Abstract
Fibrinogen and fibrin are essential for hemostasis and are major factors in thrombosis, wound healing, and several other biological functions and pathological conditions. The X-ray crystallographic structure of major parts of fibrin(ogen), together with computational reconstructions of missing portions and numerous biochemical and biophysical studies, have provided a wealth of data to interpret molecular mechanisms of fibrin formation, its organization, and properties. On cleavage of fibrinopeptides by thrombin, fibrinogen is converted to fibrin monomers, which interact via knobs exposed by fibrinopeptide removal in the central region, with holes always exposed at the ends of the molecules. The resulting half-staggered, double-stranded oligomers lengthen into protofibrils, which aggregate laterally to make fibers, which then branch to yield a three-dimensional network. Much is now known about the structural origins of clot mechanical properties, including changes in fiber orientation, stretching and buckling, and forced unfolding of molecular domains. Studies of congenital fibrinogen variants and post-translational modifications have increased our understanding of the structure and functions of fibrin(ogen). The fibrinolytic system, with the zymogen plasminogen binding to fibrin together with tissue-type plasminogen activator to promote activation to the active proteolytic enzyme, plasmin, results in digestion of fibrin at specific lysine residues. In spite of a great increase in our knowledge of all these interconnected processes, much about the molecular mechanisms of the biological functions of fibrin(ogen) remains unknown, including some basic aspects of clotting, fibrinolysis, and molecular origins of fibrin mechanical properties. Even less is known concerning more complex (patho)physiological implications of fibrinogen and fibrin.
Keywords: Fibrin formation, Fibrin structure, Fibrin properties, Fibrinogen composition, α-Helical coiled-coil, Blood clot, Fibrin polymerization, Clot mechanical properties, Molecular mechanisms of fibrinolysis, Modulation of clot structure
13.1 Introduction
Fibrinogen was first classified as a fibrous protein with keratin, myosin and epidermin, based on its wide angle X-ray diffraction pattern arising from its α-helical coiled-coil structure (Bailey et al. 1943). It is a 340-kDa glycoprotein, normally present in human blood plasma at a concentration of about 1.5–4 g/L, that is essential for hemostasis, wound healing, inflammation, angiogenesis, and several other biological functions. Fibrinogen is a soluble macromolecule, but forms an insoluble clot or gel on conversion to fibrin by the action of the serine protease thrombin, which is activated by a cascade of enzymatic reactions triggered by vessel wall injury, activated blood cells, or a foreign surface (Fig. 13.1). A mechanically stable clot is necessary to prevent blood loss (stopping bleeding is called hemostasis) and to promote wound healing. Fibrin clots are dissolved by the fibrinolytic system, acting in a series of enzymatic reactions with positive and negative feedback.
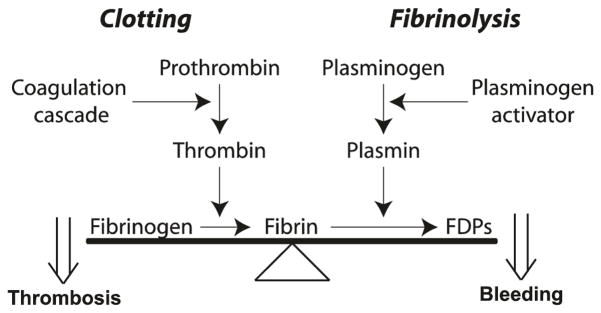
Fig. 13.1.
Basic scheme of fibrin clot formation and fibrinolysis and the balance between these processes. The clot is formed via a cascade of enzymatic reactions that activates prothrombin to the proteolytic enzyme thrombin, which converts soluble fibrinogen to make insoluble fibrin, the process referred to as blood clotting. The fibrin clot is dissolved through fibrinolysis or cleavage by the proteolytic enzyme plasmin, resulting in fibrin degradation products (FDPs). Plasmin is formed on the fibrin surface from the zymogen plasminogen by plasminogen activators. There is a balance between clotting and fibrinolysis such that excess clotting can lead to thrombosis, while excess fibrinolysis can lead to bleeding
In vivo, there is a careful balance between clotting, the conversion of fibrinogen to fibrin, and fibrinolysis, the proteolytic dissolution of the clot (Fig. 13.1). Imbalance in one direction (prevalence of fibrinolysis) can lead to bleeding while the opposite imbalance (prevalence of clotting) can cause thrombosis, or formation of a clot that blocks the flow of blood through a vessel (called a thrombus). Thrombosis, often resulting from atherosclerosis or many other pathological processes, is the most common cause of myocardial infarction, ischemic stroke, deep vein thrombosis, and other cardiovascular diseases.
In addition to fibrin clot formation, fibrinogen is also necessary for an earlier step in hemostasis (called “primary hemostasis”), the aggregation of platelets leading to formation of a platelet “plug” at the site of vessel wall injury. The bivalent fibrinogen molecules act as bridges to link activated platelets, since the ends of rod-like fibrinogen bind with high affinity to the major adhesive receptor on platelets, the integrin αIIbβ3. Fibrinogen also binds specifically to some other cells, but none of these cellular interactions will be discussed here, though other reviews on this topic are available (Bennett 2001; Wei et al. 2009; Coller and Shattil 2008; Coller 2011).
Fibrinogen and fibrin were last reviewed in the previous series of books dedicated entirely to Fibrous Proteins in 2005 (Weisel 2005), but many other reviews have appeared during the past 10 years that have summarized various aspects of the biology and biochemistry of fibrinogen and fibrin, though none as broad in scope (Cilia La Corte et al. 2011; Wolberg 2010, 2012; Undas and Ariens 2011; Ariens 2013; Weisel and Litvinov 2013; Weisel and Dempfle 2013; Bridge et al. 2014; Lord 2007, 2011; Falvo et al. 2010). We now know more about the process of fibrin formation, modulation of clot properties, and fibrinolysis, but perhaps the greatest explosion of new knowledge has been related to the structural origin of clot mechanical properties. Of special note for fibrous proteins, old observations of the α-helix to β-sheet transition upon stretching of fibrin have been confirmed and a functional spring-like role for the α-helical coiled-coil structure has been discovered. Although most data referenced in this review are related to human fibrinogen and fibrin, the basic principles discussed here have general relevance and are beyond species-specific peculiarities.
13.2 Biochemistry of Fibrinogen, the Precursor to Fibrin
13.2.1 Biosynthesis of Fibrinogen in Hepatocytes
Fibrinogen is the product of three closely linked genes, FGA, FGB, and FGG, each specifying the primary structure of one of its three polypeptide chains, Aα, Bβ, and γ, respectively (Chung et al. 1983, 1990; Crabtree 1987). The fibrinogen genes are clustered on human chromosome 4 (Kant et al. 1985) and they translate into nascent polypeptides of pre-pro-Aα chain (644 amino acid residues), pre-pro-Bβ chain (491 residues), and pre-pro-γ chain (437 residues). An extension of an open reading frame into an alternately spliced sixth intron gives rise to a longer Aα chain (αE) in 1–2 % of molecules in the adult (more in fetal blood), so that they contain an additional domain that is homologous to the C-terminal Bβ and γ chains, resulting in a fibrinogen molecule with a molecular mass of 420 kDa compared to 340 kDa for the major fibrinogen fraction with the shorter Aα chains (Fu and Grieninger 1994). The alternative splicing of a γ chain mRNA produces 8–15 % of plasma fibrinogen molecules in which the γ chain C-terminal 400–411 dodecapeptide (γA chain) is altered by adding new amino acids from 408 to 427 (γ′ chain) (Wolfenstein and Mosesson 1981; Chung and Davie 1984; de Maat and Verschuur 2005).
Before fibrinogen is secreted from hepatocytes into blood, it needs to undergo several steps of assembly of the polypeptide chains. Following translation of each of the chains, independent translocation into the lumen of the endoplasmic reticulum, interactions of the chains with chaperone proteins that assist in the assembly and folding processes, and quality control mechanisms that distinguish properly assembled fibrinogen destined for secretion from unassembled forms that are degraded intracellularly (Redman and Xia 2001; Tamura et al. 2013). During translocation of the single polypeptides into the lumen of the endoplasmic reticulum, a signal peptide is cleaved from each chain. After full processing and assembly in the endoplasmic reticulum and Golgi organelles, there are 610, 461, and 411 amino acids in each of the common final forms of the human Aα, Bβ and γ chains, respectively, remaining from the corresponding nascent pre-pro-polypeptides. In the endoplasmic reticulum there is a progression from single chains to two-chain complexes to trimeric half molecules generated by combining a Bβ chain with the Aα-γ dimer or an Aα chain with the Bβ-γ dimer. Next, the two trimers are joined at their N-termini to form the hexamer via disulfide bridges necessary for assembly of the two half molecules (Zhang and Redman 1996). Although the distal clusters of interchain disulfide bonds (named “disulfide rings”) are not necessary for assembly of the two half molecules, they are necessary for secretion. In addition to assembly and formation of disulfide bonds, before secretion fibrinogen undergoes a number of co-translational and post-translational modifications, including N-glycosylation, these are discussed below. The structure of fibrinogen can be modified even after it has been secreted into the blood, for example by limited proteolysis and glycation.
13.2.2 Fibrinogen Metabolism
The liver is the primary source of plasma fibrinogen, with a steady state rate of synthesis of 1.7–5 g per day and a large intracellular reserve (Takeda 1966). Three quarters of human fibrinogen is present in the plasma but it is also in platelets, lymph and interstitial fluid. The normal half-life of fibrinogen is 3–5 days, but despite the numerous studies on the distribution of iodine-labeled fibrinogen, its physiological catabolic pathway is largely unknown. Coagulation and fibrinolysis accounts for only 2–3 % of fibrinogen loss in healthy individuals (Nossel 1976). Moreover, fibrinogen turnover in the presence and absence of heparin, a potent inhibitor of blood clotting, did not reveal any differences in the half-life of fibrinogen, so intravascular fibrin formation was not found to be a quantitatively significant route in fibrinogen catabolism under physiological conditions in humans (Takeda 1966; Collen et al. 1972). Another proposed pathway of fibrinogen catabolism is proteolytic degradation by plasmin; however, in the presence of tranexamic acid, an inhibitor of fibrinolysis, the half-life of labeled fibrinogen did not change (Collen et al. 1972). Nevertheless, proteolytic fibrin(ogen) degradation products appear to play a role in the regulation of fibrinogen turnover (Nham and Fuller 1986).
Fibrinogen is one of the acute phase proteins, which are up-regulated in response to injury and inflammation, followed by an up to ten-fold increase in its concentration in the blood (Crabtree 1987). The up-regulation is mediated largely by interleukin-6 and perhaps other pro-inflammatory mediators that trigger intracellular signaling pathways in hepatocytes and modulate gene expression via various transcription factors (Hantgan et al. 2000; Fish and Neerman-Arbez 2012).
There is intracellular fibrinogen stored in platelet α-granules, but its origin is controversial, as is whether it is structurally and functionally distinct from plasma fibrinogen. A separate origin is suggested by the observation that the γ chain variants of fibrinogen appear to be absent in platelets (Haidaris et al. 1989), and in one patient with a heterozygous genetically modified fibrinogen, the platelets contained only normal fibrinogen (Jandrot-Perrus et al. 1979). Since platelets originate from megakaryocytes, the latter are also a possible source of fibrinogen synthesis. However, in subjects with a low fibrinogen level in blood (hypofibrinogenemia), there are also lower levels of fibrinogen in platelets, and infusion of fibrinogen results in a subsequent increase in platelet fibrinogen (Harrison et al. 1989). Also, no fibrinogen mRNA has been found in megakaryocytes (Louache et al. 1991). Therefore, it appears that platelet and megakaryocyte fibrinogen arise primarily from αIIbβ3-mediated internalization of plasma fibrinogen (Harrison et al. 1990).
Although the liver is the primary source of plasma fibrinogen, fibrinogen is also synthesized in some extra-hepatic tissues. Only fibrinogen γ chain gene expression has been shown in vivo in bone marrow, brain, and lung (Haidaris and Courtney 1990). Epithelial cells from lung and intestine secrete small amounts of fibrinogen in a polarized manner from their basolateral face (Haidaris 1997). It is possible that lung epithelium secretes fibrinogen and incorporates it into the extracellular matrix under certain pathological conditions, contributing to fibrotic lung disease, because the amount of fibrinogen expressed in lung epithelial cells is dramatically increased after treatments with dexamethasone and interleukin-6 (Lawrence and Simpson-Haidaris 2004). Synthesis of fibrinogen by cultured granulosa cells may reflect a possible function for it in ovulation (Parrott et al. 1993). The apparent in vivo synthesis of fibrinogen by trophoblasts (Galanakis et al. 1996) and the fact that the trophoblast basement membrane consists largely of fibrin(ogen) suggest that these cells may secrete fibrinogen into their abluminal and/or interstitial environment, but the functional significance is as yet unknown. Taken together, the normal biological relevance of the synthesis of fibrinogen in extra-hepatic tissues is unclear, but it may become important under certain pathological circumstances.
13.2.3 Polypeptide Chain Composition of Fibrin(ogen)
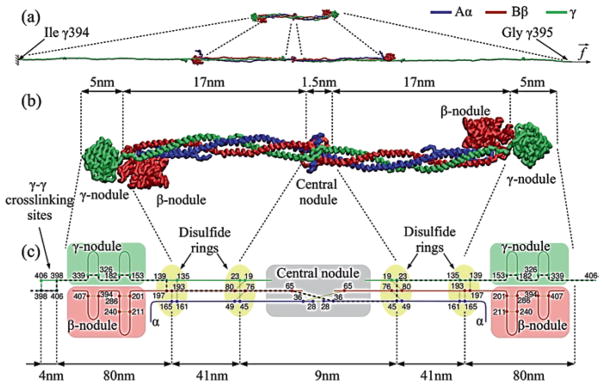
Human fibrinogen is made up of three pairs of polypeptide chains, designated Aα, Bβ and γ, with molecular masses of 66,500, 52,000, and 46,500 Da, respectively (Fig. 13.2). The co- and post-translational addition of N-linked carbohydrate to the Bβ and γ chains brings the total molecular mass to about 340 kDa. The nomenclature for the polypeptide composition of fibrinogen (Aα Bβ γ)2, arises from the designation of the small fibrinopeptides A and B (FpA and FpB) that comprise the N-terminal ends of the Aα and Bβ chains, respectively, and are cleaved by thrombin to yield the α and β chains without the fibrinopeptides. No peptides are cleaved by thrombin from the γ chains, hence the subunit composition of monomeric fibrin is (α β γ)2 and the conversion of fibrinogen into fibrin monomer can be described as (Aα Bβ γ)2 → (α β γ)2 + 2FpA + 2FpB.
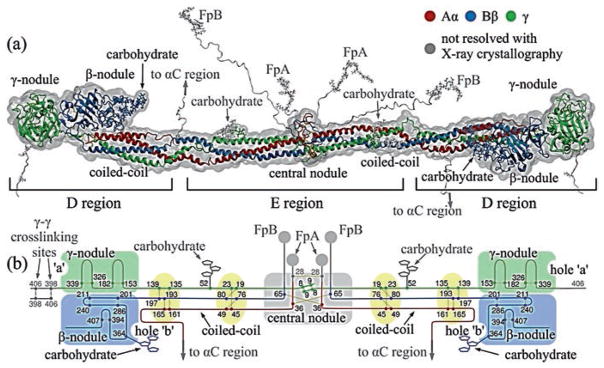
Fig. 13.2.
Fibrinogen structure. (a) The atomic resolution structure of about two-thirds of the fibrinogen molecule has been determined by X-ray crystallography (PDB Entry: 3GHG). Fibrinogen and its parts are shown with addition of portions missing from the crystal structure reconstructed computationally, namely the amino terminal ends of the Aα and Bβ chains with FpA and FpB in the central nodule and the beginning of the αC regions. (b) Schematic diagram of the polypeptide chains of fibrinogen. The Aα, Bβ and γ chains are represented by lines with lengths proportional to the number of amino acid residues in each chain and various structural regions are labeled (Zhmurov et al. 2011, with permission from Elsevier Ltd.)
All six chains are held together by 29 disulfide bonds (Henschen and McDonagh 1986) to make two symmetrical half-molecules (Fig. 13.2). There are 8, 11, and 10 cysteine residues in the Aα, Bβ, and γ chains, respectively, and the amino termini of all six chains are held together by disulfide bonds in the central globule. Unusual Cys-Pro-X-X-Cys sequences occurring twice in each chain are arranged into “disulfide ring” structures, in which all three chains are joined together at each end of the α-helical coiled-coils (Doolittle 1984). Three interchain disulfide bonds link the two halves of the molecule together, one between the two Aα chains and two between the two γ chains. A single interchain disulfide bond connects the Aα and Bβ chains within each half-molecule. The remainder of the Aα chain contains one intrachain disulfide, while the Bβ chain contains three intrachain disulfides and the γ chain contains two.
13.2.4 Overall Structure of Fibrinogen Molecules
Based on transmission electron microscopy, atomic force microscopy, and X-ray crystallographic data, the fibrinogen molecule has an elongated shape 45 nm in length and ~2–5 nm in diameter (Fig. 13.2; Hall and Slayter 1959; Fowler and Erickson 1979; Williams 1981; Weisel et al. 1985). Plasmin cleaves fibrinogen into a number of core fragments, namely fragment E comprising the central part of the molecule and two identical fragments D originating from the lateral parts of fibrinogen (Nussenzweig et al. 1961). According to the nomenclature of the corresponding proteolytic fragments, the fibrinogen molecule has two distal globular D regions and one central globular E region each containing a part of α-helical coiled-coils (Medved and Weisel 2009) (Fig. 13.2). The atomic resolution structure of more than two-thirds of fibrinogen has been accomplished through X-ray crystallography (Yee et al. 1997; Spraggon et al. 1997; Brown et al. 2000; Madrazo et al. 2001), though the structure still has not been completed, since there are missing unstructured portions, which are not resolved crystallographically. These are residues 1–26, 1–57, and 1–13 at the N-termini of the Aα, Bβ and γ chains, respectively, and residues 201–610, 459–461, and 395–411 at the C-termini of the Aα, Bβ and γ chains, respectively. These unresolved portions have been reconstructed computationally (Zhmurov et al. 2011) to gain a complete molecular structure of fibrinogen. Unlike other crystallographically unresolved parts of fibrinogen, the 390 residue-long C-termini of the Aα chains (residues 221–610), named the αC regions, were visualized using transmission electron microscopy (Veklich et al. 1993) and atomic force microscopy (Protopopova et al. 2015). The αC regions fold back from the distal ends of the triple coiled-coils to form a fourth strand and then extend outward via a flexible connector to relatively compact C-terminal domains that interact with the central globule of fibrinogen (Veklich et al. 1993; Litvinov et al. 2007b). Truncation of the αC regions affects substantially the hydrodynamic behavior of fibrinogen (Raynal et al. 2013).
13.2.5 Domain Structure of Fibrinogen
Fibrinogen is organized into domains, or independently folded structural units (Weisel 2005). From the X-ray crystallographic studies of fibrinogen, the central E region has four domains and each D region comprises seven domains (Medved and Weisel 2009; Fig. 13.2). The E region is composed of two symmetrical parts, in which the C-terminal parts of the Aα, Bβ and γ chains form a coiled-coil-E domain comprising a triple α-helical structure. The N-terminal parts of two γ chains form an asymmetric γN-domain domain in the center of the E region. Another domain, called the funnel-shaped domain, is formed on the opposite side of the E region by the parts of two Aα and two Bβ chains. The globular part of the E region without the coiled-coil-E domains is often called the central nodule. In the lateral D region, the N-terminal parts of all the Aα, Bβ and γ chains form a coiled-coil-D domain comprising a triple α-helical structure. The C-terminal parts of the β and γ chains make up the β-nodule and γ-nodule, respectively. They are also named β- or γ-modules and each is made of three domains. As determined in the crystal structure of the γ-module these domains are named A-domain (N-terminal), B-domain (central) and P-domain (C-terminal; Medved and Weisel 2009).
Although the structure of the remaining fibrinogen regions not present in the crystal structures is not as well defined, it is known from NMR studies that in human fibrinogen each αC region (residues Aα221–610) forms two relatively compact structures. Namely, the C-terminal part (residues Aα392–610) contains a distinct structure connected to the rest of the molecule via the N-terminal portion (residues Aα221–391) comprising a flexible unstructured tether. As a result, the compact part is referred to as the αC-domain and the flexible part as the αC-connector. Notably, the two unstructured BβN regions comprising the flexible N-terminal portions of the Bβ chains contain a number of functionally important binding sites (Gorkun et al. 2006).
13.2.6 α-Helical Coiled-Coils of Fibrinogen
The central nodule and end globular parts of fibrin(ogen) are joined together by 17-nm-long triple (and partially quadruple) α-helical coiled-coil connectors formed by 111 or 112 amino acid residues from each of the Aα Bβ and γ chains that are bordered by “disulfide rings” (Fig. 13.2). In the α-helical coiled-coil, three right-handed α-helices wind around each other to form a left-handed supercoil (Cohen and Parry 1990). Unlike most triple helices, each of the coiled-coils of fibrinogen has a fourth helix in the bundle containing 30 residues (AαSer166 to AαPro195) that begins at the lateral disulfide ring where the Aα chain makes a U-turn and stretches in the reverse direction for about ¼ of the length of the coiled-coil connector (Spraggon et al. 1997). The coiled-coils can bend around a central hinge point located in a non-helical segment of the γ chain adjacent to the carbohydrate attachment site γAsn52 (Marsh et al. 2013). The bent portion of the coiled-coil exposes the cleavage sites that can be hydrolyzed by plasmin and other proteases followed by degradation of fibrinogen into proteolytic fragments. It also explains the conformational flexibility of fibrinogen observed in solution and at interfaces (Kohler et al. 2015). The functional role of the coiled-coil region has been recently ascribed to the tensile deformation of fibrin fibers, namely to the ability to undergo partial unraveling and spring-like reversible extension-contraction that helps to accommodate and propagate the tensile stress along the fiber axis (Zhmurov et al. 2011). At a high extent of tensile or compressive deformation, the α-helices undergo mechanical conversion into β-sheets (Litvinov et al. 2012; Zhmurov et al. 2012; see Sect. 13.5.5).
13.2.7 Ca2+-Binding Sites in Fibrinogen
Fibrinogen has both strong and weak binding sites for calcium ions, which are important for its functions, including fibrin polymerization, and lytic stability. Although biochemical experiments originally suggested that there were three high-affinity calcium binding sites in fibrinogen (Marguerie et al. 1977), the binding sites were identified from X-ray crystallographic structures. High-affinity binding sites (named γ1) for calcium ions are present in the γ chains and are associated with four coordinating amino acid residues, namely γAsp318, γAsp320, γGly324, and γPhe322, and two strongly bound water molecules (Yee et al. 1997; Spraggon et al. 1997). Other high-affinity Ca2+-binding sites (named β1) are located in the β-nodules in loop β381–385, each of which has one coordinating water molecule (Everse et al. 1998b). The dissociation constant for calcium ions in these binding sites is high enough that both types of these sites will be fully occupied in fibrinogen at physiological Ca2+ concentrations.
Two other Ca2+-binding sites named γ2 and β2 have much lower affinities. The γ2 sites are located in the loops γ294–301 (Everse et al. 1999). Impairment of these sites by mutating residues γAsp298 and γAsp301 caused only moderate effects on the crystal structure and functional properties of fibrinogen (Kostelansky et al. 2007). It is likely that the γ2 sites are formed as a result of molecular rearrangements induced by crystal packing (Kostelansky et al. 2004a, b). The low-affinity Ca2+-binding sites β2 are formed by residues BβAsp261, BβAsp398, and γGlu132 and the backbone carbonyl oxygen of BβAsp263 (Everse et al. 1999; Kostelansky et al. 2002, 2004a, b). The β2 sites anchor the β-nodules to the coiled-coil connector and were shown to be involved in the lateral aggregation of protofibrils (Kostelansky et al. 2004b). It has been proposed that the β2 sites regulate accessibility of the tissue plasminogen activator (t-PA)-binding site in fibrin(ogen) (Doolittle and Pandi 2006). There may also be some low affinity Ca2+-binding sites associated with the sialic acid residues on the carbohydrate chains (Dang et al. 1989).
When calcium ions are bound to the γ chain high affinity sites, the γ chains are protected from enzymatic degradation (Ly and Godal 1973; Odrljin et al. 1996), similar to the way that the peptide Gly-Pro-Arg-Pro protects the molecule from plasmin digestion (Yamazumi and Doolittle 1992). Ca2+-binding does not affect FpA cleavage by thrombin or batroxobin, but appears to be important for modulating fibrin polymerization by enhancing lateral aggregation to form thicker fibers, so that mutations affecting the high-affinity Ca2+-binding site have severe functional consequences (Brennan et al. 2007). There is a conformational change in fibrin associated with FpB cleavage that is Ca2+-dependent (Dyr et al. 1989; Donovan and Mihalyi 1985; Mihalyi 1988). There is also a change in affinity of Ca2+-binding sites associated with FpB release and the accompanying conformational change. Gly-His-Arg-Pro, a knob ‘B’ mimetic peptide, binds 10-fold more strongly to fibrinogen in the presence of Ca2+ than in its absence, which may be related to the β2 site that is involved in the conformational change associated with binding of Gly-His-Arg-Pro (Everse et al. 1999).
13.2.8 Carbohydrate Moieties of Fibrinogen
Four oligosaccharide chains are linked to each molecule of fibrinogen by way of N-glycosidic bonds: two are connected to the BβAsn364 residues in the β-nodule (resolved in the crystal structure 3GHG) and the other two are connected to γAsn52 in the coiled-coils (unresolved crystallographically) (Fig. 13.2). These carbohydrate attachment sites contain the classic NXS or NXT sequences that are typical of N-glycosylation. On the other hand, the Aα chains are devoid of any carbohydrate, in spite of the presence of two NXS sequences. Variable desialylation, or removal of the terminal N-acetylneuraminic acid residue (sialyl), accounts for part of the heterogeneity of circulating fibrinogen. Fibrinogen isolated from human plasma contains equal amounts of mono- and di-sialylated carbohydrate chains but no asialo-chains (Townsend et al. 1982, 1984).
The carbohydrate on fibrinogen has striking consequences for fibrin polymerization and for clot structure. Patients with cirrhosis of the liver and some other liver diseases have fibrinogen with high levels of sialyation of their carbohydrate, resulting in fibrin networks containing thinner fibers with a higher density of branch points (Martinez et al. 1983). These results are consistent with studies using neuraminidase to remove sialic acid from the carbohydrate of normal fibrinogen, producing clots made up of thicker fibers (Dang et al. 1989). Fibrinogen synthesized in inflammatory conditions (acute phase fibrinogen) has substantially different oligosaccharide structure (Brennan 2015). Complete removal of carbohydrate has more striking effects on clot structure, resulting in clots made up of very thick fibers (Langer et al. 1988). These results suggest that both the charge and mass of the carbohydrate help to modulate the extent of lateral aggregation and that the carbohydrate moieties significantly enhance the solubility of fibrinogen. Based on computational reconstructions, it has been recently proposed that the bulky carbohydrate moieties can potentially affect the ‘B-b’ knob-hole interactions either by tethering the knob ‘B’ (γAsn52) or obstructing the hole ‘b’ (BβAsn364), or both (Zhmurov et al. in preparation).
13.3 Molecular Mechanisms of the Conversion of Fibrinogen to Fibrin
13.3.1 General Remarks
Conversion of fibrinogen to fibrin is one of the major consequences of the enzymatic cascade of blood coagulation that is essential for stopping bleeding (hemostasis), as well as in vascular obstruction or thrombosis. It occurs in two major steps: enzymatic and non-enzymatic (Fig. 13.3). At the enzymatic step, there is thrombin-catalyzed cleavage of the fibrinopeptides of fibrinogen to form the fibrin monomer. Thrombin is a highly specific serine protease upon activation of its zymogen, prothrombin, normally present in the blood. At the non-enzymatic stage, the monomeric fibrin self-assembles spontaneously to yield fibrin oligomers that lengthen to make two-stranded protofibrils. Protofibrils aggregate both laterally and longitudinally to form fibers that branch to yield a three-dimensional gelled network called a clot. Finally, the fibrin polymer is stabilized via covalent crosslinking by a plasma transglutaminase, Factor XIIIa, to form a mechanically and chemically more stable mature fibrin clot.
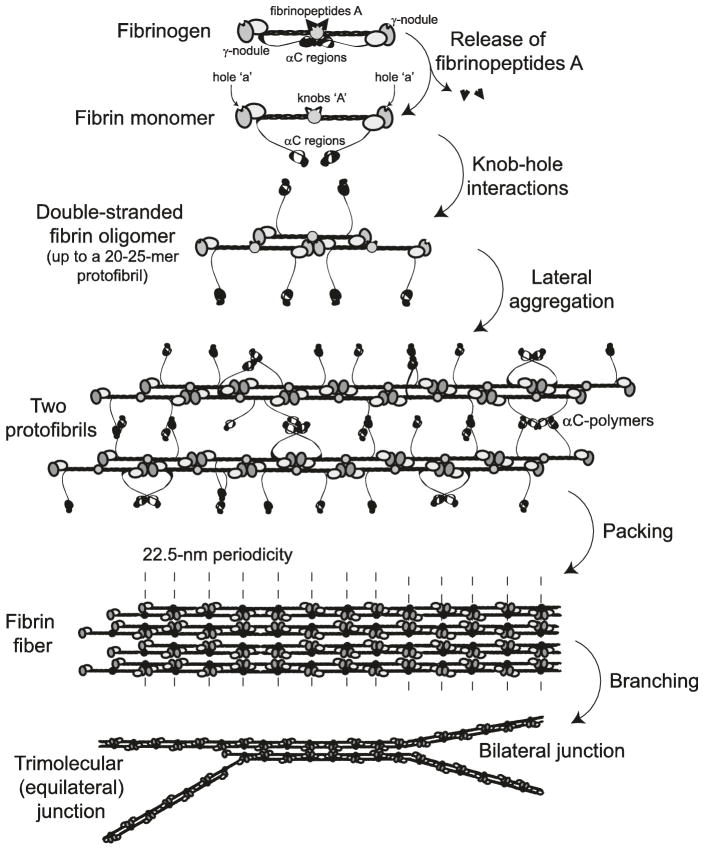
Fig. 13.3.
Schematic diagram of fibrin polymerization. Fibrinopeptides in the central nodule cover knobs that are complementary to holes that are always exposed at the ends of the protein. When the fibrinopeptides are removed by thrombin, knob-hole interactions occur, giving rise to oligomers (a trimer is shown), which elongate to produce the two-stranded protofibrils made up of half-staggered molecules. The protofibrils aggregate laterally to make fibers, a process enhanced by interactions of the αC regions and formation of the αC-polymers. The fiber has a 22.5 nm periodicity as a result of half-staggering of 45-nm molecules. At the bottom of the diagram, branch points have been initiated by the divergence of two protofibrils (right) and splitting of each strand of a single protofibril (left) (Weisel and Litvinov 2013; Weisel and Dempfle 2013)
13.3.2 Enzymatic Release of Fibrinopeptides from Fibrinogen
Fibrin polymerization is triggered when thrombin cleaves FpA and FpB from the N-terminal portions of the Aα and Bβ chains of fibrinogen, respectively, producing monomeric fibrin. FpA (residues 1–16) is cleaved at the AαArg16-Gly17 peptide bond, while FpB (residues 1–14) is cleaved at the BβArg14-Gly15 bond, albeit more slowly than FpA. With polymerization, the rate of release of FpBs increases, reaching maximum when polymerization is almost complete, indicating that they are preferentially released from fibrin polymer (Erickson and Fowler 1983; Weisel et al. 1993). In surface-attached fibrinogen, unlike in solution, FpBs are cleaved at a faster rate than FpAs, depending on fibrinogen surface density and orientation (Riedel et al. 2011), indicating that the conformation of fibrinogen determines the ability of thrombin to access and cleave FpAs and FpBs differentially. This distinction in the cleavage rate of FpA and FpB is based on the spatial restrictions of the binding of thrombin to fibrinogen, implying that the N-termini of the Aα chain containing FpA are more accessible to the active site of thrombin (Pechik et al. 2006). When thrombin cleavage sites are mutated at positions AαArg16 or BβArg14 (in dysfibrinogenemias or recombinant fibrinogens) the release of FpA or FpB is precluded, leading to impaired fibrin formation (Galanakis et al. 1989; Moen et al. 2003).
13.3.3 ‘A-a’ Knob-Hole Interactions in Fibrin
After cleavage of FpAs, the α chains have new N-terminal sequences Gly-Pro-Arg-(GPR) named knobs ‘A’ (Medved and Weisel 2009). During fibrin polymerization, knobs ‘A’ interact with holes or pockets ‘a’ that are always open in the γ-nodules of the interacting fibrin molecules; the binding of knobs ‘A’ to holes ‘a’ is called the ‘A-a’ interaction (Figs. 13.3 and 13.4). The ‘A-a’ interactions have been characterized biophysically at the single-molecule level as quite strong, highly specific, and stable intermolecular associations (Litvinov et al. 2005).
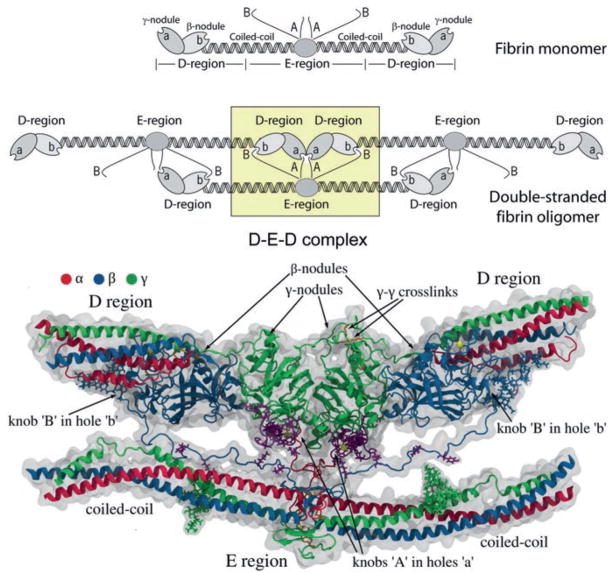
Fig. 13.4.
Complementary binding sites or knob-hole interactions in fibrin polymerization. Top. Schematic diagram of knob-hole interactions. Knobs ‘A’ and ‘B’ in the central domain of a fibrin monomer are complementary to holes ‘a’ and ‘b’ that are always exposed at the ends of the protein. When the fibrinopeptides are removed by thrombin, exposing the knobs, knob-hole interactions occur, giving rise to the trimer shown and eventually to the two-stranded protofibril made up of half-staggered molecules. Bottom. Atomic resolution structure of the knob-hole interactions. The γ- and β-nodules near the ends of the molecule contain the holes ‘a’ and ‘b’, respectively, that are complementary to the knobs ‘A’ and ‘B’ in the central nodule. Most of these structures were derived from X-ray crystallographic data, although the disordered and/or flexible N-terminal regions of the α and β chains were derived from computational modeling (with permission from Elsevier Ltd.)
Structures of fragment D (corresponding to the lateral D regions containing the γ-nodule) co-crystallized with the peptide GPRP (synthetic knob ‘A’ mimetic) clearly indicated that this ligand was located in hollows present in the γ-nodule (Everse et al. 1998b; Kostelansky et al. 2002), evidence that these are holes ‘a’. The hot spots of holes ‘a’ directly involved in the binding of the GPRP peptide were identified as residues γTrp315-Trp330, γTrp335-Asn365, and γPhe295-Thr305. However, using molecular dynamics simulations, it has been shown that during fibrin oligomerization the D:E interface includes binding sites beyond the ‘A-a’ knob-hole associations, confirming the existence of intermolecular binding sites ‘A’ and ‘a’ that are not limited to knobs ‘A’ and holes ‘a’ (Zhmurov et al. 2016). Nevertheless, release of FpA and formation of knobs ‘A’ are necessary and sufficient to induce fibrin polymerization, resulting in formation of the so-called desA-fibrin. The existence of constitutively open holes ‘a’ in fibrinogen and fibrin is also essential for fibrin polymerization. If the holes ‘a’ are obstructed by the GPRP peptide (Everse et al. 1998b) or compromised by a replacement of the most important residue γAsp364 (Okumura et al. 1997), fibrin polymerization is abrogated. Overall, these and other data suggest that fibrin polymerization and clot formation are driven by the ‘A-a’ interactions.
13.3.4 Fibrin Oligomers and Protofibrils
Fibrin polymerization begins when two fibrin monomer molecules formed after cleavage of FpA interact to form a half-staggered dimer in which knob ‘A’ binds hole ‘a’, and there are two ‘A-a’ knob-hole interactions holding the two monomers together (Erickson and Fowler 1983). A third fibrin molecule added to a half-staggered dimer forms an end-to-end connection where the two adjacent molecules touch each other and the lateral D regions of two molecules form the D:D interface that comprises the junction between monomers in each of two strands in fibrin oligomers (Everse et al. 1998a; Fig. 13.4). The D:D interface comprises residues γ275–309 (Everse et al. 1998b) and is very weak, because it yields first upon forced stretching of fibrin(ogen) oligomers (Zhmurov et al. 2011). It was found that the D:D interactions involve residues γ275, γ308, and γ309 that are essential for elongation of fibrin strands (Marchi et al. 2006; Bowley et al. 2009). Fibrin monomers can add longitudinally to form longer two-stranded fibrin oligomers of varying length. Lateral interactions between two strands of fibrin oligomers are mediated by the central E region of one fibrin molecule and two lateral D regions of two other molecules (Fig. 13.4). The D-E-D complex is held together mainly by the ‘A-a’ knob-hole bonds and by additional interactions at the D:E and D:D interfaces (Kononova et al. 2013; Zhmurov et al. in preparation).
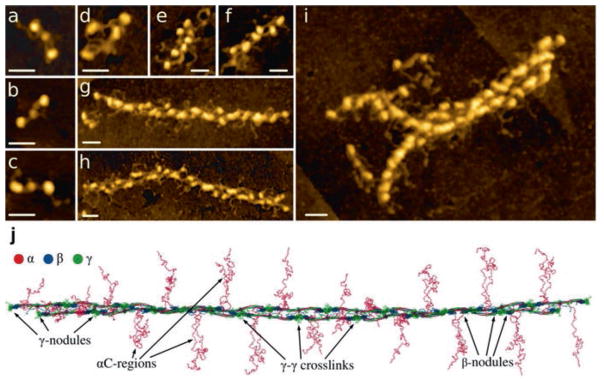
Fibrin monomers continue to add longitudinally to the oligomers, which lengthen further to make two-stranded protofibrils (Erickson and Fowler 1983), a critically important intermediate product of fibrin polymerization (Fig. 13.5). Protofibrils are usually about 0.5–0.6 μm in length, which corresponds to ~20–25 monomers, and they are long enough to self-interact and aggregate laterally (Erickson and Fowler 1983; Chernysh et al. 2011). Oligomers and protofibrils have been visualized by transmission electron microscopy (Erickson and Fowler 1983; Medved et al. 1990; Weisel et al. 1993; Chernysh et al. 2011; Huang et al. 2014), light microscopy (Chernysh and Weisel 2008; Chernysh et al. 2011), and atomic force microscopy (Yermolenko et al. 2011; Protopopova et al. 2015). In the presence of the fibrinogen γ′, which is a γ chain splice variant, protofibril formation is partially impaired by likely because of electrostatic repulsion (Cooper et al. 2003; Gersh et al. 2009; Allan et al. 2012).
Fig. 13.5.
Atomic force microscopy images of fibrinogen, fibrin oligomers, and protofibrils and reconstruction of a protofibril model. (a–i). Images by high-resolution atomic force microscopy (Published with permission and thanks to Drs. Anna D. Protopopova, Nikolay Barinov, Dmitry Klinov). All magnification bars = 30 nm. (a–c). Fibrin monomer with visible αC regions. (d). Fibrin dimer. (e). Fibrin trimer. (f). Fibrin tetramer. (g–h). Fibrin protofibrils. (i). Two protofibrils aggregating laterally. On the left, the two protofibrils are diverging, creating a branch-point. (j). Reconstruction of a twisted fibrin protofibril based on the X-ray crystallographic structure of fibrinogen (PDB Entry: 3GHG). The molecules are shown with addition of missing parts of the crystal structure reconstructed from molecular dynamics simulations, including the full-length αC regions. ‘A-a’ knob-hole bonds that are the major basis of fibrin polymerization are as in Fig. 13.4 (Published with permission and thanks to Dr. Artem Zhmurov)
13.3.5 Lateral Aggregation of Protofibrils
Protofibrils aggregate laterally to form more or less thick fibers, in which the half-staggered molecular packing gives rise to a 22.5-nm periodicity corresponding to half the length of the fibrin molecule (Fig. 13.3). This longitudinally ordered molecular packing of fibrin fibers was visualized by transmission electron microscopy of negatively contrasted specimens and atomic force microscopy as regular cross-striations (Weisel 1986; Yermolenko et al. 2011) (Fig. 13.10b). The mechanisms, structural motifs involved, and driving forces of the protofibrils’ lateral aggregation are mostly still unknown. Importantly, protofibrils self-associate laterally only after they reach a certain threshold length, implying that the bonds mediating the interactions between protofibrils are weak and cooperative along the axis of the protofibril. At present, the structures that have been shown or presumed to participate in inter-protofibril lateral aggregation are the following: knobs ‘B’ and holes ‘b’, the αC regions, the C-terminal parts of the γ chains, adjacent β-nodules (Yang et al. 2000), the coiled-coils (Okumura et al. 2006), and N-glycans at residues βAsn364 and γAsn52 (Langer et al. 1988).
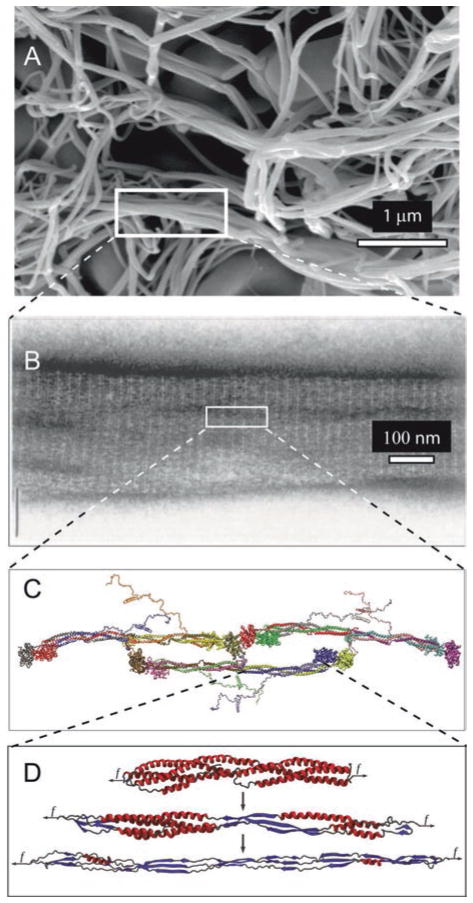
Fig. 13.10.
Unfolding of the coiled-coils of fibrin. (a). scanning electron micrograph of a fibrin clot, with a box enclosing part of a fiber. (b). A transmission electron micrograph of a negatively contrasted fibrin fiber showing the ultrastructure, with the 22.5 nm repeat arising from the half-staggering of 45-nm molecules. (c). A fibrin trimer from X-ray crystallographic data and molecular dynamics simulations of regions not present in the crystal structure. (d). Fibrin α-helical coiled coils undergoing a forced transition from α-helix to β-sheet. The mechanical transition from α-helical coiled coils to β-sheets in the fibrin(ogen) molecule was characterized using molecular dynamics simulations of their forced elongation and theoretical modeling (Adapted with permission from Zhmurov et al. 2012. Copyright 2012 American Chemical Society)
Because some other structural proteins that self-assemble have similar intermolecular interactions in vitro as in vivo (Weisel et al. 1978), useful mechanistic information about lateral aggregation of protofibrils has been gleaned from the interfaces formed between D regions in crystals. Thus, a mechanism for lateral aggregation has been proposed based on crystal packing via direct association of β-nodules of neighboring protofibrils, employing residues β330–375 (Yang et al. 2000). Some data suggest that lateral aggregation of protofibrils could be mediated by the residues βAla68 located in the N-terminal portion of the β chain (Mullin et al. 2000) and βGly15, the N-terminal residue of knob ‘B’, no matter whether FpB is released or not (Hirota-Kawadobora et al. 2003).
The packing in fibrin generally seems to be paracrystalline (Weisel et al. 1983; Weisel 1986), meaning that fibrin is more regularly organized axially than laterally, but there is also evidence for regularity in lateral packing (Torbet et al. 1981; Caracciolo et al. 2003; Yeromonahos et al. 2010). In addition, it appears that bundling of twisted protofibrils proceeds in such a way that the resulting fibers are also twisted (Medved et al. 1990). To maintain the 22.5-nm repeat, protofibrils that are newly added to the outside of a fiber must be stretched as their path length increases. This may comprise a thermodynamic mechanism to control the diameter of fibers, as the lateral aggregation stops when the protofibril stretching energy surpasses the energy of bonding.
An alternative model of fibrin formation and unusual structure is based on the formation of ultrathin fibrin sheets spanning channels on a plastic substrate, but the physiological relevance of these results is not yet known (O’Brien et al. 2008). Another proposed mechanism of the early stages of fibrin assembly implies that short thin fibrin branches form at an initial phase of polymerization, in which single-bonded “Y-ladder” polymers rapidly elongate before undergoing a delayed transition to the double-stranded fibrils (Rocco et al. 2014).
13.3.6 Role of ‘B-b’ Knob-Hole Interactions
When FpB is cleaved off, the β chain acquires a new N-terminal sequence Gly-His-Arg-Pro (GHRP), comprising knob ‘B’ capable of binding to hole ‘b’ located in the β-nodule (Fig. 13.4). The peptide GHRP, which reproduces the structure of knob ‘B’, has a much lower equilibrium binding affinity for fibrinogen (Kd = 140 μM) compared to the peptide GPRP (Kd = 25 μM) that mimics the structure of knob ‘A’ (Everse et al. 1998b). Moreover, a much larger piece of fibrin molecule from the central part (bearing knobs ‘A’) interacted with fibrinogen (bearing holes ‘a’) with even higher affinity (Kd = 5.8 ± 1.1 μM; Geer et al. 2007), suggesting that the ‘A-a’ interactions are not limited to the Gly-Pro-Arg motif. There must be additional interface beyond the ‘A-a’ bonds that has substantially higher binding strength, which has been confirmed using structural modeling (Kononova et al. 2013; Zhmurov et al. 2016).
Despite direct evidence for the existence of ‘B-b’ interactions (Litvinov et al. 2007a), the role of the ‘B-b’ interactions in fibrin formation is not quite clear because it is supported only indirectly. If fibrin is formed by cleavage of FpA only (while FpB remains uncleaved) the network consists of thinner fibers compared to the fibrin networks formed after cleavage of FpAs and FpBs., These data suggest that in the presence of ‘B-b’ interactions the fibers are thicker due to enhanced lateral aggregation of protofibrils (Blombäck et al. 1978), despite the fact that cleavage of FpB is not necessary for lateral aggregation and fiber formation. There are rare congenital homozygous fibrinogen mutations (fibrinogens Metz and Frankfurt XIII) in which FpB can be cleaved, while FpA cannot, that still form fibrin clots under the action of thrombin at low temperature (Galanakis et al. 1993). Thus, these clots are formed exclusively via ‘B-b’ interactions, indicating that they can actually occur in fibrin. Moreover, recombinant fibrinogen variants without functional holes ‘a’ (due to mutation of the γAsp364 residue) formed fibrin under the action of thrombin (that cleaved both FpAs and FpBs) but did not form fibrin under the action of reptilase (that cleaved FpAs only; Okumura et al. 2007). On the other hand, fibrinogen without functional hole ‘b’ (due to mutation BβAsp432Ala) formed normal fibrin (Bowley et al. 2009). Therefore, ‘B-b’ bonds not only exist physically but they form during fibrin polymerization under some special conditions (e.g., when ‘A-a’ bonds cannot form), but their role in normal fibrin formation remains largely undefined. Using molecular dynamics simulations, structural and thermodynamic characteristics of the ‘B-b’ interactions has been gleaned (Kononova et al. 2013). It has been proposed that ‘B-b’ interactions have effects on the susceptibility of clots to proteolytic digestion (Doolittle and Pandi 2006). Besides the known ‘A-a’ and ‘B-b’ knob-hole interactions, experimental data suggest that the ‘A:b’ interactions are physically possible, while the ‘B:a’ interactions are unlikely to exist (Litvinov et al. 2005, 2007a). Fibrin structure and properties are affected by addition of a knob ‘B’ mimetic combined with polyethyleneglycol, thus indirectly confirming the functional importance of ‘B-b’ knob-hole interactions (Brown et al. 2015).
13.3.7 The Role of the αC Regions in Fibrin Formation
During fibrin polymerization, the protruding and flexible αC regions (see Sects. 13.2.4 and 13.2.5) can self-interact both within and between protofibrils; these αC-αC interactions can lead to formation of αC polymers that are reinforced with additional crosslinking by Factor XIIIa (Tsurupa et al. 2011; Fig. 13.3). The αC-αC polymerization involves two mechanisms. One is self-association of the αC-domains that occurs via their N-terminal subdomains by β-hairpin swapping. The second is the interaction of the C-terminal subdomain with the αC-connector (Tsurupa et al. 2012). Although they are not necessary, the αC regions are known to augment lateral aggregation (Weisel and Medved 2001; Litvinov et al. 2007b; Tsurupa et al. 2011). Fibrinogen lacking the αC regions forms clots with thinner fibers and a higher density of branch points than clots made of full-length fibrinogen (Collet et al. 2005). Furthermore, a recombinant fibrinogen construct that has the longer human αC regions replaced with the shorter chicken αC regions displayed impaired lateral aggregation of protofibrils (Ping et al. 2011).
13.3.8 Fibrin Branching and Network Architecture
As fibrin fibers thicken by lateral aggregation and grow in length, they also branch, yielding a space-filling 3D network. Electron microscopy revealed at least two different molecular mechanisms by which branch points may form (Figs. 13.3 and 13.5i). One of them known as a “bilateral junction”, originates from two protofibrils that undergo incomplete lateral aggregation but diverge into two separate protofibrils, each of them giving rise to a new fiber (Mosesson et al. 1993). The second type of branchpoint, called a “trimolecular junction” or “equilateral junction”, forms when a fibrin monomer attached to the end of a protofibril via only one ‘A-a’ bond (or one γ-nodule), such that both the monomeric molecule and the protofibril to which it is bound can grow independently, forming two strands each (Fogelson and Keener 2010). In either case, most of branch points in clots consist of three fibers of about the same diameters joined together (Ryan et al. 1999), suggesting that the type of initial branchpoint does not affect much the final network structure. Branching has been observed directly in an imaging study of the early stages of fibrin polymerization (Chernysh et al. 2011). Finally, an increase in the number of branch points in a clot normally correlated with a decrease of the fiber diameters (Ryan et al. 1999). The aggregate of data support a notion that that branching (as a part of elongation process) and lateral aggregation compete. In other words, the prevalence of lateral aggregation leads to fibrin with thicker fibers and fewer branch points, while reduced lateral aggregation will result in formation of fibrin with thinner fibers and more branch points.
13.3.9 Fibrin Structure and the Gelation Point
Formation of a fibrin clot is a transition from sol to gel upon formation of a three-dimensional filamentous network (Fig. 13.6). The gelation point determined as blood/plasma clotting time is commonly used in clinical assays as a test to reveal coagulation disorders. The gelation point occurs when only about 15–20 % of the fibrinogen is converted to fibrin, which is enough to form the gel (Chernysh and Weisel 2008). Some correlations between gelation time and final clot structure have been established (Blombäck and Okada 1982), but the formation of a stable fully branched network is not yet completed at the gelation point, so that new fibers and branch points continue to form in the gel (Chernysh and Weisel 2008). The structure of fibrin networks can be determined and quantified using scanning electron microscopy by a number of parameters, such as the fiber diameter, fiber density, number of branch points, fiber length, and the size of the pores (Fig. 13.10a). All of these parameters are variable and depend on the kinetics of fibrin polymerization. Various biophysical techniques have been applied to study the fine structure of fibrin clots that revealed the complex structural hierarchy at different spatial scales (Ryan et al. 1999; Ferri et al. 2002; Guthold et al. 2004; Evans et al. 2010; Yeromonahos et al. 2010; Magatti et al. 2013). Remarkably, the diffusion rate of proteins in fibrin clots does not depend much on the structure of the network because there are large pores. However, permeability of fibrin clots for fluid (Okada and Blombäck 1983) or nanoparticles (Spero et al. 2011) perfused through the clot depends strongly on the pore size and hence on overall clot structure. The diffusivity of the fibrin network is reduced dramatically when it is embedded with blood cells and compressed, as happens in a contracted whole blood clot (Cines et al. 2014).
Fig. 13.6.
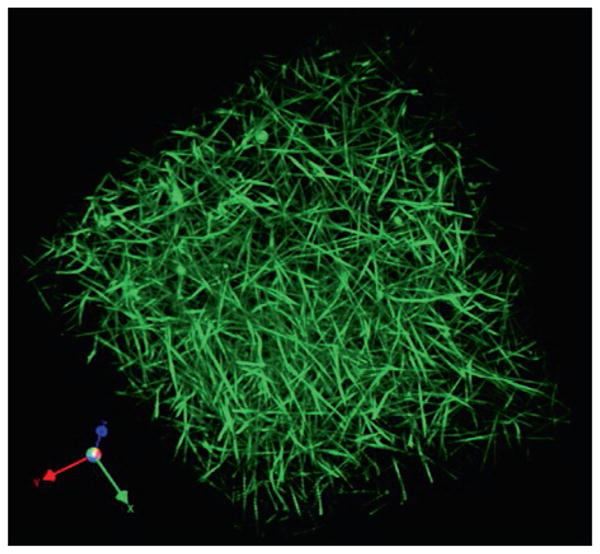
Fibrin clot network. A 3-dimensional reconstruction of a hydrated fibrin gel obtained using fluorescent confocal microscopy. Fibers are very straight under tension and branch to form a network. Fibrin(ogen) was fluorescently labeled with Alexa 488 (Brown et al. 2009)
13.3.10 Factor XIIIa-Catalyzed Covalent Crosslinking of Fibrin
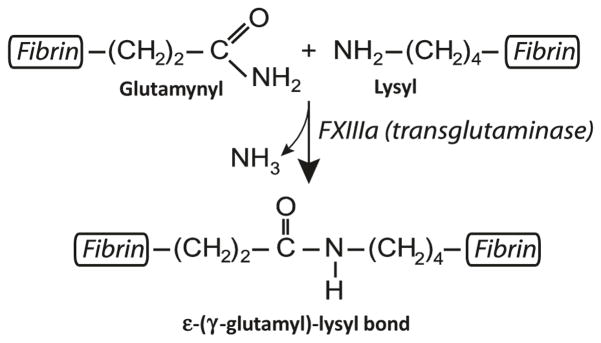
To stabilize the clot against proteolytic and mechanical insults, fibrin is covalently crosslinked by the plasma transglutaminase, Factor XIIIa, an active from of Factor XIII zymogen activated by thrombin in the presence of Ca2+. The C-terminal ends of the γ chains of fibrin(ogen) have amino acid residues comprising a crosslinking site for two end-to-end interacting molecules that form covalent isopeptide ε-(γ-glutamyl)-lysyl bonds between the γLys406 of one molecule and γGln398/399 of another molecule (Fig. 13.7). There has been a disagreement about longitudinal γ-γ-crosslinking within a strand of a protofibril versus transverse γ-γ-crosslinking between strands (Weisel 2004; Mosesson 2004), but new evidence has been provided for the longitudinal orientation of these bonds (Rosenfeld et al. 2015). Formation of the same isopeptide bonds is catalyzed at a smaller rate between the αC regions to stabilize long αC polymers (Matsuka et al. 1996). Crosslinking also occurs among α and γ chains followed by formation of α-γ-heterodimers (Standeven et al. 2007). Before crosslinking, fibrin polymerization is reversible, with fibrin being an equilibrium polymer (Chernysh et al. 2012). After crosslinking within and between protofibrils, polymerization becomes irreversible, and the clot is more stable, mechanically strong, and resistant to fibrinolysis. The crosslinked fibrin can be dissolved either by reduction of disulphide bonds that hold polypeptide chains together or by chemical/enzymatic hydrolysis of peptide bonds. A normal genetic variant of Factor XIII with a Val34Leu polymorphism forms either porous permeable clots or dense clots with reduced permeability at various fibrinogen levels (Lim et al. 2003). A synthetic hemostatic polymer has been recently designed that stabilizes fibrin clots chemically, thus mimicking the crosslinking effect of Factor XIIIa (Chan et al. 2015).
Fig. 13.7.
Formation of isopeptide bond catalyzed by Factor XIIIa. The chemical reaction catalyzed by Factor XIIIa, yielding insoluble fibrin crosslinked by ε-(γ-glutamyl)-lysine bonds
13.4 Variations and Modulation of Fibrin(ogen) Structure and Properties
13.4.1 Genetic Polymorphisms of Fibrinogen
Variants of fibrinogen are present in the blood as a result of several common polymorphisms or normal alternative primary structures. The most abundant fibrinogen variants contain two types of γ chains, γA and γ ′, that result from an alternative polyadenylation signal in intron 9 of the FGG gene (Francis et al. 1980). About 8–15 % of total fibrinogen contains the γ′ chain, of which the majority is in the heterodimeric γA/γ′ form with a homodimer γ′/γ′ comprising only about 1 % (Chung and Davie 1984). The presence of γ′ chains was shown to slow down lateral aggregation of protofibrils and alter fibrin formation and structure (Ajjan et al. 2009; Domingues et al. 2015; Muthard et al. 2015). An increase in plasma fibrinogen γ′ concentration is associated with the risk of myocardial infarction and other thrombotic states (Mannila et al. 2007; Lovely et al. 2002; Uitte de Willige et al. 2005, 2009). Fibrinogen polymorphisms with functional consequences can occur in the polypeptide chains other than the γ chain. αE fibrinogen (see Sect. 13.2.1) has a reduced rate of fibrin polymerization and forms thinner and more branched fibers than fibrin networks containing the α chains (Mosesson et al. 2004). The fibrinogen Aα chain gene FGA polymorphism 2224G/A has been associated with reduced clot permeability (Mannila et al. 2006), while the Ala312 allele of FGA 6534/Thr312Ala was associated with increased clot stiffness (Standeven et al. 2003), and the Lys448 allele of the fibrinogen Bβ chain gene (FGB) polymorphism BβArg448Lys resulted in a compact fibrin network structure resistant to lysis (Ajjan et al. 2008). The other most widespread polymorphisms in the fibrinogen genes occur in the noncoding regions and can result in changes in plasma fibrinogen levels. There are many more examples of strong associations between fibrinogen polymorphisms, clot structure and properties, and disease (Ariëns et al. 2002; Scott et al. 2004).
13.4.2 Post-translational Modifications and Heterogeneity of Fibrinogen
There are many molecular forms of fibrinogen present in blood, as originally detected from variations in biochemical properties and gel electrophoretic behavior. It has been estimated that fibrinogen may occur in more than a million non-identical forms in a healthy individual as a result of the many combinations of modified or inherently polymorphic sites (Henschen-Edman 2001). Several of these genetically determined variations have already been mentioned, but there are also post-translational heterogeneities originating from multiple biochemical reactions that accompany various physiological and especially pathological conditions, such as inflammation or ischemia. These reactions can modify the fibrinogen molecule in many ways, such as phosphorylation at specific seryl and threonyl sites, prolyl hydroxylation, tyrosyl sulfation, asparaginyl or glutaminyl deamidation, N-terminal pyroglutamate formation from glutaminyl precursors, oxidation of methionine, histidine and tryptophan residues, tyrosine nitration, modifications of cysteine residues, formation of dityrosine and carbonyl groups, etc. The C-terminal portion of the Aα chain is especially susceptible to limited cleavage by intracellular and extracellular proteolytic enzymes, but some digestion also occurs at specific sites in the Bβ and γ chains, such that lower molecular weight forms are commonly present in plasma fibrinogen. Alternative N-glycosylation can be another source of fibrinogen heterogeneity because it may result in formation of oligosaccharides with a variable structure (Brennan 2015). Acquired abnormal fibrinogen variants occur in patients with several conditions via non-enzymatic reactions, including glycation of lysine residues in uncompensated diabetes mellitus (Dunn and Ariens 2004) or homocysteinylation in hyperhomocysteinemia (Sauls et al. 2003). Fibrinogen derivatives can form in pathological conditions associated with thrombin generation, activation of fibrinolytic enzymes, or immune reactions, for example crosslinked fibrin(ogen) degradation products form when fibrinolytic activity in the blood is excessive, and antibody-fibrinogen complexes are formed in some autoimmune diseases. Individuals treated with acetylsalicylic acid (aspirin) have acetylated lysine residues (Ajjan et al. 2009). Oxidative stress has been widely implicated in physiological processes such as aging, in various disease pathogenesis, including arterial and venous thrombosis. Proteins are major targets for oxidants, and fibrinogen is a common target for oxidative post-translational modifications (Martinez et al. 2013; Rosenfeld et al. 2014). Neutrophils and monocytes, the most prevalent leukocytes in the sites of inflammation and venous thrombi, generate nitrating metabolic intermediates capable of nitration of fibrinogen (Heffron et al. 2009; Martinez et al. 2012). Tobacco smokers tend to have nitration of tyrosine and oxidation of methionine, histidine or tryptophan residues (Parastatidis et al. 2008). Many of these chemically modified forms are associated with differences in functional and structural properties of fibrinogen and fibrin, including a thrombogenic phenotype associated with increased risk of arterial and venous thrombosis (Nowak et al. 2007; Parastatidis et al. 2008; Paton et al. 2010; Sauls et al. 2006; Undas et al. 2006; Weigandt et al. 2012).
13.4.3 Hereditary Fibrinogen Defects (Dysfibrinogenemias, Afibrinogenemia, and Hypofibrinogenemia)
Dysfibrinogenemias are characterized by inherent structural changes in the fibrinogen molecule that commonly result in alterations in clotting or other functional aspects of the protein (Casini et al. 2016). Traditionally, particular defective fibrinogen variants are named after the city of their discovery or where the patient lived. Most congenital defects are rare but have led to important insights into fibrin(ogen) structure-function relationships. Many of these mutations have been described in reviews (Galanakis 1993; Roberts et al. 2001; Matsuda and Sugo 2001; de Moerloose and Neerman-Arbez 2009; Asselta et al. 2006; de Moerloose et al. 2013; Asselta et al. 2015; Casini et al. 2015). In addition, the website, http://www.geht.org/databaseang/fibrinogen, maintains a database of fibrinogen mutations and their functional consequences.
Mutations that give rise to dysfibrinogenemias are commonly caused by single base mutations that lead to the substitution of a single amino acid residue, but other mutations can give rise to a stop codon, resulting in a truncation of one of the chains. In addition, base additions or deletions may occur, with consequences for fibrinogen structure and function. Such mutations can cause a predisposition to thrombosis, or bleeding, or be asymptomatic, depending on their functional effects. They can affect fibrinopeptide cleavage, fibrin polymerization, Factor XIIIa-catalyzed crosslinking, susceptibility to fibrinolysis or integrin αIIbβ3-mediated platelet aggregation. In homozygous forms of the dysfibrinogenemias, the mutations occur in all fibrinogen molecules so only abnormal homodimers are present in the blood, but these mutations are rare, so most dysfibrinogenemias are heterozygous. As a result, the pool of fibrinogen molecules in most subjects with a dysfibrinogenemia consists of various proportions of normal homodimers, mutant homodimers and heterodimers.
Most dysfibrinogenemias are likely to be undetected by clotting assays, but some of the most commonly found alterations are in the N-terminal part of the Aα chain, in the regions responsible for thrombin binding or cleavage or in or near knob ‘A’, i.e. the AαGly17-Pro18-Arg19 motif. One such mutation is a substitution of AαArg16 (the last residue of FpA) by His or Cys, the former causing delayed release of FpA, with the latter completely abrogating its release. With no FpA cleavage, FpB is released slowly and clots made of desB-fibrin form, but only at lower temperatures (Shainoff and Dardik 1979). Mutations of AαGly17, AαPro18, AαArg19 or AαVal20 result in defective polymerization because of alterations of knobs ‘A.’
Mutations of the Bβ chain are less common. Substitution of Cys for BβGly at position 15 results in slow polymerization from delayed release of FpB (Sugo et al. 2000). Mutation of BβAla68 to Thr results in defective binding of thrombin to fibrin and consequent thrombosis (Koopman et al. 1992), since fibrin is a physiological absorbent of thrombin and was initially called antithrombin I (Mosesson 2007).
In the γ chain, many mutations in the C-terminal portion have been identified, particularly at position γArg275 (Cote et al. 1998). This residue makes contacts at the D:D interface, so that mutations affect fibrin polymerization. Mutations in or near the hole ‘a’ are common but patients with most of these substitutions are asymptomatic, presumably because they are heterozygous.
Some point mutations are responsible for congenital hypo- and afibrinogenemia, as a result of defects in molecular processing, assembly, secretion, and domain stability of fibrinogen. Afibrinogenemia is an autosomal recessive disorder characterized by the complete absence of detectable fibrinogen in the blood and hypofibrinogenemia is characterized by a reduced level of fibrinogen. Analysis of the three fibrinogen genes in affected individuals has led to the identification of several causative mutations (Brennan et al. 2001; Neerman-Arbez 2001), mostly as a result of mutations in the FBA gene. The analysis of the degree of severity of the fibrinogen disorders associated with truncation of the Aα chain suggest that formation of the distal disulfide ring of the coiled-coil is essential for assembly and secretion of fibrinogen molecules.
13.4.4 Environmental Conditions of Fibrin Formation
Fibrin formation, structure, and properties both in vitro and in vivo are strongly affected by external factors, such as ionic strength, composition, pH and various endogenous and exogenous substances, e.g., polyphosphate, mono-, oligo- and polysaccharides, peptides, lipids, proteins, nucleic acids, medications, as well as many other normal and pathological, natural and artificial compounds present in blood and injured tissues. Thrombin activity in blood has a profound effect on fibrin, with a high thrombin activity resulting in clots with thinner fibers, a higher density of branch points, and smaller pores, while a low thrombin activity results in thicker fibers with fewer branch points and larger pores. Most of these structural variations are based on the kinetics of individual steps of fibrin polymerization (Weisel and Nagaswami 1992). The structure of fibrin networks is often affected by physical factors, such as hydrodynamic flow or a strong magnetic field that result in formation of oriented anisotropic fibrin fibers (Gersh et al. 2010; Campbell et al. 2010). Fibrin structure and properties are greatly influenced by the presence of blood cells, namely activated platelets and erythrocytes (Aleman et al. 2014; Malecki et al. 2015) that form a natural and very active environment for clot formation. In addition to whole cells, the effects of circulating cell-derived microparticles on the fibrin clot structure and properties have been recently demonstrated (Zubairova et al. 2015). Fibrin can interact with other components of the extracellular matrix, both filamentous and non-filamentous, that not only affects fibrin structure but imparts additional mechanical and chemical stability (Maquart and Monboisse 2014). The structure of fibrin clots can be directly related to clinical conditions associated with thrombosis (Collet et al. 2006; Zalewski et al. 2015).
Here is a list of plasma proteins that bind specifically to fibrinogen or fibrin or both, with some indications of the biological significance:
| Actin | modulation of clot structure and properties (Talens et al. 2012) |
| Albumin | modulation of lateral aggregation and clot structure (Galanakis et al. 1987; Talens et al. 2012) |
| α1-Antitrypsin | local regulation of proteases involved in coagulation or fibrinolysis (Talens et al. 2012) |
| α2-Antiplasmin | local regulation of fibrinolysis (Tsurupa et al. 2010) |
| Apolipoproteins A-IV, A-I, J, E | associated with high-density lipoproteins; the physio logic role of binding to fibrin(ogen) is not clear (Talens et al. 2012) |
| Carboxypeptidase N | a local fibrinolysis inhibitor (Talens et al. 2012) |
| CD44 | mediates tumor metastasis at the sites of fibrin deposition (Alves et al. 2009) |
| Coagulation Factor Xa | a negative feedback for thrombin generation (Iino et al. 1995) |
| Coagulation Factor VIII | platelet-attached soluble fibrin mediates binding of Factor VIII (Gilbert et al. 2015) |
| Coagulation Factor XIII | crosslinking of fibrin and multiple plasma proteins (Weisel and Dempfle 2013) |
| Complement C3 | delayed fibrinolysis (Howes et al. 2012) |
| Factor H-related proteins-associated lipoprotein particles (FALP) | the physiologic role of fibrinogen on FALP is not clear (Park and Wright 2000) |
| Ferritin | some fibrinogens circulate in the form of a complex with ferritin (Takahashi et al. 2013) |
| Fibroblast Growth Factor-2 | augmented angiogenesis and cell proliferation at the sites of fibrin deposition (Sahni et al. 1999) |
| Fibulin | interferes with the fibrin assembly (Tran et al. 1995) |
| Haptoglobin | associated with high-density lipoproteins; the physiologic role of binding to fibrin(ogen) is not clear (Talens et al. 2012) |
| Hepatocyte-derived fibrinogen related protein-1 | liver cell growth regulation (Talens et al. 2012) |
| Immunoglobulins | modulate fibrin structure, localize immune and inflam matory reactions (Talens et al. 2012) |
| Interleukin-1β | modulation of inflammation (Sahni et al. 2004) |
| Lipoprotein(a) | modulation of fibrinolysis (Weisel and Litvinov 2014) |
| α2-Macroglobulin | local regulation of proteases involved in coagulation or fibrinolysis (Talens et al. 2012) |
| Myosin | modulation of fibrinolysis (Kolev et al. 2003) |
| Plasma fibronectin | platelet adhesion and thrombus formation (Gailit and Ruoslahti 1988; Talens et al. 2012) |
| Plasminogen | promotion of fibrinolysis (Weisel and Litvinov 2014) |
| Plasminogen activator inhibitor-1 | modulation of fibrinolysis (Smolarczyk et al. 2005) |
| Serum amyloid P | associated with high-density lipoproteins; the physiologic role of binding to fibrin(ogen) is not clear (Talens et al. 2012) |
| Thrombin | elimination of active thrombin from the circulation (Weisel and Dempfle 2013) |
| Thrombin-activatable fibrinolysis inhibitor (TAFI) | modulation of fibrinolysis (Valnickova and Enghild 1998) |
| Thrombospondin | modulation of fibrinolysis (Bacon-Baguley et al. 1990) |
| Tissue-type plasminogen activator (t-PA) | promotion of fibrinolysis (Medved et al. 2001) |
| Vascular Endothelial Growth Factor (VEGF) | promotion of angiogenesis at the sites of fibrin deposition (Sahni and Francis 2000) |
| VE-cadherin | suppression of inflammation by inhibiting leukocyte transmigration at the sites of fibrin deposition (Yakovlev et al. 2011) |
| Very low density lipoprotein receptor (VLDLR) | promotion of inflammation and transendothelial migration of leukocytes at the sites of fibrin deposition (Yakovlev and Medved 2015) |
| Vitronectin | platelet adhesion and thrombus formation (Schvartz et al. 2002; Podor et al. 2002) |
| von Willebrand Factor | platelet adhesion and thrombus formation (Miszta et al. 2014) |
13.4.5 Fibrin Formation Under Hydrodynamic Flow
Blood flow is one of the most important physical factors that affects profoundly formation of the fibrin network, its structure and properties in vivo (Swieringa et al. 2016). Clot formation in static conditions stops when all soluble fibrinogen is converted to insoluble fibrin. Therefore, in normal human plasma with a fibrinogen concentration of about 3 g/L, a fibrin clot will consist of only 0.3 % protein and 99.7 % liquid by mass. Alternatively, under flow conditions more fibrinogen is added to the forming clot, so the clot will contain more protein and have a different structure with much denser, thicker and bundled fibers (Silvain et al. 2011; Neeves et al. 2010). Fibrin formed under flow conditions also has some fibrin fibers orientated along the direction of flow (Gersh et al. 2010; Campbell et al. 2010; Whittaker and Przyklenk 2009), which changes clot mechanical properties and affects its susceptibility to enzymatic lysis (Campbell et al. 2010; Varju et al. 2011). Furthermore, it has been proposed from microfluidics experiments that the shear forces of blood flow determine the likelihood of embolization, namely the rupture of a piece of a clot that is carried by the blood stream to block another vessel (Colace et al. 2012; Brass and Diamond 2016).
13.5 Fibrin Mechanical Properties and Their Structural Origins
13.5.1 General Remarks
Fibrin mechanics is an important and rapidly developing field because fibrin networks form at sites of vascular injuries and perform a mechanical task of stemming blood flow by forming a gel under hydrodynamic blood shear (Campbell et al. 2010; Flamm and Diamond 2012), contraction of platelets (Lam et al. 2011), contraction of adjacent muscles, and pulsation of a vessel wall (Gasser et al. 2008; Ashton et al. 2009). Therefore, the outcomes of many bleeding and thrombotic disorders, including thromboembolism, are largely determined by the mechanical behavior of fibrin networks (Tran et al. 2013). In addition, fibrin has been used as a biomaterial for numerous purposes of surgical repairs and tissue engineering to stop or control bleeding or to form a provisional fibrin matrix for growing blood vessels and tissue regeneration, all of which strongly depend on the mechanical properties of fibrin gels.
13.5.2 Viscoelastic Properties of Fibrin
Fibrin is a viscoelastic polymer, which means that it has both elastic and viscous properties. The elasticity (or stiffness) is characterized by reversible mechanical deformation, while viscosity (or plasticity) is characterized by a slow irreversible deformation (creep) induced by force. Viscoelastic biomaterials differ in the relative degrees of both elastic and viscous properties. The elastic response of the fibrin clot is characterized by the shear storage modulus, G′, corresponding to the part of shear stress that is in phase with strain. The viscous response of the clot to applied shear is measured by the shear loss modulus, G″, where strain lags stress. The storage and loss moduli determine how the clot responds to the forces to which it is subjected. The ratio G″/G′ is often used to characterize relative viscosity and stiffness of a fibrin clot.
Clots derived from the blood of subjects with pulmonary embolism showed accelerated establishment of viscoelastic properties compared to healthy donors (Martinez et al. 2014). In addition, the stiffness of clots formed from the blood of patients who have had heart attacks at an early age is 50 % greater than that of controls (Collet et al. 2006). The clot fractal dimension, based on viscoelastic properties of incipient blood clots, has been used as a biomarker of prothrombotic clot microstructure (Lawrence et al. 2015; Davies et al. 2015). Platelets sense the stiffness of the underlying fibrin/fibrinogen substrate so that higher substrate stiffness leads to increased platelet activation, adhesion and spreading (Qiu et al. 2014; Wufsus et al. 2015). Lastly, the stiffness of the fibrin scaffold of occlusive thrombi is a major determinant for effectiveness of their mechanical damage and removal to restore the impaired blood flow (Weiss et al. 2013).
13.5.3 Non-linear Elasticity and High Extensibility of Fibrin
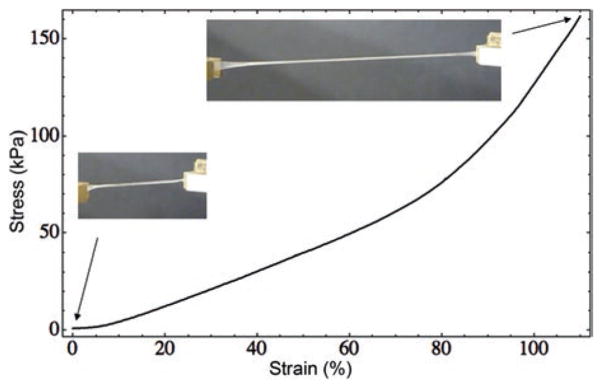
The elasticity of fibrin clots is generally characterized by a stress-strain curve, in which an applied stress (force/area) is plotted against the degree of induced deformation (strain) (Fig. 13.8). At low strains or deformations of fibrin, stress is directly proportional to strain and the slope of the curve (the elastic modulus) is constant. At larger strains, the linearity is broken and the slope of the curve increases dramatically, so that the elastic modulus or stiffness of the clot increases up to one order of magnitude or more (Janmey et al. 1983). This non-linearity is called strain hardening or strain stiffening and is a fundamental property of biological gel-like structures (Storm et al. 2005).
Fig. 13.8.
Stress-strain curve of a fibrin clot. Representative stress-strain curve of a cylindrical fibrin clot reaching greater than a two-fold longitudinal stretch. As the strain increases, the stress on the clot increases linearly until a strain of ~80 % is reached, at which point the sample hardens and enters a new regime with a steeper slope, corresponding to increased stiffness or strain hardening or stiffening. Insets show photographs of the initial clot and stretched clot
Fibrin is a highly extensible polymer, which means that under stress blood clots will tend to stretch rather than break. Plasma clots stabilized with Factor XIIIa could be stretched to over three times their relaxed length before breaking (Brown et al. 2009). The extraordinary extensibility, unusual visoelasticity, including strain stiffening, has been demonstrated and quantified at the level of individual fibers. This can be observed when a fibrin fiber is laterally stretched with a tip of an atomic force microscope, so that crosslinked and uncrosslinked fibrin fibers are stretched to about 2.5 and 3.3 times their original length before rupturing (Liu et al. 2006, 2010; Guthold et al. 2007). The propagation of strain-stiffening throughout the entire fibrin gel is largely determined by the fine structure and non-linear elastic properties of individual fibrin fibers (Hudson et al. 2015; Piechocka et al. 2010). As fibers are stretched, they become stiffer than any surrounding fibers at lower strains, which allow the more strained, stiffer fibers to distribute the strain load to the less strained fibers and reduce strain concentrations (Hudson et al. 2010). In addition to shear and tension, the non-linear elasticity of fibrin has been observed also in response to compressive deformations (Kim et al. 2014).
There are a number of environmental factors that modulate fibrin stiffness both in vitro and in vivo. The main physiological modulator of fibrin mechanics is Factor XIIIa, which catalyzes fibrin crosslinking (Fig. 13.7) and increases the elastic modulus of fibrin several-fold, apparently by fiber compaction (Kurniawan et al. 2014). Based on the in vitro effects of zinc that reduces fibrin clot stiffness, it was suggested that zinc released from activated platelets may modulate clot strength and stability in cooperation with Factor XIIIa. Fibrin elasticity can be modulated by other physical influences (Kotlarchyk et al. 2011; Munster et al. 2013) or biochemical modifications as well as by blood components and cells incorporated into the fibrin network (Rojas et al. 2009; Weigandt et al. 2012; Lauricella et al. 2013; Jansen et al. 2013; Henderson et al. 2015).
The tunable non-linear elasticity of fibrin may be important biologically because it allows fibrin clots to be compliant at smaller strains and then become stiffer at larger deformations that could otherwise threaten clot integrity and make them prone to embolization. In addition, the complex mechanical behavior of fibrin is important for the interaction between cells and extracellular matrix (Wen and Janmey 2013). Since mechanical stress makes fibrin more resistant to fibrinolysis (Varju et al. 2011; Bucay et al. 2015), fibrin elasticity may be a significant determinant of susceptibility of clots and thrombi to enzymatic lysis (Rottenberger et al. 2013; Longstaff et al. 2013).
13.5.4 Multiscale Structural Mechanics of Fibrin Clots
It has been shown that fibrin mechanics are governed by a structural hierarchy, implying that fibrin deformation is accompanied by multiple structural rearrangements at different scales, namely the molecular level, individual fibers, fiber network, and the whole clot (Brown et al. 2009; Purohit et al. 2011; Piechocka et al. 2010; Yeromonahos et al. 2010).
At the macroscopic scale (10−2 m), in addition to their large extensibility, fibrin clots also display a dramatic decrease in volume when they are stretched (Brown et al. 2009). The shrinkage of the stretched clot is due to water expulsion and network densification, as confirmed by an approximately tenfold increase in the protein content in clots stretched threefold. This observation might be related to the phenomenon of negative normal stress observed for networks of semiflexible polymers (Guo et al. 2009). An alternative explanation is that the volume change is associated with a molecular structural transition that occurs in stretched fibrin fibers (Brown et al. 2009; Purohit et al. 2011).
At the network scale (10−5 m), when strain is applied, the fibers begin to align along the direction of strain (Brown et al. 2009; Litvinov et al. 2012; Purohit et al. 2011), the fibers become thinner, closer together, and bundle (Müller et al. 1984; Brown et al. 2009). A dramatic rearrangement of the fibrin network was also observed in response to compressive deformation (Kim et al. 2014): the fibrin network density increased, fibers reoriented in the compression plane and shorter fiber segments were formed as a result of fiber crisscrossing. These transformations of the network structure are accompanied by a dramatic increase of fibrin elasticity.
At the fiber scale (10−6 m), in response to compression or shear, individual fibrin fibers in the network begin to buckle and bend in the direction of deformation (Lindstrom et al. 2013; Kim et al. 2014). Because buckling and subsequent bending make fibers more compliant, the stiffness of the network in the transverse shear direction gradually decreases with compression.
13.5.5 Molecular Structural Origins of Fibrin Mechanical Properties
At the molecular level, fibrin has the potential to elongate more than 5-fold, from 45 nm to about 250 nm (Fig. 13.9). The most important structural changes during fibrin deformations include unfolding of coiled-coils (Brown et al. 2007; Lim et al. 2008; Litvinov et al. 2012), the γ-nodules (Averett et al. 2008, 2009), and extension of the αC regions. In addition, other studies point to a role of network branch points (Carlisle et al. 2010) and the γA/γ′ splice variants (Gersh et al. 2009; Allan et al. 2012). Based on the known crystal structure of a folded fibrin(ogen) molecule (Fig. 13.9), it could be predicted that full hypothetical unfolding of compact structures would result in a ~4.7-fold elongation (Zhmurov et al. 2011; Hudson et al. 2013), which characterizes a great potential contribution of molecular unfolding in large deformations of fibrin.
Fig. 13.9.
Unfolding of fibrin(ogen). A schematic representation of the fibrin(ogen) molecule in the naturally folded (b) and fully unfolded states ((a) and (c)). The molecule is constrained at the C-terminal part of one γ chain, and mechanical force is applied to the C-terminal part of the other γ chain. (a) Shows full unfolding without much detail, while the structural details are given in (c), showing schematically the lengths of the central nodule, γ-nodules, β-nodules, coiled-coils, taking into account the disulfide bonds. Dimensions are shown in the compact crystal structure (b), and the contour lengths of various structural elements are shown in the fully unfolded state (c), assuming a contour length per residue of 0.38 nm (Zhmurov et al. 2011, with permission of Elsevier Ltd.)
The first direct experimental observation for an α-helix to β-strand conversion of the coiled-coils accompanying extension of fibrin was wide-angle X-ray scattering of squeezed fibrin films (Bailey et al. 1943). Unlike the extensively studied α-helix to β-strand transition in keratin, this preliminary result was not pursued until recently. The secondary structure changes during deformations of fibrin polymers studied using Fourier Transform infrared spectroscopy showed that both extension and compression of a hydrated fibrin clot are accompanied by the transition of a triple α-helix to β-sheet (Litvinov et al. 2012). This structural transition has been confirmed and analyzed in detail using full-atom molecular dynamics simulations of extensional mechanical unfolding of a fibrin(ogen) molecule (Zhmurov et al. 2012; Fig. 13.10).
Based on the paracrystalline molecular packing within a fibrin fiber that results in a 22.5-nm periodicity, it has been hypothesized that forced elongation and unfolding of fibrin molecules during stretching must result in an increase of this repeat. However, small angle X-ray scattering revealed that the position of the peak corresponding to the 22.5-nm spacing does not change significantly as the clot is stretched. Instead, there was an increase in sample disorder, consistent with an increasing number of molecules unfolding non-uniformly in response to the large strain, as expected for a two-state system in which some molecules extend completely while others remain folded (Brown et al. 2009).
Single-stranded fibrinogen oligomers have been prepared and the unfolding of fibrinogen domains has been measured by single-molecule atomic force microscopy (Brown et al. 2007; Zhmurov et al. 2011). In addition, computer-based modeling of the experimental data enabled the visualization of the various structural transitions and identification of relationships to the forces observed in the experimental or simulated force-extension curves. Molecular elongation of fibrin(ogen) is largely determined by the combined sequential unfolding transitions in the C-terminal γ chain nodules and limited reversible extension-contraction of the α-helical coiled-coil connectors. The coiled-coils act as molecular springs to take up the slack as other domains unfold.
The role of the αC polymers in fibrin mechanics, underestimated in the past, has been studied intensively more recently (Houser et al. 2010; Falvo et al. 2010; Ping et al. 2011; Helms et al. 2012; Averett et al. 2012). It has been shown that the transglutaminase-catalyzed covalent crosslinking of the α chains contributes substantially to the fibrin clot stiffness and elasticity (Collet et al. 1999; Piechocka et al. 2010; Helms et al. 2012; Duval et al. 2014; Kurniawan et al. 2014), including the fast elastic recoil of stretched fibrin fibers (Hudson et al. 2013). Based on the exceptional role of the αC regions in fibrin mechanics, a structural model of fibrinogen has been proposed that captures the stress-strain behavior of individual fibrin fibers (Averett et al. 2012).
13.5.6 Fibrin as a Biomaterial
A new impetus to study fibrin mechanics came from its biomedical applications as a hydrogel (Janmey et al. 2009). Fibrin has been extensively used as a biopolymer scaffold in tissue engineering, taking advantage of its unique biological and physical characteristics, including its porosity, deformability, elasticity and biodegradability (Ahmed et al. 2008). Fibrin alone, or in combination with other materials, has been used as a biological scaffold for stem or primary cells to regenerate adipose tissue, bone, cardiac tissue, cartilage, liver, nervous tissue, ocular tissue, skin, tendons, and ligaments. Although fibrin can be used as the only biomaterial, it has been shown that the addition of collagen to fibrin can provide extra stiffness and durability of the forming biomaterial (Lai et al. 2012). Gels based on fibrin, alone or in combination with gelatin, collagen, or elastin, have been used to optimize cellular activities, including differentiation, proliferation, and changes in morphology (Shevchenko et al. 2010; Chaterji et al. 2007; Dikovsky et al. 2006; Natesan et al. 2011; Kuehn et al. 2014; Brougham et al. 2015). The ability of cells to modify their phenotype and behavior in response to variations in the stiffness of a fibrin-containing substrate (called mechanosensing) has been used, e.g., in regulation of neurite outgrowth in fibrin gels (Man et al. 2011) and modulation of the secretory activity of endothelial cells and mesenchymal stem cells (Rao et al. 2012). Fibrin clots with longitudinally aligned fibrin fibers could serve as a scaffold for longitudinal axonal regrowth in cases of traumatic peripheral nerve injuries (Gessmann et al. 2016).
Fibrin sealants formed by mixing fibrinogen and thrombin are widely used to stop bleeding (Corral et al. 2016). Importantly, various commercially available fibrin sealants have diverse mechanical properties (Hickerson et al. 2011) and glues with distinct structure and stiffness had different hemostatic efficacy (Fortelny et al. 2011).
13.6 Lytic Stability of Fibrin
13.6.1 Molecular Mechanisms of Fibrinolysis
After a clot has formed in vivo and fulfilled its hemostatic function, it is normally dissolved by the fibrinolytic system, to restore the impaired blood flow (Weisel and Litvinov 2008, 2014; Longstaff and Kolev 2015). The main enzyme involved is plasmin (Pn), a serine protease derived from its inactive precursor, plasminogen (Plg), through the action of activators. Pn also cleaves several other substrates, including extracellular matrix proteins, and activates some proteases and growth factors. So, in addition to fibrinolysis, Plg and Pn are involved in several other physiological and pathophysiological processes, such as wound healing, inflammation, cell migration, angiogenesis, embryogenesis, ovulation, tumor growth and metastasis, and atherosclerosis.
There is a complex system of biochemical reactions comprising fibrinolysis and its regulatory mechanisms (Fig. 13.11). Fibrinolysis occurs first by the conversion of Plg to Pn by a Plg activator, primarily on the fibrin surface, and then by the degradation of fibrin by Pn. Any Pn that dissociates from fibrin is rapidly inactivated by inhibitors. The activators of Plg are the serine proteases tissue-type plasminogen activator (t-PA) or urokinase-type plasminogen activator and bacterial proteins that acquire proteolytic activity after the interaction with human Plg or Pn, streptokinase and staphylokinase. t-PA and staphylokinase are fibrin-selective, remaining bound to fibrin and protected from rapid inhibition, while streptokinase and two-chain urokinase-type plasminogen activator are non-fibrin-selective enzymes, activating both Plg in the circulating blood and fibrin-bound Plg. Attachment of Plg and t-PA to fibrin is mediated by the C-terminal lysine residues of fibrin and the specific lysine-binding motifs on the Plg and t-PA molecules. Because Pn cleaves at lysine residues, the new C-terminal lysines provide additional binding sites for Plg and t-PA, as a positive feedback mechanism. The number of t-PA and Plg binding sites on fibrin and the rate of lysis can be increased by ultrasound-induced perturbation of fibrin clots, which may be of practical importance for thrombolytic therapy (Chernysh et al. 2015).
Fig. 13.11.
Schematic diagram of fibrinolysis on the fibrin clot surface and in the liquid phase. Schematic representation of the major reactions of fibrinolysis and their regulation on a fibrin clot surface and in the surrounding plasma milieu. The grey highlighted area represents a fibrin clot surface (solid phase) surrounded by the blood plasma (liquid phase). Black arrows show the biochemical conversions involving proteolytic cleavage. T-like symbols indicate inhibitory effects. Abbreviations and functions of molecules: Lys-Fibrin C-terminal lysine residues on fibrin to which Plg and t-PA bind selectively, Plg plasminogen, bound to the C-terminal lysine residues on fibrin and free in plasma, Pn plasmin, formed on fibrin (by the action of t-PA) and in plasma (by the action of tcu-PA) from Plg. Pn cleaves fibrin and fibrinogen, activates scu-PA and TAFI, t-PA tissue-type Plg activator, fibrin-selective Plg activator, bound to fibrin via the C-terminal lysine residues on fibrin, scu-PA single-chain urokinase-type Plg activator (inactive), tcu-PA two-chain u-PA (active), non-fibrin-selective Plg activator, PAI-1 plasminogen activator inhibitor-1, blocks both t-PA and tcu-PA, TAFIa thrombin-activatable fibrinolysis inhibitor (enzymatically active form) that splits off the C-terminal lysine residues from fibrin, thus preventing binding of Plg and t-PA to fibrin, α2-AP α2-antiplasmin, direct Pn inhibitor, forms circulating Pn-α2-AP complexes, FDP fibrin(ogen) degradation products, resulting from cleavage of fibrin or fibrinogen by Pn, D-dimer a proteolytic fragment (degradation product) that is formed by Pn only from crosslinked fibrin
Enzymatic lysis of fibrin results in formation of soluble degradation products. Basically, the fibrin degradation products generated by plasmin are very similar to the proteolytic fragments of fibrinogen, namely intermediate fragments X and Y and the terminal fragments D and E, with the difference based on the presence or absence of fibrinopeptides (Marder and Budzynski 1975). What makes fibrin degradation products formed in vivo different from those of fibrinogen is the non-covalent interactions and covalent Factor XIIIa-mediated crosslinking of fibrin, which results in formation of various soluble oligomeric structures (Veklich et al. 1998; Horan and Francis 2001). The smallest crosslinked fibrin degradation product is D-dimer formed of two D regions of the adjacent fibrin molecules connected by a γ-γ bond. Because the only source of D-dimer is crosslinked fibrin, a high level of D-dimer-containing products in blood is widely used by clinicians as a laboratory sign of intravascular fibrin deposition occurring during local thrombosis or disseminated intravascular coagulation (Bates 2012). Fibrin degradation products comprise fibrin(ogen) fragments with incomplete sets of binding sites that competitively inhibit fibrin polymerization and can result in formation of large oligomeric structures containing non-polymerized fibrin molecules named “soluble fibrin complexes” or “soluble fibrin”.
13.6.2 Modulators of Fibrinolysis
There are a number of biochemical reactions that moderate the activity of the profibrinolytic components, such as Plg activator inhibitor-1, a potent inhibitor of t-PA and u-PA, and α2-antiplasmin, which directly inhibits Pn (Fig. 13.11). Thrombin-activatable fibrinolysis inhibitor cleaves C-terminal lysine residues from partially degraded fibrin and thus inhibits fibrinolysis by preventing the lysine-dependent binding of Plg to fibrin. Lipoprotein(a) has a structural homology with Plg and, therefore can compete with Plg for binding to lysine residues and impair fibrinolysis (Angles-Cano et al. 2001).
The effectiveness of fibrinolysis results from the combination of regulated enzymatic activity and the physical characteristics of the fibrin scaffold, such as the density of fibers and branch points, pore size, and fiber diameter. In general, the rate of lysis appears to be faster for clots made up of thicker fibers than for clots made up of thinner fibers, but also depends on other biophysical properties of the clot (Weisel 2007). In addition, platelet aggregation and clot retraction have dramatic effects on fibrinolysis (Collet et al. 2002). Stretching also affects the rate of lysis of clots (Varju et al. 2011).
13.6.3 Internal and External Fibrinolysis
A variety of systems have been used to study fibrinolysis experimentally, and each has strengths and weaknesses and is suited to the investigation of different aspects of lysis. With each of these systems, there are often several biochemical or structural methods that can be used to quantify rates of lysis.
The process that mimics the physiological process of fibrinolysis has been called internal or intrinsic lysis. To simulate this process in vitro, either t-PA mixed with plasma or t-PA and Plg mixed with fibrinogen have been clotted, so that the clot is formed and then dissolved as the t-PA activates Plg to Pn on the fibrin surface. The process of lysis has been followed by measurement of the decrease of turbidity or by confocal or other light microscopy or biochemically by the appearance of lysis products (Gabriel et al. 1992; Collet et al. 2003).
The process that mimics clinical thrombolysis is called external or extrinsic fibrinolysis. For therapeutic thrombolysis, t-PA is introduced into the vasculature, so that it circulates and binds to thrombi, activating Plg to Pn on the fibrin surface. In vitro, t-PA can be introduced at the edge of a pre-formed clot in a chamber, commonly made of a light microscope slide and cover slip, so that lysis can be observed either by eye or by light microcopy (Sakharov and Rijken 1995; Collet et al. 2000). In the external lysis system just described, the t-PA enters the clot only by diffusion. However, in thrombolysis the t-PA is delivered by flowing blood, so there usually is permeation or perfusion into the thrombus. Thus, a variation of external lysis is to allow the t-PA to permeate into the clot. The results can be quantified by the measurement of digestion products released or by observation of changes in clot structure as a function of time.
13.7 Conclusions
During the past 10 years, all of these different facets of research have contributed to the vigorous flowering of new information about fibrinogen and fibrin. It is now well established that fibrin is essential for hemostasis and is a major factor in thrombosis, inflammation and infection. Many of the molecular mechanisms of the biological processes of hemostasis and how they go awry in thrombosis have been determined. The binding of fibrinogen to the integrin αIIbβ3 and its role in platelet aggregation continues to represent a model system to study cell adhesion. In addition, fibrin has become increasingly used as a versatile and unique biomaterial.
In spite of the breadth and depth of research on fibrin(ogen) in the past 10 years, many mysteries remain. For example, molecular mechanisms of lateral aggregation and branching in fibrin polymerization are largely unknown. Since the preponderance of research on fibrinogen and fibrin has been on mammalian systems, much remains unknown about their roles in other animals. Many of the binding partners of fibrin(ogen) have been identified but their functions are mostly unknown. Basic mechanisms of the catabolism of fibrinogen remain to be discovered. The burgeoning field of fibrin mechanics will continue to thrive. Much remains to be learned about biological roles of fibrinogen beyond hemostasis. In the future, research will increasingly investigate major aspects of the role of fibrin(ogen) in more complex biological processes, such as wound healing and inflammation. The diverse array of powerful genetic and synthetic tools in various model systems, together with new super-resolution, single-molecule and single-cell imaging and manipulation methods, will drive this research. One thing is certain, fibrinogen and fibrin will continue to surprise us in the future, as they have in the past.
Acknowledgments
Some of the authors’ work mentioned here was supported by NIH grants NHLBI HL090774 and UO1HL116330, and NSF grant DMR1505662. We thank Drs. Oleg V. Gorkun and Lubica Rauova for careful reading of the manuscript and helpful suggestions.
Abbreviations
- FpA and FpB
fibrinopeptides A and B
- GHRP
Gly-His-Arg-Pro
- GPRP
Gly-Pro-Arg-Pro
- NXS or NXT
Asn-X-Ser or Asn-X-Thr
- Plg
plasminogen
- Pn
plasmin
- t-PA
tissue-type Plg activator
References
- Ahmed TE, Dare EV, Hincke M. Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng Part B Rev. 2008;14:199–215. doi: 10.1089/ten.teb.2007.0435. [DOI] [PubMed] [Google Scholar]
- Ajjan R, Lim BC, Standeven KF, Harrand R, Dolling S, Phoenix F, Greaves R, Abou-Saleh RH, Connell S, Smith DA, Weisel JW, Grant PJ, Ariens RA. Common variation in the C-terminal region of the fibrinogen beta-chain: effects on fibrin structure, fibrinolysis and clot rigidity. Blood. 2008;111:643–650. doi: 10.1182/blood-2007-05-091231. [DOI] [PubMed] [Google Scholar]
- Ajjan RA, Standeven KF, Khanbhai M, Phoenix F, Gersh KC, Weisel JW, Kearney MT, Ariens RA, Grant PJ. Effects of aspirin on clot structure and fibrinolysis using a novel in vitro cellular system. Arterioscler Thromb Vasc Biol. 2009;29:712–717. doi: 10.1161/ATVBAHA.109.183707. [DOI] [PubMed] [Google Scholar]
- Aleman MM, Walton BL, Byrnes JR, Wolberg AS. Fibrinogen and red blood cells in venous thrombosis. Thromb Res. 2014;133(Suppl 1):S38–S40. doi: 10.1016/j.thromres.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan P, Uitte De Willige S, Abou-Saleh RH, Connell SD, Ariens RA. Evidence that fibrinogen gamma’ directly interferes with protofibril growth: implications for fibrin structure and clot stiffness. J Thromb Haemost. 2012;10:1072–1080. doi: 10.1111/j.1538-7836.2012.04717.x. [DOI] [PubMed] [Google Scholar]
- Alves CS, Yakovlev S, Medved L, Konstantopoulos K. Biomolecular characterization of CD44-fibrin(ogen) binding: distinct molecular requirements mediate binding of standard and variant isoforms of CD44 to immobilized fibrin(ogen) J Biol Chem. 2009;284:1177–1189. doi: 10.1074/jbc.M805144200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angles-Cano E, De La Pena DA, Loyau S. Inhibition of fibrinolysis by lipoprotein(a) Ann N Y Acad Sci. 2001;936:261–275. doi: 10.1111/j.1749-6632.2001.tb03514.x. [DOI] [PubMed] [Google Scholar]
- Ariens RA. Fibrin(ogen) and thrombotic disease. J Thromb Haemost. 2013;11(Suppl 1):294–305. doi: 10.1111/jth.12229. [DOI] [PubMed] [Google Scholar]
- Ariens RA, Lai T-S, Weisel JW, Greenberg CS, Grant PJ. Role of Factor XIII in fibrin clot formation and effects of genetic polymorphisms. Blood. 2002;100:743–754. doi: 10.1182/blood.v100.3.743. [DOI] [PubMed] [Google Scholar]
- Ashton JH, Vande Geest JP, Simon BR, Haskett DG. Compressive mechanical properties of the intraluminal thrombus in abdominal aortic aneurysms and fibrin-based thrombus mimics. J Biomech. 2009;42:197–201. doi: 10.1016/j.jbiomech.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselta R, Duga S, Tenchini ML. The molecular basis of quantitative fibrinogen disorders. J Thromb Haemost. 2006;4:2115–2129. doi: 10.1111/j.1538-7836.2006.02094.x. [DOI] [PubMed] [Google Scholar]
- Asselta R, Plate M, Robusto M, Borhany M, Guella I, Solda G, Afrasiabi A, Menegatti M, Shamsi T, Peyvandi F, Duga S. Clinical and molecular characterisation of 21 patients affected by quantitative fibrinogen deficiency. Thromb Haemost. 2015;113:567–576. doi: 10.1160/TH14-07-0629. [DOI] [PubMed] [Google Scholar]
- Averett LE, Geer CB, Fuierer RR, Akhremitchev BB, Gorkun OV, Schoenfisch MH. Complexity of “A-a” knob-hole fibrin interaction revealed by atomic force spectroscopy. Langmuir. 2008;24:4979–4988. doi: 10.1021/la703264x. [DOI] [PubMed] [Google Scholar]
- Averett LE, Schoenfisch MH, Akhremitchev BB, Gorkun OV. Kinetics of the multistep rupture of fibrin ‘A-a’ polymerization interactions measured using atomic force microscopy. Biophys J. 2009;97:2820–2828. doi: 10.1016/j.bpj.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averett RD, Menn B, Lee EH, Helms CC, Barker T, Guthold M. A modular fibrinogen model that captures the stress-strain behavior of fibrin fibers. Biophys J. 2012;103:1537–1544. doi: 10.1016/j.bpj.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon-Baguley T, Ogilvie ML, Gartner TK, Walz DA. Thrombospondin binding to specific sequences within the A alpha- and B beta-chains of fibrinogen. J Biol Chem. 1990;265:2317–2323. [PubMed] [Google Scholar]
- Bailey K, Astbury WT, Rudall KM. Fibrinogen and fibrin as members of the keratin-myosin group. Nature. 1943;151:716–717. [Google Scholar]
- Bates SM. D-dimer assays in diagnosis and management of thrombotic and bleeding disorders. Semin Thromb Hemost. 2012;38:673–682. doi: 10.1055/s-0032-1326782. [DOI] [PubMed] [Google Scholar]
- Bennett JS. Platelet-fibrinogen interactions. Ann N Y Acad Sci. 2001;936:340–354. doi: 10.1111/j.1749-6632.2001.tb03521.x. [DOI] [PubMed] [Google Scholar]
- Blombäck B, Okada M. Fibrin gel structure and clotting time. Thromb Res. 1982;25:51–70. doi: 10.1016/0049-3848(82)90214-6. [DOI] [PubMed] [Google Scholar]
- Blombäck B, Hessel B, Hogg D, Therkildsen L. A two-step fibrinogen-fibrin transition in blood coagulation. Nature. 1978;275:501–505. doi: 10.1038/275501a0. [DOI] [PubMed] [Google Scholar]
- Bowley SR, Okumura N, Lord ST. Impaired protofibril formation in fibrinogen gamma N308 K is due to altered D:D and “A:a” interactions. Biochemistry. 2009;48:8656–8663. doi: 10.1021/bi900239b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass LF, Diamond SL. Transport physics and biorheology in the setting of haemostasis and thrombosis. J Thromb Haemost. 2016;14(5):906–917. doi: 10.1111/jth.13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan SO. Variation of fibrinogen oligosaccharide structure in the acute phase response: possible haemorrhagic implications. BBA Clin. 2015;3:221–226. doi: 10.1016/j.bbacli.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan SO, Fellowes AP, George PM. Molecular mechanisms of hypo- and afibrinogenemia. Ann N Y Acad Sci. 2001;936:91–100. doi: 10.1111/j.1749-6632.2001.tb03496.x. [DOI] [PubMed] [Google Scholar]
- Brennan SO, Davis RL, Mosesson MW, Hernandez I, Lowen R, Alexander SJ. Congenital hypodysfibrinogenaemia (Fibrinogen Des Moines) due to a gamma320Asp deletion at the Ca2+ binding site. Thromb Haemost. 2007;98:467–469. [PubMed] [Google Scholar]
- Bridge KI, Philippou H, Ariens R. Clot properties and cardiovascular disease. Thromb Haemost. 2014;112:901–908. doi: 10.1160/TH14-02-0184. [DOI] [PubMed] [Google Scholar]
- Brougham CM, Levingstone TJ, Jockenhoevel S, Flanagan TC, O’brien FJ. Incorporation of fibrin into a collagen-glycosaminoglycan matrix results in a scaffold with improved mechanical properties and enhanced capacity to resist cell-mediated contraction. Acta Biomater. 2015;26:205–214. doi: 10.1016/j.actbio.2015.08.022. [DOI] [PubMed] [Google Scholar]
- Brown JH, Volkmann N, Jun G, Henschen-Edman AH, Cohen C. The crystal structure of modified bovine fibrinogen. Proc Natl Acad Sci U S A. 2000;97:85–90. doi: 10.1073/pnas.97.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AE, Litvinov RI, Discher DE, Weisel JW. Forced unfolding of coiled-coils in fibrinogen by single-molecule AFM. Biophys J. 2007;92:L39–L41. doi: 10.1529/biophysj.106.101261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AE, Litvinov RI, Discher DE, Purohit PK, Weisel JW. Multiscale mechanics of fibrin polymer: gel stretching with protein unfolding and loss of water. Science. 2009;325:741–744. doi: 10.1126/science.1172484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AC, Baker SR, Douglas AM, Keating M, Alvarez-Elizondo MB, Botvinick EL, Guthold M, Barker TH. Molecular interference of fibrin’s divalent polymerization mechanism enables modulation of multiscale material properties. Biomaterials. 2015;49:27–36. doi: 10.1016/j.biomaterials.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucay I, O’brien ET, Wulfe SD, Superfine R, Wolberg AS, Falvo MR, Hudson NE. Physical determinants of fibrinolysis in single fibrin fibers. PLoS One. 2015;10:e0116350. doi: 10.1371/journal.pone.0116350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell RA, Aleman M, Gray LD, Falvo MR, Wolberg AS. Flow profoundly influences fibrin network structure: implications for fibrin formation and clot stability in haemostasis. Thromb Haemost. 2010;104:1281–1284. doi: 10.1160/TH10-07-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caracciolo G, De Spirito M, Castellano AC, Pozzi D, Amiconi G, De Pascalis A, Caminiti R, Arcovito G. Protofibrils within fibrin fibres are packed together in a regular array. Thromb Haemost. 2003;89:632–636. [PubMed] [Google Scholar]
- Carlisle CR, Sparks EA, Der Loughian C, Guthold M. Strength and failure of fibrin fiber branchpoints. J Thromb Haemost. 2010;8:1135–1138. doi: 10.1111/j.1538-7836.2010.03824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casini A, Neerman-Arbez M, Ariens RA, De Moerloose P. Dysfibrinogenemia: from molecular anomalies to clinical manifestations and management. J Thromb Haemost. 2015;13:909–919. doi: 10.1111/jth.12916. [DOI] [PubMed] [Google Scholar]
- Casini A, Duval C, Pan X, Tintillier V, Biron-Andreani C, Ariens RA. Fibrin clot structure in patients with congenital dysfibrinogenaemia. Thromb Res. 2016;137:189–195. doi: 10.1016/j.thromres.2015.11.008. [DOI] [PubMed] [Google Scholar]
- Chan LW, Wang X, Wei H, Pozzo LD, White NJ, Pun SH. A synthetic fibrin cross-linking polymer for modulating clot properties and inducing hemostasis. Sci Transl Med. 2015;7:277ra229. doi: 10.1126/scitranslmed.3010383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaterji S, Kwon IK, Park K. Smart polymeric gels: redefining the limits of biomedical devices. Prog Polym Sci. 2007;32:1083–1122. doi: 10.1016/j.progpolymsci.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernysh IN, Weisel JW. Dynamic imaging of fibrin network formation correlated with other measures of polymerization. Blood. 2008;111:4854–4861. doi: 10.1182/blood-2007-08-105247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernysh IN, Nagaswami C, Weisel JW. Visualization and identification of the structures formed during early stages of fibrin polymerization. Blood. 2011;117:4609–4614. doi: 10.1182/blood-2010-07-297671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernysh IN, Nagaswami C, Purohit PK, Weisel JW. Fibrin clots are equilibrium polymers that can be remodeled without proteolytic digestion. Sci Rep. 2012;2:879. doi: 10.1038/srep00879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernysh IN, Everbach CE, Purohit PK, Weisel JW. Molecular mechanisms of the effect of ultrasound on the fibrinolysis of clots. J Thromb Haemost. 2015;13:601–609. doi: 10.1111/jth.12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung DW, Davie EW. Gamma and gamma’ chains of human fibrinogen are produced by alternative RNA splicing. Biochemist. 1984;23:4232–4236. doi: 10.1021/bi00313a033. [DOI] [PubMed] [Google Scholar]
- Chung DW, Rixon MW, Que BG, Davie EW. Cloning of fibrinogen genes and their cDNA. Ann N Y Acad Sci. 1983;408:449–456. doi: 10.1111/j.1749-6632.1983.tb23265.x. [DOI] [PubMed] [Google Scholar]
- Chung DW, Harris JE, Davie EW. Nucleotide sequences of the three genes coding for human fibrinogen. Adv Exp Med Biol. 1990;281:39–48. doi: 10.1007/978-1-4615-3806-6_3. [DOI] [PubMed] [Google Scholar]
- Cilia La Corte AL, Philippou H, Ariens RA. Role of fibrin structure in thrombosis and vascular disease. Adv Protein Chem Struct Biol. 2011;83:75–127. doi: 10.1016/B978-0-12-381262-9.00003-3. [DOI] [PubMed] [Google Scholar]
- Cines DB, Lebedeva T, Nagaswami C, Hayes V, Massefski W, Litvinov RI, Rauova L, Lowery TJ, Weisel JW. Clot contraction: compression of erythrocytes into tightly packed polyhedra and redistribution of platelets and fibrin. Blood. 2014;123:1596–1603. doi: 10.1182/blood-2013-08-523860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C, Parry DAD. Alpha-helical coiled coils and bundles: how to design an alpha-helical protein. Proteins. 1990;7:1–15. doi: 10.1002/prot.340070102. [DOI] [PubMed] [Google Scholar]
- Colace TV, Muthard RW, Diamond SL. Thrombus growth and embolism on tissue factor-bearing collagen surfaces under flow: role of thrombin with and without fibrin. Arterioscler Thromb Vasc Biol. 2012;32:1466–1476. doi: 10.1161/ATVBAHA.112.249789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collen D, Tytgat GN, Claeys H, Piessens R. Metabolism and distribution of fibrinogen. I. Fibrinogen turnover in physiological conditions in humans. Br J Haematol. 1972;22:681–700. doi: 10.1111/j.1365-2141.1972.tb05715.x. [DOI] [PubMed] [Google Scholar]
- Coller BS. Historical perspective and future directions in platelet research. J Thromb Haemost. 2011;9(Suppl 1):374–395. doi: 10.1111/j.1538-7836.2011.04356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller BS, Shattil SJ. The GPIIb/IIIa (integrin alphaIIbbeta3) odyssey: a technology-driven saga of a receptor with twists, turns, and even a bend. Blood. 2008;112:3011–3025. doi: 10.1182/blood-2008-06-077891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collet J-P, Veklich Y, Mullin JL, Gorkun OV, Lord ST, Weisel JW. The αC domains of fibrinogen affect the structure of the clot and its physical and biochemical properties. Thromb Haemost. 1999;82(Suppl):692. [Google Scholar]
- Collet J-P, Park D, Lesty C, Soria J, Soria C, Montalescot G, Weisel JW. Influence of fibrin network conformation and fibrin fiber diameter on fibrinolysis speed: dynamic and structural approaches by confocal microscopy. Arterioscler Thromb Vasc Biol. 2000;20:1354–1361. doi: 10.1161/01.atv.20.5.1354. [DOI] [PubMed] [Google Scholar]
- Collet J-P, Montalescot G, Lesty C, Weisel JW. A structural and dynamic investigation of the facilitating effect of glycoportein IIb/IIIa inhibitors in dissolving platelet-rich clots. Circ Res. 2002;90:428–434. doi: 10.1161/hh0402.105095. [DOI] [PubMed] [Google Scholar]
- Collet J-P, Lesty C, Montalescot G, Weisel JW. Dynamic changes of fibrin architecture during fibrin formation and intrinsic fibrinolysis of fibrin-rich clots. J Biol Chem. 2003;278:21331–21335. doi: 10.1074/jbc.M212734200. [DOI] [PubMed] [Google Scholar]
- Collet J-P, Moen JL, Veklich YI, Gorkun OV, Lord ST, Montalescot G, Weisel JW. The αC domains of fibrinogen affect the structure of the fibrin clot, its physical properties, and its susceptibility to fibrinolysis. Blood. 2005;106:3824–3830. doi: 10.1182/blood-2005-05-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collet J-P, Allali Y, Lesty C, Tanguy ML, Silvain J, Ankri A, Blanchet B, Dumaine R, Giannetti J, Payot L, Weisel JW, Montalescot G. Altered fibrin architecture is associated with hypofibrinolysis and premature coronary artery atherothrombosis. Arterioscler Thromb Vasc Biol. 2006;26:2567–2573. doi: 10.1161/01.ATV.0000241589.52950.4c. [DOI] [PubMed] [Google Scholar]
- Cooper AV, Standeven KF, Ariens RA. Fibrinogen gamma-chain splice variant gamma’ alters fibrin formation and structure. Blood. 2003;102:535–540. doi: 10.1182/blood-2002-10-3150. [DOI] [PubMed] [Google Scholar]
- Corral M, Ferko N, Hollmann S, Hogan A, Jamous N, Batiller J, Shen J. Clinician reported ease of use for a novel fibrin sealant patch for hemostasis: results from four randomized controlled trials. Curr Med Res Opin. 2016;32:367–375. doi: 10.1185/03007995.2015.1128405. [DOI] [PubMed] [Google Scholar]
- Cote HC, Lord ST, Pratt KP. Gamma-chain dysfibrinogenemias: molecular structure-function relationships of naturally occurring mutations in the gamma chain of human fibrinogen. Blood. 1998;92:2195–2212. [PubMed] [Google Scholar]
- Crabtree GR. The molecular biology of fibrinogen. In: Stamatoyannopoulos G, Nienhuis AW, Leder P, Majerus PE, editors. The molecular basis of blood diseases. W.B. Saunders; Philadelphia: 1987. [Google Scholar]
- Dang CV, Shin CK, Bell WR, Nagaswami C, Weisel JW. Fibrinogen sialic acid residues are low affinity calcium-binding sites that influence fibrin assembly. J Biol Chem. 1989;264:15104–15108. [PubMed] [Google Scholar]
- Davies NA, Harrison NK, Morris RH, Noble S, Lawrence MJ, D’silva LA, Broome L, Brown MR, Hawkins KM, Williams PR, Davidson S, Evans PA. Fractal dimension (df) as a new structural biomarker of clot microstructure in different stages of lung cancer. Thromb Haemost. 2015;114:1251–1259. doi: 10.1160/TH15-04-0357. [DOI] [PubMed] [Google Scholar]
- De Maat MP, Verschuur M. Fibrinogen heterogeneity: inherited and noninherited. Curr Opin Hematol. 2005;12:377–383. doi: 10.1097/01.moh.0000169287.51594.3b. [DOI] [PubMed] [Google Scholar]
- De Moerloose P, Neerman-Arbez M. Congenital fibrinogen disorders. Semin Thromb Hemost. 2009;35:356–366. doi: 10.1055/s-0029-1225758. [DOI] [PubMed] [Google Scholar]
- De Moerloose P, Casini A, Neerman-Arbez M. Congenital fibrinogen disorders: an update. Semin Thromb Hemost. 2013;39:585–595. doi: 10.1055/s-0033-1349222. [DOI] [PubMed] [Google Scholar]
- Dikovsky D, Bianco-Peled H, Seliktar D. The effect of structural alterations of PEG-fibrinogen hydrogel scaffolds on 3-D cellular morphology and cellular migration. Biomaterials. 2006;27:1496–1506. doi: 10.1016/j.biomaterials.2005.09.038. [DOI] [PubMed] [Google Scholar]
- Domingues MM, Macrae FL, Duval C, Mcpherson HR, Bridge KI, Ajjan RA, Ridger VC, Connell SD, Philippou H, Ariens RA. Thrombin and fibrinogen gamma’ impact clot structure by marked effects on intrafibrillar structure and protofibril packing. Blood. 2015;127(4):487–495. doi: 10.1182/blood-2015-06-652214. [DOI] [PubMed] [Google Scholar]
- Donovan JW, Mihalyi E. Clotting of fibrinogen. 1. Scanning calorimetric study of the effect of calcium. Biochemist. 1985;24:3434–3443. doi: 10.1021/bi00335a007. [DOI] [PubMed] [Google Scholar]
- Doolittle RF. Fibrinogen and fibrin. Annu Rev Biochem. 1984;53:195–229. doi: 10.1146/annurev.bi.53.070184.001211. [DOI] [PubMed] [Google Scholar]
- Doolittle LR, Pandi L. Binding of synthetic B knobs to fibrinogen changes the character of fibrin and inhibits its ability to activate tissue plasminogen activator and its destruction by plasmin. Biochemist. 2006;45:2657–2667. doi: 10.1021/bi0524767. [DOI] [PubMed] [Google Scholar]
- Dunn EJ, Ariens RA. Fibrinogen and fibrin clot structure in diabetes. Herz. 2004;29:470–479. doi: 10.1007/s00059-004-2607-z. [DOI] [PubMed] [Google Scholar]
- Duval C, Allan P, Connell SD, Ridger VC, Philippou H, Ariens RA. Roles of fibrin alpha-and gamma-chain specific cross-linking by FXIIIa in fibrin structure and function. Thromb Haemost. 2014;111:842–850. doi: 10.1160/TH13-10-0855. [DOI] [PubMed] [Google Scholar]
- Dyr JE, Blombäck B, Hessel B, Kornalik F. Conversion of fibrinogen to fibrin induced by preferential release of fibrinopeptide B. Biochim Biophys Acta. 1989;990:18–24. doi: 10.1016/s0304-4165(89)80006-6. [DOI] [PubMed] [Google Scholar]
- Erickson HP, Fowler WE. Electron microscopy of fibrinogen, its plasmic fragments and small polymers. Ann N Y Acad Sci. 1983;408:146–163. doi: 10.1111/j.1749-6632.1983.tb23242.x. [DOI] [PubMed] [Google Scholar]
- Evans PA, Hawkins K, Morris RH, Thirumalai N, Munro R, Wakeman L, Lawrence MJ, Williams PR. Gel point and fractal microstructure of incipient blood clots are significant new markers of hemostasis for healthy and anticoagulated blood. Blood. 2010;116:3341–3346. doi: 10.1182/blood-2010-02-269324. [DOI] [PubMed] [Google Scholar]
- Everse SJ, Spraggon G, Doolittle RF. A three-dimensional consideration of variant human fibrinogens. Thromb Haemost. 1998a;80:1–9. [PubMed] [Google Scholar]
- Everse SJ, Spraggon G, Veerapandian L, Riley M, Doolittle RF. Crystal structure of fragment double-D from human fibrin with two different bound ligands. Biochemist. 1998b;37:8637–8642. doi: 10.1021/bi9804129. [DOI] [PubMed] [Google Scholar]
- Everse SJ, Spraggon G, Veerapandian L, Doolittle RF. Conformational changes in fragments D and double-D from human fibrin(ogen) upon binding the peptide ligand Gly-His-Arg-Pro-amide. Biochemist. 1999;38:2941–2946. doi: 10.1021/bi982626w. [DOI] [PubMed] [Google Scholar]
- Falvo MR, Gorkun OV, Lord ST. The molecular origins of the mechanical properties of fibrin. Biophys Chem. 2010;152:15–20. doi: 10.1016/j.bpc.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri F, Greco M, Arcovito G, De Spirito M, Rocco M. Structure of fibrin gels studied by elastic light scattering techniques: dependence of fractal dimension, gel crossover length, fiber diameter, and fiber density on monomer concentration. Phys Rev E Stat Nonlin Soft Matter Phys. 2002;66(1 Pt 1):011913. doi: 10.1103/PhysRevE.66.011913. [DOI] [PubMed] [Google Scholar]
- Fish RJ, Neerman-Arbez M. Fibrinogen gene regulation. Thromb Haemost. 2012;108:419–426. doi: 10.1160/TH12-04-0273. [DOI] [PubMed] [Google Scholar]
- Flamm MH, Diamond SL. Multiscale systems biology and physics of thrombosis under flow. Ann Biomed Eng. 2012;40:2355–2364. doi: 10.1007/s10439-012-0557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelson AL, Keener JP. Toward an understanding of fibrin branching structure. Phys Rev E Stat Nonlinear Soft Matter Phys. 2010;81:051922. doi: 10.1103/PhysRevE.81.051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortelny RH, Petter-Puchner AH, Ferguson J, Gruber-Blum S, Brand J, Mika K, Redl H. A comparative biomechanical evaluation of hernia mesh fixation by fibrin sealant. J Surg Res. 2011;171:576–581. doi: 10.1016/j.jss.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Fowler WE, Erickson HP. Trinodular structure of fibrinogen: confirmation by both shadowing and negative-stain electron microscopy. J Mol Biol. 1979;134:241–249. doi: 10.1016/0022-2836(79)90034-2. [DOI] [PubMed] [Google Scholar]
- Francis CW, Marder VJ, Martin SE. Demonstration of a large molecular weight variant of the gamma chain of normal human plasma fibrinogen. J Biol Chem. 1980;255:5599–5604. [PubMed] [Google Scholar]
- Fu Y, Grieninger G. Fib420: a normal human variant of fibrinogen with two extended alpha chains. Proc Natl Acad Sci U S A. 1994;91:2625–2628. doi: 10.1073/pnas.91.7.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel DA, Muga K, Boothroyd EM. The effect of fibrin structure on fibrinolysis. J Biol Chem. 1992;267:24259–24263. [PubMed] [Google Scholar]
- Gailit J, Ruoslahti E. Regulation of the fibronectin receptor affinity by divalent cations. J Biol Chem. 1988;263:12927–12932. [PubMed] [Google Scholar]
- Galanakis DK. Inherited dysfibrinogenemia: emerging abnormal structure associations with pathologic and nonpathologic dysfunctions. Semin Thromb Hemost. 1993;19:386–395. doi: 10.1055/s-2007-993290. [DOI] [PubMed] [Google Scholar]
- Galanakis DK, Lane BP, Simon SR. Albumin modulates lateral assembly of fibrin polymers: evidence of enhanced fine fibril formation and of unique synergism with fibrinogen. Biochemist. 1987;26:2389–2400. doi: 10.1021/bi00382a046. [DOI] [PubMed] [Google Scholar]
- Galanakis DK, Henschen A, Peerschke EI, Kehl M. Fibrinogen Stony Brook, a heterozygous Aalpha16Arg —> Cys dysfibrinogenemia – evaluation of diminshed platelet aggregation support and of enhanced inhibition of fibrin assembly. J Clin Invest. 1989;84:295–304. doi: 10.1172/JCI114154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanakis D, Spitzer S, Scharrer I. Unusual A alpha 16Arg-->Cys dysfibrinogenaemic family: absence of normal Aalpha-chains in fibrinogen from two of four heterozygous siblings. Blood Coagul Fibrinolysis. 1993;4:67–71. [PubMed] [Google Scholar]
- Galanakis DK, Nuovo G, Spitzer S, Kaplan C, Scharrer I. Fibrinogen mRNA and antigen co-present in human trophoblasts in situ: possible implications. Thromb Res. 1996;81:263–269. doi: 10.1016/0049-3848(95)00243-x. [DOI] [PubMed] [Google Scholar]
- Gasser TC, Gorgulu G, Folkesson M, Swedenborg J. Failure properties of intraluminal thrombus in abdominal aortic aneurysm under static and pulsating mechanical loads. J Vasc Surg. 2008;48:179–188. doi: 10.1016/j.jvs.2008.01.036. [DOI] [PubMed] [Google Scholar]
- Geer CB, Tripathy A, Schoenfisch MH, Lord ST, Gorkun OV. Role of ‘B-b’ knob-hole interactions in fibrin binding to adsorbed fibrinogen. J Thromb Haemost. 2007;5:2344–2351. doi: 10.1111/j.1538-7836.2007.02774.x. [DOI] [PubMed] [Google Scholar]
- Gersh KC, Nagaswami C, Weisel JW, Lord ST. The presence of gamma’ chain impairs fibrin polymerization. Thromb Res. 2009;124:356–363. doi: 10.1016/j.thromres.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gersh KC, Edmondson KE, Weisel JW. Flow rate and fibrin fiber alignment. J Thromb Haemost. 2010;8:2826–2828. doi: 10.1111/j.1538-7836.2010.04118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessmann J, Seybold D, Peter E, Schildhauer TA, Koller M. Alignment of the fibrin network within an autologous plasma clot. Tissue Eng Part C Methods. 2016;22:30–37. doi: 10.1089/ten.tec.2015.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert GE, Novakovic VA, Shi J, Rasmussen J, Pipe SW. Platelet binding sites for factor VIII in relation to fibrin and phosphatidylserine. Blood. 2015;126:1237–1244. doi: 10.1182/blood-2015-01-620245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorkun OV, Litvinov RI, Veklich YI, Weisel JW. Interactions mediated by the N-terminus of fibrinogen’s Bbeta chain. Biochemistry. 2006;45:14843–14852. doi: 10.1021/bi061430q. [DOI] [PubMed] [Google Scholar]
- Guo YH, Hernandez I, Isermann B, Kang TB, Medved L, Sood R, Kerschen EJ, Holyst T, Mosesson MW, Weiler H. Caveolin-1-dependent apoptosis induced by fibrin degradation products. Blood. 2009;113:4431–4439. doi: 10.1182/blood-2008-07-169433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthold M, Liu W, Stephens B, Lord ST, Hantgan RR, Erie DA, Taylor RM, Superfine R. Visualization and mechanical manipulations of individual fibrin fibers suggest that fiber cross section has fractal dimension 1.3. Biophys J. 2004;87:4226–4236. doi: 10.1529/biophysj.104.042333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthold M, Liu W, Sparks EA, Jawerth LM, Peng L, Falvo M, Superfine R, Hantgan RR, Lord ST. A comparison of the mechanical and structural properties of fibrin fibers with other protein fibers. Cell Biochem Biophys. 2007;49:165–181. doi: 10.1007/s12013-007-9001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidaris PJ. Induction of fibrinogen biosynthesis and secretion from cultured pulmonary epithelial cells. Blood. 1997;89:873–882. [PubMed] [Google Scholar]
- Haidaris PJ, Courtney MA. Tissue-specific and ubiquitous expression of fibrinogen gamma-chain mRNA. Blood Coagul Fibrinolysis. 1990;1:433–437. doi: 10.1097/00001721-199010000-00011. [DOI] [PubMed] [Google Scholar]
- Haidaris PJ, Francis CW, Sporn LA, Arvan DS, Collichio FA, Marder VJ. Megakaryocyte and hepatocyte origins of human fibrinogen biosynthesis exhibit hepatocyte-specific expression of gamma chain-variant polypeptides. Blood. 1989;74:743–750. [PubMed] [Google Scholar]
- Hall CE, Slayter HS. The fibrinogen molecule: its size, shape and mode of polymerization. J Biophys Biochem Cytol. 1959;5:11–16. doi: 10.1083/jcb.5.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantgan RR, Simpson-Haidaris PJ, Francis CW, Marder VJ. Fibrinogen structure and physiology. In: RWC, Hirsh J, VJM, AWC, JNG, editors. Hemostasis and thrombosis: basic principles and clinical practice. 4. Lippincott, Williams & Wilkins; Philadelphia: 2000. [Google Scholar]
- Harrison P, Wilbourn B, Debili N, Vainchenker W, Breton-Gorius J, Lawrie AS, Masse JM, Savidge GF, Cramer EM. Uptake of plasma fibrinogen into the alpha granules of human megakaryocytes and platelets. J Clin Invest. 1989;73:1123–1129. doi: 10.1172/JCI114300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P, Savidge GF, Cramer EM. The origin and physiological relevance of alpha-granule adhesive proteins. Br J Haematol. 1990;74:125–130. doi: 10.1111/j.1365-2141.1990.tb02554.x. [DOI] [PubMed] [Google Scholar]
- Heffron SP, Parastatidis I, Cuchel M, Wolfe ML, Tadesse MG, Mohler ER, 3rd, Ischiropoulos H, Rader DJ, Reilly MP. Inflammation induces fibrinogen nitration in experimental human endotoxemia. Free Radic Biol Med. 2009;47:1140–1146. doi: 10.1016/j.freeradbiomed.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CC, Ariens RA, Uitte De Willige S, Standeven KF, Guthold M. alpha-alpha cross-links increase fibrin fiber elasticity and stiffness. Biophys J. 2012;102:168–175. doi: 10.1016/j.bpj.2011.11.4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson SJ, Xia J, Wu H, Stafford AR, Leslie BA, Fredenburgh JC, Weitz DA, Weitz JI. Zinc promotes clot stability by accelerating clot formation and modifying fibrin structure. Thromb Haemost. 2015;115(3):533–542. doi: 10.1160/TH15-06-0462. [DOI] [PubMed] [Google Scholar]
- Henschen A, Mcdonagh J. Fibrinogen, fibrin and factor XIII. In: Zwaal RFA, Hemker HC, editors. Blood coagulation. Elsevier Science; Amsterdam: 1986. [Google Scholar]
- Henschen-Edman AH. Fibrinogen non-inherited heterogeneity and its relationship to function in health and disease. Ann N Y Acad Sci. 2001;936:580–593. doi: 10.1111/j.1749-6632.2001.tb03546.x. [DOI] [PubMed] [Google Scholar]
- Hickerson WL, Nur I, Meidler R. A comparison of the mechanical, kinetic, and biochemical properties of fibrin clots formed with two different fibrin sealants. Blood Coagul Fibrinolysis. 2011;22:19–23. doi: 10.1097/MBC.0b013e32833fcbfb. [DOI] [PubMed] [Google Scholar]
- Hirota-Kawadobora M, Terasawa F, Yonekawa O, Sahara N, Shimizu E, Okumura N, Katsuyama T, Shigematsu H. Fibrinogens Kosai and Ogasa: Bbeta15Gly-->Cys (GGT-->TGT) substitution associated with impairment of fibrinopeptide B release and lateral aggregation. J Thromb Haemost. 2003;1:275–283. doi: 10.1046/j.1538-7836.2003.00052.x. [DOI] [PubMed] [Google Scholar]
- Horan JT, Francis CW. Fibrin degradation products, fibrin monomer and soluble fibrin in disseminated intravascular coagulation. Semin Thromb Hemost. 2001;27:657–666. doi: 10.1055/s-2001-18870. [DOI] [PubMed] [Google Scholar]
- Houser JR, Hudson NE, Ping L, O’brien ET, 3rd, Superfine R, Lord ST, Falvo MR. Evidence that alphaC region is origin of low modulus, high extensibility, and strain stiffening in fibrin fibers. Biophys J. 2010;99:3038–3047. doi: 10.1016/j.bpj.2010.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes JM, Richardson VR, Smith KA, Schroeder V, Somani R, Shore A, Hess K, Ajjan R, Pease RJ, Keen JN, Standeven KF, Carter AM. Complement C3 is a novel plasma clot component with anti-fibrinolytic properties. Diab Vasc Dis Res. 2012;9:216–225. doi: 10.1177/1479164111432788. [DOI] [PubMed] [Google Scholar]
- Huang L, Hsiao JP, Powierza C, Taylor RM, 2nd, Lord ST. Does topology drive fiber polymerization? Biochemistry. 2014;53:7824–7834. doi: 10.1021/bi500986z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson NE, Houser JR, O’brien ET, 3rd, Taylor RM, 2nd, Superfine R, Lord ST, Falvo MR. Stiffening of individual fibrin fibers equitably distributes strain and strengthens networks. Biophys J. 2010;98:1632–1640. doi: 10.1016/j.bpj.2009.12.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson NE, Ding F, Bucay I, O’brien ET, 3rd, Gorkun OV, Superfine R, Lord ST, Dokholyan NV, Falvo MR. Submillisecond elastic recoil reveals molecular origins of fibrin fiber mechanics. Biophys J. 2013;104:2671–2680. doi: 10.1016/j.bpj.2013.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson NE, Houser JR, O’brien ET, 3rd, Taylor RM, 2nd, Superfine R, Lord ST, Falvo MR. Stiffening of individual fibrin fibers equitably distributes strain and strengthens networks. Biophys J. 2015;98:1632–1640. doi: 10.1016/j.bpj.2009.12.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M, Takeya H, Takemitsu T, Nakagaki T, Gabazza EC, Suzuki K. Characterization of the binding of factor Xa to fibrinogen/fibrin derivatives and localization of the factor Xa binding site on fibrinogen. Eur J Biochem. 1995;232:90–97. doi: 10.1111/j.1432-1033.1995.tb20785.x. [DOI] [PubMed] [Google Scholar]
- Jandrot-Perrus M, Mosesson MW, Denninger MH, Menache D. Studies of platelet fibrinogen from a subject with a congenital plasma fibrinogen abnormality (fibrinogen Paris I) Blood. 1979;54:1109–1116. [PubMed] [Google Scholar]
- Janmey PA, Amis EJ, Ferry JD. Rheology of fibrin clots. VI. Stress relaxation, creep, and differential dynamic modulus of fine clots in large shearing deformations. J Rheol. 1983;27:135–153. [Google Scholar]
- Janmey PA, Winer JP, Weisel JW. Fibrin gels and their clinical and bioengineering applications. J R Soc Interface. 2009;6:1–10. doi: 10.1098/rsif.2008.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen KA, Bacabac RG, Piechocka IK, Koenderink GH. Cells actively stiffen fibrin networks by generating contractile stress. Biophys J. 2013;105:2240–2251. doi: 10.1016/j.bpj.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant JA, Fornace AJ, Saxe D, Simon MI, Mcbride OW, Crabtree GR. Evolution and organization of the fibrinogen locus on chromosome 4: gene duplication accompanied by transposition and inversion. Proc Natl Acad Sci U S A. 1985;185:1–19. doi: 10.1073/pnas.82.8.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim OV, Litvinov RI, Weisel JW, Alber MS. Structural basis for the nonlinear mechanics of fibrin networks under compression. Biomaterials. 2014;35:6739–6749. doi: 10.1016/j.biomaterials.2014.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler S, Schmid F, Settanni G. The internal dynamics of fibrinogen and its implications for coagulation and adsorption. PLoS Comput Biol. 2015;11:e1004346. doi: 10.1371/journal.pcbi.1004346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolev K, Tenekedjiev K, Ajtai K, Kovalszky I, Gombas J, Varadi B, Machovich R. Myosin: a noncovalent stabilizer of fibrin in the process of clot dissolution. Blood. 2003;101:4380–4386. doi: 10.1182/blood-2002-10-3227. [DOI] [PubMed] [Google Scholar]
- Kononova O, Litvinov RI, Zhmurov A, Alekseenko A, Cheng CH, Agarwal S, Marx KA, Weisel JW, Barsegov V. Molecular mechanisms, thermodynamics, and dissociation kinetics of knob-hole interactions in fibrin. J Biol Chem. 2013;288:22681–22692. doi: 10.1074/jbc.M113.472365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman J, Haverkate F, Lord ST, Grimbergen J, Mannucci PM. Molecular basis of fibrinogen Naples associated with defective thrombin binding and thrombophilia. Homozygous substitution of B beta 68 Ala —> Thr. J Clin Invest. 1992;90:238–244. doi: 10.1172/JCI115841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostelansky MS, Betts L, Gorkun OV, Lord ST. 2.8 A crystal structures of recombinant fibrinogen fragment D with and without two peptide ligands: GHRP binding to the “b” site disrupts its nearby calcium-binding site. Biochemist. 2002;41:12124–12132. doi: 10.1021/bi0261894. [DOI] [PubMed] [Google Scholar]
- Kostelansky MS, Bolliger-Stucki B, Betts L, Gorkun OV, Lord ST. B beta Glu397 and B beta Asp398 but not B beta Asp432 are required for “B:b” interactions. Biochemist. 2004a;43:2465–2474. doi: 10.1021/bi035996f. [DOI] [PubMed] [Google Scholar]
- Kostelansky MS, Lounes KC, Ping LF, Dickerson SK, Gorkun OV, Lord ST. Calcium-binding site beta 2, adjacent to the “b” polymerization site, modulates lateral aggregation of protofibrils during fibrin polymerization. Biochemist. 2004b;43:2475–2483. doi: 10.1021/bi0359978. [DOI] [PubMed] [Google Scholar]
- Kostelansky MS, Lounes KC, Ping LF, Dickerson SK, Gorkun OV, Lord ST. Probing the gamma2 calcium-binding site: studies with gammaD298,301A fibrinogen reveal changes in the gamma294-301 loop that alter the integrity of the “a” polymerization site. Biochemist. 2007;46:5114–5123. doi: 10.1021/bi602607a. [DOI] [PubMed] [Google Scholar]
- Kotlarchyk MA, Shreim SG, Alvarez-Elizondo MB, Estrada LC, Singh R, Valdevit L, Kniazeva E, Gratton E, Putnam AJ, Botvinick EL. Concentration independent modulation of local micromechanics in a fibrin gel. PLoS One. 2011;6:e20201. doi: 10.1371/journal.pone.0020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn C, Fulop T, Lakey JR, Vermette P. Young porcine endocrine pancreatic islets cultured in fibrin and alginate gels show improved resistance towards human monocytes. Pathol Biol (Paris) 2014;62:354–364. doi: 10.1016/j.patbio.2014.07.010. [DOI] [PubMed] [Google Scholar]
- Kurniawan NA, Grimbergen J, Koopman J, Koenderink GH. Factor XIII stiffens fibrin clots by causing fiber compaction. J Thromb Haemost. 2014;12:1687–1696. doi: 10.1111/jth.12705. [DOI] [PubMed] [Google Scholar]
- Lai VK, Lake SP, Frey CR, Tranquillo RT, Barocas VH. Mechanical behavior of collagen-fibrin co-gels reflects transition from series to parallel interactions with increasing collagen content. J Biomech Eng Trans ASME. 2012:134. doi: 10.1115/1.4005544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam WA, Chaudhuri O, Crow A, Webster KD, Li TD, Kita A, Huang J, Fletcher DA. Mechanics and contraction dynamics of single platelets and implications for clot stiffening. Nat Mater. 2011;10:61–66. doi: 10.1038/nmat2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer BG, Weisel JW, Dinauer PA, Nagaswami C, Bell WR. Deglycosylation of fibrinogen accelerates polymerization and increases lateral aggregation of fibrin fibers. J Biol Chem. 1988;263:15056–15063. [PubMed] [Google Scholar]
- Lauricella AM, Castanon MM, Kordich LC, Quintana IL. Alterations of fibrin network structure mediated by dermatan sulfate. J Thromb Thrombolysis. 2013;35:257–263. doi: 10.1007/s11239-012-0804-9. [DOI] [PubMed] [Google Scholar]
- Lawrence SO, Simpson-Haidaris PJ. Regulated de novo biosynthesis of fibrinogen in extra-hepatic epithelial cells in response to inflammation. Thromb Haemost. 2004;92:234–243. doi: 10.1160/TH04-01-0024. [DOI] [PubMed] [Google Scholar]
- Lawrence MJ, Sabra A, Mills G, Pillai SG, Abdullah W, Hawkins K, Morris RH, Davidson SJ, D’silva LA, Curtis DJ, Brown MR, Weisel JW, Williams PR, Evans PA. A new biomarker quantifies differences in clot microstructure in patients with venous thromboembolism. Br J Haematol. 2015;168:571–575. doi: 10.1111/bjh.13173. [DOI] [PubMed] [Google Scholar]
- Lim BC, Ariens RA, Carter AM, Weisel JW, Grant PJ. Genetic regulation of fibrin structure and function: complex gene-environment interactions may modulate vascular risk. Lancet. 2003;361:1424–1431. doi: 10.1016/S0140-6736(03)13135-2. [DOI] [PubMed] [Google Scholar]
- Lim BCB, Lee EH, Sotomayor M, Schulten K. Molecular basis of fibrin clot elasticity. Structure. 2008;16:449–459. doi: 10.1016/j.str.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Lindstrom SB, Kulachenko A, Jawerth LM, Vader DA. Finite-strain, finite-size mechanics of rigidly cross-linked biopolymer networks. Soft Matter. 2013;9:7302–7313. [Google Scholar]
- Litvinov RI, Gorkun OV, Owen SF, Shuman H, Weisel JW. Polymerization of fibrin: specificity, strength, and stability of knob-hole interactions studied at the single-molecule level. Blood. 2005;106:2944–2951. doi: 10.1182/blood-2005-05-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvinov RI, Gorkun OV, Galanakis DK, Yakovlev S, Medved L, Shuman H, Weisel JW. Polymerization of fibrin: direct observation and quantification of individual B:b knob-hole interactions. Blood. 2007a;109:130–138. doi: 10.1182/blood-2006-07-033910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvinov RI, Yakovlev S, Tsurupa G, Gorkun OV, Medved L, Weisel JW. Direct evidence for specific interactions of the fibrinogen alphaC-domains with the central E region and with each other. Biochemistry. 2007b;46:9133–9142. doi: 10.1021/bi700944j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvinov RI, Faizullin DA, Zuev YF, Weisel JW. The alpha-helix to beta-sheet transition in stretched and compressed hydrated fibrin clots. Biophys J. 2012;103:1020–1027. doi: 10.1016/j.bpj.2012.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Jawerth LM, Sparks EA, Falvo MR, Hantgan RR, Superfine R, Lord ST, Guthold M. Fibrin fibers have extraordinary extensibility and elasticity. Science. 2006;313:634. doi: 10.1126/science.1127317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Carlisle CR, Sparks EA, Guthold M. The mechanical properties of single fibrin fibers. J Thromb Haemost. 2010;8:1030–1036. doi: 10.1111/j.1538-7836.2010.03745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longstaff C, Kolev K. Basic mechanisms and regulation of fibrinolysis. J Thromb Haemost. 2015;13(Suppl 1):S98–S105. doi: 10.1111/jth.12935. [DOI] [PubMed] [Google Scholar]
- Longstaff C, Varju I, Sotonyi P, Szabo L, Krumrey M, Hoell A, Bota A, Varga Z, Komorowicz E, Kolev K. Mechanical stability and fibrinolytic resistance of clots containing fibrin, DNA, and histones. J Biol Chem. 2013;288:6946–6956. doi: 10.1074/jbc.M112.404301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord ST. Fibrinogen and fibrin: scaffold proteins in hemostasis. Curr Opin Hematol. 2007;14:236–241. doi: 10.1097/MOH.0b013e3280dce58c. [DOI] [PubMed] [Google Scholar]
- Lord ST. Molecular mechanisms affecting fibrin structure and stability. Arterioscler Thromb Vasc Biol. 2011;31:494–499. doi: 10.1161/ATVBAHA.110.213389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louache F, Debili N, Cramer EM, Breton-Gorius J, Vainchenker W. Fibrinogen is not synthesized by human megakaryocytes. Blood. 1991;77:311–316. [PubMed] [Google Scholar]
- Lovely RS, Falls LA, Al-Mondhiry HA, Chambers CE, Sexton GJ, Ni H, Farrell DH. Association of gammaA/gamma’ fibrinogen levels and coronary artery disease. Thromb Haemost. 2002;88:26–31. [PubMed] [Google Scholar]
- Ly B, Godal HC. Denaturation of fibrinogen: the protective effect of calcium. Haematologica. 1973;1:204. [Google Scholar]
- Madrazo J, Brown JH, Litvinovich S, Dominguez R, Yakovlev S, Medved L, Cohen C. Crystal structure of the central region of bovine fibrinogen (E5 fragment) at 1.4-A resolution. Proc Natl Acad Sci U S A. 2001;98:11967–11972. doi: 10.1073/pnas.211439798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magatti D, Molteni M, Cardinali B, Rocco M, Ferri F. Modeling of fibrin gels based on confocal microscopy and light-scattering data. Biophys J. 2013;104:1151–1159. doi: 10.1016/j.bpj.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malecki R, Gacka M, Kuliszkiewicz-Janus M, Jakobsche-Policht U, Kwiatkowski J, Adamiec R, Undas A. Altered plasma fibrin clot properties in essential thrombocythemia. Platelets. 2015:1–7. doi: 10.3109/09537104.2015.1042967. [DOI] [PubMed] [Google Scholar]
- Man AJ, Davis HE, Itoh A, Leach JK, Bannerman P. Neurite outgrowth in fibrin gels is regulated by substrate stiffness. Tissue Eng A. 2011;17:2931–2942. doi: 10.1089/ten.tea.2011.0030. [DOI] [PubMed] [Google Scholar]
- Mannila MN, Eriksson P, Ericsson CG, Hamsten A, Silveira A. Epistatic and pleiotropic effects of polymorphisms in the fibrinogen and coagulation factor XIII genes on plasma fibrinogen concentration, fibrin gel structure and risk of myocardial infarction. Thromb Haemost. 2006;95:420–427. doi: 10.1160/TH05-11-0777. [DOI] [PubMed] [Google Scholar]
- Mannila MN, Lovely RS, Kazmierczak SC, Eriksson P, Samnegard A, Farrell DH, Hamsten A, Silveira A. Elevated plasma fibrinogen gamma’ concentration is associated with myocardial infarction: effects of variation in fibrinogen genes and environmental factors. J Thromb Haemost. 2007;5:766–773. doi: 10.1111/j.1538-7836.2007.02406.x. [DOI] [PubMed] [Google Scholar]
- Maquart FX, Monboisse JC. Extracellular matrix and wound healing. Pathol Biol (Paris) 2014;62:91–95. doi: 10.1016/j.patbio.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Marchi RC, Carvajal Z, Boyer-Neumann C, Angles-Cano E, Weisel JW. Functional characterization of fibrinogen Bicetre II: a gamma 308 Asn-->Lys mutation located near the fibrin D:D interaction sites. Blood Coagul Fibrinolysis. 2006;17:193–201. doi: 10.1097/01.mbc.0000220241.22714.68. [DOI] [PubMed] [Google Scholar]
- Marder VJ, Budzynski AZ. Data for defining fibrinogen in its plasmic degradation products. Thromb Diath Haemorrh. 1975;33:199–207. [PubMed] [Google Scholar]
- Marguerie G, Chagniel G, Suscillon M. The binding of calcium to bovine fibrinogen. Biochim Biophys Acta. 1977;490:94–103. doi: 10.1016/0005-2795(77)90109-x. [DOI] [PubMed] [Google Scholar]
- Marsh JJ, Guan HS, Li S, Chiles PG, Tran D, Morris TA. Structural insights into fibrinogen dynamics using amide hydrogen/deuterium exchange mass spectrometry. Biochemistry. 2013;52:5491–5502. doi: 10.1021/bi4007995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J, Keane PM, Gilman PB, Palascak JE. The abnormal carbohydrate composition of the dysfibrinogenemia associated with liver disease. Ann N Y Acad Sci. 1983;408:388–396. doi: 10.1111/j.1749-6632.1983.tb23259.x. [DOI] [PubMed] [Google Scholar]
- Martinez M, Cuker A, Mills A, Lightfoot R, Fan Y, Tang WH, Hazen SL, Ischiropoulos H. Nitrated fibrinogen is a biomarker of oxidative stress in venous thromboembolism. Free Radic Biol Med. 2012;53:230–236. doi: 10.1016/j.freeradbiomed.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M, Weisel JW, Ischiropoulos H. Functional impact of oxidative posttranslational modifications on fibrinogen and fibrin clots. Free Radic Biol Med. 2013;65:411–418. doi: 10.1016/j.freeradbiomed.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez MR, Cuker A, Mills AM, Crichlow A, Lightfoot RT, Chernysh IN, Nagaswami C, Weisel JW, Ischiropoulos H. Enhanced lysis and accelerated establishment of viscoelastic properties of fibrin clots are associated with pulmonary embolism. Am J Physiol Lung Cell Mol Physiol. 2014;306:L397–L404. doi: 10.1152/ajplung.00265.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M, Sugo T. Hereditary disorders of fibrinogen. Ann N Y Acad Sci. 2001;936:65–68. doi: 10.1111/j.1749-6632.2001.tb03494.x. [DOI] [PubMed] [Google Scholar]
- Matsuka YV, Medved LV, Migliorini MM, Ingham KC. Factor XIIIa-catalyzed cross-linking of recombinant alpha C fragments of human fibrinogen. Biochemist. 1996;35:5810–5816. doi: 10.1021/bi952294k. [DOI] [PubMed] [Google Scholar]
- Medved L, Weisel JW. Recommendations for nomenclature on fibrinogen and fibrin. J Thromb Haemost. 2009;7:355–359. doi: 10.1111/j.1538-7836.2008.03242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medved L, Ugarova T, Veklich Y, Lukinova N, Weisel J. Electron microscope investigation of the early stages of fibrin assembly. Twisted protofibrils and fibers. J Mol Biol. 1990;216:503–509. doi: 10.1016/0022-2836(90)90376-W. [DOI] [PubMed] [Google Scholar]
- Medved L, Tsurupa G, Yakovlev S. Conformational changes upon conversion of fibrinogen into fibrin. The mechanisms of exposure of cryptic sites. Ann N Y Acad Sci. 2001;936:185–204. doi: 10.1111/j.1749-6632.2001.tb03505.x. [DOI] [PubMed] [Google Scholar]
- Mihalyi E. Clotting of bovine fibrinogen. Calcium binding to fibrin during clotting and its dependence on release of fibrinopeptide B. Biochemistry. 1988;27:967–976. doi: 10.1021/bi00403a020. [DOI] [PubMed] [Google Scholar]
- Miszta A, Pelkmans L, Lindhout T, Krishnamoorthy G, De Groot PG, Hemker CH, Heemskerk JW, Kelchtermans H, De Laat B. Thrombin-dependent Incorporation of von Willebrand Factor into a Fibrin Network. J Biol Chem. 2014;289:35979–35986. doi: 10.1074/jbc.M114.591677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moen JL, Gorkun OV, Weisel JW, Lord ST. Recombinant BbetaArg14His fibrinogen implies participation of N-terminus of Bbeta chain in desA fibrin polymerization. Blood. 2003;102:2466–2471. doi: 10.1182/blood-2003-01-0204. [DOI] [PubMed] [Google Scholar]
- Mosesson MW. Cross-linked gamma chains in a fibrin fibril are situated transversely between its strands: yes. J Thromb Haemost. 2004;2:388–393. doi: 10.1111/j.1538-7933.2004.00613.x. [DOI] [PubMed] [Google Scholar]
- Mosesson MW. Update on antithrombin I (fibrin) Thromb Haemost. 2007;98:105–108. [PubMed] [Google Scholar]
- Mosesson MW, Diorio JP, Siebenlist KR, Wall JS, Hainfeld JF. Evidence for a second type of fibril branch point in fibrin polymer networks, the trimolecular junction. Blood. 1993;82:1517–1521. [PubMed] [Google Scholar]
- Mosesson MW, Diorio JP, Hernandez I, Hainfeld JF, Wall JS, Grieninger G. The ultrastructure of fibrinogen-420 and the fibrin-420 clot. Biophys Chem. 2004;112:209–214. doi: 10.1016/j.bpc.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Müller MF, Ris H, Ferry JD. Electron microscopy of fine fibrin clots and fine and coarse fibrin films. J Mol Biol. 1984;174:369–384. doi: 10.1016/0022-2836(84)90343-7. [DOI] [PubMed] [Google Scholar]
- Mullin JL, Gorkun OV, Lord ST. Decreased lateral aggregation of a variant recombinant fibrinogen provides insight into the polymerization mechanism. Biochemistry. 2000;39:9843–9849. doi: 10.1021/bi000045c. [DOI] [PubMed] [Google Scholar]
- Munster S, Jawerth LM, Fabry B, Weitz DA. Structure and mechanics of fibrin clots formed under mechanical perturbation. J Thromb Haemost. 2013;11:557–560. doi: 10.1111/jth.12123. [DOI] [PubMed] [Google Scholar]
- Muthard RW, Welsh JD, Brass LF, Diamond SL. Fibrin, gamma’-fibrinogen, and transclot pressure gradient control hemostatic clot growth during human blood flow over a collagen/tissue factor wound. Arterioscler Thromb Vasc Biol. 2015;35:645–654. doi: 10.1161/ATVBAHA.114.305054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natesan S, Zhang G, Baer DG, Walters TJ, Christy RJ, Suggs LJ. A bilayer construct controls adipose-derived stem cell differentiation into endothelial cells and pericytes without growth factor stimulation. Tissue Eng A. 2011;17:941–953. doi: 10.1089/ten.tea.2010.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neerman-Arbez M. Fibrinogen gene mutations accounting for congenital afibrinogenemia. Ann N Y Acad Sci. 2001;936:496–508. doi: 10.1111/j.1749-6632.2001.tb03536.x. [DOI] [PubMed] [Google Scholar]
- Neeves KB, Illing DA, Diamond SL. Thrombin flux and wall shear rate regulate fibrin fiber deposition state during polymerization under flow. Biophys J. 2010;98:1344–1352. doi: 10.1016/j.bpj.2009.12.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nham SU, Fuller GM. Effect of fibrinogen degradation products on production of hepatocyte stimulating factor by a macrophage cell line (P388D1) Thromb Res. 1986;44:467–475. doi: 10.1016/0049-3848(86)90325-7. [DOI] [PubMed] [Google Scholar]
- Nossel H. Radioimmunoassay of fibrinopeptides in relation to intravascular coagulation and thrombosis. NEJ Med. 1976;295:428–432. doi: 10.1056/NEJM197608192950807. [DOI] [PubMed] [Google Scholar]
- Nowak P, Zbikowska HM, Ponczek M, Kolodziejczyk J, Wachowicz B. Different vulnerability of fibrinogen subunits to oxidative/nitrative modifications induced by peroxynitrite: functional consequences. Thromb Res. 2007;121:163–174. doi: 10.1016/j.thromres.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Nussenzweig V, Seligmann M, Pelmont J, Grabar P. The products of degradation of human fibrinogen by plasmin. I. Separation and physicochemical properties. Ann Inst Pasteur (Paris) 1961;100:377–389. [PubMed] [Google Scholar]
- O’Brien ET, Falvo MR, Millard D, Eastwood B, Taylor RM, Superfine R. Ultrathin self-assembled fibrin sheets. Proc Natl Acad Sci U S A. 2008;105:19438–19443. doi: 10.1073/pnas.0804865105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odrljin TM, Rybarczyk BJ, Francis CW, Lawrence SO, Hamaguchi M, Simpson-Haidaris PJ. Calcium modulates plasmin cleavage of the fibrinogen D fragment gamma chain N-terminus: mapping of monoclonal antibody J88B to a plasmin sensitive domain of the gamma chain. Biochim Biophys Acta. 1996;1298:69–77. doi: 10.1016/s0167-4838(96)00090-8. [DOI] [PubMed] [Google Scholar]
- Okada M, Blombäck B. Factors influencing fibrin gel structure studied by flow measurement. Ann N Y Acad Sci. 1983;408:233–253. doi: 10.1111/j.1749-6632.1983.tb23248.x. [DOI] [PubMed] [Google Scholar]
- Okumura N, Gorkun OV, Lord ST. Severely impaired polymerization of recombinant fibrinogen gamma-364 Asp--> His, the substitution discovered in a heterozygous individual. J Biol Chem. 1997;272:29596–29601. doi: 10.1074/jbc.272.47.29596. [DOI] [PubMed] [Google Scholar]
- Okumura N, Terasawa F, Hirota-Kawadobora M, Yamauchi K, Nakanishi K, Shiga S, Ichiyama S, Saito M, Kawai M, Nakahata T. A novel variant fibrinogen, deletion of Bbeta111Ser in coiled-coil region, affecting fibrin lateral aggregation. Clin Chim Acta. 2006;365:160–167. doi: 10.1016/j.cca.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Okumura N, Terasawa F, Fujihara N, Hirota-Kawadobora M. Markedly impaired but significant thrombin-catalyzed fibrin polymerization observed at variant fibrinogens at gamma364Asp residue is arisen from B-knob and b-hole bonding. J Thromb Haemost. 2007;5(suppl 2):P-W-389. [Google Scholar]
- Parastatidis I, Thomson L, Burke A, Chernysh I, Nagaswami C, Visser J, Stamer S, Liebler DC, Koliakos G, Heijnen HF, Fitzgerald GA, Weisel JW, Ischiropoulos H. Fibrinogen beta-chain tyrosine nitration is a prothrombotic risk factor. J Biol Chem. 2008;283:33846–33853. doi: 10.1074/jbc.M805522200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CT, Wright SD. Fibrinogen is a component of a novel lipoprotein particle: factor H-related protein (FHRP)-associated lipoprotein particle (FALP) Blood. 2000;95:198–204. [PubMed] [Google Scholar]
- Parrott JA, Whaley PD, Skinner MK. Extrahepatic expression of fibrinogen by granulosa cells: potential role in ovulation. Endocrinology. 1993;133:1645–1649. doi: 10.1210/endo.133.4.8404605. [DOI] [PubMed] [Google Scholar]
- Paton LN, Mocatta TJ, Richards AM, Winterbourn CC. Increased thrombin-induced polymerization of fibrinogen associated with high protein carbonyl levels in plasma from patients post myocardial infarction. Free Radic Biol Med. 2010;48:223–229. doi: 10.1016/j.freeradbiomed.2009.10.044. [DOI] [PubMed] [Google Scholar]
- Pechik I, Yakovlev S, Mosesson MW, Gilliland GL, Medved L. Structural basis for sequential cleavage of fibrinopeptides upon fibrin assembly. Biochemist. 2006;45:3588–3597. doi: 10.1021/bi0525369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechocka IK, Bacabac RG, Potters M, Mackintosh FC, Koenderink GH. Structural hierarchy governs fibrin gel mechanics. Biophys J. 2010;98:2281–2289. doi: 10.1016/j.bpj.2010.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping L, Huang L, Cardinali B, Profumo A, Gorkun OV, Lord ST. Substitution of the human alphaC region with the analogous chicken domain generates a fibrinogen with severely impaired lateral aggregation: fibrin monomers assemble into protofibrils but protofibrils do not assemble into fibers. Biochemistry. 2011;50:9066–9075. doi: 10.1021/bi201094v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podor TJ, Campbell S, Chindemi P, Foulon DM, Farrell DH, Walton PD, Weitz JI, Peterson CB. Incorporation of vitronectin into fibrin clots. Evidence for a binding interaction between vitronectin and gamma A/gamma’ fibrinogen. J Biol Chem. 2002;277:7520–7528. doi: 10.1074/jbc.M109677200. [DOI] [PubMed] [Google Scholar]
- Protopopova AD, Barinov NA, Zavyalova EG, Kopylov AM, Sergienko VI, Klinov DV. Visualization of fibrinogen alphaC regions and their arrangement during fibrin network formation by high-resolution AFM. J Thromb Haemost. 2015;13:570–579. doi: 10.1111/jth.12785. [DOI] [PubMed] [Google Scholar]
- Purohit PK, Litvinov RI, Brown AE, Discher DE, Weisel JW. Protein unfolding accounts for the unusual mechanical behavior of fibrin networks. Acta Biomater. 2011;7:2374–2383. doi: 10.1016/j.actbio.2011.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Brown AC, Myers DR, Sakurai Y, Mannino RG, Tran R, Ahn B, Hardy ET, Kee MF, Kumar S, Bao G, Barker TH, Lam WA. Platelet mechanosensing of substrate stiffness during clot formation mediates adhesion, spreading, and activation. Proc Natl Acad Sci U S A. 2014;111:14430–14435. doi: 10.1073/pnas.1322917111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RR, Peterson AW, Ceccarelli J, Putnam AJ, Stegemann JP. Matrix composition regulates three-dimensional network formation by endothelial cells and mesenchymal stem cells in collagen/fibrin materials. Angiogenesis. 2012;15:253–264. doi: 10.1007/s10456-012-9257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynal B, Cardinali B, Grimbergen J, Profumo A, Lord ST, England P, Rocco M. Hydrodynamic characterization of recombinant human fibrinogen species. Thromb Res. 2013;132:e48–e53. doi: 10.1016/j.thromres.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman CM, Xia H. Fibrinogen biosynthesis. Assembly, intracellular degradation, and association with lipid synthesis and secretion. Ann N Y Acad Sci. 2001;936:480–495. [PubMed] [Google Scholar]
- Riedel T, Suttnar J, Brynda E, Houska M, Medved L, Dyr JE. Fibrinopeptides A and B release in the process of surface fibrin formation. Blood. 2011;117:1700–1706. doi: 10.1182/blood-2010-08-300301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts HR, Stinchcombe TE, Gabriel DA. The dysfibringenemias. British J Haematol. 2001;114:249–257. doi: 10.1046/j.1365-2141.2001.02892.x. [DOI] [PubMed] [Google Scholar]
- Rocco M, Molteni M, Ponassi M, Giachi G, Frediani M, Koutsioubas A, Profumo A, Trevarin D, Cardinali B, Vachette P, Ferri F, Perez J. A comprehensive mechanism of fibrin network formation involving early branching and delayed single- to double-strand transition from coupled time-resolved X-ray/light-scattering detection. J Am Chem Soc. 2014;136:5376–5384. doi: 10.1021/ja5002955. [DOI] [PubMed] [Google Scholar]
- Rojas AM, Kordich L, Lauricella AM. Homocysteine modifies fibrin clot deformability: another possible explanation of harm. Biorheology. 2009;46:379–387. doi: 10.3233/BIR-2009-0459. [DOI] [PubMed] [Google Scholar]
- Rosenfeld MA, Shchegolikhin AN, Bychkova AV, Leonova VB, Biryukova MI, Kostanova EA. Ozone-induced oxidative modification of fibrinogen: role of the D regions. Free Radic Biol Med. 2014;77:106–120. doi: 10.1016/j.freeradbiomed.2014.08.018. [DOI] [PubMed] [Google Scholar]
- Rosenfeld MA, Leonova VB, Shchegolikhin AN, Bychkova AV, Kostanova EA, Biryukova MI. Covalent structure of single-stranded fibrin oligomers cross-linked by FXIIIa. Biochem Biophys Res Commun. 2015;461:408–412. doi: 10.1016/j.bbrc.2015.04.052. [DOI] [PubMed] [Google Scholar]
- Rottenberger Z, Komorowicz E, Szabo L, Bota A, Varga Z, Machovich R, Longstaff C, Kolev K. Lytic and mechanical stability of clots composed of fibrin and blood vessel wall components. J Thromb Haemost. 2013;11:529–538. doi: 10.1111/jth.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan EA, Mockros LF, Weisel JW, Lorand L. Structural origins of fibrin clot rheology. Biophys J. 1999;77:2813–2826. doi: 10.1016/S0006-3495(99)77113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahni A, Francis CW. Vascular endothelial growth factor binds to fibrinogen and fibrin and stimulates endothelial cell proliferation. Blood. 2000;96:3772–3778. [PubMed] [Google Scholar]
- Sahni A, Sporn LA, Francis CW. Potentiation of endothelial cell proliferation by fibrin(ogen)-bound fibroblast growth factor-2. J Biol Chem. 1999;274:14936–14941. doi: 10.1074/jbc.274.21.14936. [DOI] [PubMed] [Google Scholar]
- Sahni A, Guo M, Sahni SK, Francis CW. Interleukin-1beta but not IL-1alpha binds to fibrinogen and fibrin and has enhanced activity in the bound form. Blood. 2004;104:409–414. doi: 10.1182/blood-2004-01-0126. [DOI] [PubMed] [Google Scholar]
- Sakharov DV, Rijken DC. Superficial accumulation of plasminogen during plasma clot lysis. Circulation. 1995;92:1883–1890. doi: 10.1161/01.cir.92.7.1883. [DOI] [PubMed] [Google Scholar]
- Sauls DL, Wolberg AS, Hoffman M. Elevated plasma homocysteine leads to alterations in fibrin clot structure and stability: implications for the mechanism of thrombosis in hyperhomocysteinemia. J Thromb Haemost. 2003;1:300–306. doi: 10.1046/j.1538-7836.2003.00053.x. [DOI] [PubMed] [Google Scholar]
- Sauls DL, Lockhart E, Warren ME, Lenkowski A, Wilhelm SE, Hoffman M. Modification of fibrinogen by homocysteine thiolactone increases resistance to fibrinolysis: a potential mechanism of the thrombotic tendency in hyperhomocysteinemia. Biochemistry. 2006;45:2480–2487. doi: 10.1021/bi052076j. [DOI] [PubMed] [Google Scholar]
- Schvartz I, Seger D, Maik-Rachline G, Kreizman T, Shaltiel S. Truncated vitronectins: binding to immobilized fibrin and to fibrin clots, and their subsequent interaction with cells. Biochem Biophys Res Commun. 2002;290:682–689. doi: 10.1006/bbrc.2001.6273. [DOI] [PubMed] [Google Scholar]
- Scott EM, Ariens RA, Grant PJ. Genetic and environmental determinants of fibrin structure and function: relevance to clinical disease. Arterioscler Thromb Vasc Biol. 2004;24:1558–1566. doi: 10.1161/01.ATV.0000136649.83297.bf. [DOI] [PubMed] [Google Scholar]
- Shainoff JR, Dardik BN. Fibrinopeptide B and aggregation of fibrinogen. Science. 1979;204:200–202. doi: 10.1126/science.155308. [DOI] [PubMed] [Google Scholar]
- Shevchenko RV, James SL, James SE. A review of tissue-engineered skin bioconstructs available for skin reconstruction. J R Soc Interface. 2010;7:229–258. doi: 10.1098/rsif.2009.0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvain J, Collet JP, Nagaswami C, Beygui F, Edmondson KE, Bellemain-Appaix A, Cayla G, Pena A, Brugier D, Barthelemy O, Montalescot G, Weisel JW. Composition of coronary thrombus in acute myocardial infarction. J Am Coll Cardiol. 2011;57:1359–1367. doi: 10.1016/j.jacc.2010.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolarczyk K, Boncela J, Szymanski J, Gils A, Cierniewski CS. Fibrinogen contains cryptic PAI-1 binding sites that are exposed on binding to solid surfaces or limited proteolysis. Arterioscler Thromb Vasc Biol. 2005;25:2679–2684. doi: 10.1161/01.ATV.0000189305.84297.8b. [DOI] [PubMed] [Google Scholar]
- Spero RC, Sircar RK, Schubert R, Taylor RM, 2nd, Wolberg AS, Superfine R. Nanoparticle diffusion measures bulk clot permeability. Biophys J. 2011;101:943–950. doi: 10.1016/j.bpj.2011.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spraggon G, Everse SJ, Doolittle RF. Crystal structures of fragment D from human fibrinogen and its crosslinked counterpart from fibrin. Nature. 1997;389:455–462. doi: 10.1038/38947. [DOI] [PubMed] [Google Scholar]
- Standeven KF, Grant PJ, Carter AM, Scheiner T, Weisel JW, Ariens RA. Functional analysis of the fibrinogen Aalpha Thr312Ala polymorphism: effects on fibrin structure and function. Circulation. 2003;107:2326–2330. doi: 10.1161/01.CIR.0000066690.89407.CE. [DOI] [PubMed] [Google Scholar]
- Standeven KF, Carter AM, Grant PJ, Weisel JW, Chernysh I, Masova L, Lord ST, Ariens RA. Functional analysis of fibrin {gamma}-chain cross-linking by activated factor XIII: determination of a cross-linking pattern that maximizes clot stiffness. Blood. 2007;110:902–907. doi: 10.1182/blood-2007-01-066837. [DOI] [PubMed] [Google Scholar]
- Storm C, Pastore JJ, Mackintosh FC, Lubensky TC, Janmey PA. Nonlinear elasticity in biological gels. Nature. 2005;435:191–194. doi: 10.1038/nature03521. [DOI] [PubMed] [Google Scholar]
- Sugo T, Nakamikawa C, Yoshida N, Niwa K, Sameshima M, Mimuro J, Weisel JW, Nagita A, Matsuda M. End-linked homodimers in fibrinogen Osaka VI with a B beta-chain extension lead to fragile clot structure. Blood. 2000;96:3779–3785. [PubMed] [Google Scholar]
- Swieringa F, Baaten CC, Verdoold R, Mastenbroek TG, Rijnveld N, Van Der Laan KO, Breel EJ, Collins PW, Lance MD, Henskens YM, Cosemans JM, Heemskerk JW, Van Der Meijden PE. Platelet control of fibrin distribution and microelasticity in thrombus formation under flow. Arterioscler Thromb Vasc Biol. 2016;36(4):692–699. doi: 10.1161/ATVBAHA.115.306537. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Kondo T, Yoshikawa Y, Watanabe K, Orino K. The presence of heat-labile factors interfering with binding analysis of fibrinogen with ferritin in horse plasma. Acta Vet Scand. 2013;55:70. doi: 10.1186/1751-0147-55-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y. Studies of the metabolism and distribution of fibrinogen in healthy men with autologous 125-I-labeled fibrinogen. J Clin Invest. 1966;45:103–111. doi: 10.1172/JCI105314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talens S, Leebeek FW, Demmers JA, Rijken DC. Identification of fibrin clot-bound plasma proteins. PLoS One. 2012;7:e41966. doi: 10.1371/journal.pone.0041966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T, Arai S, Nagaya H, Mizuguchi J, Wada I. Stepwise assembly of fibrinogen is assisted by the endoplasmic reticulum lectin-chaperone system in HepG2 cells. PLoS One. 2013;8:e74580. doi: 10.1371/journal.pone.0074580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torbet J, Freyssinet JM, Hudry-Clergeon G. Oriented fibrin gels formed by polymerization in strong magnetic fields. Nature. 1981;289:91–93. doi: 10.1038/289091a0. [DOI] [PubMed] [Google Scholar]
- Townsend RR, Hilliker E, Li YT, Laine RA, Bell WR, Lee YC. Carbohydrate structure of human fibrinogen. Use of 300-MHz 1H-NMR to characterize glycosidase-treated glycopeptides. J Biol Chem. 1982;257:9704–9710. [PubMed] [Google Scholar]
- Townsend RR, Heller DN, Fenselau CC, Lee YC. Determination of the sialylation pattern of human fibrinogen glycopeptides with fast atom bombardment. Biochemist. 1984;23:6389–6392. doi: 10.1021/bi00321a016. [DOI] [PubMed] [Google Scholar]
- Tran H, Tanaka A, Litvinovich SV, Medved LV, Haudenschild CC, Argraves WS. The interaction of fibulin-1 with fibrinogen. A potential role in hemostasis and thrombosis. J Biol Chem. 1995;270:19458–19464. doi: 10.1074/jbc.270.33.19458. [DOI] [PubMed] [Google Scholar]
- Tran R, Myers DR, Ciciliano J, Trybus Hardy EL, Sakurai Y, Ahn B, Qiu Y, Mannino RG, Fay ME, Lam WA. Biomechanics of haemostasis and thrombosis in health and disease: from the macro- to molecular scale. J Cell Mol Med. 2013;17:579–596. doi: 10.1111/jcmm.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurupa G, Yakovlev S, Mckee P, Medved L. Noncovalent interaction of alpha(2)-antiplasmin with fibrin(ogen): localization of alpha(2)-antiplasmin-binding sites. Biochemistry. 2010;49:7643–7651. doi: 10.1021/bi1010317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurupa G, Mahid A, Veklich Y, Weisel JW, Medved L. Structure, stability, and interaction of fibrin alphaC-domain polymers. Biochemistry. 2011;50:8028–8037. doi: 10.1021/bi2008189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurupa G, Pechik I, Litvinov RI, Hantgan RR, Tjandra N, Weisel JW, Medved L. On the mechanism of alphaC polymer formation in fibrin. Biochemistry. 2012;51:2526–2538. doi: 10.1021/bi2017848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitte De Willige S, De Visser MC, Houwing-Duistermaat JJ, Rosendaal FR, Vos HL, Bertina RM. Genetic variation in the fibrinogen gamma gene increases the risk for deep venous thrombosis by reducing plasma fibrinogen gamma’ levels. Blood. 2005;106:4176–4183. doi: 10.1182/blood-2005-05-2180. [DOI] [PubMed] [Google Scholar]
- Uitte De Willige S, Standeven KF, Philippou H, Ariens RA. The pleiotropic role of the fibrinogen gamma’ chain in hemostasis. Blood. 2009;114:3994–4001. doi: 10.1182/blood-2009-05-217968. [DOI] [PubMed] [Google Scholar]
- Undas A, Ariens RA. Fibrin clot structure and function: a role in the pathophysiology of arterial and venous thromboembolic diseases. Arterioscler Thromb Vasc Biol. 2011;31:e88–e99. doi: 10.1161/ATVBAHA.111.230631. [DOI] [PubMed] [Google Scholar]
- Undas A, Brozek J, Jankowski M, Siudak Z, Szczeklik A, Jakubowski H. Plasma homocysteine affects fibrin clot permeability and resistance to lysis in human subjects. Arterioscler Thromb Vasc Biol. 2006;26:1397–1404. doi: 10.1161/01.ATV.0000219688.43572.75. [DOI] [PubMed] [Google Scholar]
- Valnickova Z, Enghild JJ. Human procarboxypeptidase U, or thrombin-activable fibrinolysis inhibitor, is a substrate for transglutaminases. Evidence for transglutaminase-catalyzed cross-linking to fibrin. J Biol Chem. 1998;273:27220–27224. doi: 10.1074/jbc.273.42.27220. [DOI] [PubMed] [Google Scholar]
- Varju I, Sotonyi P, Machovich R, Szabo L, Tenekedjiev K, Silva MM, Longstaff C, Kolev K. Hindered dissolution of fibrin formed under mechanical stress. J Thromb Haemost. 2011;9:979–986. doi: 10.1111/j.1538-7836.2011.04203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veklich YI, Gorkun OV, Medved LV, Nieuwenhuizen W, Weisel JW. Carboxyl-terminal portions of the alpha chains of fibrinogen and fibrin. Localization by electron microscopy and the effects of isolated alpha C fragments on polymerization. J Biol Chem. 1993;268:13577–13585. [PubMed] [Google Scholar]
- Veklich Y, Francis CW, White J, Weisel JW. Structural studies of fibrinolysis by electron microscopy. Blood. 1998;92:4721–4729. [PubMed] [Google Scholar]
- Wei AH, Schoenwaelder SM, Andrews RK, Jackson SP. New insights into the haemostatic function of platelets. Br J Haematol. 2009;147:415–430. doi: 10.1111/j.1365-2141.2009.07819.x. [DOI] [PubMed] [Google Scholar]
- Weigandt KM, White N, Chung D, Ellingson E, Wang Y, Fu X, Pozzo DC. Fibrin clot structure and mechanics associated with specific oxidation of methionine residues in fibrinogen. Biophys J. 2012;103:2399–2407. doi: 10.1016/j.bpj.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisel JW. The electron microscope band pattern of human fibrin: various stains, lateral order, and carbohydrate localization. J Ultrastruct Mol Struct Res. 1986;96:176–188. doi: 10.1016/0889-1605(86)90019-4. [DOI] [PubMed] [Google Scholar]
- Weisel JW. Cross-linked gamma chains in a fibrin fibril are situated transversely between its strands: no. J Thromb Haemost. 2004;2:394–399. doi: 10.1111/j.1538-7836.2004.00873.x. [DOI] [PubMed] [Google Scholar]
- Weisel JW. Fibrinogen and fibrin. In: Parry DAD, Squire J, editors. Coiled-coils, collagen & elastomers. Elsevier; San Diego: 2005. [Google Scholar]
- Weisel JW. Structure of fibrin: impact on clot stability. J Thromb Haemost. 2007;5(Suppl 1):116–124. doi: 10.1111/j.1538-7836.2007.02504.x. [DOI] [PubMed] [Google Scholar]
- Weisel JW, Dempfle C-EH. Fibrinogen structure and function. In: Marder V, Aird WC, Bennett JS, Schulman S, White GC, editors. Hemostasis and thrombosis: basic principles and clinical practice. 6. Lippincott Williams and Wilkins; Philadelphia: 2013. [Google Scholar]
- Weisel JW, Litvinov RI. The biochemical and physical process of fibrinolysis and effects of clot structure and stability on the lysis rate. Cardiovasc Hematol Agents Med Chem. 2008;6:161–180. doi: 10.2174/187152508784871963. [DOI] [PubMed] [Google Scholar]
- Weisel JW, Litvinov RI. Mechanisms of fibrin polymerization and clinical implications. Blood. 2013;121:1712–1719. doi: 10.1182/blood-2012-09-306639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisel JW, Litvinov RI. Mechanisms of fibrinolysis and basic principles of management. In: Saba HI, Roberts HR, editors. Hemostasis and thrombosis: practical guidelines in clinical management. Wiley-Blackwell; Chichester: 2014. [Google Scholar]
- Weisel JW, Medved L. The structure and function of the alpha C domains of fibrinogen. Ann N Y Acad Sci. 2001;936:312–327. doi: 10.1111/j.1749-6632.2001.tb03517.x. [DOI] [PubMed] [Google Scholar]
- Weisel JW, Nagaswami C. Computer modeling of fibrin polymerization kinetics correlated with electron microscope and turbidity observations: clot structure and assembly are kinetically controlled. Biophys J. 1992;63:111–128. doi: 10.1016/S0006-3495(92)81594-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisel JW, Warren SG, Cohen C. Crystals of modified fibrinogen: size, shape and packing of molecules. J Mol Biol. 1978;126:159–183. doi: 10.1016/0022-2836(78)90357-1. [DOI] [PubMed] [Google Scholar]
- Weisel JW, Phillips GJ, Cohen C. The structure of fibrinogen and fibrin: II. Architecture of the fibrin clot. Ann N Y Acad Sci. 1983;408:367–379. doi: 10.1111/j.1749-6632.1983.tb23257.x. [DOI] [PubMed] [Google Scholar]
- Weisel JW, Stauffacher CV, Bullitt E, Cohen C. A model for fibrinogen: domains and sequence. Science. 1985;230:1388–1391. doi: 10.1126/science.4071058. [DOI] [PubMed] [Google Scholar]
- Weisel JW, Veklich Y, Gorkun O. The sequence of cleavage of fibrinopeptides from fibrinogen is important for protofibril formation and enhancement of lateral aggregation in fibrin clots. J Mol Biol. 1993;232:285–297. doi: 10.1006/jmbi.1993.1382. [DOI] [PubMed] [Google Scholar]
- Weiss HL, Selvaraj P, Okita K, Matsumoto Y, Voie A, Hoelscher T, Szeri AJ. Mechanical clot damage from cavitation during sonothrombolysis. J Acoust Soc Am. 2013;133:3159–3175. doi: 10.1121/1.4795774. [DOI] [PubMed] [Google Scholar]
- Wen Q, Janmey PA. Effects of non-linearity on cell-ECM interactions. Exp Cell Res. 2013;319:2481–2489. doi: 10.1016/j.yexcr.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker P, Przyklenk K. Fibrin architecture in clots: a quantitative polarized light microscopy analysis. Blood Cells Mol Dis. 2009;42:51–56. doi: 10.1016/j.bcmd.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RC. Morphology of bovine fibrinogen monomers and fibrin oligomers. J Mol Biol. 1981;150:399–408. doi: 10.1016/0022-2836(81)90555-6. [DOI] [PubMed] [Google Scholar]
- Wolberg AS. Plasma and cellular contributions to fibrin network formation, structure and stability. Haemophilia. 2010;16(Suppl 3):7–12. doi: 10.1111/j.1365-2516.2010.02253.x. [DOI] [PubMed] [Google Scholar]
- Wolberg AS. Determinants of fibrin formation, structure, and function. Curr Opin Hematol. 2012;19:349–356. doi: 10.1097/MOH.0b013e32835673c2. [DOI] [PubMed] [Google Scholar]
- Wolfenstein TC, Mosesson MW. Carboxy-terminal amino acid sequence of a human fibrinogen gamma-chain variant (gamma’) Biochemist. 1981;20:6146–6149. doi: 10.1021/bi00524a036. [DOI] [PubMed] [Google Scholar]
- Wufsus AR, Rana K, Brown A, Dorgan JR, Liberatore MW, Neeves KB. Elastic behavior and platelet retraction in low- and high-density fibrin gels. Biophys J. 2015;108:173–183. doi: 10.1016/j.bpj.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovlev S, Medved L. Interaction of fibrin with the very low density lipoprotein receptor: further characterization and localization of the fibrin-binding site. Biochemistry. 2015;54:4751–4761. doi: 10.1021/acs.biochem.5b00582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovlev S, Gao Y, Cao C, Chen L, Strickland DK, Zhang L, Medved L. Interaction of fibrin with VE-cadherin and anti-inflammatory effect of fibrin-derived fragments. J Thromb Haemost. 2011;9:1847–1855. doi: 10.1111/j.1538-7836.2011.04438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazumi K, Doolittle RF. The synthetic peptide Gly-Pro-Arg-Pro-amide limits the plasmic digestion of fibrinogen in the same fashion as calcium ion. Protein Sci. 1992;1:1719–1720. doi: 10.1002/pro.5560011220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Mochalkin I, Doolittle LR. A model for fibrin formation based on crystal structures of fibrinogen and fibrin fragments complexed with specific peptides. Proc Natl Acad Sci U S A. 2000;97:14156–14161. doi: 10.1073/pnas.97.26.14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee VC, Pratt KP, Cote HC, Trong IL, Chung DW, Davie EW, Stenkamp RE, Teller DC. Crystal structure of a 30 kDa C-terminal fragment from the gamma chain of human fibrinogen. Structure. 1997;5:125–138. doi: 10.1016/s0969-2126(97)00171-8. [DOI] [PubMed] [Google Scholar]
- Yermolenko IS, Lishko VK, Ugarova TP, Magonov SN. High-resolution visualization of fibrinogen molecules and fibrin fibers with atomic force microscopy. Biomacromolecules. 2011;12:370–379. doi: 10.1021/bm101122g. [DOI] [PubMed] [Google Scholar]
- Yeromonahos C, Polack B, Caton F. Nanostructure of the fibrin clot. Biophys J. 2010;99:2018–2027. doi: 10.1016/j.bpj.2010.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalewski J, Bogaert J, Sadowski M, Woznicka O, Doulaptsis K, Ntoumpanaki M, Zabczyk M, Nessler J, Undas A. Plasma fibrin clot phenotype independently affects intracoronary thrombus ultrastructure in patients with acute myocardial infarction. Thromb Haemost. 2015;113:1258–1269. doi: 10.1160/TH14-09-0801. [DOI] [PubMed] [Google Scholar]
- Zhang JZ, Redman CM. Fibrinogen assembly and secretion. Role of intrachain disulfide loops. J Biol Chem. 1996;95:30083–30088. doi: 10.1074/jbc.271.47.30083. [DOI] [PubMed] [Google Scholar]
- Zhmurov A, Brown AE, Litvinov RI, Dima RI, Weisel JW, Barsegov V. Mechanism of fibrin(ogen) forced unfolding. Structure. 2011;19:1615–1624. doi: 10.1016/j.str.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhmurov A, Kononova O, Litvinov RI, Dima RI, Barsegov V, Weisel JW. Mechanical transition from alpha-helical coiled coils to beta-sheets in fibrin(ogen) J Am Chem Soc. 2012;134:20396–20402. doi: 10.1021/ja3076428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhmurov A, Protopopova AD, Litvinov RI, Zhukov P, Mukhitov AR, Weisel JW, Barsegov V. Structural basis of interfacial flexibility in fibrin oligomers. Structure. 2016;24:1907–1917. doi: 10.1016/j.str.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubairova LD, Nabiullina RM, Nagaswami C, Zuev YF, Mustafin IG, Litvinov RI, Weisel JW. Circulating microparticles alter formation, structure, and properties of fibrin clots. Sci Rep. 2015;5:17611. doi: 10.1038/srep17611. [DOI] [PMC free article] [PubMed] [Google Scholar]