Abstract
As our knowledge of the structure and functions of fibrinogen and fibrin has increased tremendously, several key findings have given some people a superficial impression that the biological and clinical significance of these clotting proteins may be less than earlier thought. Most strikingly, studies of fibrinogen knockout mice demonstrated that many of these mice survive to weaning and beyond, suggesting that fibrin(ogen) may not be entirely necessary. Humans with afibrinogenemia also survive. Furthermore, in recent years, the major emphasis in the treatment of arterial thrombosis has been on inhibition of platelets, rather than fibrin. In contrast to the initially apparent conclusions from these results, it has become increasingly clear that fibrin is essential for hemostasis; is a key factor in thrombosis; and plays an important biological role in infection, inflammation, immunology, and wound healing. In addition, fibrinogen replacement therapy has become a preferred, major treatment for severe bleeding in trauma and surgery. Finally, fibrin is a unique biomaterial and is used as a sealant or glue, a matrix for cells, a scaffold for tissue engineering, and a carrier and/or a vector for targeted drug delivery.
Keywords: fibrin, fibrinogen, hemostasis, thrombosis, afibrinogenemia, hypofibrinogenemia, dysfibrinogenemia
If you enjoy a pretty sight, examine this [clotted] blood with a microscope. You will see a fibrous texture, and a network of nerve-like threads
– Marcello Malpighi, 1666
Although the Koran from the seventh century said that humans were created from clots of blood (Surah 96), it was not until the seventeenth century that Marcello Malpighi described the structural component of blood clots as a white fibrous substance that we now know as fibrin.1 Since the first purification of fibrinogen from blood in the late 1930s, research on fibrin has accelerated greatly, now with more than 200,000 studies in the Medline bibliographic database with the keyword “fibrin.”
Thus, it is impossible to even summarize briefly the various functions of fibrin. Instead, we are addressing a specific attitude toward fibrin that has arisen recently. We have become increasingly aware of an underlying current of thought among some hematologists and other clinicians that minimizes the significance of fibrin in hemostasis and thrombosis. We will first define the origins of this phenomenon and then demonstrate the fallacy of this belief.
What Is Fibrin?
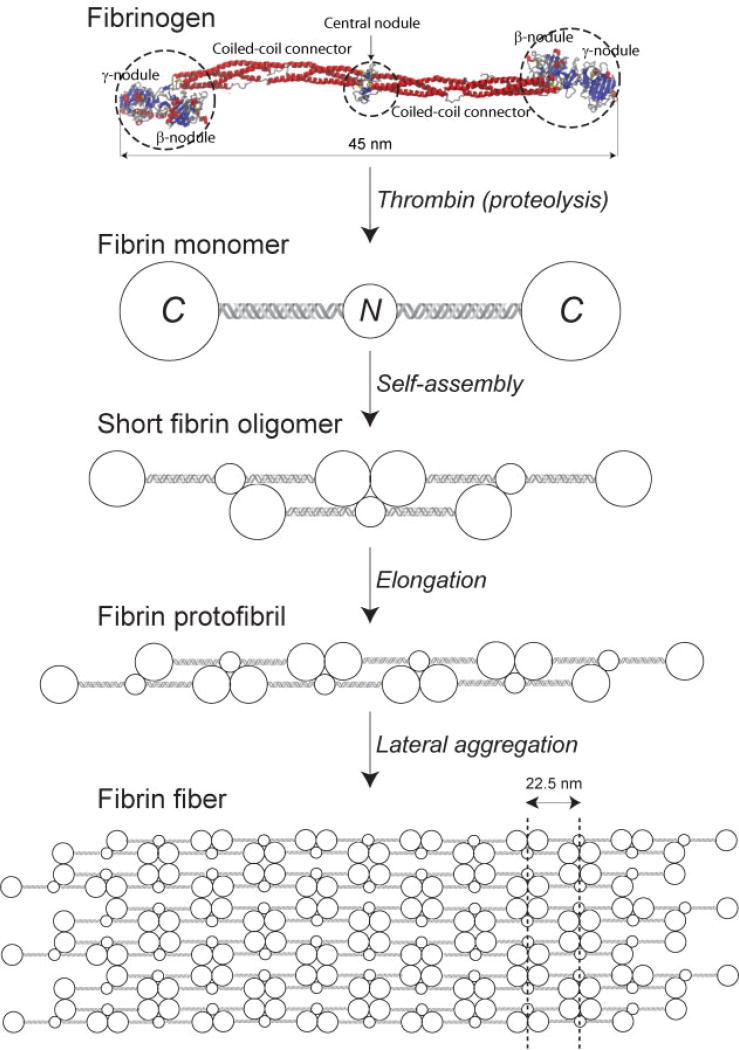
Human blood plasma normally contains around 1.5 to 3.5 g/L of fibrinogen, which is converted to insoluble fibrin via thrombin-catalyzed cleavage of two pairs of fibrinopeptides that exposes binding sites in the central nodule complementary to sites constitutively available at the ends of the molecule (Fig. 1). As a consequence, fibrin polymerization proceeds via half-staggered interactions that lead to two-stranded protofibrils aggregating laterally to make fibers, which branch to form a three-dimensional network (Fig. 2).2
Fig. 1.
Schematic representation of the major steps of fibrin polymerization, beginning with a crystallographic image of fibrinogen (PDB Entry: 3GHG), a soluble plasma protein. Fibrinogen molecule has a rod-like shape and is composed of the lateral globular parts (two β- and two γ-nodules) and one central nodule linked with the lateral portions via two triple-helical coiled-coil connectors. Limited thrombin-catalyzed cleavage of four peptide bonds in fibrinogen leads to formation of monomeric fibrin with two C-terminal globules and a central N-terminal globule containing complementary intermolecular binding sites. Self-assembly of monomeric fibrin in a half-staggered manner forms two-stranded fibrin oligomers that elongate up to the length of a protofibril comprising 20 to 25 monomeric units. Protofibrils aggregate laterally and get packed into a fiber with a regular 22.5-nm periodic cross-striation due to the half-staggered molecular structure and regular protofibril arrangement. A detailed description of fibrin formation can be found in our review article.2
Fig. 2.
Scanning electron micrographs of in vitro clots. (A) Clot from platelet-poor plasma, which is an open branched network of fibrin fibers. (B) Clot from platelet-rich plasma, with platelet aggregates connected by fibrin fibers. (C) Whole blood clot, with fibrin, platelets, and red blood cells. Magnification bar = 20 µm.
Fibrin clots are very open, porous networks, which is important for their function in hemostasis, fibrinolysis, and wound healing. The mechanical properties of fibrin are unique, in that it is a viscoelastic polymer, which means that it has both reversible elastic characteristics and irreversible plastic or viscous properties, and it undergoes strain stiffening, or increasing stiffness at high strains, which helps to prevent damage under harsh conditions, as in arterial shear. In addition, fibrin clots possess extreme extensibility and compressibility, such that they can deform greatly without rupture.3,4 The molecular and structural origins of the biochemical and mechanical properties of fibrin have been or are being determined. Furthermore, the properties of the fibrin network can be greatly modulated by a wide variety of environmental factors.
Why Some Scientists and Clinicians Think | that Fibrin Is Not Important
There have been great advances in our knowledge of fibrinogen and its conversion to fibrin. In a sense, the study of fibrin is a victim of its own success. At each major accomplishment of research, such as purification of fibrinogen, determination of its amino acid sequence at a time when that was a considerable achievement, X-ray crystallographic structure at the time the largest protein solved, cloning of recombinant fibrinogen, and establishment of transgenic mouse models, it was proclaimed that now we know all that we need to know about this molecule. Thus, there has been a feeling that whatever is subsequently learned cannot be important.
With the creation of a transgenic fibrinogen knockout mouse,5 it seemed at first that all this research came to an ignominious head. In contrast to mouse knockout models of some other key proteins, these mice mostly survived, that is, the lack of fibrinogen was not lethal, and that led some scientists and clinical hematologists at that time to conclude that fibrinogen cannot be very important.
Afibrinogenemia (i.e., the lack of fibrinogen in blood) is rare in humans, and patients with this condition can have either mild or serious bleeding problems.6 The fact that some patients do not have severe bleeding may also lead some to the superficial conclusion that fibrinogen is not really necessary for survival of humans.
As platelets are a major component of arterial thrombi and have been clearly implicated in thrombosis, they have been a primary target for antithrombotic drugs, with significant success in reducing the morbidity and mortality associated with cardiovascular disease. The emphasis on antiplatelet drugs for cardiovascular disease could leave some people with the impression that therapies involving inhibition of fibrin formation, namely anticoagulants, are no longer important for thromboembolic disease. In addition, thrombolytic drugs are increasingly being supplanted by direct mechanical removal of thrombi.
Studies of in vivo clotting by intravital microscopy of arterioles and venules in mice have demonstrated that platelets form a dense core in combination with fibrin, and that there is a more loosely packed shell of platelets without fibrin.7 Further research employing a variety of inhibitors indicated that inhibition of thrombin or the generation of fibrin has little effect on the hemostatic properties of the clot in this model.8
Clinical coagulation testing often involves measurements related to thrombin generation or activity. As a result, it may appear that thrombin is all that matters in the diagnosis of bleeding or thrombotic conditions.
Fibrin Is Necessary for Hemostasis
Bleeding Disorders
In contrast to the initial impressions from the findings mentioned earlier, there is firm evidence that fibrin is necessary for hemostasis. For example, fibrinogen knockout mice have a serious reduction in lifetime expectancy, even in the cage environment, and all females die during pregnancy.5 Although there are compensatory mechanisms that can partially make up for the lack of fibrinogen, including platelet aggregation via other ligands (perhaps plasma fibronectin or von Willebrand factor), and other unknown effects, fibrin is clearly essential. In more recent studies using transgenic mice with a fibrinogen that cannot be cleaved by thrombin but does support platelet aggregation,9 there was also a strong propensity for bleeding, which directly indicates that fibrin formation is critically important for hemostasis. The superiority of these mice over fibrinogen knockout mice in various assays and in survival indicates that fibrinogen-mediated platelet aggregation is certainly important,9 but fibrin formation is also essential for effective hemostasis. It can be concluded that platelet aggregation cannot rescue the lack of fibrin polymerization in severe bleeding, as in liver injury in this study, for example. In addition, the lack of occlusion in the carotid artery ferric chloride model with these mice suggests that fibrin polymer is important in thrombosis as well.9
Human afibrinogenemia can be quite a difficult condition to manage, with some patients exhibiting serious bleeding problems, and a woman with afibrinogenemia is unable to carry a pregnancy without treatment. This condition is most commonly treated successfully with fibrinogen replacement therapy.10,11 It should also be noted that some patients have thrombotic complications, with or without replacement therapy, probably because of the role of fibrin as an adsorbent of thrombin, as fibrin was initially called antithrombin I.12
Intravital microscopy studies of either laser injury or small puncture wounds have found that platelets play the major role in hemostasis in these small injuries.13 However, this is only one model for hemostasis, and the consequences are quite different in other parts of the vasculature and especially in more severe bleeding (see later).
Many coagulation assays measure thrombin generation, and thrombin is important for both platelet activation and fibrin polymerization, but coagulation is not just all about thrombin. In fact, most of the platelet-poor plasma assays involve measurement of the effects of the thrombin on fibrin polymerization. Many of the global clotting assays, such as thromboelastography and clot waveform analysis, focus more generally on fibrin properties.
Hemophilia A and B are diseases that result in impaired thrombin generation because of missing or mutated coagulation factor VIII or IX. Often the emphasis in diagnosis and treatment is on increasing the thrombin generation. However, it should be kept in mind that the impact of decreased thrombin generation in hemophilia is mostly on delayed and deficient fibrin formation, while platelets are less important. In other words, the bleeding diathesis in hemophilia suggests that fibrin is necessary for hemostasis, notwithstanding the existence of some hemophiliacs who rarely bleed. Finally, it remains a puzzle why the phenotype of afibrinogenemia and hemophilia are quite different.
Factor XIII deficiency is rare but often involves serious bleeding.14 Although fibrin is formed in these individuals, it is not stabilized by factor XIIIa-catalyzed covalent cross-linking and is more easily digested. As a result, these patients experience recurrent bleeding, 80% umbilical cord bleeding, 30% spontaneous intracranial hemorrhage (major cause of death), as well as bruising, nose and mouth bleeds, muscle bleeds, menorrhagia, miscarriage, and bleeding after surgery.15 These clinical symptoms imply the necessity not only of fibrin but also for it to be stabilized by cross-linking.16
Platelets play a major role in both hemostasis and thrombosis. Platelet adhesion and aggregation can be mediated by fibrinogen, monomeric fibrin, or oligomeric fibrin.17 Although most hematologists tend to think of fibrinogen as the ligand for the platelet integrin αIIbβ3, because that is what is commonly used in laboratory analyses of platelet aggregation purely for convenience, it is likely that the major ligand for platelets is some form of fibrin, not fibrinogen.18 During clotting in vivo, fibrin is being formed by thrombin on or near the platelet surface at the same time as platelets aggregate,7 and in images of clots, fibrin connects platelet aggregates.19 Thus, fibrin is important for platelet functions as well as for gelation.
Blood Clot Contraction
Fibrin is essential for blood clot contraction (or retraction), that is, spontaneous shrinkage of the clot, which plays a role in hemostasis, wound healing, and restoring the flow of blood past obstructive thrombi.20 The driving force of clot contraction is activated platelets that generate contractile forces due to intracellular interaction of actin and nonmuscle myosin IIa.21 These contractile forces are propagated through the fibrin network due to strong platelet–fibrin interactions mediated by the activated platelet adhesive receptor integrin αIIbβ3.22–24 The extent of clot contraction has been shown to be dependent on the covalent cross-linking of fibrin by factor XIIIa.25 As clot contraction progresses, there is redistribution of platelets and fibrin to the surface of the contacting clot and the mechanical compaction of erythrocytes in the core, such that they take on a polyhedral shape; hence, they have been named polyhedrocytes.26
There is strong, though indirect, evidence that clot contraction has (patho)physiological significance. Compressed polyhedrocytes, a morphological sign of clot contraction,26 were found in thrombi extracted from patients with ST-elevation myocardial infarction,27 which corroborates intravascular platelet–fibrin network contraction and suggests that polyhedrocytes may be a marker for thrombosis. These compacted erythrocytes may also be important for creating an impermeable seal that is associated with hemostasis, as transport into a contracted clot is exceedingly slow.26 They also impair the ability of fibrinolytic enzymes to infiltrate the clots and make mature clots and thrombi resistant to thrombolytic therapy.28
The importance of clot contraction in vivo perhaps can be best exemplified through disorders disrupting the generation of contractile force. Mutation of nonmuscle myosin IIa (encoded by the MYH9 gene) results in platelets being activated and binding fibrin(ogen) without the development of stress fibers or the generation of contractile force.29,30 Patients with this MYH9 mutation have an increased tendency to bleed and decreased thrombus stability. Because they have normal platelet aggregation and secretion responses to various agonists, it is thought that the bleeding tendency and decreased thrombus stability are due to the impaired ability to generate platelet contractile forces.30,31 A predisposition to bleeding and an absence of clot contraction is also present in murine MYH9 mutations, suggesting that platelet contraction is vital for a stable hemostatic plug to form in vivo independent of platelet aggregation and/or fibrin formation.31–33 This conclusion is complicated by the concurrent macrothrombocytopenia that accompanies MYH9 disorders, which also would increase bleeding, but transgenic diYF mice, which have normal platelet number and size, but impaired signaling such that they do not support contraction, also have bleeding.34,35
Fibrin is essential for clot contraction because it propagates the platelet-generated contractile forces through the entire network that comprises a clot or thrombus. The structure and rheological properties of fibrin gels are important because they determine the degree of platelet-mediated contraction. Clot properties in turn are largely governed by the fiber diameters, branching, and network density24 and the degree of factor XIIIa-mediated fibrin cross-linking.25 In addition, the importance of factor XIIIa for clot contraction36 could be due to mediating the translocation of fibrin nearby sphingomyelin-rich membrane rafts, where it is able to interact with myosin inside the platelet via αIIbβ3.25 Another effect of factor XIIIa-catalyzed fibrin cross-linking on clot contraction has been recently shown to affect retention of erythrocytes inside a contracted clot.37 Moreover, in vivo studies with factor XIII-deficient mice suggested that this mechanism can modulate thrombus size via cross-linking of fibrin’s α-chains.38,39
Collectively, these findings suggest that platelet-driven, fibrin-mediated clot contraction can play an important role in preventing blood loss, reducing the volume of the clot, and restoring blood flow past otherwise obstructive thrombi.7,40
Dysfibrinogenemias
Dysfibrinogenemia is a collective name for qualitative defects of fibrinogen that affect its functionality, almost invariably the ability to form fibrin clots, as this is usually the mode of discovery, and they can have thrombotic or bleeding consequences or be asymptomatic. Dysfibrinogenemias can be either acquired or congenital (hereditary). Congenital dysfibrinogenemia is a qualitative defect and should be distinguished from congenital hypo- or afibrinogenemia, which represent quantitative fibrinogen abnormalities.6,11,41–43 All of the acquired and congenital fibrinogen defects have clinical implications, corroborating the significance of fibrin formation in vivo.
Acquired dysfibrinogenemia most often accompanies severe liver diseases, as fibrinogen is synthesized in hepatocytes.44,45 The structural defect of fibrinogen in liver-related dysfibrinogenemia is usually caused by altered posttranslational glycosylation, leading to increased content of sialic acid in the N-glycans of the Bβ- and γ-chains that impairs fibrinogen’s clottability.46,47 Patients with liver disease have both thrombotic complications and bleeding, but the relative contribution of dysfibrinogenemia to systemic hemostatic disorders is hard to assess because multiple and sometimes opposite mechanisms are involved, such as decreased production of blood coagulation factors, including fibrinogen (hypofibrinogenemia) and thrombogenic structure and properties of fibrin clots, disseminated intravascular coagulation, accelerated fibrinolysis, thrombocytopenia, and so on.47,48 Other pathological states, such as increased immunoglobulin production (myeloma) or autoimmune disorders (systemic lupus erythematosus), are also associated with acquired dysfibrinogenemia followed by abnormal fibrin polymerization, which is caused by generation of specific antibodies against fibrinogen and/or fibrin.49 Antibodies against fibrin (ogen) can interfere with fibrinopeptide release, fibrin polymerization, or factor XIIIa-mediated cross-linking and usually correlate with abnormal coagulation tests, although there has been no consistent correlation with bleeding.50
Congenital dysfibrinogenemia is caused by a mutation within one of the three fibrinogen chain genes, inherited as autosomal dominant or codominant, except a few cases that are transmitted recessively.51 Mutations that cause dysfibrinogenemia affect the functionality of fibrinogen, cause impaired release of fibrinopeptides, delayed or enhanced polymerization, defective factor XIIIa-mediated cross-linking, decreased thrombin binding (including polymorphism of the γ-chain52), and delayed fibrinolysis. As some abnormal fibrinogens form fibrin clots that are resistant to lysis by plasmin, these fibrin(ogen) defects are associated with thromboembolic complications. Another possible reason for thrombotic events in dysfibrinogenemia is defective binding of thrombin to abnormal fibrin via nonsubstrate binding sites, which can result in elevated levels of thrombin.53
Trauma or Surgery: Fibrinogen Replacement
As a result of trauma or surgery, fibrinogen levels decrease at an early stage in severe hemorrhage, from blood loss with consumption of clotting factors and dilutional effects from treatments with colloids or crystalloids to maintain blood pressure. In addition, hyperfibrinolysis, acidosis, hypothermia, and metabolic changes affect the coagulation system. All of these factors directly and rapidly affect the polymerization of fibrin, whereas other bleeding deficiencies usually occur later in trauma. Although trauma-induced coagulopathy is not yet well understood, it may be different from disseminated intravascular coagulation, as there is no generalized intravascular blood clotting with subsequent consumption of clotting factors. Instead, there is a bleeding-related loss of coagulation factors and platelets.54 A variety of approaches have developed over the years for treating severe bleeding, and this field is now rapidly evolving. Although fresh frozen plasma had been the gold standard for treatment of trauma, it has become apparent that this approach comes with a host of problems, including more frequent cardiac overload, severe infections and respiratory complications, citrate overload, and acute lung injury.55 At the same time, there has been increasing evidence that the most efficacious method for treatment of severe bleeding in trauma is the administration of fibrinogen concentrates. In blood loss, fibrinogen levels reach a critical level sooner than any other coagulation factor, or even platelets.54 Studies from treatment of severe bleeding in animal models,56 various forms of surgery, and severe trauma have all demonstrated that fibrinogen supplements are beneficial.57–61 Even thrombocytopenia can be successfully treated with fibrinogen concentrates.62 In contrast, treatments with other clotting factors, including thrombin, or with platelets, are not as effective in stopping bleeding associated with trauma or surgery. In conclusion, fibrin is necessary to stop severe bleeding.
Fibrin has been used for at least 75 years for a whole variety of surgical and repair processes as a biodegradable tissue adhesive or sealant to stop or control bleeding.63,64 Its mechanical and other physical properties make it valuable as a hemostatic glue and for wound repair. Its biological properties, including specific and nonspecific binding of many drugs and cellular interactions, useful for cell differentiation and tissue engineering, enhance the range of useful applications. As a result, fibrin sealants are now a major product for several companies around the world.
Fibrin Is Important in Venous and Arterial Thromboses
Fibrin Is a Major Component of Thrombi
The long-standing paradigm is that arterial thrombi are platelet rich, while venous thrombi are fibrin and erythrocyte rich. This is true as a generality, but we now know that fibrin is a major component of coronary artery thrombi, and the prevalence of fibrin increases with age following occlusion and predominates in older thrombi.65 In addition, thrombi removed mechanically from patients with acute middle cerebral artery ischemic stroke also contained large percentages of fibrin.66 In summary, fibrin is a major component of arterial as well as venous thrombi (Fig. 3) and must be taken into account in treatment of thromboembolic disease.
Fig. 3.
Scanning electron micrographs of in vivo thrombi. (A) Coronary artery thrombus, with dense mesh of fibrin and platelet aggregates. Magnification bar = 10 µm. (B) Pulmonary embolus, with dense mesh of fibrin, platelet aggregates, and red blood cells. Magnification bar = 10 µm.
Many studies have shown that fibrin clots formed in vitro from the blood of patients with cardiovascular diseases and thromboses have an altered structure associated with increased mechanical stiffness and lower susceptibility to fibrinolysis.67 In the blood of patients with acute coronary disease, fibrin clots were less porous and resistant to fibrinolysis than in patients with stable coronary disease.68 Close relatives of patients with coronary artery disease also displayed prothrombotic fibrin clot structure, suggesting that there is a genetic basis for the thrombophilic phenotype of fibrin structure.69 Similar findings in fibrin architecture and properties were observed in patients with ischemic stroke,70,71 peripheral vascular disease,72 deep vein thrombosis,73 and so on.74 The common organization and properties of fibrin clots from the blood of patients with various types of thrombotic complications suggest direct participation of fibrin in the pathogenesis of both arterial and venous thrombosis.
Owing to its deposition in virtually all types of intravascular clots and thrombi, arterial or venous, nascent or matured, fibrin is a preferable target for molecular imaging of thrombosis.75,76 There are a variety of fibrin-binding probes that are widely used for thrombus imaging with many techniques such as positron emission tomography, single-photon emission computed tomography, computed X-ray tomography, magnetic resonance imaging, near-infrared fluorescence, and combinations. A probe for selective fibrin and thrombus imaging should have a high affinity and high specificity for fibrin over fibrinogen and other blood proteins, rapid clearance from the circulation to increase thrombus-to-background ratio, and small size to enable penetration into thrombi. There are several labeled short cyclic fibrin-binding peptides that meet these criteria.77,78
Disseminated Intravascular Coagulation
An extreme pathological condition in which fibrin clots play a pivotal role is disseminated intravascular coagulation, resulting from uncontrolled massive activation of the blood coagulation cascade, leading to an extensive microvascular fibrin deposition that impairs the blood supply and causes multiple organ failure.79,80 In addition to diffuse microthrombosis, acute disseminated intravascular coagulation is characterized by severe bleeding and hemorrhagic tissue necrosis as a result of consumption and proteolytic degradation of blood clotting factors (including fibrinogen), anticoagulants, and platelets, leading to profuse uncontrollable bleeding and often death. Disseminated intravascular coagulation most often complicates sepsis, major trauma, and obstetric catastrophes, but in a more or less severe form, it can accompany many diseases, including malignancies and leukemia. Fibrin deposition in the parenchyma of organs with developed microvasculature is a major microscopic finding in disseminated intravascular coagulation.81
Fibrin-Related Molecular Markers of Thrombosis
Fibrin formation in the vasculature is preceded and followed by formation of fibrin(ogen) derivatives that have been used as molecular markers of intravascular fibrin formation in laboratory diagnosis of prothrombotic and thrombotic states. Soluble fibrin precursors known as soluble fibrin monomer complexes or soluble fibrin appear in the blood as a result of increased intravascular thrombin generation and reflect a high risk of intravascular blood clotting or ongoing fibrin formation. Quantitative determination of soluble fibrin monomer complexes in the blood is based on immunoassays that distinguish between fibrinogen and thrombin-modified fibrinogen derivatives.82 The diagnostic significance of soluble fibrin monomer complexes was shown in venous thromboembolism,83 coronary arterial thrombosis,84 cardiopulmonary bypass,85 and many other conditions associated with thrombophilia and/or thrombotic states. Another set of fibrin-related molecular markers of thrombosis are called fibrin degradation products originating from enzymatic cleavage of fibrin under the action of plasmin, the main protease of the fibrinolytic system. Unlike most proteolytic fibrin(ogen) fragments that could be made either from fibrinogen or fibrin, D-dimer is produced exclusively from cross-linked fibrin, which makes a high level of D-dimer in serum indicative of intravascular fibrin deposition occurring during local thrombosis or disseminated intravascular coagulation.86 The D-dimer test has a high negative predictive value to rule out venous thromboembolism.87 Despite some limitations, the D-dimer has been widely used as a molecular marker of coagulation activation and fibrinolysis88 and, therefore, reinforces the significant role of fibrin in thrombosis.
Anticoagulants and Thrombolytics
Indirect but weighty evidence for the importance of fibrin in thrombosis is the use of anticoagulants to prevent fibrin/thrombus formation and the efficacy of thrombolytic agents that remove thrombi via fibrin dissolution. Apart from antiplatelet drugs that prevent or reduce platelet activation, anticoagulants interfere with thrombin generation and/or inhibit its activity, thus preventing both thrombin-induced platelet stimulation and conversion of fibrinogen to fibrin. Therefore, anticlotting agents are generally more potent than antiplatelet drugs in preventing thrombosis, except in some arterial diseases. In some cases, dual antiplatelet and anticoagulant therapy is recommended, despite an increased risk of bleeding complications, indicating that they are aimed at different pathogenic targets, platelet activation versus fibrin formation. Therapeutic thrombolysis or thrombolytic therapy is the dissolution of fibrin by the delivery of exogenous plasminogen activators into the circulation to remove a thrombus. It is a common, though decreasing, treatment for acute myocardial infarction and stroke, sometimes in addition to or in combination with mechanical elimination of a thrombus (sonothrombolysis, aspiration, etc.). The efficacy of thrombolytic therapy is based on the incontestable evidence that fibrin is a major component of any thrombus, providing it with integrity as well as mechanical and chemical stability.
Fibrin in Infection and Inflammation
Fibrin is essential for antimicrobial host defense.89 Responses to infection in transgenic mice with a fibrinogen that cannot polymerize to make fibrin are also impaired,8 indicating that fibrin, rather than fibrinogen, is necessary for antibacterial action to be most effective. This result could be explained by either a direct role for fibrin polymer or different cell-binding specificity of fibrinogen versus fibrin.
The leukocyte integrin Mac-1 supports the inflammatory response through engagement of fibrin(ogen). Mice lacking the Mac-1-binding motif have normal fibrinogen levels and no defects in hemostasis, but their inflammatory response is severely compromised in terms of ability to effectively clear bacterial infection, demonstrating that fibrin(ogen) is essential for innate immunity.90 Moreover, mice lacking fibrinogen or missing only the Mac-1-binding domain and challenged with collagen-induced arthritis have fewer arthritic joints than control mice, indicating that fibrin(ogen) is an important determinant of inflammatory arthritis.91
Although aspects of wound healing can occur without fibrinogen, cells from fibrinogen-deficient animals are unable to efficiently organize and migrate during wound healing.92 Fibrin(ogen) is important for appropriate cellular migration and organization during wound healing and in initially establishing wound strength and stability.
Fibrin Is a Biomaterial
On a practical basis, fibrin has achieved widespread use as a biomaterial, among both clinicians and engineers. As a major component of extracellular matrix that plays an important role in cell–matrix interactions, fibrin has been employed in various tissue engineering applications, such as engineering of pancreatic endocrine tissue93 and design of cardiac microenvironment.94 Additionally, fibrin has been shown to promote angiogenesis95,96 via sprouting from existing vasculature, which is crucial for bone regeneration and tissue transplantation.97 Fibrin has been used in vitro for engineering of provisional matrices.98 In addition, fibrin has also been used for drug delivery applications,99,100 when drug molecules or factors are loaded in the fibrin gel via impregnation and tethering to the gel through covalent linkages or affinity-based systems. However, the most widespread use of fibrin is, for various surgical applications, as a safe, easy, and effective sealant or glue to stop or control bleeding.63
What makes fibrin a useful biomaterial is a unique combination of its mechanical and biological characteristics, including easily modifiable biochemical, structural, and viscoelastic properties, as well as lack of toxic reactions and biodegradability. The biochemical and mechanical characteristics of fibrin determine how this biomaterial responds to its environment, including hydrodynamic and other forces. Fibrin clot mechanics has been studied extensively over the past decade under various experimental conditions.3,101 The mechanical properties of fibrin networks depend strongly on their structure and composition determined by the concentration of fibrinogen, thrombin activity, factor XIIIa-mediated cross-linking, and the presence of flow.102,103 One of the remarkable mechanical characteristics of fibrin is its stiffening under load as it becomes increasingly resistant to applied deformation.3,4 This strain-hardening behavior can be important for fibrin as a biomaterial, as it allows fibrin to be compliant at low strains but then stiffer at large deformations to prevent damage.
Numerous recent studies on cellular mechanosensing show that extracellular matrix mechanics has dramatic effects on cell motility, differentiation, and proliferation. In other words, mechanical characteristics and microstructure of fibrin networks largely determine functioning and behavior of supported and embedded cells and, hence, the biological properties of fibrin-built biomaterials and engineered tissues. Gels based on fibrin are being used as scaffolds104 to optimize cellular activities, including differentiation, proliferation, and morphological changes.105 In summary, fibrin has become increasingly important as a unique biomaterial.106
Conclusion
Reports of my death are greatly exaggerated.
–Mark Twain
Like this quote from Mark Twain responding to tales that he had died, rumors of fibrin’s demise as a necessary component of hemostasis and thrombosis are indeed inconsistent with reality. The involvement of fibrin in pathophysiological processes and its importance is summarized in Fig. 4. Fibrin is essential for hemostasis, as demonstrated by human afibrinogenemia, transgenic fibrinogen knockout or mutated mouse models, hemophilia and factor XIII deficiency, clot contraction, dysfibrinogenemias, and especially for severe bleeding from trauma or surgery. Fibrin is also implicated as a major factor in thrombosis, infection, and inflammation. Finally, fibrin has become increasingly useful as a versatile biomaterial with unique properties.
Fig. 4.
Formation of fibrin clots in vivo, their size, and location, as well as fine structure and properties are greatly influenced by several variable pathogenic factors shown in the upper part of the cartoon. The biological and clinical relevance of fibrin is determined by its implications in the vital interconnected patho (physiological) reactions and processes as well as by the use of fibrin polymers as a versatile biomaterial.
Acknowledgments
The authors thank Dr. Lubica Rauova for careful reading of the manuscript and valuable suggestions. The work was supported by NIH grants HL090774 and U01-HL116330.
References
- 1.Forrester JM. Malpighi’s De polypo cordis: an annotated translation. Med Hist. 1995;39(4):477–492. doi: 10.1017/s0025727300060385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weisel JW, Litvinov RI. Mechanisms of fibrin polymerization and clinical implications. Blood. 2013;121(10):1712–1719. doi: 10.1182/blood-2012-09-306639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown AE, Litvinov RI, Discher DE, Purohit PK, Weisel JW. Multiscale mechanics of fibrin polymer: gel stretching with protein unfolding and loss of water. Science. 2009;325(5941):741–744. doi: 10.1126/science.1172484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim OV, Litvinov RI, Weisel JW, Alber MS. Structural basis for the nonlinear mechanics of fibrin networks under compression. Biomaterials. 2014;35(25):6739–6749. doi: 10.1016/j.biomaterials.2014.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suh TT, Holmbäck K, Jensen NJ, et al. Resolution of spontaneous bleeding events but failure of pregnancy in fibrinogen-deficient mice. Genes Dev. 1995;9(16):2020–2033. doi: 10.1101/gad.9.16.2020. [DOI] [PubMed] [Google Scholar]
- 6.de Moerloose P, Casini A, Neerman-Arbez M. Congenital fibrinogen disorders: an update. Semin Thromb Hemost. 2013;39(6):585–595. doi: 10.1055/s-0033-1349222. [DOI] [PubMed] [Google Scholar]
- 7.Stalker TJ, Traxler EA, Wu J, et al. Hierarchical organization in the hemostatic response and its relationship to the platelet-signaling network. Blood. 2013;121(10):1875–1885. doi: 10.1182/blood-2012-09-457739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welsh JD, Muthard RW, Stalker TJ, Taliaferro J, Diamond SL, Brass LF. More than just red cells: how do hemostatic thrombi prevent the loss of plasma-borne molecules? J Thromb Haemost. 2015;13:174–174. [Google Scholar]
- 9.Prasad JM, Gorkun OV, Raghu H, et al. Mice expressing a mutant form of fibrinogen that cannot support fibrin formation exhibit compromised antimicrobial host defense. Blood. 2015;126(17):2047–2058. doi: 10.1182/blood-2015-04-639849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bornikova L, Peyvandi F, Allen G, Bernstein J, Manco-Johnson MJ. Fibrinogen replacement therapy for congenital fibrinogen deficiency. J Thromb Haemost. 2011;9(9):1687–1704. doi: 10.1111/j.1538-7836.2011.04424.x. [DOI] [PubMed] [Google Scholar]
- 11.Casini A, de Moerloose P, Neerman-Arbez M. Clinical features and management of congenital fibrinogen deficiencies. Semin Thromb Hemost. 2016 doi: 10.1055/s-0036-1571339. [DOI] [PubMed] [Google Scholar]
- 12.Mosesson MW. Update on antithrombin I (fibrin) Thromb Haemost. 2007;98(1):105–108. [PubMed] [Google Scholar]
- 13.Stalker TJ, Welsh JD, Brass LF. Shaping the platelet response to vascular injury. Curr Opin Hematol. 2014;21(5):410–417. doi: 10.1097/MOH.0000000000000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muszbek L, Katona E. Diagnosis and management of congenital and acquired factor XIII deficiencies. Semin Thromb Hemost. 2016 doi: 10.1055/s-0036-1572326. [DOI] [PubMed] [Google Scholar]
- 15.Lassila R. Clinical use of FXIII concentrates. Semin Thromb Hemost. 2016 doi: 10.1055/s-0036-1572324. [DOI] [PubMed] [Google Scholar]
- 16.Schroeder V, Kohler HP. Factor XIII, structure and function. Semin Thromb Hemost. 2016 doi: 10.1055/s-0036-1571341. [DOI] [PubMed] [Google Scholar]
- 17.Jarvis GE, Atkinson BT, Frampton J, Watson SP. Thrombin-induced conversion of fibrinogen to fibrin results in rapid platelet trapping which is not dependent on platelet activation or GPIb. Br J Pharmacol. 2003;138(4):574–583. doi: 10.1038/sj.bjp.0705095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Litvinov RI, Farrell DH, Weisel JW, Bennett JS. The platelet integrin αIIbβ3 differentially interacts with fibrin versus fibrinogen. (e-pub ahead of print) J Biol Chem. 2016 doi: 10.1074/jbc.M115.706861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collet JP, Montalescot G, Lesty C, Weisel JW. A structural and dynamic investigation of the facilitating effect of glycoprotein IIb/IIIa inhibitors in dissolving platelet-rich clots. Circ Res. 2002;90(4):428–434. doi: 10.1161/hh0402.105095. [DOI] [PubMed] [Google Scholar]
- 20.Carr ME., Jr Development of platelet contractile force as a research and clinical measure of platelet function. Cell Biochem Biophys. 2003;38(1):55–78. doi: 10.1385/CBB:38:1:55. [DOI] [PubMed] [Google Scholar]
- 21.Lam WA, Chaudhuri O, Crow A, et al. Mechanics and contraction dynamics of single platelets and implications for clot stiffening. Nat Mater. 2011;10(1):61–66. doi: 10.1038/nmat2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bennett JS. Platelet-fibrinogen interactions. Ann N Y Acad Sci. 2001;936:340–354. doi: 10.1111/j.1749-6632.2001.tb03521.x. [DOI] [PubMed] [Google Scholar]
- 23.Ehrlicher A, Hartwig JH. Cell mechanics: Contracting to stiffness. Nat Mater. 2011;10(1):12–13. doi: 10.1038/nmat2928. [DOI] [PubMed] [Google Scholar]
- 24.Wufsus AR, Rana K, Brown A, Dorgan JR, Liberatore MW, Neeves KB. Elastic behavior and platelet retraction in low- and high-density fibrin gels. Biophys J. 2015;108(1):173–183. doi: 10.1016/j.bpj.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasahara K, Kaneda M, Miki T, et al. Clot retraction is mediated by factor XIII-dependent fibrin-αIIβB3-myosin axis in platelet sphingomyelin-rich membrane rafts. Blood. 2013;122(19):3340–3348. doi: 10.1182/blood-2013-04-491290. [DOI] [PubMed] [Google Scholar]
- 26.Cines DB, Lebedeva T, Nagaswami C, et al. Clot contraction: compression of erythrocytes into tightly packed polyhedra and redistribution of platelets and fibrin. Blood. 2014;123(10):1596–1603. doi: 10.1182/blood-2013-08-523860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ząbczyk M, Sadowski M, Zalewski J, Undas A. Polyhedrocytes in intracoronary thrombi from patients with ST-elevation myocardial infarction. Int J Cardiol. 2015;179:186–187. doi: 10.1016/j.ijcard.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Skewis LR, Lebedeva T, Papkov V, et al. T2 magnetic resonance: a diagnostic platform for studying integrated hemostasis in whole blood—proof of concept. Clin Chem. 2014;60(9):1174–1182. doi: 10.1373/clinchem.2014.223735. [DOI] [PubMed] [Google Scholar]
- 29.Eckly A, Strassel C, Freund M, et al. Abnormal megakaryocyte morphology and proplatelet formation in mice with megakaryocyte-restricted MYH9 inactivation. Blood. 2009;113(14):3182–3189. doi: 10.1182/blood-2008-06-164061. [DOI] [PubMed] [Google Scholar]
- 30.Kunishima S, Saito H. Advances in the understanding of MYH9 disorders. Curr Opin Hematol. 2010;17(5):405–410. doi: 10.1097/MOH.0b013e32833c069c. [DOI] [PubMed] [Google Scholar]
- 31.Léon C, Eckly A, Hechler B, et al. Megakaryocyte-restricted MYH9 inactivation dramatically affects hemostasis while preserving platelet aggregation and secretion. Blood. 2007;110(9):3183–3191. doi: 10.1182/blood-2007-03-080184. [DOI] [PubMed] [Google Scholar]
- 32.Ono A, Westein E, Hsiao S, et al. Identification of a fibrin-independent platelet contractile mechanism regulating primary hemostasis and thrombus growth. Blood. 2008;112(1):90–99. doi: 10.1182/blood-2007-12-127001. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Conti MA, Malide D, et al. Mouse models of MYH9-related disease: mutations in nonmuscle myosin II-A. Blood. 2012;119(1):238–250. doi: 10.1182/blood-2011-06-358853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Law DA, DeGuzman FR, Heiser P, Ministri-Madrid K, Killeen N, Phillips DR. Integrin cytoplasmic tyrosine motif is required for outside-in alphaIIbbeta3 signalling and platelet function. Nature. 1999;401(6755):808–811. doi: 10.1038/44599. [DOI] [PubMed] [Google Scholar]
- 35.Stalker TJ, Welsh JD, Tomaiuolo M, et al. A systems approach to hemostasis: 3. Thrombus consolidation regulates intrathrombus solute transport and local thrombin activity. Blood. 2014;124(11):1824–1831. doi: 10.1182/blood-2014-01-550319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kasahara K, Souri M, Kaneda M, Miki T, Yamamoto N, Ichinose A. Impaired clot retraction in factor XIII A subunit-deficient mice. Blood. 2010;115(6):1277–1279. doi: 10.1182/blood-2009-06-227645. [DOI] [PubMed] [Google Scholar]
- 37.Aleman MM, Byrnes JR, Wang JG, et al. Factor XIII activity mediates red blood cell retention in venous thrombi. J Clin Invest. 2014;124(8):3590–3600. doi: 10.1172/JCI75386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Byrnes JR, Duval C, Wang Y, et al. Factor XIIIa-dependent retention of red blood cells in clots is mediated by fibrin α-chain crosslinking. Blood. 2015;126(16):1940–1948. doi: 10.1182/blood-2015-06-652263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Byrnes JR, Wolberg AS. Newly recognized roles of factor XIII in thrombosis. Semin Thromb Hemost. 2016 doi: 10.1055/s-0036-1571343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muthard RW, Diamond SL. Blood clots are rapidly assembled hemodynamic sensors: flow arrest triggers intraluminal thrombus contraction. Arterioscler Thromb Vasc Biol. 2012;32(12):2938–2945. doi: 10.1161/ATVBAHA.112.300312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Casini A, Blondon M, Lebreton A, et al. Natural history of patients with congenital dysfibrinogenemia. Blood. 2015;125(3):553–561. doi: 10.1182/blood-2014-06-582866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Casini A, Neerman-Arbez M, Ariëns RA, de Moerloose P. Dysfibrinogenemia: from molecular anomalies to clinical manifestations and management. J Thromb Haemost. 2015;13(6):909–919. doi: 10.1111/jth.12916. [DOI] [PubMed] [Google Scholar]
- 43.Neerman-Arbez M, de Moerloose P, Casini A. Laboratory and genetic investigation of mutations accounting for congenital fibrinogen disorders. Semin Thromb Hemost. 2016 doi: 10.1055/s-0036-1571340. [DOI] [PubMed] [Google Scholar]
- 44.Pluta A, Gutkowski K, Hartleb M. Coagulopathy in liver diseases. Adv Med Sci. 2010;55(1):16–21. doi: 10.2478/v10039-010-0018-3. [DOI] [PubMed] [Google Scholar]
- 45.Kopec AK, Luyendyk JP. Role of fibrin(ogen) in progression of liver disease: guilt by association? Semin Thromb Hemost. 2016 doi: 10.1055/s-0036-1579655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martinez J, Palascak JE, Kwasniak D. Abnormal sialic acid content of the dysfibrinogenemia associated with liver disease. J Clin Invest. 1978;61(2):535–538. doi: 10.1172/JCI108964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lisman T, Ariëns RAS. Alterations in fibrin structure in patients with liver diseases. Semin Thromb Hemost. 2016 doi: 10.1055/s-0036-1572327. [DOI] [PubMed] [Google Scholar]
- 48.Lisman T, Porte RJ. Rebalanced hemostasis in patients with liver disease: evidence and clinical consequences. Blood. 2010;116(6):878–885. doi: 10.1182/blood-2010-02-261891. [DOI] [PubMed] [Google Scholar]
- 49.Panzer S, Thaler E. An acquired cryoglobulinemia which inhibits fibrin polymerization in a patient with IgG kappa myeloma. Haemostasis. 1993;23(1):69–76. doi: 10.1159/000216856. [DOI] [PubMed] [Google Scholar]
- 50.Dear A, Brennan SO, Sheat MJ, Faed JM, George PM. Acquired dysfibrinogenemia caused by monoclonal production of immunoglobulin lambda light chain. Haematologica. 2007;92(11):e111–e117. doi: 10.3324/haematol.11837. [DOI] [PubMed] [Google Scholar]
- 51.Hanss M, Biot F. A database for human fibrinogen variants. Ann N Y Acad Sci. 2001;936:89–90. doi: 10.1111/j.1749-6632.2001.tb03495.x. [DOI] [PubMed] [Google Scholar]
- 52.Macrae FL, Domingues MM, Casini A, Ariëns RAS. The (patho)physiology of fibrinogen. Semin Thromb Hemost. 2016 doi: 10.1055/s-0036-1572353. [DOI] [PubMed] [Google Scholar]
- 53.de Moerloose P, Neerman-Arbez M. Congenital fibrinogen disorders. Semin Thromb Hemost. 2009;35(4):356–366. doi: 10.1055/s-0029-1225758. [DOI] [PubMed] [Google Scholar]
- 54.Fries D, Martini WZ. Role of fibrinogen in trauma-induced coagulopathy. Br J Anaesth. 2010;105(2):116–121. doi: 10.1093/bja/aeq161. [DOI] [PubMed] [Google Scholar]
- 55.Fries D. The early use of fibrinogen, prothrombin complex concentrate, and recombinant-activated factor VIIa in massive bleeding. Transfusion. 2013;53(Suppl 1):91S–95S. doi: 10.1111/trf.12041. [DOI] [PubMed] [Google Scholar]
- 56.Martini J, Maisch S, Pilshofer L, Streif W, Martini W, Fries D. Fibrinogen concentrate in dilutional coagulopathy: a dose study in pigs. Transfusion. 2014;54(1):149–157. doi: 10.1111/trf.12241. [DOI] [PubMed] [Google Scholar]
- 57.Fries D, Innerhofer P, Schobersberger W. Time for changing coagulation management in trauma-related massive bleeding. Curr Opin Anaesthesiol. 2009;22(2):267–274. doi: 10.1097/ACO.0b013e32832678d9. [DOI] [PubMed] [Google Scholar]
- 58.Haas T, Fries D, Velik-Salchner C, Reif C, Klingler A, Innerhofer P. The in vitro effects of fibrinogen concentrate, factor XIII and fresh frozen plasma on impaired clot formation after 60% dilution. Anesth Analg. 2008;106(5):1360–1365. doi: 10.1213/01.ane.0b013e3181684339. [DOI] [PubMed] [Google Scholar]
- 59.Innerhofer P, Westermann I, Tauber H, et al. The exclusive use of coagulation factor concentrates enables reversal of coagulopathy and decreases transfusion rates in patients with major blunt trauma. Injury. 2013;44(2):209–216. doi: 10.1016/j.injury.2012.08.047. [DOI] [PubMed] [Google Scholar]
- 60.Martini J, Cabrales P, Fries D, Intaglietta M, Tsai AG. Effects of fibrinogen concentrate after shock/resuscitation: a comparison between in vivo microvascular clot formation and thromboelastometry. Crit Care Med. 2013;41(11):e301–e308. doi: 10.1097/CCM.0b013e31828a4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Samama CM. Fibrinogen concentrates for acquired fibrinogen deficiencies? Semin Thromb Hemost. 2016 doi: 10.1055/s-0036-1572325. [DOI] [PubMed] [Google Scholar]
- 62.Velik-Salchner C, Haas T, Innerhofer P, et al. The effect of fibrinogen concentrate on thrombocytopenia. J Thromb Haemost. 2007;5(5):1019–1025. doi: 10.1111/j.1538-7836.2007.02481.x. [DOI] [PubMed] [Google Scholar]
- 63.Gazzeri R, Fiore C, Galarza M. Role of EVICEL fibrin sealant to assist hemostasis in cranial and spinal epidural space: a neurosurgical clinical study. Surg Technol Int. 2015;26:364–369. [PubMed] [Google Scholar]
- 64.Scognamiglio F, Travan A, Rustighi I, et al. Adhesive and sealant interfaces for general surgery applications. J Biomed Mater Res B Appl Biomater. 2015 doi: 10.1002/jbm.b.33409. [DOI] [PubMed] [Google Scholar]
- 65.Silvain J, Collet JP, Nagaswami C, et al. Composition of coronary thrombus in acute myocardial infarction. J Am Coll Cardiol. 2011;57(12):1359–1367. doi: 10.1016/j.jacc.2010.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liebeskind DS, Sanossian N, Yong WH, et al. CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke. 2011;42(5):1237–1243. doi: 10.1161/STROKEAHA.110.605576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Collet JP, Allali Y, Lesty C, et al. Altered fibrin architecture is associated with hypofibrinolysis and premature coronary atherothrombosis. Arterioscler Thromb Vasc Biol. 2006;26(11):2567–2573. doi: 10.1161/01.ATV.0000241589.52950.4c. [DOI] [PubMed] [Google Scholar]
- 68.Undas A, Wiek I, Stêpien E, Zmudka K, Tracz W. Hyperglycemia is associated with enhanced thrombin formation, platelet activation, and fibrin clot resistance to lysis in patients with acute coronary syndrome. Diabetes Care. 2008;31(8):1590–1595. doi: 10.2337/dc08-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mills JD, Ariëns RA, Mansfield MW, Grant PJ. Altered fibrin clot structure in the healthy relatives of patients with premature coronary artery disease. Circulation. 2002;106(15):1938–1942. doi: 10.1161/01.cir.0000033221.73082.06. [DOI] [PubMed] [Google Scholar]
- 70.Undas A, Podolec P, Zawilska K, et al. Altered fibrin clot structure/function in patients with cryptogenic ischemic stroke. Stroke. 2009;40(4):1499–1501. doi: 10.1161/STROKEAHA.108.532812. [DOI] [PubMed] [Google Scholar]
- 71.Undas A, Slowik A, Wolkow P, Szczudlik A, Tracz W. Fibrin clot properties in acute ischemic stroke: relation to neurological deficit. Thromb Res. 2010;125(4):357–361. doi: 10.1016/j.thromres.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 72.Bhasin N, Ariëns RA, West RM, Parry DJ, Grant PJ, Scott DJ. Altered fibrin clot structure and function in the healthy first-degree relatives of subjects with intermittent claudication. J Vasc Surg. 2008;48(6):1497–1503. 1503.e1. doi: 10.1016/j.jvs.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 73.Undas A, Zawilska K, Ciesla-Dul M, et al. Altered fibrin clot structure/function in patients with idiopathic venous thromboembolism and in their relatives. Blood. 2009;114(19):4272–4278. doi: 10.1182/blood-2009-05-222380. [DOI] [PubMed] [Google Scholar]
- 74.Undas A. How to assess fibrinogen levels and fibrin clot in clinical practice. Semin Thromb Hemost. 2016 doi: 10.1055/s-0036-1579636. [DOI] [PubMed] [Google Scholar]
- 75.Ciesienski KL, Yang Y, Ay I, et al. Fibrin-targeted PET probes for the detection of thrombi. Mol Pharm. 2013;10(3):1100–1110. doi: 10.1021/mp300610s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nair SA, Kolodziej AF, Bhole G, Greenfield MT, McMurry TJ, Caravan P. Monovalent and bivalent fibrin-specific MRI contrast agents for detection of thrombus. Angew Chem Int Ed Engl. 2008;47(26):4918–4921. doi: 10.1002/anie.200800563. [DOI] [PubMed] [Google Scholar]
- 77.Kolodziej AF, Nair SA, Graham P, et al. Fibrin specific peptides derived by phage display: characterization of peptides and conjugates for imaging. Bioconjug Chem. 2012;23(3):548–556. doi: 10.1021/bc200613e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Overoye-Chan K, Koerner S, Looby RJ, et al. EP-2104R: a fibrin-specific gadolinium-Based MRI contrast agent for detection of thrombus. J Am Chem Soc. 2008;130(18):6025–6039. doi: 10.1021/ja800834y. [DOI] [PubMed] [Google Scholar]
- 79.Levi M, van der Poll T. A short contemporary history of disseminated intravascular coagulation. Semin Thromb Hemost. 2014;40(8):874–880. doi: 10.1055/s-0034-1395155. [DOI] [PubMed] [Google Scholar]
- 80.Semeraro N, Ammollo CT, Semeraro F, Colucci M. Sepsis, thrombosis and organ dysfunction. Thromb Res. 2012;129(3):290–295. doi: 10.1016/j.thromres.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 81.Cotovio M, Monreal L, Armengou L, Prada J, Almeida JM, Segura D. Fibrin deposits and organ failure in newborn foals with severe septicemia. J Vet Intern Med. 2008;22(6):1403–1410. doi: 10.1111/j.1939-1676.2008.0178.x. [DOI] [PubMed] [Google Scholar]
- 82.Hamano A, Tanaka S, Takeda Y, Umeda M, Sakata Y. A novel monoclonal antibody to fibrin monomer and soluble fibrin for the detection of soluble fibrin in plasma. Clin Chim Acta. 2002;318(1–2):25–32. doi: 10.1016/s0009-8981(01)00779-3. [DOI] [PubMed] [Google Scholar]
- 83.Misaki T, Kitajima I, Kabata T, et al. Changes of the soluble fibrin monomer complex level during the perioperative period of hip replacement surgery. J Orthop Sci. 2008;13(5):419–424. doi: 10.1007/s00776-008-1266-y. [DOI] [PubMed] [Google Scholar]
- 84.Ieko M, Naito S, Yoshida M, et al. Plasma soluble fibrin monomer complex as a marker of coronary thrombotic events in patients with acute myocardial infarction. Tohoku J Exp Med. 2009;219(1):25–31. doi: 10.1620/tjem.219.25. [DOI] [PubMed] [Google Scholar]
- 85.Bonk R, Trowbridge C, Stammers A, et al. Soluble fibrin monomer complex and cardiopulmonary bypass. J Extra Corpor Technol. 2009;41(3):157–160. [PMC free article] [PubMed] [Google Scholar]
- 86.Kabrhel C, Mark Courtney D, Camargo CA, Jr, et al. Factors associated with positive D-dimer results in patients evaluated for pulmonary embolism. Acad Emerg Med. 2010;17(6):589–597. doi: 10.1111/j.1553-2712.2010.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wells PS, Anderson DR, Rodger M, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med. 2003;349(13):1227–1235. doi: 10.1056/NEJMoa023153. [DOI] [PubMed] [Google Scholar]
- 88.Bates SM. D-dimer assays in diagnosis and management of thrombotic and bleeding disorders. Semin Thromb Hemost. 2012;38(7):673–682. doi: 10.1055/s-0032-1326782. [DOI] [PubMed] [Google Scholar]
- 89.Ko Y-P, Flick MJ. Fibrinogen and bacterial infection. Semin Thromb Hemost. 2016 doi: 10.1055/s-0036-1579635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Flick MJ, Du X, Witte DP, et al. Leukocyte engagement of fibrin (ogen) via the integrin receptor alphaMbeta2/Mac-1 is critical for host inflammatory response in vivo. J Clin Invest. 2004;113(11):1596–1606. doi: 10.1172/JCI20741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Flick MJ, LaJeunesse CM, Talmage KE, et al. Fibrin(ogen) exacerbates inflammatory joint disease through a mechanism linked to the integrin alphaMbeta2 binding motif. J Clin Invest. 2007;117(11):3224–3235. doi: 10.1172/JCI30134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Drew AF, Liu H, Davidson JM, Daugherty CC, Degen JL. Wound-healing defects in mice lacking fibrinogen. Blood. 2001;97(12):3691–3698. doi: 10.1182/blood.v97.12.3691. [DOI] [PubMed] [Google Scholar]
- 93.Riopel M, Trinder M, Wang R. Fibrin, a scaffold material for islet transplantation and pancreatic endocrine tissue engineering. Tissue Eng Part B Rev. 2015;21(1):34–44. doi: 10.1089/ten.TEB.2014.0188. [DOI] [PubMed] [Google Scholar]
- 94.Davis ME, Hsieh PC, Grodzinsky AJ, Lee RT. Custom design of the cardiac microenvironment with biomaterials. Circ Res. 2005;97(1):8–15. doi: 10.1161/01.RES.0000173376.39447.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Allen P, Melero-Martin J, Bischoff J. Type I collagen, fibrin and PuraMatrix matrices provide permissive environments for human endothelial and mesenchymal progenitor cells to form neovascular networks. J Tissue Eng Regen Med. 2011;5(4):e74–e86. doi: 10.1002/term.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martineau L, Doillon CJ. Angiogenic response of endothelial cells seeded dispersed versus on beads in fibrin gels. Angiogenesis. 2007;10(4):269–277. doi: 10.1007/s10456-007-9079-8. [DOI] [PubMed] [Google Scholar]
- 97.Itosaka H, Kuroda S, Shichinohe H, et al. Fibrin matrix provides a suitable scaffold for bone marrow stromal cells transplanted into injured spinal cord: a novel material for CNS tissue engineering. Neuropathology. 2009;29(3):248–257. doi: 10.1111/j.1440-1789.2008.00971.x. [DOI] [PubMed] [Google Scholar]
- 98.Bensaïd W, Triffitt JT, Blanchat C, Oudina K, Sedel L, Petite H. A biodegradable fibrin scaffold for mesenchymal stem cell transplantation. Biomaterials. 2003;24(14):2497–2502. doi: 10.1016/s0142-9612(02)00618-x. [DOI] [PubMed] [Google Scholar]
- 99.Ahmad E, Fatima MT, Hoque M, Owais M, Saleemuddin M. Fibrin matrices: The versatile therapeutic delivery systems. Int J Biol Macromol. 2015;81:121–136. doi: 10.1016/j.ijbiomac.2015.07.054. [DOI] [PubMed] [Google Scholar]
- 100.Gonçalves NR, Whelan R, Foxe JJ, Lalor EC. Towards obtaining spatiotemporally precise responses to continuous sensory stimuli in humans: a general linear modeling approach to EEG. Neuroimage. 2014;97:196–205. doi: 10.1016/j.neuroimage.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 101.Piechocka IK, Bacabac RG, Potters M, Mackintosh FC, Koenderink GH. Structural hierarchy governs fibrin gel mechanics. Biophys J. 2010;98(10):2281–2289. doi: 10.1016/j.bpj.2010.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ariëns RA, Lai TS, Weisel JW, Greenberg CS, Grant PJ. Role of factor XIII in fibrin clot formation and effects of genetic polymorphisms. Blood. 2002;100(3):743–754. doi: 10.1182/blood.v100.3.743. [DOI] [PubMed] [Google Scholar]
- 103.Kurniawan NA, Grimbergen J, Koopman J, Koenderink GH. Factor XIII stiffens fibrin clots by causing fiber compaction. J Thromb Haemost. 2014;12(10):1687–1696. doi: 10.1111/jth.12705. [DOI] [PubMed] [Google Scholar]
- 104.Shevchenko RV, James SL, James SE. A review of tissue-engineered skin bioconstructs available for skin reconstruction. J R Soc Interface. 2010;7(43):229–258. doi: 10.1098/rsif.2009.0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dikovsky D, Bianco-Peled H, Seliktar D. The effect of structural alterations of PEG-fibrinogen hydrogel scaffolds on 3-D cellular morphology and cellular migration. Biomaterials. 2006;27(8):1496–1506. doi: 10.1016/j.biomaterials.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 106.Janmey PA, Winer JP, Weisel JW. Fibrin gels and their clinical and bioengineering applications. J R Soc Interface. 2009;6(30):1–10. doi: 10.1098/rsif.2008.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]