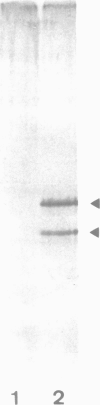
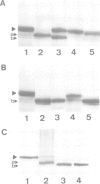
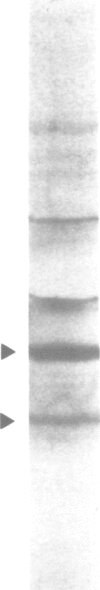
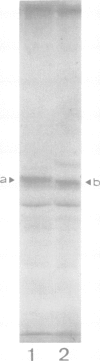
Abstract
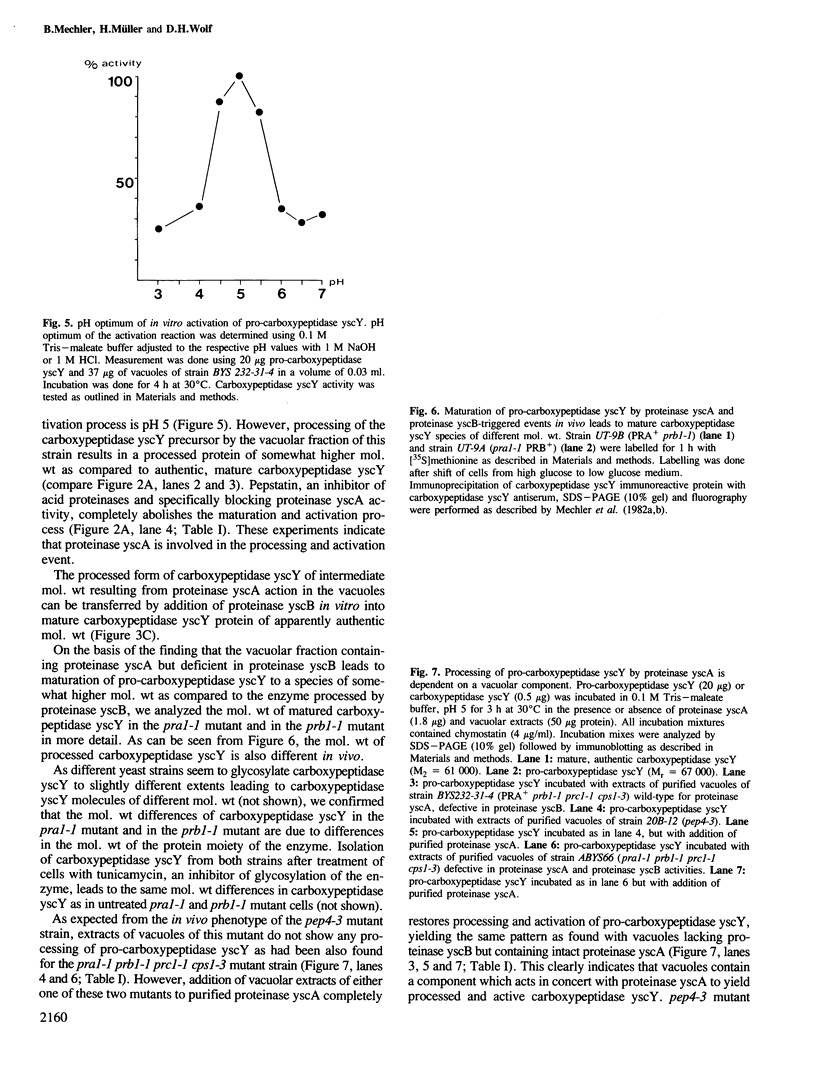
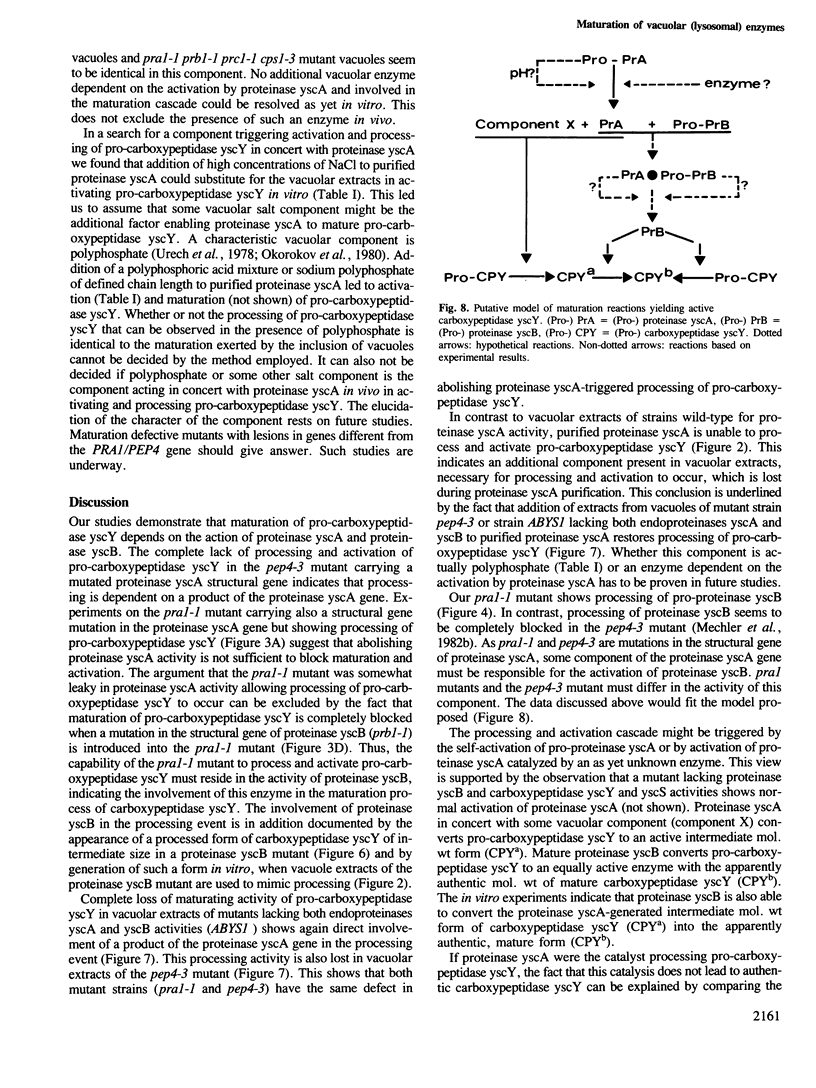
Studies were performed to unravel the activation and maturation mechanism of vacuolar (lysosomal) proteinases in Saccharomyces cerevisiae. In vivo and in vitro studies show that proteinase yscA and proteinase yscB are involved in the activation and processing event of pro-carboxypeptidase yscY. Processing and activation of pro-carboxypeptidase yscY by proteinase yscA depends on an additional factor contained in the vacuolar fraction. Comparable activation can be mimicked by sodium polyphosphate. Optimum pH for processing by this proteinase yscA-triggered event is 5. The proteinase yscA-triggered maturation process of pro-carboxypeptidase yscY leads to an intermediate mol. wt form of the enzyme which is, however, fully active. Proteinase yscB transfers the intermediate mol. wt form of the original precursor to the apparently authentic, mature and active carboxypeptidase yscY. An activation and maturation scheme is devised.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achstetter T., Emter O., Ehmann C., Wolf D. H. Proteolysis in eukaryotic cells. Identification of multiple proteolytic enzymes in yeast. J Biol Chem. 1984 Nov 10;259(21):13334–13343. [PubMed] [Google Scholar]
- Achstetter T., Wolf D. H. Hormone processing and membrane-bound proteinases in yeast. EMBO J. 1985 Jan;4(1):173–177. doi: 10.1002/j.1460-2075.1985.tb02333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achstetter T., Wolf D. H. Proteinases, proteolysis and biological control in the yeast Saccharomyces cerevisiae. Yeast. 1985 Dec;1(2):139–157. doi: 10.1002/yea.320010203. [DOI] [PubMed] [Google Scholar]
- Ammerer G., Hunter C. P., Rothman J. H., Saari G. C., Valls L. A., Stevens T. H. PEP4 gene of Saccharomyces cerevisiae encodes proteinase A, a vacuolar enzyme required for processing of vacuolar precursors. Mol Cell Biol. 1986 Jul;6(7):2490–2499. doi: 10.1128/mcb.6.7.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz H., Hinze H., Holzer H. Isolation and properties of two inhibitors of proteinase B from yeast. J Biol Chem. 1974 Jul 25;249(14):4515–4521. [PubMed] [Google Scholar]
- Hasilik A., Tanner W. Biosynthesis of the vacuolar yeast glycoprotein carboxypeptidase Y. Conversion of precursor into the enzyme. Eur J Biochem. 1978 Apr 17;85(2):599–608. doi: 10.1111/j.1432-1033.1978.tb12275.x. [DOI] [PubMed] [Google Scholar]
- Hemmings B. A., Zubenko G. S., Hasilik A., Jones E. W. Mutant defective in processing of an enzyme located in the lysosome-like vacuole of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1981 Jan;78(1):435–439. doi: 10.1073/pnas.78.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinze H., Betz H., Saheki T., Holzer H. Formation of a complex between yeast proteinases A and B. Hoppe Seylers Z Physiol Chem. 1975 Aug;356(8):1259–1264. doi: 10.1515/bchm2.1975.356.2.1259. [DOI] [PubMed] [Google Scholar]
- Jones E. W. Proteinase mutants of Saccharomyces cerevisiae. Genetics. 1977 Jan;85(1):23–33. doi: 10.1093/genetics/85.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E. W. The synthesis and function of proteases in Saccharomyces: genetic approaches. Annu Rev Genet. 1984;18:233–270. doi: 10.1146/annurev.ge.18.120184.001313. [DOI] [PubMed] [Google Scholar]
- Jones E. W., Zubenko G. S., Parker R. R. PEP4 gene function is required for expression of several vacuolar hydrolases in Saccharomyces cerevisiae. Genetics. 1982 Dec;102(4):665–677. doi: 10.1093/genetics/102.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kominami E., Hoffschulte H., Holzer H. Purification and properties of proteinase B from yeast. Biochim Biophys Acta. 1981 Sep 15;661(1):124–135. doi: 10.1016/0005-2744(81)90091-7. [DOI] [PubMed] [Google Scholar]
- Matile P., Wiemken A. The vacuole as the lysosome of the yeast cell. Arch Mikrobiol. 1967 Feb 20;56(2):148–155. doi: 10.1007/BF00408765. [DOI] [PubMed] [Google Scholar]
- Mechler B., Müller M., Müller H., Meussdoerffer F., Wolf D. H. In vivo biosynthesis of the vacuolar proteinases A and B in the yeast Saccharomyces cerevisiae. J Biol Chem. 1982 Oct 10;257(19):11203–11206. [PubMed] [Google Scholar]
- Mechler B., Müller M., Müller H., Wolf D. H. In vivo biosynthesis of vacuolar proteinases in proteinase mutants of Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1982 Aug;107(3):770–778. doi: 10.1016/0006-291x(82)90590-3. [DOI] [PubMed] [Google Scholar]
- Mechler B., Wolf D. H. Analysis of proteinase A function in yeast. Eur J Biochem. 1981 Dec;121(1):47–52. doi: 10.1111/j.1432-1033.1981.tb06427.x. [DOI] [PubMed] [Google Scholar]
- Okorokov L. A., Lichko L. P., Kulaev I. S. Vacuoles: main compartments of potassium, magnesium, and phosphate ions in Saccharomyces carlsbergenis cells. J Bacteriol. 1980 Nov;144(2):661–665. doi: 10.1128/jb.144.2.661-665.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. H., Hunter C. P., Valls L. A., Stevens T. H. Overproduction-induced mislocalization of a yeast vacuolar protein allows isolation of its structural gene. Proc Natl Acad Sci U S A. 1986 May;83(10):3248–3252. doi: 10.1073/pnas.83.10.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saheki T., Holzer H. Comparisons of the tryptophan synthase inactivating enzymes with proteinases from yeast. Eur J Biochem. 1974 Mar 1;42(2):621–626. doi: 10.1111/j.1432-1033.1974.tb03377.x. [DOI] [PubMed] [Google Scholar]
- Stevens T. H., Rothman J. H., Payne G. S., Schekman R. Gene dosage-dependent secretion of yeast vacuolar carboxypeptidase Y. J Cell Biol. 1986 May;102(5):1551–1557. doi: 10.1083/jcb.102.5.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens T., Esmon B., Schekman R. Early stages in the yeast secretory pathway are required for transport of carboxypeptidase Y to the vacuole. Cell. 1982 Sep;30(2):439–448. doi: 10.1016/0092-8674(82)90241-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urech K., Dürr M., Boller T., Wiemken A., Schwencke J. Localization of polyphosphate in vacuoles of Saccharomyces cerevisiae. Arch Microbiol. 1978 Mar;116(3):275–278. doi: 10.1007/BF00417851. [DOI] [PubMed] [Google Scholar]
- Wolf D. H. Cellular control in the eukaryotic cell through action of proteinases: the yeast Saccharomyces cerevisiae as a model organism. Microbiol Sci. 1986 Apr;3(4):107-11, 114. [PubMed] [Google Scholar]
- Wolf D. H., Ehmann C. Studies on a proteinase B mutant of yeast. Eur J Biochem. 1979 Aug 1;98(2):375–384. doi: 10.1111/j.1432-1033.1979.tb13197.x. [DOI] [PubMed] [Google Scholar]
- Woolford C. A., Daniels L. B., Park F. J., Jones E. W., Van Arsdell J. N., Innis M. A. The PEP4 gene encodes an aspartyl protease implicated in the posttranslational regulation of Saccharomyces cerevisiae vacuolar hydrolases. Mol Cell Biol. 1986 Jul;6(7):2500–2510. doi: 10.1128/mcb.6.7.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubenko G. S., Mitchell A. P., Jones E. W. Septum formation, cell division, and sporulation in mutants of yeast deficient in proteinase B. Proc Natl Acad Sci U S A. 1979 May;76(5):2395–2399. doi: 10.1073/pnas.76.5.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]