Abstract
Purpose of review
Premature ovarian failure (POF) is diagnosed by amenorrhea before 40 years of age. Owing to exhaustion of follicles in POF ovaries, egg donation is the only option. Although menstrual cycles cease in POF patients, some of them still contain residual dormant follicles in ovaries. Recently, we developed a new infertility treatment and named it as in-vitro activation (IVA), which enables POF patients to conceive using their own eggs by activation of residual dormant follicles. Here, we summarize data showing the potential of IVA as a new infertility treatment for POF patients.
Recent findings
Transgenic mouse studies revealed that the stimulation of phosphatidylinositol-3-kinase-AKT-forkhead box O3 pathway activated dormant primordial follicles. In murine and human ovaries, the phosphatase and tensin homolog inhibitors and phosphatidylinositol-3-kinase activators were demonstrated to activate dormant primordial follicles in in-vitro cultures. Subsequent studies showed that ovarian fragmentation suppressed Hippo signaling pathway, leading to ovarian follicle growth. Combining these two methods in an IVA approach followed by ovarian tissue autotransplantation, successful follicle growth, and pregnancies were reported in POF patients. Currently, two healthy babies were delivered, together with two additional pregnancies.
Summary
IVA treatment is a potential infertility therapy for POF patients who have residual follicles.
Keywords: in-vitro activation, infertility treatment, premature ovarian failure
INTRODUCTION
Premature ovarian failure (POF) is a disease in which the follicles in the ovaries rapidly decrease with none or few residual follicles in women younger than 40 years [1▪]. When the number of residual follicles decreases below the threshold (less than 1000), the regular activation of follicles no longer occurs and recruitment of developing follicles arrests, leading to anovulation and amenorrhea. POF occurs in 1% of women caused by autoimmune ovarian damage or because of genetic aberrations involving the X chromosome, autosomes, or some specific genes [2]. Furthermore, iatrogenic factors, including ovarian surgery, radiation, and chemotherapeutic interventions could also cause POI. Because POF patients show arrest of spontaneous ovulation at an early age, they are resistant to traditional infertility treatments. To date, although in cancer patients before germ cell-damaging chemo or radiation therapy, cryopreservation of ovarian tissues, mature oocytes, and embryos are available for potential options [3], the most effective infertility treatments for POF patients involve IVF and embryo transfer (IVF-ET) with the use of donor eggs from young women [3,4]. Despite still having varying amounts of residual dormant follicles in ovaries of some POF patients, these follicles are difficult to grow spontaneously, and thus the patients unlikely conceive with their own eggs. Recently, we developed a new infertility treatment and named it as in-vitro activation (IVA), which enables POF patients to conceive using their own eggs by artificial activation of residual dormant follicles. In this review, we summarize the potential use of IVA as a new infertility treatment in POF patients.
ACTIVATION OF DORMANT PRIMORDIAL FOLLICLES
Primordial follicles are dormant in ovaries. A number of intraovarian factors, including kit ligand, neurotrophins, vascular endothelial growth factor, bone morphogenetic protein 4, BMP7, leukemia inhibitory factor, basic fibroblast growth factor, and keratinocyte growth factor, have been shown to be important for primordial follicle activation from the dormant state [5▪]. Although the exact mechanisms how a limited number of follicles are activated among a huge number of dormant follicles are still unknown, recent studies demonstrated the intracellular signaling mechanisms important for activation of dormant primordial follicles [6].
Among diverse intracellular signaling systems, the important roles of the phosphatidylinositol-3-kinase (PI3K)-Akt-forkhead box O3 (FOXO3) pathway in primordial follicle activation are underscored by transgenic animal studies. Kit ligand binds its cognate tyrosine kinase receptor, c-kit and stimulates PI3K, resulting in the conversion of the lipid second messenger PIP2 (phosphatidylinositol (4, 5) bisphosphate) into PIP3 (phosphatidylinositol (3, 4, 5) triphosphate). Then, PIP3 stimulates phosphatidylinositol-dependent kinase 1 (PDK1), followed by AKT activation. Translocation of AKT to the nucleus inhibits the activity of a transcriptional factor, FOXO3. Phosphatase and tensin homolog (PTEN) negatively regulates this pathway by dephosphorylating PIP3 to PIP2 [7].
In FOXO3 deficient mice, all dormant primordial follicles were activated spontaneously at early neonatal stage and ovarian follicles were depleted during early life, resembling the POF phenotype [8]. Similar phenotypes were detected in mutant mice with oocyte-specific PTEN deletion [9]. Furthermore, inducible deletion of PTEN in the oocyte of adult mice also exhibited stimulation of AKT phosphorylation and nuclear export of FOXO3 proteins, resulting in activation of primordial follicles [10].
Mammalian target of rapamycin (mTOR) is the catalytic subunit of two structurally distinct complexes, mTORC1 and mTORC2. The rapamycin-sensitive mTORC1 positively regulates cell growth and proliferation, whereas the activity of mTORC1 is suppressed by tuberous sclerosis complex (Tsc) 1 and 2 complexes [11]. Studies using mice lacking Tsc1 or Tsc2 genes in oocytes demonstrated spontaneous activation of dormant primordial follicles [12,13]. Furthermore, mTOR activators were also shown to promote activation of primordial follicles [14▪] and stimulated secondary follicle growth [15▪]. Interestingly, double deletion of Tsc1 and PTEN leads to synergistically enhanced primordial follicle activation, when compared with singly mutated mice [13]. These findings indicate the essential and cooperative roles of PI3K-AKT and mTOR signaling pathways in regulating primordial follicle dormancy [16].
Diverse intraovarian factors involved in activation of primordial follicles are likely to converge on common intracellular signaling pathways. Thus, we attempted to activate dormant primordial follicles pharmacologically through manipulating the functions of intracellular signaling pathways. Short-term culture of murine ovaries with a PTEN inhibitor and a PI3K-stimulating phosphopeptide could activate dormant primordial follicles by increasing AKT activity, leading to nuclear exclusion of FOXO3 in primordial oocytes. Following transplantation of the activated ovaries under kidney capsules of ovariectomized host mice, primordial follicles develop to the preovulatory stage, and mature oocytes exhibiting normal epigenetic patterns of imprinted genes were obtained. After IVF-ET, healthy pups were delivered [17]. The normality of the progeny was proven by long-term follow-up without showing abnormalities in reproductive activities and increases in chronic diseases [18], indicating that pharmacological activation of dormant primordial follicles could be a safe and effective way to generate mature oocytes in POF patients. In humans, in-vitro treatment of ovarian cortices with a PTEN inhibitor also activated dormant primordial follicles. After xenotransplantation to severe combined immunodeficiency mice for 6 months, preovulatory follicles were developed at 6 months after grafting and could generate mature oocytes in response to human chorionic gonadotropin (hCG) stimulation [17].
STIMULATION OF FOLLICLE GROWTH BY SUPPRESSION OF HIPPO SIGNALING PATHWAY
For infertility treatments in patients with polycystic ovarian syndrome, ovarian wedge resection and ovarian drilling have been shown to induce follicle growth. In cancer patients who underwent sterilizing treatment, ovarian cortices are fragmented to allow better freezing and grafting for fertility preservation and subsequent grafting of fragmented cortices is associated with follicle growth. These procedures include a common intervention, namely disruption of ovarian cortices, suggesting induction of follicle growth by alterations in mechanical tensions. To test this hypothesis, we cut rodent ovaries into three pieces, and transplanted them under kidney capsules of adult hosts. As compared with paired intact ovaries, fragmentation of ovaries increased ovarian weights because of promotion of secondary follicle growth [19▪].
The Hippo signaling pathway is an important intracellular signaling system in controlling cell proliferation and determining organ size and is conserved in all metazoan animals [20–22]. Hippo signaling consists of several negative growth regulators acting in a serine/threonine kinase cascade that eventually phosphorylates and inactivates key transcriptional coactivators, Yes-associated protein (YAP) and TAZ (Transcriptional coactivator with PDZ-binding motif) by nuclear export. Thus, disruption of Hippo signaling leads to decreases in YAP phosphorylation followed by increases in nuclear levels of YAP. In conjunction with TEAD (Transcription factors containing the TEA/ATTS DNA binding domain) transcriptional factors, nuclear YAP induces several CCN growth factors and baculoviral inhibitors of apoptosis repeat containing (BIRC) apoptosis inhibitors. CCN and BIRC proteins, in turn, stimulate cell growth, survival, and proliferation [20]. The Hippo signaling pathway is regulated mainly by a network of upstream components involved in regulating cell adhesion, shape, and polarity [23]. Actin is a multifunctional protein that forms microfilaments maintaining important cellular processes. Rapid changes in the polymerization of globular actin (G-actin) to the filamentous form (F-actin) mediate cell adhesion, shape maintenance, and locomotion. F-actin formation in the stress fiber has been shown to disrupt Hippo signaling leading to nuclear YAP accumulation [24].
In murine and human ovaries, we found the expression of key Hippo signaling genes (YAP, TAZ, MST1/2, SAV1, and LATS1/2) in ovarian follicles at different stages [19▪]. Furthermore, we demonstrated that fragmentation of ovaries induced a transient increase in the polymerization of G-actin to F-actin, decreases in phospho-YAP levels, and increases in nuclear localization of YAP, resulting in the upregulation of downstream CCN growth factors and BIRC apoptosis inhibitors [19▪].
Treatment of murine ovaries with Jasplakinolide, an actin polymerization-promoting cyclic peptide, or sphingosine-1-phosphate, a follicular fluid constituent known to promote actin polymerization, increased the conversion of G-actin to the F-actin, followed by increased nuclear YAP and expression of downstream CCN growth factor leading to follicle growth [25▪]. We also demonstrated the important roles of YAP in ovarian fragmentation-induced follicle growth by using verteporfin [19▪], a small molecule capable of inhibiting interactions between YAP and TEAD transcriptional factors [26]. In human, CCN growth factors were also increased in ovarian cortices after cutting thawed human cortical strips to small cubes [19▪], indicating essential roles of CCN growth factors in ovarian follicle growth.
IN-VITRO ACTIVATION AS A NEW INFERTILITY TREATMENT IN PREMATURE OVARIAN FAILURE PATIENTS
The chance to conceive in POF patients is rare because of exhaustion of ovarian follicles leading to anovulation. Indeed, only a 1.5% pregnancy rate was found in controlled trials [27]. Furthermore, extensive studies of a cohort of 358 young POI patients (26.6 ±7.9 years of age at time of diagnosis) revealed a spontaneous pregnancy rate of 4.4% during 13 years of observation [28]. Therefore, egg donation has been commonly used to treat infertility in POF patients.
Although diverse hormone therapies and ovulation induction treatments have been prescribed for POF patients, these infertility treatments have a limited success. Thus, the development of a new method for treating infertility that would enable POF patients to conceive using their own eggs has been much anticipated. In our basic and translational studies, treatment with PI3K activators stimulated murine and human dormant primordial follicles by stimulating the PI3K-AKT-FOXO3 pathway [17,19▪]. Furthermore, ovarian fragmentation induces disruption of Hippo signaling pathway to promoted secondary follicle growth in mice and human [19▪]. These findings provide the basis to develop the IVA approach, a new infertility treatment by activation of dormant primordial and restrained secondary/preantral follicles to start/ resume growth in POF patients.
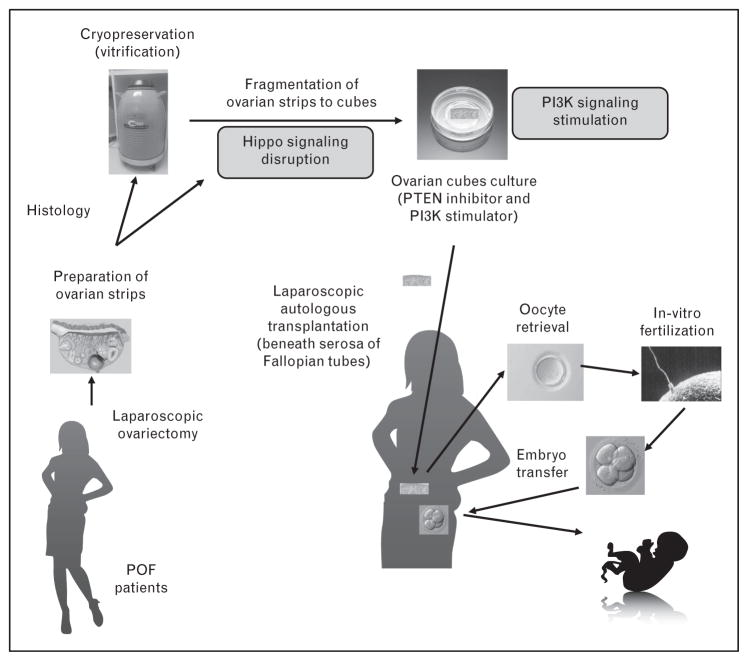
After confirmation of the safety of IVA by our [17] and other [18] groups, we then applied this technique followed by IVF-ET in POF patients with approval from the ethical committee and informed written consent from the patients (Fig. 1) [19▪]. Because treatment with PI3K activators stimulates dormant primordial follicles, whereas ovarian fragmentation stimulates secondary follicle growth, ovarian fragmentation, and PI3K activator treatment were combined to activate residual follicles in ovaries of POF patients [19▪]. One or both ovaries from POF patients were removed using laparoscopic surgery. Because Fallopian tubes are used later as sites for ovarian transplantation, the surgeon must carefully remove the ovaries without damaging Fallopian tubes. Of note, because the blood flows are poor around POF ovaries, the ovaries can be usually removed without requiring extensive hemostasis by electrocoagulation.
FIGURE 1.
A scheme for in-vitro activation. Under laparoscopic surgery, one or both ovaries from POF patients were removed and cut into cortical strips before vitrification. Using small portion of ovarian cortices, histological analyses were performed to detect residual follicles. After thawing of cryopreserved ovarian tissues, the ovarian strips were fragmented into 1–2 mm cubes and cultured with PI3K stimulators for 2 days. After cultures, cubes were washed and autografted beneath serosa of Fallopian tubes under laparoscopic surgery. Follicle growth was promoted by FSH treatment. When antral follicles reached preovulatory stage, mature oocytes were retrieved and fertilized with husbands’ sperm in vitro followed by embryo transfer. Modified from [19▪]. hCG, human chorionic gonadotropin; PI3K, phosphatidylinositol-3-kinase; POF, premature ovarian failure.
Ovarian cortices where residual follicles were localized were immediately dissected by removing medulla followed by cutting into small strips (0.5–1 ×0.5–1 cm, 1–2 mm thickness) [19▪]. For cryopreservation of ovarian tissues, a vitrification method was used [29▪]. To identify residual follicles in ovarian cortices of POF patients, 10–20% volumes of the cortices from each cortical strip were dissected and subjected to histological analyses before cryopreservation. This histological assessment of residual follicles was effective to predict successful follicle growth of IVA treatment, because none of the patients without residual follicles (n =17/37) based on the histological analysis showed follicle growth during ~1 year of observation after transplantation in previous studies [29▪]. Although the cryopreservation process of the ovarian tissue is not essential for IVA, it provides the following benefits. In POF, the number of residual follicles continues to diminish with age [30]. If one ovary is cryopreserved at an earlier stage of POF, patients could undergo additional noninvasive infertility therapies before the decision for second surgery for ovary grafting. Furthermore, cryopreservation provides sufficient time to complete histological analyses to evaluate the presence of residual follicles before a decision for autotransplantation.
Frozen ovarian strips were then thawed and fragmented into small cubes of 1–2 mm in size. These ovarian cubes were subjected to in-vitro culture by treatment with PI3K activators for two days [19▪]. After culture, the ovarian tissues were thoroughly washed and an autologous transplantation beneath the serosa of Fallopian tubes was performed under laparoscopic surgery. The sites beneath the serosa of Fallopian tubes were swollen by preinjection with saline, followed by cutting the serosa and making a pouch between serosa and Fallopian tube to insert ovarian cubes. Then, approximately 20–80 ovarian cubes each were inserted beneath the serosa of the two Fallopian tubes, followed by closure of the serosa using sutures or coverage by an oxidized regeneration cellulose to avoid cube loss from the incision site [19▪,29▪]. This transplantation site was selected because of high vascularization, convenience for transvaginal ultrasound monitoring, and ease for oocyte retrieval for IVF-ET.
After autologous transplantation of ovarian cubes, follicle growth was monitored weekly or biweekly by measurement of serum estrogen and gonadotrophin levels supported by transvaginal ultrasound to detect growing antral follicles. Suppression of endogenous gonadotrophins is considered to be important in the induction of follicle growth in POF patients likely by restoring the responsiveness of residual follicles to exogenous gonadotrophin stimulation [30] and by inhibiting early luteinization during follicle growth. We treated patients with estrogen together with GnRH agonist to suppress elevated endogenous gonadotrophins before exogenous gonadotrophin stimulation [19▪,29▪]. Follicle growth was stimulated by injecting recombinant FSH daily. In some cases, GnRH antagonist cetrorelix acetate was used to avoid the premature LH surge. When follicles matured, oocyte maturation was triggered by hCG. At 36 h later, oocytes were retrieved under transvaginal ultrasound guidance and then IVF was performed [19▪,29▪]. Follicle growth was found in about 50% of patients with residual follicles. In some cases, preovulatory follicles were detected within weeks or a few months after grafting, suggesting the growth of residual secondary follicles in response to Hippo signal disruption [19▪]. In contrast, some preovulatory follicles were detected after 6 months or longer, likely because of the activation of dormant primordial follicles [17]. After IVF and embryo transfer, three pregnancies were achieved based on serum hCG. Although one was a miscarriage, two healthy IVA babies have been born with the first one being more than 3 years of age now. Two other pregnancies by two other centers have also been achieved [31▪].
CONCLUSION
The IVA approach leads to a new infertility treatment strategy for POF patients to conceive their own genetic children [31▪]. IVA could also be effective for treating infertility in patients with diminishing ovarian reserve, including aging women and cancer survivors. It is important to note that IVA has a potential to increase the quantity of mature oocytes for infertility treatment but does not alter age-associated decline in oocyte quality. Although these observations may have profound clinical implications, spontaneous recovery of menstrual cycles, and subsequent pregnancies have been reported in POF patients [28]. Thus, controlled studies would be required before IVA can be advocated for more widespread clinical use.
To improve the efficiency of IVA, it is important to develop a noninvasive method to predict the presence of residual follicles before first surgery of ovariectomy. Although shorter duration from initial POF diagnosis to IVA therapy is a valid parameter [29▪], establishment of more sensitive and specific assays is needed. Because a substantial number of ovarian follicles are lost in association with grafting of ovarian tissue [3], it is important to develop new methods to minimize such loss by improvement of the method for cryopreservation of ovarian tissue as well as by promoting neovascularization of grafted ovaries.
KEY POINTS.
The IVA approach leads to a new infertility treatment strategy for POF patients to conceive their own genetic children.
The IVA is effective in POF patients who have residual follicles, but there is no established method to predict the presence of residual follicles before IVA treatment.
In addition to POF patients, IVA is suitable for treating infertility in patients with diminishing ovarian reserve and in cancer survivors following germ cell-damaging therapies.
Acknowledgments
Financial support and sponsorship
This work was supported by Grant-In-Aid for Scientific Research (Challenging Exploratory Research: 15K15613, and Innovative Areas, Mechanisms regulating gamete formation in animals: 26114510) and by research funds from the Grant for Fertility Innovation, Smoking Research Foundation, the Mochida Memorial Foundation for Medical and Pharmaceutical Research and the Takeda Science Foundation.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1▪.Kovanci E, Schutt AK. Premature ovarian failure: clinical presentation and treatment. Obstet Gynecol Clin North Am. 2015;42:153–161. doi: 10.1016/j.ogc.2014.10.004. This is the latest article to learn general knowledge for POF. This article summarizes the latest clinical presentation and treatment of patients with POF. [DOI] [PubMed] [Google Scholar]
- 2.De Vos M, Devroey P, Fauser BC. Primary ovarian insufficiency. Lancet. 2010;376:911–921. doi: 10.1016/S0140-6736(10)60355-8. [DOI] [PubMed] [Google Scholar]
- 3.Donnez J, Dolmans MM. Fertility preservation in women. Nat Rev Endocrinol. 2013;9:735–749. doi: 10.1038/nrendo.2013.205. [DOI] [PubMed] [Google Scholar]
- 4.Sauer MV, Paulson RJ, Lobo RA. A preliminary report on oocyte donation extending reproductive potential to women over 40. N Engl J Med. 1990;323:1157–1160. doi: 10.1056/NEJM199010253231702. [DOI] [PubMed] [Google Scholar]
- 5▪.Hsueh AJ, Kawamura K, Cheng Y, et al. Intraovarian control of early folliculogenesis. Endocr Rev. 2015;36:1–24. doi: 10.1210/er.2014-1020. This is the first review article regarding IVA. This article describes the molecular basis of IVA and its clinical applications including suggestions for future research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adhikari D, Liu K. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr Rev. 2009;30:438–464. doi: 10.1210/er.2008-0048. [DOI] [PubMed] [Google Scholar]
- 7.Reddy P, Shen L, Ren C, et al. Activation of Akt (PKB) and suppression of FKHRL1 in mouse and rat oocytes by stem cell factor during follicular activation and development. Dev Biol. 2005;281:160–170. doi: 10.1016/j.ydbio.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Castrillon DH, Miao L, Kollipara R, et al. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–218. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- 9.Reddy P, Liu L, Adhikari D, et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319:611–613. doi: 10.1126/science.1152257. [DOI] [PubMed] [Google Scholar]
- 10.John GB, Gallardo TD, Shirley LJ, et al. Foxo3 is a PI3K-dependent molecular switch controlling the initiation of oocyte growth. Dev Biol. 2008;321:197–204. doi: 10.1016/j.ydbio.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adhikari D, Flohr G, Gorre N, et al. Disruption of Tsc2 in oocytes leads to overactivation of the entire pool of primordial follicles. Mol Hum Reprod. 2009;15:765–770. doi: 10.1093/molehr/gap092. [DOI] [PubMed] [Google Scholar]
- 13.Adhikari D, Zheng W, Shen Y, et al. Tsc/mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles. Hum Mol Genet. 2010;19:397–410. doi: 10.1093/hmg/ddp483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14▪.Sun X, Su Y, He Y, et al. New strategy for in vitro activation of primordial follicles with mTOR and PI3K stimulators. Cell Cycle. 2015;14:721–731. doi: 10.1080/15384101.2014.995496. This is the first article to indicate a potential of mTOR activators for primordial follicle activation. This article suggests a new infertility treatment with activation of dormant primordial follicles by activating both mTOR and PI3K pathways in POF patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15▪.Cheng Y, Kim J, Li XX, Hsueh AJ. Promotion of ovarian follicle growth following mTOR activation: synergistic effects of AKT stimulators. PLoS One. 2015;10:e0117769. doi: 10.1371/journal.pone.0117769. This is the first article to demonstrate stimulation of ovarian follicle growth using mTOR activators. This article highlights that mTOR activators and AKT stimulators show synergistic effects to promote secondary follicle growth in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adhikari D, Liu K. mTOR signaling in the control of activation of primordial follicles. Cell Cycle. 2010;9:1673–1674. doi: 10.4161/cc.9.9.11626. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Kawamura K, Cheng Y, et al. Activation of dormant ovarian follicles to generate mature eggs. Proc Natl Acad Sci U S A. 2011;107:10280–10284. doi: 10.1073/pnas.1001198107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adhikari D, Gorre N, Risal S, et al. The safe use of a PTEN inhibitor for the activation of dormant mouse primordial follicles and generation of fertilizable eggs. PLoS One. 2012;7:e39034. doi: 10.1371/journal.pone.0039034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19▪.Kawamura K, Cheng Y, Suzuki N, et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci U S A. 2013;110:17474–17479. doi: 10.1073/pnas.1312830110. This is the first clinical trial to demonstrate the efficacy of IVA in POF patients. This article describes the development of IVA treatment by PI3K signal activation and Hippo signal disruption. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan D. Hippo signaling in organ size control. Genes Dev. 2007;21:886–897. doi: 10.1101/gad.1536007. [DOI] [PubMed] [Google Scholar]
- 21.Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development. 2011;138:9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hergovich A. Mammalian Hippo signalling: a kinase network regulated by protein-protein interactions. Biochem Soc Trans. 2012;40:124–128. doi: 10.1042/BST20110619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson R, Halder G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat Rev Drug Discov. 2014;13:63–79. doi: 10.1038/nrd4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wada K, Itoga K, Okano T, et al. Hippo pathway regulation by cell morphology and stress fibers. Development. 2011;138:3907–3914. doi: 10.1242/dev.070987. [DOI] [PubMed] [Google Scholar]
- 25▪.Cheng Y, Feng Y, Jansson L, et al. Actin polymerization-enhancing drugs promote ovarian follicle growth mediated by the Hippo signaling effector YAP. FASEB J. 2015;9:2423–2430. doi: 10.1096/fj.14-267856. The article demonstrates the mechanisms by which actin polymerization-enhancing drugs promote ovarian follicle growth. This article focuses on disruption of Hippo signaling pathway by actin polymerization-enhancing drugs in follicle growth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu-Chittenden Y, Huang B, Shim JS, et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26:1300–1305. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Kasteren YM, Schoemaker J. Premature ovarian failure: a systematic review on therapeutic interventions to restore ovarian function and achieve pregnancy. Hum Reprod Update. 1999;5:483–492. doi: 10.1093/humupd/5.5.483. [DOI] [PubMed] [Google Scholar]
- 28.Bidet M, Bachelot A, Bissauge E, et al. Resumption of ovarian function and pregnancies in 358 patients with premature ovarian failure. J Clin Endocrinol Metab. 2011;96:3864–3872. doi: 10.1210/jc.2011-1038. [DOI] [PubMed] [Google Scholar]
- 29▪.Suzuki N, Yoshioka N, Takae S, et al. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod. 2015;30:608–615. doi: 10.1093/humrep/deu353. This is a second article showing the outcome of clinical trial of IVA. This article highlights an importance of cryopreservation of ovarian tissue and prediction of residual follicles in POF ovaries. [DOI] [PubMed] [Google Scholar]
- 30.Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360:606–614. doi: 10.1056/NEJMcp0808697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31▪.Kawamura K, Cheng Y, Sun YP, et al. Ovary transplantation: to activate or not to activate. Hum Reprod. 2015;30:2457–2460. doi: 10.1093/humrep/dev211. This is a commentary to support a strategy of IVA to treat infertile POF patients. This article emphasizes the effectiveness of IVA on reproduction but not on endocrine functions. [DOI] [PubMed] [Google Scholar]