Nox1 overactivity induces dephosphorylation of actin-binding protein cofilin, PI3K-dependent activation of myotubularin-related protein 6 (MTMR6) followed by cytoskeletal rearrangements and increased LDL uptake [113]. Thus, TSP1-mediated stimulation of ROS-dependent signaling and oxidative stress have numerous pathogenic consequences, which promote atherogenesis.
6. TSPs in Myocardial Infarction
Myocardial injury initiates the post-MI tissue repair response aimed to restore cardiac conduction and contractility, blood supply, and replace necrotic cardiomyocytes in the infarct with a scar [114]. After MI, cardiomyocyte necrosis occurs early in post-MI remodeling of the infarct area while apoptosis occurs in the infarct and distant cardiac regions both in the early and late stages of remodeling [115]. In the early stage of cardiac repair, infiltrated inflammatory cells release MMPs, particularly MMP-2/9, to degrade ECM in the injured myocardial area and adjacent regions [116]. In parallel with ECM destruction, transformation of cardiac fibroblasts to myofibroblasts associated with their proliferation and migration to the site of injury begins. In response to profibrotic signals, myofibroblasts produce collagen and other ECM components, which are deposited in the cell-free infarcted zone that was cleared by macrophages from necrotic cells [117]. In the proliferative stage of myocardial repair, the collagen amount quickly rises in the injured region while collagen fibers undergo cross-linking in the maturation stage [118]. Collagen can be also accumulated in the non-infarcted area that can initiate reactive myocardial hypertrophy [116].
Compared to pressure overload, post-MI reperfusion in TSP1-deficient mice leads to more intensive and long-term heart inflammation in the infarct border zone and excessive remodeling [55]. These observations allow to suggest that TSP1 exerts a barrier function in the infarct border zone to limit propagation of inflammation and fibrosis into the non-injured cardiac regions. The mechanisms of this effect are not well studied. TGF-β-dependent up-regulation of TSP1 production may be involved in this process [55,56]. TSP1 also enhances apoptosis of activated T cells through CD47-dependent activation of proapoptotic Bcl-2 family member BNIP3 that primarily links it to inflammation [119,120].
TSP1 inhibits both eNOS-dependent NO production and NO-mediated signaling that stimulates vascular relaxation and angiogenesis [107]. These data may suggest for a putative contribution of TSP1 to heart ischemia and/or MI [121]. However, TSP1 also exhibit cardioprotective effects by activating TGF-β, an anti-inflammatory cytokine [56]. Indeed, inhibition of CD47 represents a more attractive therapeutic target than inhibition of TSP1. In ischemic mouse models, exposure to CD47-blocking agents results in significant improvement of tissue survival and decreased vasculopathy, an evidence of the therapeutic value of CD47 suppression to treat cardiovascular disease [122]. In MI or ischemia, down-regulation of CD47 may have unpleasant sequela such as diminished apoptosis of inflammatory macrophages, which can result in elevated levels of proinflammatory cytokines [123]. Enhanced TSP1 production may be involved in NO resistance observed in aging and ischemic heart disease [122]. Indeed, therapeutic targeting of vessel NO signaling through TSP1/CD47 can be valuable [121].
Compared with TSP1, our knowledge of a role of TSP2 in MI is constrained. TSP2 and TSP4 do not involved to the control of NO signaling [106]. However, TSP2 shares with TSP1 many anti-angiogenic properties, promotes CD36-mediated apoptosis of ECs, and initiates cell cycle arrest [68]. TSP2-deficient mice exhibited increased angiogenesis that was associated with MMP-9 up-regulation [123]. Similarly, TSP2 deficiency was shown to induce enhanced angiogenesis and delayed skin wound contraction accompanied with increased MMP-2/9 and soluble VEGF production [124]. These findings indicate that anti-angiogenic effects TSP2 can be released through multiple mechanisms. TSP2 deletion in mouse was shown to induce increased vascularity and defects in connective tissue formation due to impaired collagen fibrillogenesis [125,126] indicating that cardiac TSP2 may be involved in post-injury heart remodeling and repair through the control of fibrillogenesis [127]. In favor of the involvement of TSP2 into cardiac repair, the ability of TSP2 to positively modulate function of human cardiomyocyte progenitor cells (hCMPCs) in hypoxic conditions was demonstrated [128]. In mice, short-term exposure to hypoxia stimulates migratory and invasive properties of hCMPCs while prolonged exposure activates proliferation, angiogenesis, and blocks migration likely due to TSP2 actions [129]. Limitation of migratory activity of hCMPCs is necessary to induce proliferation and differentiation of progenitor cells into cardiomyocytes in the infarcted region.
Post-MI and in cardiac hypertrophy, TSP4 expression was shown to be chronically up-regulated in the heart, especially in the left ventricle [45]. TSP4 mRNA levels directly correlated with the rate of left ventricular remodeling indicating the role of TSP4 in post-MI cardiac remodeling [130]. TSP4 plays a cardioprotective role by diminishing cardiac ER stress. Furthermore, cardiac TSP4 overproduction is protective against MI [63]. Further studies showed that the type III repeat domain and the C-terminal domain of TSP2 are involved in Atf6α binding and regulation of ER stress response [131]. ATF6α is an ER stress-regulated transcription factor that drives expression of ER chaperons needed to initiate the unfolded protein response and prevent ER stress [132]. TSP4 cardiac-specific transgenic mice was resistant to myocardial infarction (MI) while TSP4-deficient mice exert cardiac maladaptation.
In the pilot genetic study, an association of the TMP1 N700S and TMP4 A387P variants with higher risk familial premature MI was demonstrated. The TMP2 T/G 3′UTR polymorphism was associated with lower risk of familial MI [34]. A global meta-analysis confirmed association with CAD only for the TMP1 N700S and TMP4 A387P polymorphisms but not for the TMP2 T/G 3′UTR [41]. The TSP4 A387P polymorphism was associated with increased coronary risk in post-MI subjects who had elevated levels of high density lipoprotein (HDL) cholesterol and C-reactive protein (CRP), an inflammatory marker [39]. Accordingly, the TT genotype of the TMP2 T/G 3′UTR variant showed association with plaque erosion independently of age, gender, and cigarette smoking in cases of sudden death [133]. Lesional erosion that is induced by intimal injury and does not lead to plaque rupture is a frequent cause of sudden death [134]. However, the results of case-control studies were contradictory since no significant association of the TMP1 N700S, TMP2 T/G 3’UTR, and TMP4 A387P genetic variants, and with both CAD and MI were found in other studies [135,136,137,138]. Possible reasons of such an inconsistency may be referred to the different patients’ selection criteria, insufficient size of the population samples tested, racial differences, etc. For example, the frequency of the TSP1 N700S variant was reported to be extremely low in the Chinese Han population [138]. This therefore underlines the need to recruit case-control cohorts of a larger size to provide a sufficient statistical power that is critical to the success of genetic association studies to detect causal genes of human complex diseases such as CAD.
7. TMPs in Cardiac Hypertrophy
Cardiac hypertrophy can be induced by chronic pressure overload (for instance, by essential hypertension) and is characterized by extensive growth of cardiac muscle cells, proliferation of cardiac fibroblasts, increased ECM deposition (i.e., fibrosis), and intensive cell death. Heart fibrosis occurs due to the massive production and deposition of collagens type I and type III, which exceeds their degradation. Fibrosis is resulted from the up-regulation of collagen synthesis, down-regulation of collagen destruction, or both [139]. In heart hypertrophy, cardiac matrix composition is altered due to collagen redistribution and increased cross-linking that can lead to changes in ECM functional properties [140]. MMPs is the most frequent type of enzymes involved in matrix remodeling. Except for matrix degradation, MMPs also promote ECM synthesis by liberating growth factors and other profibrotic messengers from the matrix [141]. In the heart, chronic hypertension stimulates apoptosis of cardiomyocytes [142] and inflammation [143].
In hypertensive cardiac disease, levels of TSP1, TSP2, and TSP4 are elevated [45,59,144]. Pressure overload stimulates heart expression of TSP1 and TSP4 [45,144]. TSP2 up-regulation was observed in hypertrophic hearts of rats that overexpressed renin and further progressed to heart failure [59].
In mice, TSP1 deficiency led to early onset of heart hypertrophy and enhanced late dilatation in response to pressure overload. Degenerative morphological changes in cardiomyocytes were observed due to the sarcomeric loss and rupture of sarcolemma. Cardiac remodeling was abnormal and accompanied with abundant infiltration of defective fibroblasts. The fibroblast-to-myofibroblast transdifferentiation was impaired. Collagen synthesis was reduced due to the perturbed TGF-β signaling. Furthermore, myocardial production of MMP-3 and MMP-9 was up-regulated [144]. Indeed, TSP1 loss in the heart leads to adverse consequences by impairing response to pressure overload and inducing aberrant tissue remodeling. TSP1 activation in the pressure-overloaded myocardium is critical in the control of the fibroblast phenotype and heart remodeling through up-regulation of TGF-β-dependent pathway and cardiac matrix preservation through inhibition of MMPs. However, no significant changes in inflammatory responses was detected [144] that is rather paradoxical since TGF-β exerts anti-inflammatory properties. In an ischemia-reperfusion model, TSP1 deletion was associated with prolonged post-MI inflammatory response and increased release of proinflammatory factors such as chemokine (C-C motif) ligand 2 (CCL2), chemokine (C-X-C motif) ligand 10 (CXCL10), interleukin (IL)-1β, IL-6, macrophage inflammatory protein-1α (MIP-1α) [55]. In experimental diabetic cardiomyopathy complicated with abdominal aortic coartaction, implementation of LKSL, a peptide that antagonizes TSP1-dependent TGF-β activation, had beneficial actions on the myocardium by inhibiting TGF-β-driven fibrosis [52]. Hence, from the pharmacological point of view, antagonizing TSP1-dependent activation of TGF-β looks more attractive and efficient than blockade of TSP1 signaling in heart hypertrophy.
Reduced vascularity because of decreased angiogenesis is supposed to promote progression of heart hypertrophy to heart failure [145]. The inhibitory actions of TSP1 on cancer angiogenesis were broadly investigated. However, there are contradictory results of studies that examine microvascular effects of TSP1 in the heart. Global deletion of TSP1 was reported to induce the development of dense cardiac capillary network and higher cardiac mass [146]. These findings were not confirmed [144]. Up-regulation of MMPs in TSP1-deficient mice may substantially contribute to enhanced angiogenesis [144].
In response to vasoactive stress, TSP1-deficient mice responded by increased heart rate and changes in blood pressure by elevation of central diastolic and mean arterial blood pressure and reduction of peripheral blood pressure and pulse pressure. In response to epinephrine, the hypertensive response was diminished in both TSP1-deficient or CD47-deficient mice [105]. These data indicate an important role of TSP1 and its receptor CD47 in the acute regulation of blood pressure, which possess vasoconstrictor effects to hold global hemodynamics under vasoactive stress. By inhibiting NO-dependent vasorelaxation, TSP1 maintains blood pressure under stressful conditions [107]. Since CD47 is crucially involved in TSP1-mediated regulation of blood pressure, pharmaceutical targeting of the TSP1/CD47 mechanism may be useful for treatment of hypertension.
In cardiac hypertrophy, TSP2 and TSP4 activities are rather oriented to the control of matrix composition. In pressure overload, TSP2-lacking mice developed cardiac rupture or heart failure accompanied with higher activities of MMP-2/9 indicating that TSP2 contributes to the control of cardiac integrity in hypertrophic hearts [59]. The up-regulation of TSP2 was recognized as a useless effort to rescue the integrity in pressure overload. In response to experimentally induced transverse aortic constriction (TAC), TSP4-deficient mice exhibited advanced heart hypertrophy and fibrosis along with left ventricular dilation, decreased systolic and impaired diastolic function. These cardiac changes resembled age-dependent hypertrophy and fibrosis [50]. TAC-induced interstitial fibrosis was accompanied with up-regulated collagen production, increased MMP expression, lowered microvessel density and was not associated with apoptosis of cardiac muscle cells and inflammation [147]. However, it is unclear whether decrease in myocardial capillary density is the effect of fibrosis or angiogenesis.
TSP4-lacking hearts failed the ability to respond properly to acute pressure overload by enhanced heart contractility or by activation of stretch-response pathways (Akt- and ERK1/2-dependent). In addition to missing capacity to reply normally to acute pressure overload, TSP4-deficient mice also failed the ability to perform an appropriate adaptive response to chronic press overload that induces cardiac dilation, greater myocardial mass, and decline in heart function. However, no changes in interstitial fibrosis was detected. Pressure overload affected cardiac contractility of a whole cardiac muscle, not in separate cardiomyocytes [49]. Therefore, TSP4 can serve as a cardiomyocyte-interstitial mechano-sensing molecule, which regulates adaptive myocardial contractile reactions in response to acute stress. Stable and stressed ECM is likely to negatively regulate function of cardiac muscle cells, which is confronted by TSP4 in normal conditions [49].
8. TSPs in Heart Failure
The aberrant remodeling leads to myocardial overwork that if untreated can progress to heart failure. Structural changes associated with right or left heart failure are differentiated [148]. Right failure is characterized by intensive collagen degradation and cross-linking disruption and off-center hypertrophy. In left failure, cardiomyocyte hypertrophy is concentric while interstitial collagen content and cross-linking is increased [149]. Heart remodeling is accompanied by substantial cardiomyocyte loss, which is supposed to represent one of the major mechanisms of heart failure progression. Apoptosis, necrosis, and autophagy mediate the cardiomyocyte death [150,151]. Cardiac inflammation also contributes to heart failure through enhanced production of TNF-α and other inflammatory cytokines that exhibit adverse effects on the myocardium [152].
TSP1 levels were decreased in human failing hearts and positively correlated with TGF-β levels indicating that cardiac fibrosis is an attribute of early stages of heart failure [153]. In rats with artificially induced heart failure, TSP1 production was up-regulated [154,155]. The TSP1/CD47 signaling seems to play a central role in promoting left ventricular hypertrophy and heart failure [155]. Up-regulation of histone deacetylase 3 (HDAC3) and Ca2+/calmodulin protein kinase II (CaMKII) stimulates hypertrophy of the left ventricle. Accordingly, blockade of either CD47, HDAC3 or CaMKII has beneficial cardiac effects by reducing hypertrophy and softening heart failure [155] thereby underlining a value of the TSP1/CD47 pathway in the pathogenesis of heart failure.
In a murine model of age-related heart failure, a modulatory role was shown for miR-18/19, both are members of the aging-associated miRNA cluster 17–92 that targets TSP1 and connective tissue growth factor (CTGF) [156]. In age-related heart failure, expression of cluster 17–92 miRNA members was down-regulated. Accordingly, TSP1 and CTGF were increased. Importantly, these expression changes occurred only in cardiomyocytes, not in fibroblasts. Indeed, miR-18/19 protect heart against age-related heart failure. With aging, expression of the cluster 17–92 declines while expression of TSP1 and GTCF grows, a phenomenon that predisposes to heart failure [156].
In aged mice, TSP2 production is also increased and is attributed to the ECM surrounding cardiac muscle cells [54]. In older animals, TSP2 deficiency was associated with severe dilated cardiomyopathy, cardiac fibrosis, altered systolic function, and inflammation. Cardiomyocytes were subjected to increased cellular stress and death. Cardiac capillary density was not affected. MMP-2 expression was up-regulated and tissue transglutaminase-2 was down-regulated that led to aberrant cross-linking. Cardiac expression of the TSP2 transgene prevented dilated cardiomyopathy in aged rats [54].
The cardioprotective action of TSP2 was confirmed in a model of doxorubicin-induced cardiomyopathy. Deletion of TSP2 in mice caused higher death rate in response to doxorubicin while survived animals has diminished heart function associated with intensive apoptosis of cardiac muscle cells and ECM destruction because of MMP-1/9 activation [51]. As shown in a model of viral myocarditis-induced heart failure, TSP2 deficiency also stimulates heart inflammation suggesting for the involvement of TSP2 in the regulation of inflammatory response [157]. Lack of TSP2 is related to reduced activation of regulatory T cells, advanced necrosis and fibrosis, heart dilation, and diminished systolic function. TSP2 overexpression prevents heart failure and reduces mortality through attenuating heart inflammation, inflammation, and virus-induced cardiac death. TSP2 was also up-regulated in the myocardial biopsy samples from subjects affected with viral myocarditis [157]. Overall, these data demonstrate protective effects of TSP2 against heart failure.
The up-regulation of cardiac TSP4 expression was observed in the heart of rats with pressure overload-induced heart failure [45,158] and in rats with heart failure induced by the volume overload after aortocaval fistula [159]. Interestingly, there were no significant changes in systolic function and expression of genes responsible for Ca2+ homeostasis, neurohumoral regulation, contractility, and cytoskeleton organization during transition from left ventricular hypertrophy to heart failure [158]. Only, expression of ECM proteins such as TSP4 and matrix Gla protein was elevated indicating that progression from hypertrophy to heart failure is regulated by ECM remodeling. In the heart of TSP4-deficient mice subjected to TAC to increase left ventricle load, massive ECM depositions, higher cardiac mass, decreased microvessel density, abnormal heart function, and inflammation, but no signs of apoptosis were observed [50]. These observations show that increase of cardiac TSP4 expression is an adaptive response to pressure overload. TSP4 display cardioprotective effects by regulating myocardial remodeling in pressure overload to prevent progression to heart failure.
9. TSPs in Calcific Aortic Valve Disease
The ECM and factors that control ECM composition and remodeling are implicated in the pathogenesis of calcific aortic valve disease (CAVD). Inflammation and angiogenesis, both are regulated by TSPs, also closely linked to the pathogenesis of aortic stenosis [160]. CAVD is characterized by increased proliferation of myofibroblasts, neovasculogenesis, and valvular calcification. Expression of TSPs 1–4 was detected in stenotic valves, with up-regulation of TSP2 [65]. TSP2 expression was permanently increased in neovessels during progression from early valve remodeling to adverse stenosis indicating a role of TSP2 in the control of CAVD-associated neovascularization [65]. Further studies are required to discover a precise mechanism underlining a role of TSP2 in calcified aortic valves.
10. TSPs in other Pathologies
The involvement of TSP1 to hypoxia-induced pulmonary hypertension was shown. This disorder is characterized by increased pressure in the pulmonary artery, pulmonary vein, and lung vasculature. Pulmonary hypertension is accompanied by narrowing of lung-associated vessels due to thickening of the tunica intima and tunica media as a result of pathogenic vascular remodeling. Increased workload of the heart causes hypertrophy of the right ventricle that can finally progress to the right heart failure [161].
Chronic lung ischemia induces overexpression of TSP1 in the pulmonary artery as was shown in a pig model of experimental pulmonary hypertension. TSP1 overactivity correlated with increased death of ECs and endothelial dysfunction likely due to the proapoptotic effects of TSP1 [162]. Similarly, up-regulated levels of various matricellular proteins including TSP1, TSP2, and TSP4 were detected in the right ventricle of monocrotaline-induced pulmonary hypertensive rats [163]. In humans affected with pulmonary hypertension, elevated levels of circulating TSH1 were also reported [164]. Deletion of TSP1 in mice was related to increased arterial VSMC hyperplasia, proliferation, and growth, less advanced vascular remodeling, lowered right ventricular hypertrophy and right ventricle systolic pressure compared with wild-type counterparts exposed to chronic hypoxia. In fact, TSP1-deficient animals showed increased resistance to hypoxia-induced pulmonary hypertension [165].
Mechanistically, TSP1-induced activation of TGF-β promotes VSMC hyperplasia and proliferation in the pulmonary artery and lung arteries of less caliber [166]. In hypoxia-induced human pulmonary artery VSMCs, TSP1 activation also up-regulates production of the NADPH oxidase subunit, Nox4, that can be inhibited by the peroxisome proliferator-activated receptor γ (PPARγ) or its agonist, rosiglitazone [167]. In hypoxic VSMCs, TGF-β acts in an autocrine manner stimulating insulin-like growth factor binding protein-3 (IGFBP-3) activation via the Akt/PI3K mechanism. IGFBP-3 then increases Nox4 expression, which induces VSMC proliferation and transformation of cardiac fibroblasts to myofibroblasts through ROS-dependent signaling and therefore aggravates lung arterial thickening and right ventricular hypertrophy [168,169,170]. Cardiovascular protective effects of PPARγ are mediated through inhibition of hypoxia-induced binding of the transcription factor NF-κB to the Nox4 promoter that prevents transcription [171]. On the other hand, hypoxia was shown to down-regulate PPARγ in pulmonary arterial VSMCs via the ERK1/2- NF-κB-Nox4 mechanism [172]. In summary, these observations suggest for a pathogenic role of both TSP1 and TGF-β in pulmonary hypertension that cooperate in induction of abnormal tissue remodeling associated with increased arterial VSMC hyperplasia/proliferation and hypertrophy of the cardiac right ventricle.
In addition to the involvement of coronary atherosclerosis and post-MI cardiac remodeling, a role of TSPs in cerebrovascular ischemic disease and ischemic stroke was reported. After stroke, increased production of TSP1 and TSP2 was observed in experimental models of ischemic stroke [173,174]. The post-stroke activation of TSPs is necessary to promote an adaptive response to brain injury in order to the recover synaptic plasticity and motor function [175] and regulate angiogenic and platelet-mediated prothrombotic mechanisms [176]. In the resolution phase of the repair of brain infarct, TSP-1/CD36 interaction is important to activate clearance of dead and apoptotic cells by macrophages in response to stimulation by IL-4 or monocyte colony-stimulating factor (M-CSF) [174,177]. Thus, these data indicate protective effects of TSP1 and TSP2 on brain function during healing of ischemic cerebral injury.
TSP1 plays a protective role in non-ischemic neurological pathology such as Alzheimer’s disease (AD), fragile X syndrome, and Down syndrome, both are associated with serious mental impairments and reduced synaptic plasticity. Decreased expression of TSP1 was shown in a subset of cortical pyramidal neurons and astrocytes that are prone to AD [178,179]. TSP1 was shown to protect neurons against β-amyloid-induced synaptic degeneration [179]. On the other hand, β-amyloid inhibits release of TSP1 by astrocytes that in turn attenuates expression of synaptic proteins such as synaptophysin and PSD95 followed by aberrations in the morphology of dendritic spines and reduction of synaptic plasticity [180]. Similar abnormalities such as spine malformations and reduced synaptic density were observed in Down syndrome astrocytes, astrocytes from animal models of fragile X syndrome, and astrocytes from TSP-deficient mice indicating a pathological role of TSP1 deficits [181,182]. In the AD brain, prostaglandin E2, an inflammatory messenger, reduces astrocytic expression of TSP1 by induction of miR-135 that targets the TSP1 mRNA. Binding of prostaglandin E2 to its receptor EP4 leads to protein kinase A (PKA)-dependent stimulation of the CCAAT/enhancer-binding protein δ (CEBPD), a transcriptional coactivator that up-regulates expression of miR-135 in astrocytes [183]. In summary, these findings suggest for a key role of TSP1 in astrogenesis and maturation, spine development, and synaptogenesis. Thus, decreased TSP1 activity is linked to neurodegenerative, neurodevelopmental, and mental pathology associated with dysfunction of dendritic spines and aberrations in synaptic plasticity.
Thrombospondins may contribute to the pathogenesis of congenital heart defects (i.e., congenital heart disease) associated with structural heart anomalies presented at birth. In children with congenital ventricular septal defect characterized by the perforation of the ventricular septum, serum levels of TSP1 were dramatically increased and showed positive correlation with the risk of ventricular septal defect [184] thereby suggesting for a potential value for early diagnosis of this cardiac defect. A pathologic role of TSP1 up-regulation in the ventricular septal defect is unclear but may be related to the constitutive impairment of the TGF-β signaling associated with alterations of migration of neural crest cells [185] that contribute to the formation of the septum part, which separates the pulmonary circulation from the aorta [186].
Depletion of the Hect domain E3 ubiquitin ligase Nedd4 in mice resulted in detrimental abnormalities in heart development associated with the formation of double-outlet right ventricle and atrioventricular cushion defects that was fatal for developing embryos [187]. TSP1 expression was markedly up-regulated in Nedd4-deficient mice. Nedd4 is involved in the ubiquitination of a variety of ion channels, membrane transporters, growth factors and their receptors [188] followed by proteosomal degradation. Interestingly, VEGFR2 is a Nedd4 substrate [189] whose degradation is promoted by TSP1. Indeed, Nedd4-deficient mice experience adverse problems in cardiogenesis and vasculogenesis associated with letal developmental deviations likely due to the defects in protein trafficking machinery, ER stress, and heart malformations due to enhanced and deregulated growth factor signaling. In addition, Nedd4 deficiency may induce the premature control loss of the activity of sodium channels such as cardiac voltage-gated channel Nav1.5 [190] and peripheral neuronal channels Nav1.2 and Nav1.7 [191], which is rather lethal because of the inability to support heart conduction/contractility and cardiac/neuronal connectivity in a proper manner.
Some cardiac congenital aberrations such as for example the Holt-Oram syndrome are accompanied by alterations in electrical conduction [192]. The role of TSPs in the regulation of myocardial electrophysiology and contraction is widely unclear. Direct evidence for the involvement to the cardiac muscle contractility was obtained only for TSP4 that modulates heart contraction in response to stress induced by enhanced blood flow [49]. In TSP1-deficient mice, no difference in heart contractility was observed compared to the wild-type counterparts [193]. Although TSPs are able to bind many Ca2+ cations, this property may be primarily essential for Ca2+-dependent signaling. However, there are some evidence in favor of a potential involvement of TPSs in the control of muscle contraction. As mentioned above, the TSP1/CD47 mechanism is implicated in CaKMII-mediated cardiac hypertrophy [155]. In differentiated SMCs, CaKMII controls cell contractility [194]. In peripheral sensory nerves, painful nerve injury interrupts Ca2+ signaling by stimulating plasma membrane Ca2+-ATPase (PMCA) activity and inhibiting sarco-endoplasmic reticulum Ca2+-ATPase (SERCA) activity that results in depletion of ER-associated Ca2+ depots and increase of cytoplasmic Ca2+ levels. Injury-induced TSP4 up-regulation resembles effects of painful injury on Ca2+ homeostasis by reducing Ca2+ current (ICa) via high-voltage-activated Ca2+ channels and stimulating ICa through low-voltage-activated Ca2+ channels in dorsal root ganglion neurons. This leads to the PMCA activation, SERCA depression, increase of store-operated Ca2+ influx, and Ca2+ signaling disruption through TSP4-dependent stimulation of the voltage-gated Ca2+ channel α2δ1 subunit (Cavα2δ1) and PKC-mediated signaling [195]. It would be interesting to examine whether TPSs contribute to the regulation of cardiomyocyte-specific Ca2+ handling and activity of SERCA and CaKMII that are primarily involved in the myocardial contractility and electric conductivity.
11. Therapeutic Potential of Thrombospondins
The multidomain structural architecture of TSP molecules defines their diverse functions and pleiotropic actions. TSPs have a capability to bind a variety of proteins such as cytokines, growth factors, receptors, and proteases that emphasizes their function in a tissue- and cell type-specific manner [23]. So far, the antiangiogenic activity of TSP1 and TSP2 in cancers inflamed an interest to use these molecules for anti-cancer therapy [196]. Peptides mimicking the anti-angiogenic domains of TSPs and recombinant proteins were developed [197,198].
As known, in TSP1 and TSP2, the anti-angiogenic function is related to the properdin (type-1) repeats located at the N-terminal stalk region [22]. Small peptides derived from this region exhibited only weak inhibitory effects on angiogenesis. However, a single D-amino acid substitution (D-isoleucine) of a particular properdin-region heptapeptide was found to strengthen the anti-angiogenic activity by 1000-fold [199]. Finally, a potent anti-angiogenic TSP1 mimetic nonapeptide analog of this substituted heptapeptide named ABT-510 was constructed [200]. In preclinical studies, ABT-510 showed an ability to efficiently inhibit VEGF-induced migration of microvascular ECs and exhibited anti-angiogenic and anti-tumor activity in several mouse and human xenograft models [201]. In humans, ABT-510 alone or in combination with cytotoxic agents was tested in several Phase I clinical trials to treat a variety of advanced solid and soft cancers [202,203,204,205,206,207]. Overall, ABT-510 administration was safe and well-tolerated, with modest adverse effects with injection-site reactions and fatigue as the most frequent. The anti-tumor effect of ABT-510 varied depending on the cancer type. In principal, treatment with ABT-510 alone had a modest efficiency. However, combinational therapy with cytotoxic agents improved the efficiency of anti-cancer therapy. In Phase II trials [208,209,210], ABT-510 monotherapy led to stabilization of tumor growth and inhibition of tumor expression of proangiogenic factors. In overall, treatment with ABT-510 alone showed a moderate efficiency in Phase II clinical studies by providing only a modest prolongation of overall survival of patients. Thus, based on the results of Phase II trials, clinical application of ABT-510 in combination with other anti-cancer agents was recommended.
In the context of cardiovascular therapy, anti-angiogenic approaches tested in tumors may be helpful in graft atherosclerosis. Mapping of salutary and deleterious effects to different TSP domains will provide an option to construct other TSP-targeting agents for widespread cardiovascular pathology such as MI and heart failure. In heart hypertrophy, atherosclerosis, heart failure, and MI, the down-regulation of the TSP/CD47 axis to enhance angiogenesis and restore NO-dependent signaling would be beneficial [121]. CD47 blockade with a monoclonal antibody was preclinically tested in animal models of ischemia resulted in improvement of angiogenesis and great increases in tissue survival [109,211].
So far, assessment of therapeutic effects CD47 blockade with a monoclonal antibody undergoes transition from the preclinical phase to clinical evaluation. The main purpose of these Phase I clinical trials is to check biosafety/tolerability of a CD47 antibody CC-90002 and find an optimal dose for treatment of advanced hematological neoplasms in combination with Rituximab, an anti-CD20 monoclonal antibody (trial NCT02367196) or for monotherapy of acute myeloid leukemia and high-risk myelodysplastic syndrome (trial NCT02641002). Another humanized anti-CD47 monoclonal antibody, Hu5F9-G4, will be clinically tested alone for treatment of recurrent/refractory acute myeloid leukemia (trial NCT02678338) and advanced solid malignancy or lymphoma (trial NCT02216409) [212]. At present, patients are enrolled for these clinical studies. Expected beneficial effects of this immunotherapy involve the inhibition of tumor angiogenesis and invasion, decrease of tumor-induced macrophage apoptosis and functional impairment, and depletion of CD47-expressing cancer stem cells that are key contributors to tumor relapse and chemoresistance [213]. In a case of the evident tolerability to antibodies, these trials will proceed to the Phase II.
In order to target a profibrotic activity of TSP1 through the activation of TGF-β, the activation sequence (LKSL) in the TSP1 molecule essential for the interaction with the latency-associated peptide (LAP) was mapped [214] and an LKSL peptide was developed [215]. The peptide antagonizes TSH1 binding to LAP and inhibits TGF-β liberation from the latent complex with LAT. The anti-fibrotic activity of the LKSL peptide was demonstrated in various animal models including experimental models of liver fibrosis [216], unilateral ureteral obstruction [217], diabetic nephropathy [218], and post-hemorrhagic hydrocephalus [219]. Regarding cardiovascular pathology, cardioprotective effects of LKSL peptide-mediated inhibition of TGF-β activation were observed in TAC-induced cardiomyopathy in type 1 diabetic rats. Diabetic rats treated with the LKSL peptide did not develop cardiac fibrosis and had improved heart function [52]. However, in a recent study, detrimental effects of implication of this peptide to treat angiotensin II-induced abdominal aortic aneurysm in ApoE-deficient mice were observed [220]. LKSL-dependent suppression of TGF-β activation further aggravated abdominal aortic aneurysm associated with increase of aortic diameter, adverse atherosclerosis within the aortic arch, and aortic elastin fragmentation due to down-regulation of the TGF-β-target gene lysyl oxidase-like 1 (LOXL1), an enzyme involved in cross-linking of elastin [221]. These data suggest for protective role of TGF-β against aortic aneurism. Inhibition of TGF-β signaling may therefore have deleterious consequences by impairing vascular ECM repair and promoting aortic infiltration of inflammatory cells. However, in overall, LKSL-mediated suppression of TSH1-dependent TGF-β activation showed beneficial results in inhibition of tissue fibrosis and should be further explored to prevent or diminish the advanced profibrotic response in hypertrophic hearts or in post-MI cardiac repair.
Since the regenerative potential of human heart is limited, MI-induced loss of cardiomyocytes can result in heart failure and death. Stem cell therapy has emerged as a promising strategy for healing cardiac injury, directly or indirectly, and seems to offer functional benefits to patients. Cardiac stem cell therapy involves using of hematopoietic, mesenchymal, and cardiac stem cells for regenerative purposes. However, a common challenge is to increase the retention and survival of engrafted cells at the injured site in order to strengthen their chances for proliferation and differentiation to functional cardiomyocytes [222]. To enhance the regenerative and prosurvival capacity, stem cells are subjected to ischemic/pharmacological preconditioning before transplantation. For example, regenerative properties of CD34+ hematopoietic progenitor cells from diabetic patients with atherosclerosis are frequently reduced and impaired. Treatment of CD34+ progenitors with TSP1-derived peptide RFYVVMWK promotes expression of TSP-1, integrins, and P-selectin that in turn increases adhesion capability and retention of the autologous progenitor cell engraft although do not affect apoptosis and viability [223]. Hypoxic exposure of adipose tissue-derived mesenchymal cells from aged mice improve their functionality by decreasing expression of anti-angiogenic, prothrombotic, and profibrotic molecules such as TSP-1, plasminogen activator inhibitor-1 (PAI-1), and TGF-β [224]. Preconditioning of mesenchymal stem cells with oxytocin significantly stimulates their therapeutic potential, angiogenic properties, and resistance to hypoxia and apoptosis through induction of various prosurvival and anti-apoptotic factors including TSP-1 [225].
12. Conclusions
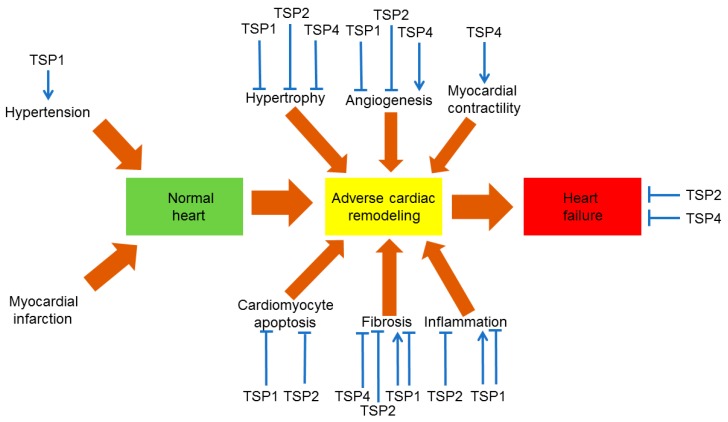
Effects of TSPs on the mechanisms of cardiac remodeling are represented in Figure 2. Table 1 recapitulates TSP-dependent actions in the cardiovascular system. In summary, TSP1, TSP2, and TSP4 possess protective properties against heart hypertrophy since their deletion in animal models of pressure overload results in aberrant remodeling [49,50,59,144,147]. Lack of TSP1 and TSP2 activates MMP production and causes dilation of the left ventricle whereas altered TSP1-dependent TGF-β activation disturbs conversion of fibroblasts to myofibroblasts and down-regulates cardiac matrix synthesis [144]. In a murine pressure overload model, loss of TSP4 resulted in increased cardiac mass and fibrosis [50]. TSP4 also protects from abnormal heart remodeling through induction of stretch-mediated enhancement of myocardial contraction in pressure overload and prevention of the ER stress in cardiomyocytes [49,63]. On the basis of the role in heart hypertrophy, TSP1 and TSP2 would be suggested to influence post-MI remodeling but their effects are needed to be evaluated.
Figure 2.
The role of thrombospondins (TSPs) in cardiovascular pathology. TSP1, TSP2, and TSP4 are the best preclinically studied TSPs in experimental models of cardiovascular pathology involving knockout or overexpression of these TSPs. Sharp arrows define stimulatory effects. Other type of arrows defines inhibitory effects.
Table 1.
The role of thrombospondins in cardiovascular physiology and pathology
| Characteristics | TSP1 | TSP2 | TSP3 | TSP4 | TSP5 |
|---|---|---|---|---|---|
| Expression in the vascular wall | Yes | Yes | Yes | Yes | Yes |
| Expression in the atherosclerotic plaque | Yes | Yes | Yes | Yes | Yes |
| Cardiac expression | Yes | Yes | No? | Yes | Unknown |
| Angiogenesis in the myocardium | Inhibition | Inhibition | Unknown | Activation | Unknown |
| Up-regulated expression in cardiac remodeling | Yes | Yes | Yes | Yes | Yes |
| Inhibition of MMP-2/3/9 | Yes | Yes | No | No | No |
| Cardiac fibrosis | Activation/Inhibition | Inhibition | Unknown | Inhibition | Unknown |
| VSMC proliferation/hyperplasia | Activation | Activation | Unknown | No effect | Inhibition |
| Blood pressure | Vasoconstriction | Vasoconstriction | Unknown | Unknown | Unknown |
| Inflammation | Activation/Inhibition | Inhibition | Unknown | Activation (moderate) | Unknown |
| Effects on macrophages | Stimulation of phagocytosis Foam cell formation | Unknown | Unknown | Recruitment to the plaque | Unknown |
| Plaque progression | Activation | Unknown | Unknown | Activation | Unknown |
| Oxidative stress | Activation | Unknown | Unknown | Unknown | Unknown |
| Cardiomyocyte apoptosis | Inhibition | Inhibition | Unknown | Unknown | Unknown |
| Cardiac contractility | No effect | Unknown | Unknown | Activation | Unknown |
| Cardiac hypertrophy | Inhibition | Inhibition | Unknown | Inhibition | Unknown |
| Heart failure | Inhibition? | Inhibition | Unknown | Inhibition | Unknown |
Abbreviations: MMP, matrix metalloproteinase; TSP, thrombospondin; VSMC, vascular smooth muscle cell.
In failing hearts, TSP2 and TSP4 protect cardiac ECM from adverse remodeling. In TSP-deficient ageing heart, systolic function is altered while fibrinogenesis and cardiac dilatation are activated [54]. Also, an advanced cardiac muscle cell death, inflammation, MMP-2 activation and aberrant collagen cross-linking was observed [54]. TSP2 possess cardioprotective properties against heart failure in viral myocarditis by repressing inflammation, fibrotic response, and cardiomyocyte death [157]. In doxorubicin-induced cardiomyopathy, the anti-hypertrophic activity of TSP2 is related to the inhibition of apoptosis of cardiac muscle cells and preserving ECM from the damage [51]. As in a case of TSP2, TSP4 deletion results in fibrosis, altered diastolic function, and depressed systolic function denoting the involvement of TSP4 in the regulation of ECM composition [50]. Finally, TSP4 appears to possess the proatherosclerotic activity since ApoE-deficient mice lacking TSP4 had reduced macrophage accumulation in the plaques due to reduced proinflammatory activation of ECs and decreased recruitment of inflammatory leukocytes to the endothelium [31].
So far, the most comprehensive functional assessment of the physiological and pathological roles was done only for TSP1, a founder member of the TSP family. Less complete results were obtained for TSP2 and TSP4. From the scientific literature, there is a profound data deficit about the function of TSP3 and TSP5. Although TSP3 is expressed by VSMCs at significant levels, its expression in the homeostatic myocardium seems to be absent [42]. However, stimulation of cardiac fibroblasts with a peptide matricryptin generated by a limited proteolysis of collagen Iα1 by MMP-2/9 results in the induction of TSP3 expression. Along with TSP3, matricryptin induces production of many ECM structural and regulatory proteins that contribute to post-MI cardiac repair and promote scar formation and angiogenesis [226]. This effect of matricryptin has a therapeutic promise and therefore should be further evaluated. Since TSP1 and TSP2 inhibit proteolytic activation of MMP-2 and MMP-9 [227], these TSPs can potentially suppress cardiac expression of TSP3. Therefore, assessing reciprocal TSP regulation would be intriguing. Thus, future prospects in the thrombospondin-related research may ultimately concern investigation of TSP3 and TSP5 functions. Further, TSPs have a variety of binding partners and the number of TSP ligands is growing. However, regulatory mechanisms of binding these ligands are widely unknown. Actually, ligand binding is spatially and temporally regulated, and it would be of great interest to reveal these regulatory patterns and recognize their functional significance.
Overall, studies involving knockout mice indicate that deficiency of TSP1, TSP2, and TSP4 appears to be deleterious in cardiovascular pathology. Therefore, enhancing of cardiac TSP-dependent signaling in stressful settings such as pressure overload may be profitable. Precise analysis of the relationship between the TSP structure and function, identification of new receptors, and functional mapping of various domains will be useful for the development of novel drugs to target TSPs and promote the gain in TSP-dependent signaling pathways.
Acknowledgments
This work was supported by Russian Science Foundation (Grant # 14–15–00112).
Author contributions
Dimitry A. Chistiakov, Alexandra A. Melnichenko, and Veronika A. Myasoedova drafted the paper. Andrey V. Grechko and Alexander N. Orekhov corrected the paper. Alexander N. Orekhov finally approved the paper. All authors read the final version and approved submission.
Conflicts of Interest
All authors state that they have no conflict of interests.
References
- 1.Chistiakov D.A., Sobenin I.A., Orekhov A.N. Vascular extracellular matrix in atherosclerosis. Cardiol. Rev. 2013;21:270–288. doi: 10.1097/CRD.0b013e31828c5ced. [DOI] [PubMed] [Google Scholar]
- 2.Lewinsohn A.D., Anssari-Benham A., Lee D.A., Taylor P.M., Chester A.H., Yacoub M.H., Screen H.R. Anisotropic strain transfer through the aortic valve and its relevance to the cellular mechanical environment. Proc. Inst. Mech. Eng. H. 2011;225:821–830. doi: 10.1177/0954411911406340. [DOI] [PubMed] [Google Scholar]
- 3.Okech W., Abberton K.M., Kuebel J.M., Hocking D.C., Sarelius I.H. Extracellular matrix fibronectin mediates an endothelial cell response to shear stress via the heparin-binding, matricryptic RWRPK sequence of FNIII1H. Am. J. Physiol. Heart Circ. Physiol. 2016;311:H1063–H1071. doi: 10.1152/ajpheart.00126.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casals G., Fernández-Varo G., Melgar-Lesmes P., Marfà S., Reichenbach V., Morales-Ruiz M., Jiménez W. Factors involved in extracellular matrix turnover in human derived cardiomyocytes. Cell Physiol. Biochem. 2013;32:1125–1136. doi: 10.1159/000354513. [DOI] [PubMed] [Google Scholar]
- 5.Luu N.T., Glen K.E., Egginton S., Rainger G.E., Nash G.B. Integrin-substrate interactions underlying shear-induced inhibition of the inflammatory response of endothelial cells. Thromb. Haemost. 2013;109:298–308. doi: 10.1160/TH12-06-0400. [DOI] [PubMed] [Google Scholar]
- 6.Von Bary C., Makowski M., Preissel A., Keithahn A., Warley A., Spuentrup E., Buecker A., Lazewatsky J., Cesati R., Onthank D., et al. MRI of coronary wall remodeling in a swine model of coronary injury using an elastin-binding contrast agent. Circ. Cardiovasc. Imaging. 2011;4:147–155. doi: 10.1161/CIRCIMAGING.109.895607. [DOI] [PubMed] [Google Scholar]
- 7.Li J., Philip J.L., Xu X., Theccanat T., Abdur Razzaque M., Akhter S.A. β-Arrestins regulate human cardiac fibroblast transformation and collagen synthesis in adverse ventricular remodeling. J. Mol. Cell Cardiol. 2014;76:73–83. doi: 10.1016/j.yjmcc.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mujumdar V.S., Tyagi S.C. Temporal regulation of extracellular matrix components in transition from compensatory hypertrophy to decompensatory heart failure. J. Hypertens. 1999;17:261–270. doi: 10.1097/00004872-199917020-00011. [DOI] [PubMed] [Google Scholar]
- 9.Sakamuri S.S., Takawale A., Basu R., Fedak P.W., Freed D., Sergi C., Oudit G.Y., Kassiri Z. Differential impact of mechanical unloading on structural and nonstructural components of the extracellular matrix in advanced human heart failure. Transl. Res. 2016;172:30–44. doi: 10.1016/j.trsl.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Bornstein P. Thrombospondins as matricellular modulators of cell function. J. Clin. Investig. 2001;107:929–934. doi: 10.1172/JCI12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurihara K., Sato I. Distribution of tenascin-C and -X, and soft X-ray analysis of the mandibular symphysis during mandible formation in the human fetus. Okajimas Folia Anat. Jpn. 2004;81:49–55. doi: 10.2535/ofaj.81.49. [DOI] [PubMed] [Google Scholar]
- 12.Huang W., Zhang L., Niu R., Liao H. Tenascin-R distinct domains modulate migration of neural stem/progenitor cells in vitro. In Vitro Cell Dev. Biol. Anim. 2009;45:10–14. doi: 10.1007/s11626-008-9145-6. [DOI] [PubMed] [Google Scholar]
- 13.Gillan L., Matei D., Fishman D.A., Gerbin C.S., Karlan B.Y., Chang D.D. Periostin secreted by epithelial ovarian carcinoma is a ligand for αVβ3 and alphaVbeta5 integrins and promotes cell motility. Cancer Res. 2002;62:5358–5364. [PubMed] [Google Scholar]
- 14.Hoersch S., Andrade-Navarro M.A. Periostin shows increased evolutionary plasticity in its alternatively spliced region. BMC Evol. Biol. 2010;10:30. doi: 10.1186/1471-2148-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hakuno D., Kimura N., Yoshioka M., Mukai M., Kimura T., Okada Y., Yozu R., Shukunami C., Hiraki Y., Kudo A., et al. Periostin advances atherosclerotic and rheumatic cardiac valve degeneration by inducing angiogenesis and MMP production in humans and rodents. J. Clin. Investig. 2010;120:2292–2306. doi: 10.1172/JCI40973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steitz S.A., Speer M.Y., McKee M.D., Liaw L., Almeida M., Yang H., Giachelli C.M. Osteopontin inhibits mineral deposition and promotes regression of ectopic calcification. Am. J. Pathol. 2002;161:2035–2046. doi: 10.1016/S0002-9440(10)64482-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKee M.D., Addison W.N., Kaartinen M.T. Hierarchies of extracellular matrix and mineral organization in bone of the craniofacial complex and skeleton. Cell. Tissues Organs. 2005;181:176–188. doi: 10.1159/000091379. [DOI] [PubMed] [Google Scholar]
- 18.Wang K.X., Denhardt D.T. Osteopontin: Role in immune regulation and stress responses. Cytokine Growth Factor Rev. 2008;19:333–345. doi: 10.1016/j.cytogfr.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Midura R.J., Midura S.B., Su X., Gorski J.P. Separation of newly formed bone from older compact bone reveals clear compositional differences in bone matrix. Bone. 2011;49:1365–1374. doi: 10.1016/j.bone.2011.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen C.C., Lau L.F. Functions and mechanisms of action of CCN matricellular proteins. Int. J. Biochem. Cell Biol. 2009;41:771–783. doi: 10.1016/j.biocel.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dobaczewski M., Gonzalez-Quesada C., Frangogiannis N.G. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J. Mol. Cell. Cardiol. 2010;48:504–511. doi: 10.1016/j.yjmcc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carlson C.B., Lawler J., Mosher D.F. Structures of thrombospondins. Cell Mol. Life Sci. 2008;65:672–686. doi: 10.1007/s00018-007-7484-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adams J.C., Lawler J. The thrombospondins. Cold Spring Harb. Perspect. Biol. 2011;3:a009712. doi: 10.1101/cshperspect.a009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKenzie P., Chadalavada C., Bohrer J., Adams J.C. Phylogenomic analysis of vertebrate thrombospondins, reveals fish-specific paralogues, ancestral gene relationships and a tetrapod innovation. BMC Evol. Biol. 2006;6:33. doi: 10.1186/1471-2148-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bentley A.A., Adams J.C. The evolution of thrombospondins and their ligand-binding activities. Mol. Biol. Evol. 2010;27:2187–2197. doi: 10.1093/molbev/msq107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy-Ullrich J.E., Iozzo R.V. Thrombospondins in physiology and disease: New tricks for old dogs. Matrix Biol. 2012;31:152–154. doi: 10.1016/j.matbio.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rath G.M., Schneider C., Dedieu S., Rothhut B., Soula-Rothhut M., Ghoneim C., Sid B., Morjani H., El Btaouri H., Martiny L. The C-terminal CD47/IAP-binding domain of thrombospondin-1 prevents camptothecin- and doxorubicin-induced apoptosis in human thyroid carcinoma cells. Biochim. Biophys. Acta. 2006;1763:1125–1134. doi: 10.1016/j.bbamcr.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Bornstein P., Armstrong L.C., Hankenson K.D., Kyriakides T.R., Yang Z. Thrombospondin 2, a matricellular protein with diverse functions. Matrix Biol. 2000;19:557–568. doi: 10.1016/S0945-053X(00)00104-9. [DOI] [PubMed] [Google Scholar]
- 29.Kyriakides T.R., Bornstein P. Matricellular proteins as modulators of wound healing and the foreign body response. Thromb. Haemost. 2003;90:986–992. doi: 10.1160/TH03-06-0399. [DOI] [PubMed] [Google Scholar]
- 30.Schellings M.W., Van Almen G.C., Sage E.H., Heymans S. Thrombospondins in the heart: Potential functions in cardiac remodeling. J. Cell Commun. Signal. 2009;3:201–213. doi: 10.1007/s12079-009-0070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frolova E.G., Pluskota E., Krukovets I., Burke T., Drumm C., Smith J.D., Blech L., Febbraio M., Bornstein P., Plow E.F., et al. Thrombospondin-4 regulates vascular inflammation and atherogenesis. Circ. Res. 2010;107:1313–1325. doi: 10.1161/CIRCRESAHA.110.232371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mustonen E., Ruskoaho H., Rysä J. Thrombospondins, potential drug targets for cardiovascular diseases. Basic Clin. Pharmacol. Toxicol. 2013;112:4–12. doi: 10.1111/bcpt.12026. [DOI] [PubMed] [Google Scholar]
- 33.Muppala S., Xiao R., Krukovets I., Verbovetsky D., Yendamuri R., Habib N., Raman P., Plow E., Stenina-Adognravi O. Thrombospondin-4 mediates TGF-β-induced angiogenesis. Oncogene. 2017 doi: 10.1038/onc.2017.140. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Topol E.J., McCarthy J., Gabriel S., Moliterno D.J., Rogers W.J., Newby L.K., Freedman M., Metivier J., Cannata R., O’Donnell C.J., et al. Single nucleotide polymorphisms in multiple novel thrombospondin genes may be associated with familial premature myocardial infarction. Circulation. 2001;104:2641–2644. doi: 10.1161/hc4701.100910. [DOI] [PubMed] [Google Scholar]
- 35.Cui J., Randell E., Renouf J., Sun G., Han FY., Younghusband B., Xie Y.G. Gender dependent association of thrombospondin-4 A387P polymorphism with myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 2004;24:e183–e1844. doi: 10.1161/01.ATV.0000147304.67100.ee. [DOI] [PubMed] [Google Scholar]
- 36.Cui J., Randell E., Renouf J., Sun G., Green R., Han F.Y., Xie Y.G. Thrombospondin-4 1186G>C (A387P) is a sex-dependent risk factor for myocardial infarction: A large replication study with increased sample size from the same population. Am. Heart J. 2006;152:543.e1–543.e5. doi: 10.1016/j.ahj.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Wessel J., Topol E.J., Ji M., Meyer J., McCarthy J.J. Replication of the association between the thrombospondin-4 A387P polymorphism and myocardial infarction. Am. Heart J. 2004;147:905–909. doi: 10.1016/j.ahj.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 38.Zwicker J.I., Peyvandi F., Palla R., Lombardi R., Canciani M.T., Cairo A., Ardissino D., Bernardinelli L., Bauer K.A., Lawler J., et al. The thrombospondin-1 N700S polymorphism is associated with early myocardial infarction without altering von Willebrand factor multimer size. Blood. 2006;108:1280–1283. doi: 10.1182/blood-2006-04-015701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corsetti J.P., Ryan D., Moss A.J., McCarthy J., Goldenberg I., Zareba W., Sparks C.E. Thrombospondin-4 polymorphism (A387P) predicts cardiovascular risk in postinfarction patients with high HDL cholesterol and C-reactive protein levels. Thromb. Haemost. 2011;106:1170–1178. doi: 10.1160/TH11-03-0206. [DOI] [PubMed] [Google Scholar]
- 40.Stenina O.I., Ustinov V., Krukovets I., Marinic T., Topol E.J., Plow E.F. Polymorphisms A387P in thrombospondin-4 and N700S in thrombospondin-1 perturb calcium binding sites. FASEB J. 2005;19:1893–1895. doi: 10.1096/fj.05-3712fje. [DOI] [PubMed] [Google Scholar]
- 41.Zhang X.J., Wei C.Y., Li W.B., Zhang L.L., Zhou Y., Wang Z.H., Tang M.X., Zhang W., Zhang Y., Zhong M. Association between single nucleotide polymorphisms in thrombospondins genes and coronary artery disease: A meta-analysis. Thromb. Res. 2015;136:45–51. doi: 10.1016/j.thromres.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 42.Adolph K.W. Relative abundance of thrombospondin 2 and thrombospondin 3 mRNAs in human tissues. Biochem. Biophys. Res. Commun. 1999;258:792–796. doi: 10.1006/bbrc.1999.0710. [DOI] [PubMed] [Google Scholar]
- 43.Zhao X.M., Hu Y., Miller G.G., Mitchell R.N., Libby P. Association of thrombospondin-1 and cardiac allograft vasculopathy in human cardiac allografts. Circulation. 2001;103:525–531. doi: 10.1161/01.CIR.103.4.525. [DOI] [PubMed] [Google Scholar]
- 44.Sezaki S., Hirohata S., Iwabu A., Nakamura K., Toeda K., Miyoshi T., Yamawaki H., Demircan K., Kusachi S., Shiratori Y., et al. Thrombospondin-1 is induced in rat myocardial infarction and its induction is accelerated by ischemia/reperfusion. Exp. Biol. Med. 2005;230:621–630. doi: 10.1177/153537020523000904. [DOI] [PubMed] [Google Scholar]
- 45.Mustonen E., Aro J., Puhakka J., Ilves M., Soini Y., Leskinen H., Ruskoaho H., Rysä J. Thrombospondin-4 expression is rapidly upregulated by cardiac overload. Biochem. Biophys. Res. Commun. 2008;373:186–191. doi: 10.1016/j.bbrc.2008.05.164. [DOI] [PubMed] [Google Scholar]
- 46.Stenina-Adognravi O. Thrombospondins: Old players, new games. Curr. Opin. Lipidol. 2013;24:401–419. doi: 10.1097/MOL.0b013e3283642912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reinecke H., Robey T.E., Mignone J.L., Muskheli V., Bornstein P., Murry C.E. Lack of thrombospondin-2 reduces fibrosis and increases vascularity around cardiac cell grafts. Cardiovasc. Pathol. 2013;22:91–95. doi: 10.1016/j.carpath.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vanhoutte D., van Almen G.C., Van Aelst L.N., Van Cleemput J., Droogné W., Jin Y., Van de Werf F., Carmeliet P., Vanhaecke J., Papageorgiou A.P., et al. Matricellular proteins and matrix metalloproteinases mark the inflammatory and fibrotic response in human cardiac allograft rejection. Eur. Heart J. 2013;34:1930–1941. doi: 10.1093/eurheartj/ehs375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cingolani O.H., Kirk J.A., Seo K., Koitabashi N., Lee D.I., Ramirez-Correa G., Bedja D., Barth A.S., Moens A.L., Kass D.A. Thrombospondin-4 is required for stretch-mediated contractility augmentation in cardiac muscle. Circ. Res. 2011;109:1410–1414. doi: 10.1161/CIRCRESAHA.111.256743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frolova E.G., Sopko N., Blech L., Popovic Z.B., Li J., Vasanji A., Drumm C., Krukovets I., Jain M.K., Penn M.S., Plow E.F., et al. Thrombospondin-4 regulates fibrosis and remodeling of the myocardium in response to pressure overload. FASEB J. 2012;26:2363–2373. doi: 10.1096/fj.11-190728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Almen G.C., Swinnen M., Carai P., Verhesen W., Cleutjens J.P., D’hooge J., Verheyen F.K., Pinto Y.M., Schroen B., Carmeliet P., et al. Absence of thrombospondin-2 increases cardiomyocyte damage and matrix disruption in doxorubicin-induced cardiomyopathy. J. Mol. Cell Cardiol. 2011;51:318–328. doi: 10.1016/j.yjmcc.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 52.Belmadani S., Bernal J., Wei C.C., Pallero M.A., Dell’italia L., Murphy-Ullrich J.E., Berecek K.H. A thrombospondin-1 antagonist of transforming growth factor-β activation blocks cardiomyopathy in rats with diabetes and elevated angiotensin II. Am. J. Pathol. 2007;171:777–789. doi: 10.2353/ajpath.2007.070056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gonzalez-Quesada C., Cavalera M., Biernacka A., Kong P., Lee D.W., Saxena A., Frunza O., Dobaczewski M., Shinde A., Frangogiannis N.G. Thrombospondin-1 induction in the diabetic myocardium stabilizes the cardiac matrix in addition to promoting vascular rarefaction through angiopoietin-2 upregulation. Circ. Res. 2013;113:1331–1344. doi: 10.1161/CIRCRESAHA.113.302593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Swinnen M., Vanhoutte D., Van Almen G.C., Hamdani N., Schellings M.W., D’hooge J., Van der Velden J., Weaver M.S., Sage E.H., Bornstein P., et al. Absence of thrombospondin-2 causes age-related dilated cardiomyopathy. Circulation. 2009;120:1585–1597. doi: 10.1161/CIRCULATIONAHA.109.863266. [DOI] [PubMed] [Google Scholar]
- 55.Frangogiannis N.G., Ren G., Dewald O., Zymek P., Haudek S., Koerting A., Winkelmann K., Michael L.H., Lawler J., Entman M.L. Critical role of endogenous thrombospondin-1 in preventing expansion of healing myocardial infarcts. Circulation. 2005;111:2935–2942. doi: 10.1161/CIRCULATIONAHA.104.510354. [DOI] [PubMed] [Google Scholar]
- 56.Chatila K., Ren G., Xia Y., Huebener P., Bujak M., Frangogiannis N.G. The role of the thrombospondins in healing myocardial infarcts. Cardiovasc. Hematol. Agents Med. Chem. 2007;5:21–27. doi: 10.2174/187152507779315813. [DOI] [PubMed] [Google Scholar]
- 57.Cai H., Yuan Z., Fei Q., Zhao J. Investigation of thrombospondin-1 and transforming growth factor-β expression in the heart of aging mice. Exp. Ther. Med. 2012;3:433–436. doi: 10.3892/etm.2011.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun H., Zhao Y., Bi X., Li S., Su G., Miao Y., Ma X., Zhang Y., Zhang W., Zhong M. Valsartan blocks thrombospondin/transforming growth factor/Smads to inhibit aortic remodeling in diabetic rats. Diagn. Pathol. 2015;10:18. doi: 10.1186/s13000-015-0246-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schroen B., Heymans S., Sharma U., Blankesteijn W.M., Pokharel S., Cleutjens J.P., Porter J.G., Evelo C.T., Duisters R., van Leeuwen R.E., Janssen B.J., et al. Thrombospondin-2 is essential for myocardial matrix integrity: Increased expression identifies failure-prone cardiac hypertrophy. Circ. Res. 2004;95:515–522. doi: 10.1161/01.RES.0000141019.20332.3e. [DOI] [PubMed] [Google Scholar]
- 60.Orr A.W., Elzie C.A., Kucik D.F., Murphy-Ullrich J.E. Thrombospondin signaling through the calreticulin/LDL receptor-related protein co-complex stimulates random and directed cell migration. J. Cell Sci. 2003;116:2917–2927. doi: 10.1242/jcs.00600. [DOI] [PubMed] [Google Scholar]
- 61.Pallero M.A., Elzie C.A., Chen J., Mosher D.F., Murphy-Ullrich J.E. Thrombospondin 1 binding to calreticulin-LRP1 signals resistance to anoikis. FASEB J. 2008;22:3968–3979. doi: 10.1096/fj.07-104802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yan Q., Murphy-Ullrich J.E., Song Y. Structural insight into the role of thrombospondin-1 binding to calreticulin in calreticulin-induced focal adhesion disassembly. Biochemistry. 2010;49:3685–3694. doi: 10.1021/bi902067f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lynch J.M., Maillet M., Vanhoutte D., Schloemer A., Sargent M.A., Blair N.S., Lynch K.A., Okada T., Aronow B.J., Osinska H., et al. A thrombospondin-dependent pathway for a protective ER stress response. Cell. 2012;149:1257–1268. doi: 10.1016/j.cell.2012.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Frolova E.G., Drazba J., Krukovets I., Kostenko V., Blech L., Harry C., Vasanji A., Drumm C., Sul P., Jenniskens G.J., et al. Control of organization and function of muscle and tendon by thrombospondin-4. Matrix Biol. 2014;37:35–48. doi: 10.1016/j.matbio.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pohjolainen V., Mustonen E., Taskinen P., Näpänkangas J., Leskinen H., Ohukainen P., Peltonen T., Aro J., Juvonen T., Satta J., et al. Increased thrombospondin-2 in human fibrosclerotic and stenotic aortic valves. Atherosclerosis. 2012;220:66–71. doi: 10.1016/j.atherosclerosis.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 66.Streit M., Riccardi L., Velasco P., Brown L.F., Hawighorst T., Bornstein P., Detmar M. Thrombospondin-2: A potent endogenous inhibitor of tumor growth and angiogenesis. Proc. Natl. Acad. Sci. USA. 1999;96:14888–14893. doi: 10.1073/pnas.96.26.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lawler J. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. J. Cell. Mol. Med. 2002;6:1–12. doi: 10.1111/j.1582-4934.2002.tb00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lawler J., Detmar M. Tumor progression: The effects of thrombospondin-1 and -2. Int. J. Biochem. Cell Biol. 2004;36:1038–1045. doi: 10.1016/j.biocel.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 69.Zhang X., Lawler J. Thrombospondin-based antiangiogenic therapy. Microvasc. Res. 2007;74:90–99. doi: 10.1016/j.mvr.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Almog N., Henke V., Flores L., Hlatky L., Kung A.L., Wright R.D., Berger R., Hutchinson L., Naumov G.N., Bender E., et al. Prolonged dormancy of human liposarcoma is associated with impaired tumor angiogenesis. FASEB J. 2006;20:947–949. doi: 10.1096/fj.05-3946fje. [DOI] [PubMed] [Google Scholar]
- 71.Almog N. Molecular mechanisms underlying tumor dormancy. Cancer Lett. 2010;294:139–146. doi: 10.1016/j.canlet.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 72.Almog N., Ma L., Raychowdhury R., Schwager C., Erber R., Short S., Hlatky L., Vajkoczy P., Huber P.E., Folkman J., et al. Transcriptional switch of dormant tumors to fast-growing angiogenic phenotype. Cancer Res. 2009;69:836–844. doi: 10.1158/0008-5472.CAN-08-2590. [DOI] [PubMed] [Google Scholar]
- 73.Bhattacharyya S., Sul K., Krukovets I., Nestor C., Li J., Adognravi O.S. Novel tissue-specific mechanism of regulation of angiogenesis and cancer growth in response to hyperglycemia. J. Am. Heart Assoc. 2012;1:e005967. doi: 10.1161/JAHA.112.005967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krukovets I., Legerski M., Sul P., Stenina-Adognravi O. Inhibition of hyperglycemia-induced angiogenesis and breast cancer tumor growth by systemic injection of microRNA-467 antagonist. FASEB J. 2015;29:3726–3736. doi: 10.1096/fj.14-267799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Silverstein R.L., Febbraio M. CD36-TSP-HRGP interactions in the regulation of angiogenesis. Curr. Pharm. Des. 2007;13:3559–3567. doi: 10.2174/138161207782794185. [DOI] [PubMed] [Google Scholar]
- 76.Frieda S., Pearce A., Wu J., Silverstein R.L. Rec mbinant GST/CD36 fusion proteins define a thrombospondin binding domain. Evidence for a single calcium-dependent binding site on CD36. J. Biol. Chem. 1995;270:2981–2986. doi: 10.1074/jbc.270.7.2981. [DOI] [PubMed] [Google Scholar]
- 77.Simantov R., Silverstein R.L. CD36: A critical anti-angiogenic receptor. Front. Biosci. 2003;8:s874–s882. doi: 10.2741/1168. [DOI] [PubMed] [Google Scholar]
- 78.Simantov R., Febbraio M., Silverstein R.L. The antiangiogenic effect of thrombospondin-2 is mediated by CD36 and modulated by histidine-rich glycoprotein. Matrix Biol. 2005;24:27–34. doi: 10.1016/j.matbio.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 79.Kaur S., Martin-Manso G., Pendrak M.L., Garfield S.H., Isenberg J.S., Roberts D.D. Thrombospondin-1 inhibits VEGF receptor-2 signaling by disrupting its association with CD47. J. Biol. Chem. 2010;285:38923–38932. doi: 10.1074/jbc.M110.172304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bazzazi H., Isenberg J.S., Popel A.S. Inhibition of VEGFR2 activation and its downstream signaling to ERK1/2 and calcium by thrombospondin-1 (TSP1): In silico investigation. Front. Physiol. 2017 doi: 10.3389/fphys.2017.00048. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Isenberg J.S., Ridnour L.A., Dimitry J., Frazier W.A., Wink D.A., Roberts D.D. CD47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1. J. Biol. Chem. 2006;281:26069–26080. doi: 10.1074/jbc.M605040200. [DOI] [PubMed] [Google Scholar]
- 82.Isenberg J.S., Jia Y., Fukuyama J., Switzer C.H., Wink D.A., Roberts D.D. Thrombospondin-1 inhibits nitric oxide signaling via CD36 by inhibiting myristic acid uptake. J. Biol. Chem. 2007;282:15404–15415. doi: 10.1074/jbc.M701638200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Isenberg J.S., Hyodo F., Matsumoto K., Romeo M.J., Abu-Asab M., Tsokos M., Kuppusamy P., Wink D.A., Krishna M.C., Roberts D.D. Thrombospondin-1 limits ischemic tissue survival by inhibiting nitric oxide-mediated vascular smooth muscle relaxation. Blood. 2007;109:1945–1952. doi: 10.1182/blood-2006-08-041368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Isenberg J.S., Annis D.S., Pendrak M.L., Ptaszynska M., Frazier W.A., Mosher D.F., Roberts D.D. Differential interactions of thrombospondin-1, -2, and -4 with CD47 and effects on cGMP signaling and ischemic injury responses. J. Biol. Chem. 2009;284:1116–1125. doi: 10.1074/jbc.M804860200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao Y., Brandish P.E., Ballou D.P., Marletta M.A. A molecular basis for nitric oxide sensing by soluble guanylate cyclase. Proc. Natl. Acad. Sci. USA. 1999;96:14753–14758. doi: 10.1073/pnas.96.26.14753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Murad F., Rapoport R.M., Fiscus R. Role of cyclic-GMP in relaxations of vascular smooth muscle. J. Cardiovasc. Pharmacol. 1985;7:S111–S118. doi: 10.1097/00005344-198500073-00013. [DOI] [PubMed] [Google Scholar]
- 87.Isenberg J.S., Romeo M.J., Yu C., Yu C.K., Nghiem K., Monsale J., Rick M.E., Wink D.A., Frazier W.A., Roberts D.D. Thrombospondin-1 stimulates platelet aggregation by blocking the antithrombotic activity of nitric oxide/cGMP signaling. Blood. 2008;111:613–623. doi: 10.1182/blood-2007-06-098392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Koom Y.K., Kim J.M., Kim S.Y., Koo J.Y., Oh D., Park S., Yun-Choi H.S. Elevated plasma concentration of NO and cGMP may be responsible for the decreased platelet aggregation and platelet leukocyte conjugation in platelets hypo-responsive to catecholamines. Platelets. 2009;20:555–565. doi: 10.3109/09537100903337419. [DOI] [PubMed] [Google Scholar]
- 89.Miller T.W., Kaur S., Ivins-O’Keefe K., Roberts D.D. Thrombospondin-1 is a CD47-dependent endogenous inhibitor of hydrogen sulfide signaling in T cell activation. Matrix Biol. 2013;32:316–324. doi: 10.1016/j.matbio.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Csányi G., Yao M., Rodríguez A.I., Al Ghouleh I., Sharifi-Sanjani M., Frazziano G., Huang X., Kelley E.E., Isenberg J.S., Pagano P.J. Thrombospondin-1 regulates blood flow via CD47 receptor-mediated activation of NADPH oxidase 1. Arterioscler. Thromb. Vasc. Biol. 2012;32:2966–2973. doi: 10.1161/ATVBAHA.112.300031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mayer B., Kleschyov A.L., Stessel H., Russwurm M., Münzel T., Koesling D., Schmidt K. Inactivation of soluble guanylate cyclase by stoichiometric S-nitrosation. Mol. Pharmacol. 2009;75:886–891. doi: 10.1124/mol.108.052142. [DOI] [PubMed] [Google Scholar]
- 92.Tsai A.L., Berka V., Sharina I., Martin E. Dynamic ligand exchange in soluble guanylyl cyclase (sGC): Implications for sGC regulation and desensitization. J. Biol. Chem. 2011;286:43182–43192. doi: 10.1074/jbc.M111.290304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Muppala S., Frolova E., Xiao R., Krukovets I., Yoon S., Hoppe G., Vasanji A., Plow E., Stenina-Adognravi O. Proangiogenic properties of thrombospondin-4. Arterioscler. Thromb. Vasc. Biol. 2015;35:1975–1986. doi: 10.1161/ATVBAHA.115.305912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stenina O.I., Krukovets I., Wang K., Zhou Z., Forudi F., Penn M.S., Topol E.J., Plow E.F. Increased expression of thrombospondin-1 in vessel wall of diabetic Zucker rat. Circulation. 2003;107:3209–3215. doi: 10.1161/01.CIR.0000074223.56882.97. [DOI] [PubMed] [Google Scholar]
- 95.Chen D., Asahara T., Krasinski K., Witzenbichler B., Yang J., Magner M., Kearney M., Frazier W.A., Isner J.M., Andrés V. Antibody blockade of thrombospondin accelerates reendothelialization and reduces neointima formation in balloon-injured rat carotid artery. Circulation. 1999;100:849–854. doi: 10.1161/01.CIR.100.8.849. [DOI] [PubMed] [Google Scholar]
- 96.Moura R., Tjwa M., Vandervoort P., Van Kerckhoven S., Holvoet P., Hoylaerts M.F. Thrombospondin-1 deficiency accelerates atherosclerotic plaque maturation in ApoE−/− mice. Circ. Res. 2008;103:1181–1189. doi: 10.1161/CIRCRESAHA.108.185645. [DOI] [PubMed] [Google Scholar]
- 97.Stenina O.I., Plow E.F. Counterbalancing forces: What is thrombospondin-1 doing in atherosclerotic lesions? Circ. Res. 2008;103:1053–1055. doi: 10.1161/CIRCRESAHA.108.188870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Siegel-Axel D.I., Runge H., Seipel L., Riessen R. Effects of cerivastatin on human arterial smooth muscle cell growth and extracellular matrix expression at varying glucose and low-density lipoprotein levels. J. Cardiovasc. Pharmacol. 2003;41:422–433. doi: 10.1097/00005344-200303000-00011. [DOI] [PubMed] [Google Scholar]
- 99.Reed M.J., Iruela-Arispe L., O’Brien E.R., Truong T., LaBell T., Bornstein P., Sage E.H. Expression of thrombospondins by endothelial cells. Injury is correlated with TSP-1. Am. J. Pathol. 1995;147:1068–1080. [PMC free article] [PubMed] [Google Scholar]
- 100.Riessen R., Fenchel M., Chen H., Axel D.I., Karsch K.R., Lawler J. Cartilage oligomeric matrix protein (thrombospondin-5) is expressed by human vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2001;21:47–54. doi: 10.1161/01.ATV.21.1.47. [DOI] [PubMed] [Google Scholar]
- 101.Wang L., Wang X., Kong W. ADAMTS-7, a novel proteolytic culprit in vascular remodeling. Sheng Li Xue Bao. 2010;62:285–294. [PubMed] [Google Scholar]
- 102.Wang L., Zheng J., Bai X., Liu B., Liu C.J., Xu Q., Zhu Y., Wang N., Kong W., Wang X. ADAMTS-7 mediates vascular smooth muscle cell migration and neointima formation in balloon-injured rat arteries. Circ. Res. 2009;104:688–698. doi: 10.1161/CIRCRESAHA.108.188425. [DOI] [PubMed] [Google Scholar]
- 103.Gao A.G., Lindberg F.P., Finn M.B., Blystone S.D., Brown E.J., Frazier W.A. Integrin-associated protein is a receptor for the C-terminal domain of thrombospondin. J. Biol. Chem. 1996;271:21–24. doi: 10.1074/jbc.271.1.21. [DOI] [PubMed] [Google Scholar]
- 104.Yao M., Roberts D.D., Isenberg J.S. Thrombospondin-1 inhibition of vascular smooth muscle cell responses occurs via modulation of both cAMP and cGMP. Pharmacol. Res. 2011;63:13–22. doi: 10.1016/j.phrs.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Isenberg J.S., Frazier W.A., Krishna M.C., Wink D.A., Roberts D.D. Enhancing cardiovascular dynamics by inhibition of thrombospondin-1/CD47 signaling. Curr. Drug Targets. 2008;9:833–841. doi: 10.2174/138945008785909338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Isenberg J.S., Qin Y., Maxhimer J.B., Sipes J.M., Despres D., Schnermann J., Frazier W.A., Roberts D.D. Thrombospondin-1 and CD47 regulate blood pressure and cardiac responses to vasoactive stress. Matrix Biol. 2009;28:110–119. doi: 10.1016/j.matbio.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bauer E.M., Qin Y., Miller T.W., Bandle R.W., Csanyi G., Pagano P.J., Bauer P.M., Schnermann J., Roberts D.D., Isenberg J.S. Thrombospondin-1 supports blood pressure by limiting eNOS activation and endothelial-dependent vasorelaxation. Cardiovasc. Res. 2010;88:471–481. doi: 10.1093/cvr/cvq218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Soto-Pantoja D.R., Kaur S., Roberts D.D. CD47 signaling pathways controlling cellular differentiation and responses to stress. Crit. Rev. Biochem. Mol. Biol. 2015;50:212–230. doi: 10.3109/10409238.2015.1014024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Roberts D.D., Miller T.W., Rogers N.M., Yao M., Isenberg J.S. The matricellular protein thrombospondin-1 globally regulates cardiovascular function and responses to stress via CD47. Matrix Biol. 2012;31:162–169. doi: 10.1016/j.matbio.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yao M., Rogers N.M., Csányi G., Rodriguez A.I., Ross M.A., St Croix C., Knupp H., Novelli E.M., Thomson A.W., Pagano P.J., et al. Thrombospondin-1 activation of signal-regulatory protein-α stimulates reactive oxygen species production and promotes renal ischemia reperfusion injury. J. Am. Soc. Nephrol. 2014;25:1171–1186. doi: 10.1681/ASN.2013040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tong X., Khandelwal A.R., Qin Z., Wu X., Chen L., Ago T., Sadoshima J., Cohen R.A. Role of smooth muscle Nox4-based NADPH oxidase in neointimal hyperplasia. J. Mol. Cell Cardiol. 2015;89:185–194. doi: 10.1016/j.yjmcc.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 112.Tong X., Khandelwal A.R., Wu X., Xu Z., Yu W., Chen C., Zhao W., Yang J., Qin Z., Weisbrod R.M., et al. Pro-atherogenic role of smooth muscle Nox4-based NADPH oxidase. J. Mol. Cell Cardiol. 2016;92:30–40. doi: 10.1016/j.yjmcc.2016.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Csányi G., Feck D.M., Ghoshal P., Singla B., Lin H., Nagarajan S., Meijles D.N., Al Ghouleh I., Cantu-Medellin N., Kelley E.E., et al. CD47 and Nox1 mediate dynamic fluid-phase macropinocytosis of native LDL. Antioxid. Redox. Signal. 2017;26:886–901. doi: 10.1089/ars.2016.6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tiyyagura S.R., Pinney S.P. Left ventricular remodeling after myocardial infarction: Past, present, and future. Mt. Sinai J. Med. 2006;73:840–851. [PubMed] [Google Scholar]
- 115.Buja L.M., Vela D. Cardiomyocyte death and renewal in the normal and diseased heart. Cardiovasc Pathol. 2008;17:349–374. doi: 10.1016/j.carpath.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 116.Jugdutt B.I. Ventricular remodeling after infarction and the extracellular collagen matrix: When is enough enough? Circulation. 2003;108:1395–1403. doi: 10.1161/01.CIR.0000085658.98621.49. [DOI] [PubMed] [Google Scholar]
- 117.Chistiakov D.A., Orekhov A.N., Bobryshev Y.V. The role of cardiac fibroblasts in post-myocardial heart tissue repair. Exp. Mol. Pathol. 2016;101:231–240. doi: 10.1016/j.yexmp.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 118.Holmes J.W., Borg T.K., Covell J.W. Structure and mechanics of healing myocardial infarcts. Annu. Rev. Biomed. Eng. 2005;7:223–253. doi: 10.1146/annurev.bioeng.7.060804.100453. [DOI] [PubMed] [Google Scholar]
- 119.Lamy L., Ticchioni M., Rouquette-Jazdanian A.K., Samson M., Deckert M., Greenberg A.H., Bernard A. CD47 and the 19 kDa interacting protein-3 (BNIP3) in T cell apoptosis. J. Biol. Chem. 2003;278:23915–23921. doi: 10.1074/jbc.M301869200. [DOI] [PubMed] [Google Scholar]
- 120.Lamy L., Foussat A., Brown E.J., Bornstein P., Ticchioni M., Bernard A. Interactions between CD47 and thrombospondin reduce inflammation. J. Immunol. 2007;178:5930–5939. doi: 10.4049/jimmunol.178.9.5930. [DOI] [PubMed] [Google Scholar]
- 121.Isenberg J.S., Roberts D.D., Frazier W.A. CD47: A new target in cardiovascular therapy. Arterioscler. Thromb. Vasc. Biol. 2008;28:615–621. doi: 10.1161/ATVBAHA.107.158154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Isenberg J.S., Hyodo F., Pappan L.K., Abu-Asab M., Tsokos M., Krishna M.C., Frazier W.A., Roberts D.D. Blocking thrombospondin-1/CD47 signaling alleviates deleterious effects of aging on tissue responses to ischemia. Arterioscler. Thromb. Vasc. Biol. 2007;27:2582–2588. doi: 10.1161/ATVBAHA.107.155390. [DOI] [PubMed] [Google Scholar]
- 123.Mirochnik Y., Kwiatek A., Volpert O.V. Thrombospondin and apoptosis: Molecular mechanisms and use for design of complementation treatments. Curr. Drug Targets. 2008;9:851–862. doi: 10.2174/138945008785909347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Krady M.M., Zeng J., Yu J., MacLauchlan S., Skokos E.A., Tian W., Bornstein P., Sessa W.C., Kyriakides T.R. Thrombospondin-2 modulates extracellular matrix remodeling during physiological angiogenesis. Am. J. Pathol. 2008;173:879–891. doi: 10.2353/ajpath.2008.080128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Maclauchlan S., Skokos E.A., Agah A., Zeng J., Tian W., Davidson J.M., Bornstein P., Kyriakides T.R. Enhanced angiogenesis and reduced contraction in thrombospondin-2-null wounds is associated with increased levels of matrix metalloproteinases-2 and -9, and soluble VEGF. J. Histochem. Cytochem. 2009;57:301–313. doi: 10.1369/jhc.2008.952689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kyriakides T.R., Zhu Y.H., Smith L.T., Bain S.D., Yang Z., Lin M.T., Danielson K.G., Iozzo R.V., LaMarca M., McKinney C.E., et al. Mice that lack thrombospondin 2 display connective tissue abnormalities that are associated with disordered collagen fibrillogenesis, an increased vascular density, and a bleeding diathesis. J. Cell Biol. 1998;140:419–430. doi: 10.1083/jcb.140.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kyriakides T.R., Leach K.J., Hoffman A.S., Ratner B.D., Bornstein P. Mice that lack the angiogenesis inhibitor, thrombospondin 2, mount an altered foreign body reaction characterized by increased vascularity. Proc. Natl. Acad. Sci. USA. 1999;96:4449–4454. doi: 10.1073/pnas.96.8.4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bornstein P., Kyriakides T.R., Yang Z., Armstrong L.C., Birk D.E. Thrombospondin 2 modulates collagen fibrillogenesis and angiogenesis. J. Investig. Dermatol. Symp. Proc. 2000;5:61–66. doi: 10.1046/j.1087-0024.2000.00005.x. [DOI] [PubMed] [Google Scholar]
- 129.Van Oorschot A.A., Smits A.M., Pardali E., Doevendans P.A., Goumans M.J. Low oxygen tension positively influences cardiomyocyte progenitor cell function. J. Cell Mol. Med. 2011;15:2723–2734. doi: 10.1111/j.1582-4934.2011.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mustonen E., Leskinen H., Aro J., Luodonpää M., Vuolteenaho O., Ruskoaho H., Rysä J. Metoprolol treatment lowers thrombospondin-4 expression in rats with myocardial infarction and left ventricular hypertrophy. Basic Clin. Pharmacol. Toxicol. 2010;107:709–717. doi: 10.1111/j.1742-7843.2010.00564.x. [DOI] [PubMed] [Google Scholar]
- 131.Brody M.J., Schips T.G., Vanhoutte D., Kanisicak O., Karch J., Maliken B.D., Blair N.S., Sargent M.A., Prasad V., Molkentin J.D. Dissection of thrombospondin-4 domains involved in intracellular adaptive endoplasmic reticulum stress-responsive signaling. Mol. Cell Biol. 2015;36:2–12. doi: 10.1128/MCB.00607-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jin J.K., Blackwood E.A., Azizi K., Thuerauf D.J., Fahem A.G., Hofmann C., Kaufman R.J., Doroudgar S., Glembotski C.C. ATF6 decreases myocardial ischemia/reperfusion damage and links ER stress and oxidative stress signaling pathways in the heart. Circ. Res. 2017;120:862–875. doi: 10.1161/CIRCRESAHA.116.310266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Burke A., Creighton W., Tavora F., Li L., Fowler D. Decreased frequency of the 3′UTR T>G single nucleotide polymorphism of thrombospondin-2 gene in sudden death due to plaque erosion. Cardiovasc. Pathol. 2010;19:e45–e49. doi: 10.1016/j.carpath.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 134.Farb A., Burke A.P., Tang A.L., Liang T.Y., Mannan P., Smialek J., Virmani R. Coronary plaque erosion without rupture into a lipid core. A frequent cause of coronary thrombosis in sudden coronary death. Circulation. 1996;93:1354–1363. doi: 10.1161/01.CIR.93.7.1354. [DOI] [PubMed] [Google Scholar]
- 135.Koch W., Hoppmann P., de Waha A., Schömig A., Kastrati A. Polymorphisms in thrombospondin genes and myocardial infarction: A case-control study and a meta-analysis of available evidence. Hum. Mol. Genet. 2008;17:1120–1126. doi: 10.1093/hmg/ddn001. [DOI] [PubMed] [Google Scholar]
- 136.Ashokkumar M., Anbarasan C., Saibabu R., Kuram S., Raman S.C., Cherian K.M. An association study of thrombospondin 1 and 2 SNPs with coronary artery disease and myocardial infarction among South Indians. Thromb. Res. 2011;128:e49–e53. doi: 10.1016/j.thromres.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 137.Zhou X., Huang J., Chen J., Zhao J., Yang W., Wang X., Gu D. Thrombospondin-4 A387P polymorphism is not associated with coronary artery disease and myocardial infarction in the Chinese Han population. Clin. Sci. 2004;106:495–500. doi: 10.1042/CS20030322. [DOI] [PubMed] [Google Scholar]
- 138.Zhou X., Huang J., Chen J., Zhao J., Ge D., Yang W., Gu D. Genetic association analysis of myocardial infarction with thrombospondin-1 N700S variant in a Chinese population. Thromb. Res. 2004;113:181–186. doi: 10.1016/j.thromres.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 139.Burlew B.S., Weber K.T. Connective tissue and the heart. Functional significance and regulatory mechanisms. Cardiol. Clin. 2000;18:435–442. doi: 10.1016/S0733-8651(05)70154-5. [DOI] [PubMed] [Google Scholar]
- 140.Fomovsky G.M., Thomopoulos S., Holmes J.W. Contribution of extracellular matrix to the mechanical properties of the heart. J. Mol. Cell Cardiol. 2010;48:490–496. doi: 10.1016/j.yjmcc.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Spinale F.G. Matrix metalloproteinases: Regulation and dysregulation in the failing heart. Circ. Res. 2002;90:520–530. doi: 10.1161/01.RES.0000013290.12884.A3. [DOI] [PubMed] [Google Scholar]
- 142.Dorn G.W., 2nd Apoptotic and non-apoptotic programmed cardiomyocyte death in ventricular remodelling. Cardiovasc. Res. 2009;81:465–473. doi: 10.1093/cvr/cvn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xia Y., Lee K., Li N., Corbett D., Mendoza L., Frangogiannis N.G. Characterization of the inflammatory and fibrotic response in a mouse model of cardiac pressure overload. Histochem. Cell Biol. 2009;131:471–481. doi: 10.1007/s00418-008-0541-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xia Y., Dobaczewski M., Gonzalez-Quesada C., Chen W., Biernacka A., Li N., Lee D.W., Frangogiannis N.G. Endogenous thrombospondin 1 protects the pressure-overloaded myocardium by modulating fibroblast phenotype and matrix metabolism. Hypertension. 2011;58:902–911. doi: 10.1161/HYPERTENSIONAHA.111.175323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rohini A., Agrawal N., Koyani C.N., Singh R. Molecular targets and regulators of cardiac hypertrophy. Pharmacol. Res. 2010;61:269–280. doi: 10.1016/j.phrs.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 146.Malek M.H., Olfert I.M. Global deletion of thrombospondin-1 increases cardiac and skeletal muscle capillarity and exercise capacity in mice. Exp. Physiol. 2009;94:749–760. doi: 10.1113/expphysiol.2008.045989. [DOI] [PubMed] [Google Scholar]
- 147.Moens A.L., Cingolani O., Arkenbout E., Kass D.A. Exacerbated cardiac remodeling to pressure-overload in mice lacking thrombospondin-4. Eur. J. Heart Fail. 2008;7:149–150. doi: 10.1016/S1567-4215(08)60419-1. [DOI] [Google Scholar]
- 148.Bronzwaer J.G., Paulus W.J. Diastolic and systolic heart failure: Different stages or distinct phenotypes of the heart failure syndrome? Curr. Heart Fail. Rep. 2009;6:281–286. doi: 10.1007/s11897-009-0038-0. [DOI] [PubMed] [Google Scholar]
- 149.Chatterjee K., Massie B. Systolic and diastolic heart failure: Differences and similarities. J. Card. Fail. 2007;13:569–576. doi: 10.1016/j.cardfail.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 150.Diwan A., Dorn G.W., 2nd Decompensation of cardiac hypertrophy: Cellular mechanisms and novel therapeutic targets. Physiology. 2007;22:56–64. doi: 10.1152/physiol.00033.2006. [DOI] [PubMed] [Google Scholar]
- 151.Nishida K., Otsu K. Cell death in heart failure. Circ. J. 2008;72:A17–A21. doi: 10.1253/circj.CJ-08-0669. [DOI] [PubMed] [Google Scholar]
- 152.Shan K., Kurrelmeyer K., Seta Y., Wang F., Dibbs Z., Deswal A., Lee-Jackson D., Mann D.L. The role of cytokines in disease progression in heart failure. Curr. Opin. Cardiol. 1997;12:218–223. doi: 10.1097/00001573-199705000-00002. [DOI] [PubMed] [Google Scholar]
- 153.Batlle M., Pérez-Villa F., Lázaro A., García-Pras E., Vallejos I., Sionis A., Castel M.A., Roig E. Decreased expression of thrombospondin-1 in failing hearts may favor ventricular remodeling. Transplant. Proc. 2009;41:2231–2233. doi: 10.1016/j.transproceed.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 154.Muñoz-Pacheco P., Ortega-Hernández A., Caro-Vadillo A., Casanueva-Eliceiry S., Aragoncillo P., Egido J., Fernández-Cruz A., Gómez-Garre D. Eplerenone enhances cardioprotective effects of standard heart failure therapy through matricellular proteins in hypertensive heart failure. J. Hypertens. 2013;31:2309–2319. doi: 10.1097/HJH.0b013e328364abd6. [DOI] [PubMed] [Google Scholar]
- 155.Sharifi-Sanjani M., Shoushtari A.H., Quiroz M., Baust J., Sestito S.F., Mosher M., Ross M., McTiernan C.F., St Croix C.M., Bilonick R.A., et al. Cardiac CD47 drives left ventricular heart failure through Ca2+-CaMKII-regulated induction of HDAC3. J. Am. Heart Assoc. 2014;3:e000670. doi: 10.1161/JAHA.113.000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Van Almen G.C., Verhesen W., Van Leeuwen R.E., Van de Vrie M., Eurlings C., Schellings M.W., Swinnen M., Cleutjens J.P., Van Zandvoort M.A., Heymans S., et al. MicroRNA-18 and microRNA-19 regulate CTGF and TSP-1 expression in age-related heart failure. Aging Cell. 2011;10:769–779. doi: 10.1111/j.1474-9726.2011.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Papageorgiou A.P., Swinnen M., Vanhoutte D., VandenDriessche T., Chuah M., Lindner D., Verhesen W., De Vries B., D’hooge J., Lutgens E., et al. Thrombospondin-2 prevents cardiac injury and dysfunction in viral myocarditis through the activation of regulatory T-cells. Cardiovasc. Res. 2012;94:115–124. doi: 10.1093/cvr/cvs077. [DOI] [PubMed] [Google Scholar]
- 158.Rysä J., Leskinen H., Ilves M., Ruskoaho H. Distinct upregulation of extracellular matrix genes in transition from hypertrophy to hypertensive heart failure. Hypertension. 2005;45:927–933. doi: 10.1161/01.HYP.0000161873.27088.4c. [DOI] [PubMed] [Google Scholar]
- 159.Melenovsky V., Benes J., Skaroupkova P., Sedmera D., Strnad H., Kolar M., Vlcek C., Petrak J., Benes J., Jr., Papousek F., et al. Metabolic characterization of volume overload heart failure due to aorto-caval fistula in rats. Mol. Cell Biochem. 2011;354:83–96. doi: 10.1007/s11010-011-0808-3. [DOI] [PubMed] [Google Scholar]
- 160.Yetkin E., Waltenberger J. Molecular and cellular mechanisms of aortic stenosis. Int. J. Cardiol. 2009;135:4–13. doi: 10.1016/j.ijcard.2009.03.108. [DOI] [PubMed] [Google Scholar]
- 161.Rawat D.K., Alzoubi A., Gupte R., Chettimada S., Watanabe M., Kahn A.G., Okada T., McMurtry I.F., Gupte S.A. Increased reactive oxygen species, metabolic maladaptation, and autophagy contribute to pulmonary arterial hypertension-induced ventricular hypertrophy and diastolic heart failure. Hypertension. 2014;64:1266–1274. doi: 10.1161/HYPERTENSIONAHA.114.03261. [DOI] [PubMed] [Google Scholar]
- 162.Sage E., Mercier O., Van den Eyden F., De Perrot M., Barlier-Mur A.M., Dartevelle P., Eddahibi S., Herve P., Fadel E. Endothelial cell apoptosis in chronically obstructed and reperfused pulmonary artery. Respir. Res. 2008;9:19. doi: 10.1186/1465-9921-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Imoto K., Okada M., Yamawaki H. Expression profile of matricellular proteins in hypertrophied right ventricle of monocrotaline-induced pulmonary hypertensive rats. J. Vet. Med. Sci. 2017;79:1096–1102. doi: 10.1292/jvms.17-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kaiser R., Frantz C., Bals R., Wilkens H. The role of circulating thrombospondin-1 in patients with precapillary pulmonary hypertension. Respir. Res. 2016;17:96. doi: 10.1186/s12931-016-0412-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ochoa C.D., Yu L., Al-Ansari E., Hales C.A., Quinn D.A. Thrombospondin-1 null mice are resistant to hypoxia-induced pulmonary hypertension. J. Cardiothorac. Surg. 2010;5:32. doi: 10.1186/1749-8090-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kumar R., Mickael C., Kassa B., Gebreab L., Robinson J.C., Koyanagi D.E., Sanders L., Barthel L., Meadows C., Fox D., et al. TGF-β activation by bone marrow-derived thrombospondin-1 causes Schistosoma- and hypoxia-induced pulmonary hypertension. Nat. Commun. 2017;8:15494. doi: 10.1038/ncomms15494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Green D.E., Kang B.Y., Murphy T.C., Hart C.M. Peroxisome proliferator-activated receptor gamma (PPARγ) regulates thrombospondin-1 and Nox4 expression in hypoxia-induced human pulmonary artery smooth muscle cell proliferation. Pulm. Circ. 2012;2:483–491. doi: 10.4103/2045-8932.105037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cucoranu I., Clempus R., Dikalova A., Phelan P.J., Ariyan S., Dikalov S., Sorescu D. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ. Res. 2005;97:900–907. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 169.Sturrock A., Cahill B., Norman K., Huecksteadt T.P., Hill K., Sanders K., Karwande S.V., Stringham J.C., Bull D.A., Gleich M., Kennedy T.P., Hoidal J.R. Transforming growth factor-beta1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;290:L661–L673. doi: 10.1152/ajplung.00269.2005. [DOI] [PubMed] [Google Scholar]
- 170.Ismail S., Sturrock A., Wu P., Cahill B., Norman K., Huecksteadt T., Sanders K., Kennedy T., Hoidal J. NOX4 mediates hypoxia-induced proliferation of human pulmonary artery smooth muscle cells: The role of autocrine production of transforming growth factor-β-1 and insulin-like growth factor binding protein-3. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;296:L489–L499. doi: 10.1152/ajplung.90488.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lu X., Murphy T.C., Nanes M.S., Hart C.M. PPARγ regulates hypoxia-induced Nox4 expression in human pulmonary artery smooth muscle cells through NF-κB. Am. J. Physiol. Lung Cell Mol. Physiol. 2010;299:L559–L566. doi: 10.1152/ajplung.00090.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lu X., Bijli K.M., Ramirez A., Murphy T.C., Kleinhenz J., Hart C.M. Hypoxia downregulates PPARγ via an ERK1/2-NF-κB-Nox4-dependent mechanism in human pulmonary artery smooth muscle cells. Free Radic. Biol. Med. 2013;63:151–160. doi: 10.1016/j.freeradbiomed.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lin T.N., Kim G.M., Chen J.J., Cheung W.M., He Y.Y., Hsu C.Y. Differential regulation of thrombospondin-1 and thrombospondin-2 after focal cerebral ischemia/reperfusion. Stroke. 2003;34:177–186. doi: 10.1161/01.STR.0000047100.84604.BA. [DOI] [PubMed] [Google Scholar]
- 174.Woo M.S., Yang J., Beltran C., Cho S. Cell surface CD36 protein in monocyte/macrophage contributes to phagocytosis during the resolution phase of ischemic stroke in mice. J. Biol. Chem. 2016;291:23654–23661. doi: 10.1074/jbc.M116.750018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liauw J., Hoang S., Choi M., Eroglu C., Choi M., Sun G.H., Percy M., Wildman-Tobriner B., Bliss T., Guzman R.G., Barres B.A., Steinberg G.K. Thrombospondins 1 and 2 are necessary for synaptic plasticity and functional recovery after stroke. J. Cereb. Blood Flow Metab. 2008;28:1722–1732. doi: 10.1038/jcbfm.2008.65. [DOI] [PubMed] [Google Scholar]
- 176.Jurk K., Jahn U.R., Van Aken H., Schriek C., Droste D.W., Ritter M.A., Bernd Ringelstein E., Kehrel B.E. Platelets in patients with acute ischemic stroke are exhausted and refractory to thrombin, due to cleavage of the seven-transmembrane thrombin receptor (PAR-1) Thromb. Haemost. 2004;91:334–344. doi: 10.1160/TH03-01-0044. [DOI] [PubMed] [Google Scholar]
- 177.Yesner L.M., Huh H.Y., Pearce S.F., Silverstein R.L. Regulation of monocyte CD36 and thrombospondin-1 expression by soluble mediators. Arterioscler. Thromb. Vasc. Biol. 1996;16:1019–1025. doi: 10.1161/01.ATV.16.8.1019. [DOI] [PubMed] [Google Scholar]
- 178.Buée L., Hof P.R., Roberts D.D., Delacourte A., Morrison J.H., Fillit H.M. Immunohistochemical identification of thrombospondin in normal human brain and in Alzheimer’s disease. Am. J. Pathol. 1992;141:783–788. [PMC free article] [PubMed] [Google Scholar]
- 179.Son S.M., Nam D.W., Cha M.Y., Kim K.H., Byun J., Ryu H., Mook-Jung I. Thrombospondin-1 prevents amyloid beta-mediated synaptic pathology in Alzheimer’s disease. Neurobiol. Aging. 2015;36:3214–3227. doi: 10.1016/j.neurobiolaging.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 180.Rama Rao K.V., Curtis K.M., Johnstone J.T., Norenberg M.D. Amyloid-β inhibits thrombospondin 1 release from cultured astrocytes: Effects on synaptic protein expression. J. Neuropathol. Exp. Neurol. 2013;72:735–744. doi: 10.1097/NEN.0b013e31829bd082. [DOI] [PubMed] [Google Scholar]
- 181.Garcia O., Torres M., Helguera P., Coskun P., Busciglio J. A role for thrombospondin-1 deficits in astrocyte-mediated spine and synaptic pathology in Down‘s syndrome. PLoS ONE. 2010;5:e14200. doi: 10.1371/journal.pone.0014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cheng C., Lau S.K., Doering L.C. Astrocyte-secreted thrombospondin-1 modulates synapse and spine defects in the fragile X mouse model. Mol. Brain. 2016;9:74. doi: 10.1186/s13041-016-0256-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ko C.Y., Chu Y.Y., Narumiya S., Chi J.Y., Furuyashiki T., Aoki T., Wang S.M., Chang W.C., Wang J.M. CCAAT/enhancer-binding protein δ/miR135a/thrombospondin 1 axis mediates PGE2-induced angiogenesis in Alzheimer’s disease. Neurobiol. Aging. 2015;36:1356–1368. doi: 10.1016/j.neurobiolaging.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 184.Long J., Liu S., Zeng X., Yang X., Huang H., Zhang Y., Chen J., Xu Y., Huang D., Qiu X. Population study confirms serum proteins’ change and reveals diagnostic values in congenital ventricular septal defect. Pediatr. Cardiol. 2017 doi: 10.1007/s00246-017-1641-6. in press. [DOI] [PubMed] [Google Scholar]
- 185.Wang J., Nagy A., Larsson J., Dudas M., Sucov H.M., Kaartinen V. Defective ALK5 signaling in the neural crest leads to increased postmigratory neural crest cell apoptosis and severe outflow tract defects. BMC Dev. Biol. 2006;6:51. doi: 10.1186/1471-213X-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gao Z., Kim G.H., Mackinnon A.C., Flagg A.E., Bassett B., Earley J.U., Svensson E.C. Ets1 is required for proper migration and differentiation of the cardiac neural crest. Development. 2010;137:1543–1551. doi: 10.1242/dev.047696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Fouladkou F., Lu C., Jiang C., Zhou L., She Y., Walls J.R., Kawabe H., Brose N., Henkelman R.M., Huang A., Bruneau B.G., Rotin D. The ubiquitin ligase Nedd4–1 is required for heart development and is a suppressor of thrombospondin-1. J. Biol. Chem. 2010;285:6770–6780. doi: 10.1074/jbc.M109.082347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yang B., Kumar S. Nedd4 and Nedd4–2: Closely related ubiquitin-protein ligases with distinct physiological functions. Cell Death Differ. 2010;17:68–77. doi: 10.1038/cdd.2009.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Murdaca J., Treins C., Monthouël-Kartmann M.N., Pontier-Bres R., Kumar S., Van Obberghen E., Giorgetti-Peraldi S. Grb10 prevents Nedd4-mediated vascular endothelial growth factor receptor-2 degradation. J. Biol. Chem. 2004;279:26754–26761. doi: 10.1074/jbc.M311802200. [DOI] [PubMed] [Google Scholar]
- 190.Van Bemmelen M.X., Rougier J.S., Gavillet B., Apothéloz F., Daidié D., Tateyama M., Rivolta I., Thomas M.A., Kass R.S., Staub O., et al. Cardiac voltage-gated sodium channel Nav1.5 is regulated by Nedd4–2 mediated ubiquitination. Circ. Res. 2004;95:284–291. doi: 10.1161/01.RES.0000136816.05109.89. [DOI] [PubMed] [Google Scholar]
- 191.Fotia A.B., Ekberg J., Adams D.J., Cook D.I., Poronnik P., Kumar S. Regulation of neuronal voltage-gated sodium channels by the ubiquitin-protein ligases Nedd4 and Nedd4–2. J. Biol. Chem. 2004;279:28930–28935. doi: 10.1074/jbc.M402820200. [DOI] [PubMed] [Google Scholar]
- 192.Moskowitz I.P., Pizard A., Patel V.V., Bruneau B.G., Kim J.B., Kupershmidt S., Roden D., Berul C.I., Seidman C.E., Seidman J.G. The T-Box transcription factor Tbx5 is required for the patterning and maturation of the murine cardiac conduction system. Development. 2004;131:4107–4116. doi: 10.1242/dev.01265. [DOI] [PubMed] [Google Scholar]
- 193.Murhy-Ullrich J.E., Sage E.H. Revisiting the matricellular concept. Matrix Biol. 2014;37:1–14. doi: 10.1016/j.matbio.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Marganski W.A., Gangopadhyay S.S., Je H.D., Gallant C., Morgan K.G. Targeting of a novel Ca+2/calmodulin-dependent protein kinase II is essential for extracellular signal-regulated kinase-mediated signaling in differentiated smooth muscle cells. Circ. Res. 2005;97:541–549. doi: 10.1161/01.RES.0000182630.29093.0d. [DOI] [PubMed] [Google Scholar]
- 195.Guo Y., Zhang Z., Wu H.E., Luo Z.D., Hogan Q.H., Pan B. Increased thrombospondin-4 after nerve injury mediates disruption of intracellular calcium signaling in primary sensory neurons. Neuropharmacology. 2017;117:292–304. doi: 10.1016/j.neuropharm.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Patra D., Sandell L.J. Antiangiogenic and anticancer molecules in cartilage. Expert Rev. Mol. Med. 2012;14:e10. doi: 10.1017/erm.2012.3. [DOI] [PubMed] [Google Scholar]
- 197.Rusnati M., Urbinati C., Bonifacio S., Presta M., Taraboletti G. Thrombospondin-1 as a paradigm for the development of antiangiogenic agents endowed with multiple mechanisms of action. Pharmaceuticals. 2010;3:1241–1278. doi: 10.3390/ph3041241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Henkin J., Volpert O.V. Therapies using anti-angiogenic peptide mimetics of thrombospondin-1. Expert Opin. Ther. Targets. 2011;15:1369–1386. doi: 10.1517/14728222.2011.640319. [DOI] [PubMed] [Google Scholar]
- 199.Dawson D.W., Volpert O.V., Pearce S.F., Schneider A.J., Silverstein R.L., Henkin J., Bouck N.P. Three distinct D-amino acid substitutions confer potent antiangiogenic activity on an inactive peptide derived from a thrombospondin-1 type 1 repeat. Mol. Pharmacol. 1999;55:332–338. doi: 10.1124/mol.55.2.332. [DOI] [PubMed] [Google Scholar]
- 200.Westphal J.R. Technology evaluation: ABT-510, Abbott. Curr. Opin. Mol. Ther. 2004;6:451–457. [PubMed] [Google Scholar]
- 201.Amato R.J. Renal cell carcinoma: Review of novel single-agent therapeutics and combination regimens. Ann. Oncol. 2005;16:7–15. doi: 10.1093/annonc/mdi002. [DOI] [PubMed] [Google Scholar]
- 202.Hoekstra R., de Vos F.Y., Eskens F.A., Gietema J.A., Van der Gaast A., Groen H.J., Knight R.A., Carr R.A., Humerickhouse R.A., Verweij J., et al. Phase I safety, pharmacokinetic, and pharmacodynamic study of the thrombospondin-1-mimetic angiogenesis inhibitor ABT-510 in patients with advanced cancer. J. Clin. Oncol. 2005;23:5188–5197. doi: 10.1200/JCO.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 203.Hoekstra R., de Vos F.Y., Eskens F.A., de Vries E.G., Uges D.R., Knight R., Carr R.A., Humerickhouse R., Verweij J., Gietema J.A. Phase I study of the thrombospondin-1-mimetic angiogenesis inhibitor ABT-510 with 5-fluorouracil and leucovorin: A safe combination. Eur. J. Cancer. 2006;42:467–472. doi: 10.1016/j.ejca.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 204.Gietema J.A., Hoekstra R., De Vos F.Y., Uges D.R., Van der Gaast A., Groen H.J., Loos W.J., Knight R.A., Carr R.A., Humerickhouse R.A., et al. A phase I study assessing the safety and pharmacokinetics of the thrombospondin-1-mimetic angiogenesis inhibitor ABT-510 with gemcitabine and cisplatin in patients with solid tumors. Ann. Oncol. 2006;17:1320–1327. doi: 10.1093/annonc/mdl102. [DOI] [PubMed] [Google Scholar]
- 205.Gordon M.S., Mendelson D., Carr R., Knight R.A., Humerickhouse R.A., Iannone M., Stopeck A.T. A phase 1 trial of 2 dose schedules of ABT-510, an antiangiogenic, thrombospondin-1-mimetic peptide, in patients with advanced cancer. Cancer. 2008;113:3420–3429. doi: 10.1002/cncr.23953. [DOI] [PubMed] [Google Scholar]
- 206.Nabors L.B., Fiveash J.B., Markert J.M., Kekan M.S., Gillespie G.Y., Huang Z., Johnson M.J., Meleth S., Kuo H., Gladson C.L., et al. A phase 1 trial of ABT-510 concurrent with standard chemoradiation for patients with newly diagnosed glioblastoma. Arch. Neurol. 2010;67:313–319. doi: 10.1001/archneurol.2010.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Uronis H.E., Cushman S.M., Bendell J.C., Blobe G.C., Morse M.A., Nixon A.B., Dellinger A., Starr M.D., Li H., Meadows K., et al. A phase I study of ABT-510 plus bevacizumab in advanced solid tumors. Cancer Med. 2013;2:316–324. doi: 10.1002/cam4.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Markovic S.N., Suman V.J., Rao R.A., Ingle J.N., Kaur J.S., Erickson L.A., Pitot H.C., Croghan G.A., McWilliams R.R., Merchan J., et al. A phase II study of ABT-510 (thrombospondin-1 analog) for the treatment of metastatic melanoma. Am. J. Clin. Oncol. 2007;30:303–309. doi: 10.1097/01.coc.0000256104.80089.35. [DOI] [PubMed] [Google Scholar]
- 209.Ebbinghaus S., Hussain M., Tannir N., Gordon M., Desai A.A., Knight R.A., Humerickhouse R.A., Qian J., Gordon G.B., Figlin R. Phase 2 study of ABT-510 in patients with previously untreated advanced renal cell carcinoma. Clin. Cancer Res. 2007;13:6689–6695. doi: 10.1158/1078-0432.CCR-07-1477. [DOI] [PubMed] [Google Scholar]
- 210.Baker L.H., Rowinsky E.K., Mendelson D., Humerickhouse R.A., Knight R.A., Qian J., Carr R.A., Gordon G.B., Demetri G.D. Randomized, phase II study of the thrombospondin-1-mimetic angiogenesis inhibitor ABT-510 in patients with advanced soft tissue sarcoma. J. Clin. Oncol. 2008;26:5583–5588. doi: 10.1200/JCO.2008.17.4706. [DOI] [PubMed] [Google Scholar]
- 211.Isenberg J.S., Romeo M., Abu-Asab M., Tsokos M., Oldenborg A., Pappan L., Wink D.A., Frazier W.A., Roberts D.D. Increasing survival of ischemic tissue by targeting CD47. Circ. Res. 2007;100:712–720. doi: 10.1161/01.RES.0000259579.35787.4e. [DOI] [PubMed] [Google Scholar]
- 212.Liu J., Wang L., Zhao F., Tseng S., Narayanan C., Shura L., Willingham S., Howard M., Prohaska S., Volkmer J., et al. Pre-clinical development of a humanized anti-CD47 antibody with anti-cancer therapeutic potential. PLoS ONE. 2015;10:e0137345. doi: 10.1371/journal.pone.0137345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Naujokat C. Monoclonal antibodies against human cancer stem cells. Immunotherapy. 2014;6:290–308. doi: 10.2217/imt.14.4. [DOI] [PubMed] [Google Scholar]
- 214.Ribeiro S.M., Poczatek M., Schultz-Cherry S., Villain M., Murphy-Ullrich J.E. The activation sequence of thrombospondin-1 interacts with the latency-associated peptide to regulate activation of latent transforming growth factor-beta. J. Biol. Chem. 1999;274:13586–13593. doi: 10.1074/jbc.274.19.13586. [DOI] [PubMed] [Google Scholar]
- 215.Murphy-Ullrich J.E., Poczatek M. Activation of latent TGF-β by thrombospondin-1: Mechanisms and physiology. Cytokine Growth Factor Rev. 2000;11:59–69. doi: 10.1016/S1359-6101(99)00029-5. [DOI] [PubMed] [Google Scholar]
- 216.Kondou H., Mushiake S., Etani Y., Miyoshi Y., Michigami T., Ozono K. A blocking peptide for transforming growth factor-β1 activation prevents hepatic fibrosis in vivo. J. Hepatol. 2003;39:742–748. doi: 10.1016/S0168-8278(03)00377-5. [DOI] [PubMed] [Google Scholar]
- 217.Xie X.S., Li F.Y., Liu H.C., Deng Y., Li Z., Fan J.M. LSKL, a peptide antagonist of thrombospondin-1, attenuates renal interstitial fibrosis in rats with unilateral ureteral obstruction. Arch. Pharm. Res. 2010;33:275–284. doi: 10.1007/s12272-010-0213-6. [DOI] [PubMed] [Google Scholar]
- 218.Lu A., Miao M., Schoeb T.R., Agarwal A., Murphy-Ullrich J.E. Blockade of TSP1-dependent TGF-β activity reduces renal injury and proteinuria in a murine model of diabetic nephropathy. Am. J. Pathol. 2011;178:2573–2586. doi: 10.1016/j.ajpath.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Liao F., Li G., Yuan W., Chen Y., Zuo Y., Rashid K., Zhang J.H., Feng H., Liu F. LSKL peptide alleviates subarachnoid fibrosis and hydrocephalus by inhibiting TSP1-mediated TGF-β1 signaling activity following subarachnoid hemorrhage in rats. Exp. Ther. Med. 2016;12:2537–2543. doi: 10.3892/etm.2016.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Krishna S.M., Seto S.W., Jose R.J., Biros E., Moran C.S., Wang Y., Clancy P., Golledge J. A peptide antagonist of thrombospondin-1 promotes abdominal aortic aneurysm progression in the angiotensin II-infused apolipoprotein-E-deficient mouse. Arterioscler. Thromb. Vasc. Biol. 2015;35:389–398. doi: 10.1161/ATVBAHA.114.304732. [DOI] [PubMed] [Google Scholar]
- 221.Liu X., Zhao Y., Gao J., Pawlyk B., Starcher B., Spencer J.A., Yanagisawa H., Zuo J., Li T. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat. Genet. 2004;36:178–182. doi: 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- 222.Hao M., Wang R., Wang W. Cell therapies in cardiomyopathy: Current status of clinical trials. Anal. Cell Pathol. 2017;2017:9404057. doi: 10.1155/2017/9404057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Cointe S., Rhéaume É., Martel C., Blanc-Brude O., Dubé E., Sabatier F., Dignat-George F., Tardif J.C., Bonnefoy A. Thrombospondin-1-derived peptide RFYVVMWK improves the adhesive phenotype of CD34+ cells from atherosclerotic patients with type 2 diabetes. Cell Transplant. 2017;26:327–337. doi: 10.3727/096368916X693329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Efimenko A., Starostina E., Kalinina N., Stolzing A. Angiogenic properties of aged adipose derived mesenchymal stem cells after hypoxic conditioning. J. Transl. Med. 2011;9:10. doi: 10.1186/1479-5876-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Noiseux N., Borie M., Desnoyers A., Menaouar A., Stevens L.M., Mansour S., Danalache B.A., Roy D.C., Jankowski M., Gutkowska J. Preconditioning of stem cells by oxytocin to improve their therapeutic potential. Endocrinology. 2012;153:5361–5372. doi: 10.1210/en.2012-1402. [DOI] [PubMed] [Google Scholar]
- 226.Lindsey M.L., Iyer R.P., Zamilpa R., Yabluchanskiy A., DeLeon-Pennell K.Y., Hall M.E., Kaplan A., Zouein F.A., Bratton D., Flynn E.R., Cannon P.L., et al. A novel collagen matricryptin reduces left ventricular dilation post-myocardial infarction by promoting scar formation and angiogenesis. J. Am. Coll. Cardiol. 2015;66:1364–1374. doi: 10.1016/j.jacc.2015.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Bein K., Simons M. Thrombospondin type 1 repeats interact with matrix metalloproteinase 2. Regulation of metalloproteinase activity. J. Biol. Chem. 2000;275:32167–32173. doi: 10.1074/jbc.M003834200. [DOI] [PubMed] [Google Scholar]