Abstract
Cell outer membranes contain glycosphingolipids and protein receptors, which are integrated into glycoprotein microdomains, known as lipid rafts, that float freely in the membrane bilayer. These structures play an important role in assembling signaling molecules (e.g., Rac-1, RhoH, and Lyn) together with surface receptors, such as the CXCR4 receptor for α-chemokine stromal-derived factor 1 (SDF-1), the α1β4 integrin receptor (VLA-4) for vascular cell adhesion molecule-1 (VCAM-1), and the c-kit receptor for stem cell factor (SCF), which together regulate several aspects of hematopoietic stem/progenitor cell (HSPC) biology. Here, we discuss the role of lipid raft integrity in the retention and quiescence of normal HSPCs in bone marrow niches as well as in regulating HSPC mobilization and homing. We will also discuss the pathological consequences of the defect in lipid raft integrity seen in paroxysmal nocturnal hemoglobinuria (PNH) and the emerging evidence for the involvement of lipid rafts in hematological malignancies.
Keywords: Hematopoietic stem cells, stem cell mobilization, stem cell homing, lipid rafts, PNH, complement cascade, proteolytic enzymes, lipolytic microenvironment
Introduction
The cell (or cytoplasmic) membrane is a selectively permeable structure that separates the interior of the cell from the outside environment.1 It consists of a phospholipid bilayer with embedded proteins that is held together via non-covalent interactions between the hydrophobic tails. Under physiological conditions, phospholipid molecules in the cell membrane are in a liquid crystalline state; however, the cytoplasmic membranes of cells also contain combinations of glycosphingolipids and protein receptors organized into glycoprotein microdomains, called lipid rafts.2,3 Two types of lipid rafts have been proposed to occur in cell membranes: planar lipid rafts and invaginated lipid rafts, called caveolae.4,5 Planar lipid rafts and caveolae are examples of cholesterol-enriched microdomains in the cell membrane, and cholesterol can be envisioned as a kind of molecular glue that holds the components of lipid rafts together and is important for their integrity.
A vast amount of evidence has accumulated that lipid rafts play an important role in several processes regulating cell biology.4–10 Specifically, from an historical point of view, these microscopic cholesterol-enriched structures are important in assembling signaling molecules together with cell surface receptors and have been identified as playing a primary role in signaling through the immunoglobulin E receptor, T cell antigen receptor, and B cell antigen receptor.11–13 Moreover, recent evidence indicates that lipid rafts are also involved in signaling through many other receptors that have intrinsic tyrosine kinase activity (e.g., c-kit, insulin receptor, and epidermal growth factor receptor) as well as G protein-coupled receptors (e.g., CXCR4).6,14 Lipid rafts also play an important role in cell adhesion and ensure the proper function of integrin receptors (e.g., VLA-4) and their assembly with the cytoskeleton. Moreover, lipid rafts are located in cell membrane areas from which important cell–cell communication structures known as extracellular microvesicles (ExMV) are generated.15,16 Finally, lipid rafts are also often involved in intracellular entry of both non-enveloped and enveloped viruses.17
Evidence has also accumulated that lipid rafts regulate several aspects of hematopoietic stem/progenitor cell (HSPC) biology. In this review, we will discuss the role of lipid rafts in the retention and quiescence of normal HSPCs in bone marrow niches as well as in regulating their migration, mobilization, and homing. Underpinning these roles are the CXCR4 receptor for α-chemokine stromal-derived factor 1 (SDF-1), the α1β4 integrin receptor (VLA-4) for vascular adhesion molecule 1 (VCAM-1), as well as the complement cascade-inhibiting cell surface receptors CD55 and CD59. The absence of CD55 and CD59 on the cell surface combined with a defect in lipid raft formation and their association with CXCR4 and VLA-4 is responsible for the onset of paroxysmal nocturnal hemoglobinuria (PNH),18,19 which can be considered as a disease model of lipid raft integrity. Lipid rafts are also required for c-kit signaling, which regulates cell survival and proliferation after stimulation by stem cell factor (SCF).6 Evidence also indicates that lipid rafts may play a role in hematological malignancies.20–24
Visualization and detection of the presence of lipid rafts in cell membranes
Generally, lipid rafts are small microdomains ranging from 10–200 nm in size, which is below the diffraction limit of classical light microscopes, and thus they cannot be directly visualized by conventional light microscopy. This problem could potentially be solved by using a super-resolution microscopy, such as stimulated emission depletion (STED) or structured illumination microscopy. Currently, lipid rafts can be visualized and studied by employing fluorescence microscopy after labelling of the cell surface with fluorescence-conjugated cholera toxin B subunit, which binds to a lipid raft constituent and marker, the ganglioside GM1.25,26 Examples of other optical techniques for detecting the presence of lipid rafts in cell membranes include fluorescence correlation and cross-correlation spectroscopy (FCS/FCCS) or fluorescence resonance energy transfer (FRET), which detect either the mobility of the fluorochrome in the membrane or its distribution.3
Lipid rafts can also be studied by employing western blotting after separation of cell membrane fractions enriched in lipid rafts and colocalizing these fractions with proteins that are components of lipid rafts (e.g., CXCR4, VLA-4, c-kit, and Lyn).6,14,25,26 Another experimental strategy is to deplete cholesterol from lipid rafts by employing i) methyl-β-cyclodextrin (MβCD), ii) inhibitors of cholesterol synthesis (statins), or iii) drugs that sequester cholesterol, such as nystatin and amphotericin.25,26 These membrane cholesterol-targeted experimental manipulations destroy lipid raft structure and negatively affect its biological effects.
The integrity of lipid rafts may also be perturbed by exposing cell membranes to phospholipase C, a granulocyte-derived enzyme that targets glycosylophosphatidylinositol glycolipid anchor (GPI-A), which is attached during posttranslantional modification to the C-terminus of certain proteins that are included in lipid rafts.27 The most important of these proteins are the complement cascade inhibitors CD55 and CD59; the truncated isoform of the homing molecule VCAM-1, which is a ligand for the VLA-4 receptor expressed on murine HSPCs; as well as other GPI-anchored cell surface proteins, such as uPAR, the Thy-1 marker, acetylocholinesterase, and placental alkaline phosphatases.28,29 These proteins are thought to be preferentially located in lipid rafts, suggesting a high level of organization within these plasma membrane microdomains.
Interestingly, while CXCR4 and VLA-4 do not contain GPI-A within their molecular structure, a defect in GPI-A expression due to mutation of the PIGA gene (which is responsible for GPI-A biosynthesis and is mutated in PNH patient cells18,19), GPI-A depletion from HSPCs after exposure to phospholipase Cβ2 (PLC-β2), or cholesterol depletion in membrane lipid rafts by MβCD all lead to impaired function of CXCR4 and VLA-4 receptors, which are so important for bone marrow retention and migration of HSPCs.30–34 Similarly, it has been demonstrated that survival and proliferation signals mediated in HSPCs upon stimulation by SCF requires the presence of the c-kit receptor in membrane lipid rafts.6
All the experimental strategies described above have been employed to study the role of lipid rafts in regulating several aspects of HSPC biology.
Lipid rafts and the retention of HSPCs in bone marrow niches
HSPCs are retained in BM hematopoietic niches due to active interactions between CXCR4 and VLA-4 expressed on their surface and the corresponding ligands, SDF-1 and VCAM-1, present on cells that comprise hematopoietic stem cell niches.30–36 While in mice the truncated isoform of VCAM-1, as mentioned above, is a GPI-anchored protein, SDF-1, which is mainly expressed in CXCL12 (another name for SDF-1)-abundant reticular (CAR) cells in the BM microenvironment as well as by osteoblasts in osteoblastic niches and endothelial cells in endothelial stem cell niches, is not associated with GPI-A.29–37.
Importantly, since both CXCR4 and VLA-4 receptors are lipid raft-associated proteins, their optimal biological function depends on their inclusion in these small membrane domains (Figure 1 panel A). Specifically, their inclusion in lipid rafts is required for optimal association with members of the Rho guanosine triphosphate (GTPase) subfamily of the Ras superfamily, such as RhoH and Rac-1, which are crucial in regulating the actin cytoskeleton as well as adhesion and chemotaxis of HSPCs.7,14 Interestingly, it has been postulated that the calveolin proteins, present in caveolae, and flotillin proteins, expressed in planar membrane lipid rafts, have the ability to recruit a variety of signaling molecules into lipid rafts.4,5
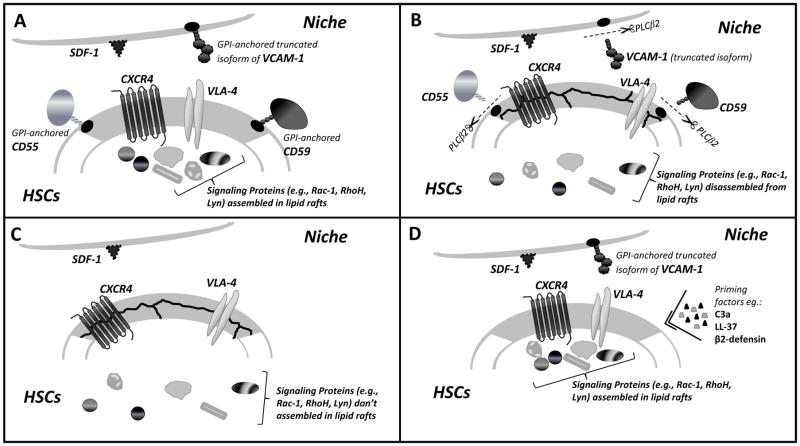
Figure 1.
Panel A. The role of lipid rafts in retention of HSPCs in stem cell niches. Membrane lipid rafts, shown as gray areas in the cell membrane, assemble with several cell surface receptors involved in retention of HSPCs in bone marrow niches (e.g., CXCR4 and VLA-4) or inhibition of the complement cascade (CD55 and CD59). As shown, CD55, CD59 and the murine truncated isoform of VCAM-1 are GPI-anchored proteins. While the role of lipid rafts is well described for cell membranes present in HSPCs, more studies are needed to determine whether bone marrow-retention ligands for HSPCs in stem cell niches, such as SDF-1 and VCAM-1, are also concentrated in lipid rafts in cells lining stem cell niches (e.g., osteoblasts, endothelial cells, and CAR cells). Panels B and C. Disassembly of lipid rafts and the egress of HSPCs into blood. Lipid raft disassembly results in the weakening of CXCR4–SDF-1 and VLA-4–VCAM-1 retention signals for HSPCs in bone marrow stem cell niches and facilitates mobilization. This effect is observed during pharmacological mobilization due to the release of phospholipase Cβ2 from granulocytes and monocytes (panel B) or in paroxysmal nocturnal hemoglobinuria (PNH) patients due to lack of expression of GPI-A and thus lack of GPI-anchored proteins (panel C). Disassembly of lipid rafts in HSPCs, as shown, weakens the interaction of both receptors with downstream signaling molecules involved in bone marrow retention. While in the case of HSPC mobilization a pivotal role is played by enzymatic digestion of GPI-anchored proteins by PLC-Cβ2, PNH results from an acquired mutation for the gene encoding GPI-A. Panel D. CXCR4 and VLA-4 inclusion in lipid rafts facilitates homing of HSPCs. Incorporation of the crucial BM homing receptors CXCR4 and VLA-4 into lipid rafts may be enhanced by exposure of HSPCs to certain molecules related to innate immunity, such as the C3a complement cascade cleavage fragment, LL-37 (cathelicidin), or β2-defensin, released from bone marrow stromal cells after radiochemotherapy-induced conditioning for transplantation. Incorporation of the CXCR4 receptor into lipid rafts sensitizes the responsiveness of HSPCs to an SDF-1 gradient. Colocalization of VLA-4 in membrane lipid rafts also plays an important role in tethering HSPCs to VCAM-1 in BM niches. As shown, lipid rafts facilitate the interaction of both receptors expressed on HSPCs with several downstream signaling molecules involved in migration, homing, and cell survival.
For simplicity, the interaction of lipid raft-associated c-kit receptor, which inhibits apoptosis and regulates proliferation of HSPCs, with its ligand, membrane-bound or soluble SCF, is not shown in the figure.
An important biological effect of the assembly of several receptors, adhesion molecules, and signaling molecules into lipid rafts is maintaining the dormancy of HSPCs and preventing them from undergoing apoptosis. As demonstrated in an elegant study, 8 lipid raft clustering plays an important role in regulating dormancy of the most primitive hematopoietic stem cells (HSCs). Specifically, while CD34–Kit+Sca-1+Lin– HSCs freshly isolated from murine BM lack lipid raft clustering, in CD34+ KSL Kit+Sca-1+Lin– hematopoietic progenitor cells, the c-kit receptor is condensed in lipid raft clusters.8 Moreover cytokine stimulation promoted lipid raft formation and activated downstream signaling pathways, including PI3K–Akt–FOXO.8 This latter effect is supported by the observation that the integrity of lipid rafts causes sustained nuclear accumulation of FOXO transcription factors, which regulate genes involved in metabolism and redox control that antagonize apoptosis in dormant stem cells.8 On the other hand, as reported by another group of investigators, c-kit receptor recruitment to lipid rafts is required for optimal activation of PI3K–Akt and Src family kinase signaling pathways and SCF-mediated proliferation.6 Based on this finding, lipid raft reorganization appears to be indispensable for CD34–Kit+Sca-1+Lin– HSCs to move from the hibernation state back into the cell cycle.8 In support of this latter notion, when stimulated with cytokines in the presence of the membrane lipid raft inhibitor MβCD, the vast majority of CD34–Kit+Sca-1+Lin– HSCs freshly isolated from murine BM remained in the G0 phase of the cell cycle.8
While most of the studies with lipid rafts and hematopoietic cells were performed on cells isolated from BM niches, more work is needed to understand the role of lipid rafts in regulating HSPCs when these cells reside in their specific niches. Moreover, while there are several studies on the role of lipid rafts expressed on the surface of HSPCs, lipid rafts could also play a role in integrating homing molecules on the surface of cells lining the stem cell niches (e.g., osteoblasts or CAR cells) to provide proper signals for HSPCs. Thus, studies are needed to investigate whether, in addition to VCAM-1, optimal exposure of HSPCs to membrane-bound SCF or SDF-1 also depends on lipid raft assembly on osteoblasts or CAR cells.
Lipid raft disassembly and mobilization of HSPCs
The retention of HSPCs seems to be an active process involving the normal function of lipid rafts in counteracting the gradients of bioactive phosphosphingolipids, such as sphingosine-1-phosphate (S1P) and ceramide-1-phosphate (C1P), which are potent chemotactic factors for HSPCs in blood. Even under steady-state conditions, their levels are sufficient to promote egress from BM into PB.38–42
The number of HSPCs increases in circulating blood in response to infection, tissue or organ damage, and strenuous exercise and can be enhanced up to 100 fold by pharmacology-induced mobilization due to administration of certain pro-mobilizing drugs, such as granulocyte-colony stimulating factor (G-CSF), the CXCR4 inhibitor plerixafor (AMD3100), or the VLA-4 inhibitor BIO4860.30–36,43
While the phenomenon of stem cell mobilization is still not completely understood, it is known that an early event involves activation of the complement cascade and induction of a proteolytic microenvironment in bone marrow due to release of several proteolytic enzymes from granulocytes and monocytes residing in marrow tissue (e.g., elastase, cathepsin G, and the metalloproteinases MMP-2 and MMP-9).30–36 These proteolytic enzymes collectively digest CXCR4–SDF-1 and VCAM-1–VLA-4 proteins and thus attenuate the membrane lipid raft-dependent receptor–ligand interaction axes that retain HSPCs in their bone marrow niches.34 Moreover, as recently reported, proteins of the membrane type 1 MMP (MT1-MMP) protein subfamily are incorporated into membrane lipid rafts in response to G-CSF stimulation and contribute to conversion of pro-metalloproteinase 2 into MMP-2, which facilitates the HSPC mobilization process.44
On the other hand, it is well known that, in addition to proteolytic enzymes, granulocytes and monocytes are sources of phospholipase Cβ2 (PLC-β2), and we observed its release from neutrophils during the mobilization process. PLC-β2 is an enzyme that targets GPI-A, which is important for maintaining the integrity of lipid rafts and optimal signaling from CXCR4 and VLA-4 receptors, and removes several types of GPI-anchored molecules, such as the truncated isoform of VCAM-1, CD55, CD59, and uPAR, from the cell membrane.27
Thus, PLC-β2 released during the mobilization of HSPCs facilitates stem cell detachment from their niches by perturbing the membrane lipid raft integrity required for optimal bone marrow-retention signaling through the CXCR4 and VLA-4 receptors (Figure 1 panel B). An additional PLC-β2 pro-mobilizing effect in mice is due to removal of the GPI-anchored truncated isoform of VCAM-1, which is involved in BM retention by cells comprising the stem cell niche, and due to decreasing expression of the complement inhibitors CD55 and CD59 on the surface of cells, which leads to enhanced activation of the mobilization-promoting complement cascade in the BM microenvironment.27 As already reported, we directly confirmed the pro-mobilizing role of this lipolytic enzyme, which is involved in the disintegration of membrane lipid rafts, by employing PLCβ2−/− mice.27
Based on this mechanism, disassembly of lipid rafts due to PLCβ2-mediated enzymatic removal of GPI-anchored proteins from cell membranes (Figure 1 panel B) or lack of GPI-A due to an acquired mutation in the PIG-A gene, as seen in PNH (Figure 1 panel C), facilitates the egress of stem cells from bone marrow into circulating blood.
The role of lipid rafts in the homing of HSPCs
Homing of HSPCs from circulating blood to bone marrow is the reverse process to mobilization and precedes HSPC engraftment.33 A crucial role in this process is played by chemoattractants released in the bone marrow microenvironment in response to myeloablative conditioning by radio or chemotherapy, which is employed to eliminate recipient hematopoiesis.
It is widely accepted that SDF-1 is a major chemoattractant that is upregulated in BM conditioned by myeloblative treatment. Despite the important role of this α-chemokine, several observations indicate the involvement of other factors in this process.46–48 Interestingly, we observed that the proteolytic microenvironment induced by myeloablative conditioning for transplantation may also enzymatically degrade the SDF-1 protein, which suggests the involvement of other supportive mechanisms.38,49
To explain these observations, we have to consider the presence of alternative homing pathways. These alternative pathways include, on the one hand, the involvement of bioactive phosphosphingolipids (S1P and C1P) and extracellularly released nucleotides (e.g., UTP and ATP), which may support or even replace SDF-1–CXCR4-mediated homing, as seen in transplants of CXCR4−/− fetal liver HSPCs.41,49,50 Another possibility highly relevant to the topic of this review is the modulation of lipid raft integrity by certain soluble factors released in the bone marrow microenvironment after conditioning for transplantation. Specifically, certain small molecules related to inflammation, such as cleavage fragments (C3a and desArgC3a) of the third component of the complement cascade (C3); cathelicidin (LL-37) and β2-defensin, released by bone marrow stromal cells; as well as hyaluronic acid, fibronectin, and soluble VCAM-1, ICAM-1, and uPAR, may enhance the responsiveness of HSPCs to a shallow SDF-1 gradient.14,25,26,51,52 This phenomenon is known in the literature as the HSPC priming effect (Figure 1 panel D).
At the molecular level, this priming phenomenon is based on the fact that at least some of the priming factors (e.g., LL-37 and C3a) promote physical incorporation of the CXCR4 receptor into membrane lipid rafts.14,25,26 This has been demonstrated in cells exposed to C3a or LL-37 by the presence of both these receptors (CXCR4 and VLA-4) in membrane fractions enriched for lipid rafts. Specifically, western blotting and confocal microscopy were employed to show colocalization of CXCR4 and VLA-4 with a marker of lipid rafts, the ganglioside GM1.14,25,26 This priming phenomenon facilitates optimal signaling and the chemotactic responsiveness of HSPCs to an SDF-1 gradient. At the same time, however, the same phenomenon occurs in the case of VLA-4, which, if incorporated into lipid rafts, assures optimal retention of HSPCs in the bone marrow microenvironment by interacting with VCAM-1 in the stem cell niche.
Based on what has been presented so far, the disassembly of receptors and signaling molecules from lipid rafts should promote egress of HSPCs from bone marrow into blood, and, by contrast, their physical assembly into lipid rafts is important in homing and retention of HSPCs in bone marrow. Therefore, strategies that enhance incorporation of CXCR4 and VLA-4 into membrane lipid rafts could find practical application in enhancing the homing of HSPCs.14,25,26,41
Optimal homing is particularly important in cases when the number of HSPCs is limited and is crucial, for example, in umbilical cord blood (UCB) transplants. To explore this possibility, a clinical trial to prime UCB by a short ex vivo exposure to C3a before infusion into the patient has recently been initiated.53 Another interesting strategy to enhance homing of HSPCs by modulation of lipid raft-mediated mechanisms has recently been proposed by another group.54 Specifically, a short, mild heat treatment (39.5°C) primes human CD34+ umbilical cord blood cells for migration up an SDF-1 gradient and enhances engraftment of these cells in an immunodeficient mouse model. This treatment was associated with increased expression of CXCR4 on CD34+ cells and enhanced colocalization of CXCR4 within lipid raft domains with Rac1, a GTPase that is crucial for cell migration and adhesion, with CXCR4 recruitment to the lipid raft essential for this effect. Based on this intriguing observation, mild heating of UCB-derived HSPCs before transplantation may be a simple and inexpensive strategy to enhance engraftment.54
PNH as a model lipid raft integrity disorder
As mentioned above, a defect in lipid raft formation and integrity plays a major role in paroxysmal nocturnal hemoglobinuria (PNH). From the clinical and molecular points of view, it is an acquired hemolytic anemia and stem cell disease due to a somatic mutation in the PIGA gene, which is responsible for GPI-A biosynthesis.18 PNH-affected HSPCs, in which GPI-A is missing, produce red blood cells, leucocytes, and platelets, which are susceptible to complement-mediated lysis because of the lack of CD55 and CD59 complement cascade inhibitory proteins on erythroid cells.18 Over time, the mutated stem cell clone that produces GPI-A-deficient cells repopulates the entire hematopoietic system and outgrows normal HSPCs.
Since GPI-A is not a potential tumor suppressor gene, the reason why PNH-affected HSPCs expand over time in BM and outgrow normal hematopoietic cells is still poorly understood. To explain this phenomenon, several theories have been proposed but none explains the pathogenesis of this disorder in a fully convincing way.18
Based on our work with lipid rafts and knowing that the presence of GPI-A is critical in lipid raft integrity and thus optimal CXCR4- and VLA-4-based retention of HSPCs in bone marrow, we hypothesized that PNH starts with a primary lipid raft formation defect that, in the course of this disease, precipitates other events involved in its pathogenesis (Figure 1 panel C).19
By employing direct visualization using confocal microscopy, we have also demonstrated that BM-purified CD34+ PNH cells show a defect in incorporation of both CXCR4 and VLA-4 into membrane lipid rafts, respond weakly to SDF-1 stimulation, and show impaired adhesion and chemotaxis in response to an SDF-1 gradient.55 By contrast, these cells respond robustly to an S1P gradient. In addition to primary patient cells, similar results were obtained with the human GPI-A-deficient Jurkat T cell line.55
Our findings explain the expansion of PNH-mutated HSPCs in BM in a novel way.19 Specifically, since PNH-mutated HSPCs have defective adhesion due to a defect in lipid raft integrity, they are more mobile in the BM microenvironment and over time expand and outcompete normal HSPCs from their stem cell niches. These cells, however, respond normally to S1P, which under physiological concentrations is a major chemotactic factor for HSPCs.38–40 Of note, erythrocytes are highly enriched in this active phospho-sphingolipid and release it into the blood during lysis. The S1P level is already high in blood under steady-state conditions and is additionally increased during complement-mediated hemolysis.19,55
The role of lipid rafts in hematopoietic malignancies
Evidence has accumulated that lipid rafts also play an important role in malignant hematopoiesis. Thus, it is important to explore in more detail whether changes in the composition of lipid rafts between normal and malignant hematopoietic cells could be used in developing new adjuvant anti-leukemic therapies.20–22 It has been demonstrated, for example, that constitutive localization of death receptor 4 (DR4) is mandatory for TRAIL-induced apoptosis in B cell hematopoietic malignancies. In a similar way, the induction of apoptosis in acute myeloid leukemia cells by CD44 ligation with A3D8 antibody also requires the presence of CD44 in lipid rafts.21,22 Furthermore, differences between individuals with B cell lymphomas with respect to the therapeutic effect of rituximab may also depend on the membrane cholesterol content, which promotes lipid raft formation. Specifically, rituximab may, in some patients, have a paradoxical anti-apoptotic effect related to recruitment and activation of Syk and Akt kinases in membrane lipid rafts.24 Cholesterol depletion in such cases leads to an enhanced therapeutic effect by rituximab. Thus, modulation of lipid rafts could provide a new strategy for enhancing the anti-lymphoma action of this antibody.24
Finally, an important question to be addressed is also how certain drugs or dietary habits truly influence lipid raft integrity. For example, it has been proposed that omega-3 polyunsaturated fatty acids affect formation of membrane lipid rafts.56,57 It would also be interesting to see whether certain pro-mobilization effects of endothelial progenitor cells and other stem cells in patients treated with statins could be explained by the effect of these drugs on cholesterol availability for proper lipid raft formation and thus retention of stem cells in BM niches.58 Similarly, statins administered to leukemic patients could also potentially modulate the responsiveness of leukemic blasts to therapeutic agents, and further studies are needed to address the significance of this phenomenon.24 As discussed above, lipid rafts could also be crucial in maintaining the dormancy of normal HSPCs and in inhibiting apoptosis.
Overall, we expect that ex vivo strategies based on enhancement of lipid raft formation in HSPCs prepared for transplantation would be beneficial for better homing and subsequent engraftment of HSPCs. On the other hand, strategies facilitating lipid raft disassembly would improve mobilization and thus the harvesting of HSPCs for transplantation. In the case of malignant hematopoietic cells, any strategies to modulate lipid raft expression should be individually assessed, depending on the mechanism by which they modulate the therapeutic response to potential treatments.20–22,24,59–62
Future studies and conclusions
The mechanisms behind lipid raft formation and their role in biological processes need further clarification. While a vast amount of direct and indirect evidence indicates their presence in cell membranes, there is no consensus about their ultimate size, the timescale of their existence, and even their existence.
The overall concept of lipid rafts is still evolving, and more investigations are needed to fully understand their structure and function. The membrane lipid raft model is mainly built upon the existence of line tension, which minimizes the free energy between immiscible disordered and ordered liquid phases. This line tension is observed in membrane models, but so far it has not been readily observed in cell systems. There is also still a lack of consensus on lipid raft size and the timescale of their existence. It has even been proposed that some of the effects of cholesterol depletion, which is used to determine lipid raft function, may be related to the disruption of another lipid, PI(4,5)P2, that regulates the cytoskeleton.63 Interestingly, it has even been proposed that proteins and not lipids are primarily involved in the formation of lipid rafts.56
Despite all these concerns, membrane lipid rafts seem to explain several biological processes that play an important role in the retention of HSPCs in BM niches. Their integrity is also crucial in maintaining HSPC quiescence and in homing of transplanted cells into the BM microenvironment. On the other hand, the disintegration of lipid rafts, which is induced after administration of mobilizing agents or by mutation of GPI-A, as seen in PNH patients, facilitates egress of HSPCs into the blood. Based on this effect, pharmacological modulation of lipid raft function may lead to development of better HSPC mobilization and homing protocols. Further studies are also needed to better understand the roles of lipid rafts in drug resistance in hematopoietic malignancies and in differentially regulating long-term hematopoietic stem cells compared with more differentiated hematopoietic progenitors.6,8
Acknowledgments
This work was supported by NIH grants 2R01 DK074720 and R01HL112788 and the Stella and Henry Endowment to MZR.
Footnotes
Conflicts of interest.
None.
References
- 1.Goñi FM. The basic structure and dynamics of cell membranes: an update of the Singer-Nicolson model. Biochim Biophys Acta. 2014;1838:1467–1476. doi: 10.1016/j.bbamem.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Róg T, Vattulainen I. Cholesterol, sphingolipids, and glycolipids: What do we know about their role in raft-like membranes? Chem Phys Lipids. 2014;184:82–104. doi: 10.1016/j.chemphyslip.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Deleu M, Crowet JM, Nasir MN, Lins L. Complementary biophysical tools to investigate lipid specificity in the interaction between bioactive molecules and the plasma membrane: A review. Biochim Biophys Acta. 2014;1838:3171–3190. doi: 10.1016/j.bbamem.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 4.Reeves VL, Thomas CM, Smart EJ. Lipid rafts, caveolae and GPI-linked proteins. Adv Exp Med Biol. 2012;729:3–13. doi: 10.1007/978-1-4614-1222-9_1. [DOI] [PubMed] [Google Scholar]
- 5.Staubach S, Hanisch FG. Lipid rafts: signaling and sorting platforms of cells and their roles in cancer. Expert Rev Proteomics. 2011;8:263–277. doi: 10.1586/epr.11.2. [DOI] [PubMed] [Google Scholar]
- 6.Jahn T, Leifheit E, Gooch S, Sindhu S, Weinberg K. Lipid rafts are required for Kit survival and proliferation signals. Blood. 2007;110:1739–1747. doi: 10.1182/blood-2006-05-020925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chae HD, Lee KE, Williams DA, Gu Y. Cross-talk between RhoH and Rac1 in regulation of actin cytoskeleton and chemotaxis of hematopoietic progenitor cells. Blood. 2008;111:2597–2605. doi: 10.1182/blood-2007-06-093237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamazaki S, Iwama A, Takayanagi S, Morita Y, Eto K, Ema H, et al. Cytokine signals modulated via lipid rafts mimic niche signals and induce hibernation in hematopoietic stem cells. EMBO J. 2006;25:3515–3523. doi: 10.1038/sj.emboj.7601236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee MY, Ryu JM, Lee SH, Park JH, Han HJ. Lipid rafts play an important role for maintenance of embryonic stem cell self-renewal. J Lipid Res. 2010;51:2082–2089. doi: 10.1194/jlr.M001545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan JL. Cell biology. Integrins, rafts, Rac, and Rho. Science. 2004;303:773–774. doi: 10.1126/science.1094376. [DOI] [PubMed] [Google Scholar]
- 11.Sheets ED, Holowka D, Baird B. Membrane organization in immunoglobulin E receptor signaling. Curr Opin Chem Biol. 1999;3:95–99. doi: 10.1016/s1367-5931(99)80017-9. [DOI] [PubMed] [Google Scholar]
- 12.Janes PW, Ley SC, Magee AI, Kabouridis PS. The role of lipid rafts in T cell antigen receptor (TCR) signalling. Semin Immunol. 2000;12:23–34. doi: 10.1006/smim.2000.0204. [DOI] [PubMed] [Google Scholar]
- 13.Gupta N, DeFranco AL. Lipid rafts and B cell signaling. Semin Cell Dev Biol. 2007;18:616–626. doi: 10.1016/j.semcdb.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wysoczynski M, Reca R, Ratajczak J, Kucia M, Shirvaikar N, Honczarenko M, et al. Incorporation of CXCR4 into membrane lipid rafts primes homing-related responses of hematopoietic stem/progenitor cells to an SDF-1 gradient. Blood. 2005;105:40–48. doi: 10.1182/blood-2004-04-1430. [DOI] [PubMed] [Google Scholar]
- 15.Bauer N, Wilsch-Bräuninger M, Karbanová J, Fonseca AV, Strauss D, Freund D, et al. Haematopoietic stem cell differentiation promotes the release of prominin-1/CD133-containing membrane vesicles--a role of the endocytic-exocytic pathway. EMBO Mol Med. 2011;3:398–409. doi: 10.1002/emmm.201100147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan SS, Yin Y, Lee T, Lai RC, Yeo RWY, Zhang B, et al. Therapeutic MSC exosomes are derived from lipid raft microdomains in the plasma membrane. J Extracell Vesicles. 2013;2 doi: 10.3402/jev.v2i0.22614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ziello JE, Huang Y, Jovin IS. Cellular endocytosis and gene delivery. Mol Med. 2010;16:222–229. doi: 10.2119/molmed.2009.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brodsky RA. How do PIG-A mutant paroxysmal nocturnal hemoglobinuria stem cells achieve clonal dominance? Expert Rev Hematol. 2009;2:353–356. doi: 10.1586/ehm.09.35. [DOI] [PubMed] [Google Scholar]
- 19.Ratajczak J, Kucia M, Mierzejewska K, Liu R, Kim CH, Natarajan N, et al. A novel view of paroxysmal nocturnal hemoglobinuria pathogenesis: more motile PNH hematopoietic stem/progenitor cells displace normal HSPCs from their niches in bone marrow due to defective adhesion, enhanced migration and mobilization in response to erythrocyte-released sphingosine-1 phosphate gradient. Leukemia. 2012;26:1722–1725. doi: 10.1038/leu.2012.46. [DOI] [PubMed] [Google Scholar]
- 20.Marconi M, Ascione B, Ciarlo L, Vona R, Garofalo T, Sorice M, et al. Constitutive localization of DR4 in lipid rafts is mandatory for TRAIL-induced apoptosis in B-cell hematologic malignancies. Cell Death Dis. 2013;4:e863. doi: 10.1038/cddis.2013.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qian H, Xia L, Ling P, Waxman S, Jing Y. CD44 ligation with A3D8 antibody induces apoptosis in acute myeloid leukemia cells through binding to CD44s and clustering lipid rafts. Cancer Biol Ther. 2012;13:1276–1283. doi: 10.4161/cbt.21784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uddin S, Al-Kuraya KS. Localization of death receptor 4 in lipid rafts sensitizes chronic lymphocytic leukemia to chemotherapeutic drug mediated apoptosis. Leuk Lymphoma. 2011;52:1176–1177. doi: 10.3109/10428194.2011.569963. [DOI] [PubMed] [Google Scholar]
- 23.Osterhues A, Liebmann S, Schmid M, Buk D, Huss R, Graeve L, et al. Stem cells and experimental leukemia can be distinguished by lipid raft protein composition. Stem Cells Dev. 2006;15:677–686. doi: 10.1089/scd.2006.15.677. [DOI] [PubMed] [Google Scholar]
- 24.Nozaki Y, Mitsumori T, Yamamoto T, Kawashima I, Shobu Y, Hamanaka S, et al. Rituximab activates Syk and AKT in CD20-positive B cell lymphoma cells dependent on cell membrane cholesterol levels. Exp Hematol. 2013;41:687–696. doi: 10.1016/j.exphem.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Ratajczak MZ, Reca R, Wysoczynski M, Kucia M, Baran JT, Allendorf DJ, et al. Transplantation studies in C3-deficient animals reveal a novel role of the third complement component (C3) in engraftment of bone marrow cells. Leukemia. 2004;18:1482–1490. doi: 10.1038/sj.leu.2403446. [DOI] [PubMed] [Google Scholar]
- 26.Wu W, Kim CH, Liu R, Kucia M, Marlicz W, Greco N, et al. The bone marrow-expressed antimicrobial cationic peptide LL-37 enhances the responsiveness of hematopoietic stem progenitor cells to an SDF-1 gradient and accelerates their engraftment after transplantation. Leukemia. 2012;26:736–745. doi: 10.1038/leu.2011.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borkowska S, Poniewierska-Baran A, Schneider G, Suszynska M, Ratajczak J, Kucia M, et al. Novel evidence that, in addition to proteolytic enzymes, lipolytic enzymes are involved in mobilization of hematopoietic stem/progenitor cells (HSPCs) - an important pro-mobilizing role identified for hematopoietic-specific phospholipase C (PLCβ2) Blood. 2014;124:S711. (abstract 2448) [Google Scholar]
- 28.Kinashi T, St Pierre Y, Springer TA. Expression of glycophosphatidylinositol-anchored and -non-anchored isoforms of vascular cell adhesion molecule 1 in murine stromal and endothelial cells. J Leukoc Biol. 1995;57:168–173. doi: 10.1002/jlb.57.1.168. [DOI] [PubMed] [Google Scholar]
- 29.Tjwa M, Sidenius N, Moura R, Jansen S, Theunissen K, Andolfo A, et al. Membrane-anchored uPAR regulates the proliferation, marrow pool size, engraftment, and mobilization of mouse hematopoietic stem/progenitor cells. J Clin Invest. 2009;119:1008–1018. doi: 10.1172/JCI36010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonig H, Papayannopoulou T. Hematopoietic stem cell mobilization: updated conceptual renditions. Leukemia. 2013;27:24–31. doi: 10.1038/leu.2012.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greenbaum AM, Link DC. Mechanisms of G-CSF-mediated hematopoietic stem and progenitor mobilization. Leukemia. 2011;25:211–217. doi: 10.1038/leu.2010.248. [DOI] [PubMed] [Google Scholar]
- 32.Lapidot T, Kollet O. The brain-bone-blood triad: traffic lights for stem-cell homing and mobilization. Hematology Am Soc Hematol Educ Program. 2010;2010:1–6. doi: 10.1182/asheducation-2010.1.1. [DOI] [PubMed] [Google Scholar]
- 33.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–1910. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- 34.Lévesque JP, Helwani FM, Winkler IG. The endosteal ‘osteoblastic’ niche and its role in hematopoietic stem cell homing and mobilization. Leukemia. 2010;24:1979–1992. doi: 10.1038/leu.2010.214. [DOI] [PubMed] [Google Scholar]
- 35.Ratajczak MZ. A novel view of the adult bone marrow stem cell hierarchy and stem cell trafficking. Leukemia. 2015 doi: 10.1038/leu.2014.346. e-pub ahead of print 9 December 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ratajczak MZ, Kim CH, Wojakowski W, Janowska-Wieczorek A, Kucia M, Ratajczak J. Innate immunity as orchestrator of stem cell mobilization. Leukemia. 2010;24:1667–1675. doi: 10.1038/leu.2010.162. [DOI] [PubMed] [Google Scholar]
- 37.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 38.Ratajczak MZ, Lee H, Wysoczynski M, Wan W, Marlicz W, Laughlin MJ, et al. Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex. Leukemia. 2010;24:976–985. doi: 10.1038/leu.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Golan K, Vagima Y, Ludin A, Itkin T, Cohen-Gur S, Kalinkovich A, et al. S1P promotes murine progenitor cell egress and mobilization via S1P1-mediated ROS signaling and SDF-1 release. Blood. 2012;119:2478–2488. doi: 10.1182/blood-2011-06-358614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Juarez JG, Harun N, Thien M, Welschinger R, Baraz R, Pena AD, et al. Sphingosine-1-phosphate facilitates trafficking of hematopoietic stem cells and their mobilization by CXCR4 antagonists in mice. Blood. 2012;119:707–716. doi: 10.1182/blood-2011-04-348904. [DOI] [PubMed] [Google Scholar]
- 41.Ratajczak MZ, Kim CH, Abdel-Latif A, Schneider G, Kucia M, Morris AJ, et al. A novel perspective on stem cell homing and mobilization: review on bioactive lipids as potent chemoattractants and cationic peptides as underappreciated modulators of responsiveness to SDF-1 gradients. Leukemia. 2012;26:63–72. doi: 10.1038/leu.2011.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Massberg S, Schaerli P, Knezevic-Maramica I, Köllnberger M, Tubo N, Moseman EA, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rettig MP, Ansstas G, DiPersio JF. Mobilization of hematopoietic stem and progenitor cells using inhibitors of CXCR4 and VLA-4. Leukemia. 2012;26:34–53. doi: 10.1038/leu.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shirvaikar N, Marquez-Curtis LA, Shaw AR, Turner AR, Janowska-Wieczorek A. MT1-MMP association with membrane lipid rafts facilitates G-CSF--induced hematopoietic stem/progenitor cell mobilization. Exp Hematol. 2010;38:823–835. doi: 10.1016/j.exphem.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 45.Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- 46.Ma Q, Jones D, Springer TA. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 1999;10:463–471. doi: 10.1016/s1074-7613(00)80046-1. [DOI] [PubMed] [Google Scholar]
- 47.Christopherson KW, 2nd, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305:1000–1003. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- 48.Onai N, Zhang Yy, Yoneyama H, Kitamura T, Ishikawa S, Matsushima K. Impairment of lymphopoiesis and myelopoiesis in mice reconstituted with bone marrow-hematopoietic progenitor cells expressing SDF-1-intrakine. Blood. 2000;96:2074–2080. [PubMed] [Google Scholar]
- 49.Kim CH, Wu W, Wysoczynski M, Abdel-Latif A, Sunkara M, Morris A. Conditioning for hematopoietic transplantation activates the complement cascade and induces a proteolytic environment in bone marrow: a novel role for bioactive lipids and soluble C5b-C9 as homing factors. Leukemia. 2012;26:106–116. doi: 10.1038/leu.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rossi L, Manfredini R, Bertolini F, Ferrari D, Fogli M, Zini R, et al. The extracellular nucleotide UTP is a potent inducer of hematopoietic stem cell migration. Blood. 2007;109:533–542. doi: 10.1182/blood-2006-01-035634. [DOI] [PubMed] [Google Scholar]
- 51.Hoggatt J, Pelus LM. Eicosanoid regulation of hematopoiesis and hematopoietic stem and progenitor trafficking. Leukemia. 2010;24:1993–2002. doi: 10.1038/leu.2010.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Avigdor A, Goichberg P, Shivtiel S, Dar A, Peled A, Samira S, et al. CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34+ stem/progenitor cells to bone marrow. Blood. 2004;103:2981–2989. doi: 10.1182/blood-2003-10-3611. [DOI] [PubMed] [Google Scholar]
- 53.Brunstein CG, McKenna DH, DeFor TE, Sumstad D, Paul P, Weisdorf DJ, et al. Complement fragment 3a priming of umbilical cord blood progenitors: safety profile. Biol Blood Marrow Transplant. 2013;19:1474–1479. doi: 10.1016/j.bbmt.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Capitano ML, Hangoc G, Cooper S, Broxmeyer HE. Mild heat treatment primes human CD34+ cord blood cells for migration towards SDF-1a and enhances engraftment in an NSG mouse model. Stem Cells. 2015 doi: 10.1002/stem.1988. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mierzejewska K, Rodriguez C, Sharma VR, Kucia M, Maciejewski JP, Ratajczak J, et al. Novel Evidence That PNH Affected Cells Residing in Bone Marrow (BM) Due to Impaired Incorporation of CXCR4 and VLA-4 Into Membrane Lipid Rafts Show Defective SDF-1- and VCAM-1-Mediated Retention in BM What Leads to Their Increased Motility and Impaired Interaction with the BM Stem Cell Niches. Blood. 2012;120:S508. (abstract 1256) [Google Scholar]
- 56.Edidin M. The state of lipid rafts: from model membranes to cells. Annu Rev Biophys Biomol Struct. 2003;32:257–283. doi: 10.1146/annurev.biophys.32.110601.142439. [DOI] [PubMed] [Google Scholar]
- 57.Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol. 2013;75:645–662. doi: 10.1111/j.1365-2125.2012.04374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stravodimou A, Voutsadakis IA. Statin use and peripheral blood progenitor cells mobilization in patients with multiple myeloma. Clin Transl Oncol. 2014;16:85–90. doi: 10.1007/s12094-013-1046-9. [DOI] [PubMed] [Google Scholar]
- 59.Caliceti C, Zambonin L, Rizzo B, Fiorentini D, Vieceli Dalla Sega F, Hrelia S, et al. Role of plasma membrane caveolae/lipid rafts in VEGF-induced redox signaling in human leukemia cells. Biomed Res Int. 2014 doi: 10.1155/2014/857504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.zum Büschenfelde CM, Wagner M, Lutzny G, Oelsner M, Feuerstacke Y, Decker T, et al. Recruitment of PKC-βII to lipid rafts mediates apoptosis-resistance in chronic lymphocytic leukemia expressing ZAP-70. Leukemia. 2010;24:141–152. doi: 10.1038/leu.2009.216. [DOI] [PubMed] [Google Scholar]
- 61.Tabe Y, Jin L, Iwabuchi K, Wang RY, Ichikawa N, Miida T, et al. Role of stromal microenvironment in nonpharmacological resistance of CML to imatinib through Lyn/CXCR4 interactions in lipid rafts. Leukemia. 2012;26:883–892. doi: 10.1038/leu.2011.291. [DOI] [PubMed] [Google Scholar]
- 62.Gmeiner WH, Jennings-Gee J, Stuart CH, Pardee TS. Thymineless death in F10-treated AML cells occurs via lipid raft depletion and Fas/FasL co-localization in the plasma membrane with activation of the extrinsic apoptotic pathway. Leuk Res. 2014 doi: 10.1016/j.leukres.2014.11.006. e-pub ahead of print 29 November 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Caroni P. Actin cytoskeleton regulation through modulation of PI(4,5)P2 rafts. The EMBO Journal. 2001;20:4332–4336. doi: 10.1093/emboj/20.16.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]