Abstract
Background
Platelet-rich plasma (PRP) has gained growing popularity in use in spinal fusion procedures in the last decade. Substantial intraoperative blood loss is frequently accompanied with spinal fusion, and it is unknown whether blood harvested intraoperatively qualifies for PRP preparation.
Material/Methods
Whole blood was harvested intraoperatively and venous blood was collected by venipuncture. Then, we investigated the platelet concentrations in whole blood and PRP, the concentration of growth factors in PRP, and the effects of PRP on the proliferation and viability of human bone marrow-derived mesenchymal stem cells (HBMSCs).
Results
Our results revealed that intraoperatively harvested whole blood and whole blood collected by venipuncture were similar in platelet concentration. In addition, PRP formulations prepared from both kinds of whole blood were similar in concentration of platelet and growth factors. Additional analysis showed that the similar concentrations of growth factors resulted from the similar platelet concentrations of whole blood and PRP between the two groups. Moreover, these two kinds of PRP formulations had similar effects on promoting cell proliferation and enhancing cell viability.
Conclusions
Therefore, intraoperatively harvested whole blood may be a potential option for preparing PRP spinal fusion.
MeSH Keywords: Cell Proliferation; Osteoarthritis, Spine; Platelet-Rich Plasma
Background
Spinal fusion is a widely used procedure for spinal columns that demonstrate pathological instability. Although autogenous bone graft is still the gold standard for spinal fusion, there are concerns arising from associated donor site morbidity and inadequate tissue availability [1]. Allografts and xenografts have been used as alternative methods. However, these methods suffer from risks of disease transmission and rejection [2]. To overcome these problems, bone tissue engineering has been introduced in an attempt to provide an alternative option.
Platelet-rich plasma (PRP) is an autologous blood product that contains concentrated platelets, and these platelets can release growth factors at concentrations significantly higher than baseline blood levels, including platelet-derived growth factor (PDGF), transforming growth factor (TGF), vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF), fibroblast growth factor (FGF), and epidermal growth factor (EGF) [3–5]. Many of these growth factors are universally known to have beneficial effects on osteogenesis, and the use of PRP has been demonstrated to promote bone tissue regeneration [6–10]. Therefore, PRP is considered a promising option for autogenous bone graft used in spinal fusion [11].
PRP preparation usually begins with autologous venous blood collection. However, substantial intraoperative blood loss is frequently accompanied with spinal fusion, and the additional blood loss from venous blood collection may increase the need for transfusion, which may overwhelm the advantage of PRP as an autograft [12,13]. Therefore, the addition of venous blood collection has been a challenge in the use of PRP for spinal fusion. Since intraoperative blood loss in spinal fusion is more than the blood needed for PRP preparation, preparing PRP from blood harvested intraoperatively might be a potential solution. The objective of this study was to compare the characteristics of PRP prepared from blood harvested intraoperatively to blood from venipuncture, in an attempt to develop an option to provide a safe, simple, and cost-efficient approach for the preparation of PRP used in spinal fusion.
Material and Methods
Participant recruitment
This study was performed in accordance with the principles of Declaration of Helsinki. The Independent Ethics Committee of Shanghai East Hospital Affiliated to Tongji University approved the protocols of this study. Written informed consent was obtained from each volunteer.
Between March 2013 and October 2015, a total of 20 patients treated with spinal fusion in our department were recruited for intraoperative blood donation (group A), and 20 healthy volunteers were recruited for venous blood donation (group B). The inclusion criterion was adults who agreed to participate in the study. The exclusion criteria were platelet concentration lower than 150×109/L, a medical history of relevant diseases, use of any medications known to affect platelet function or concentration for 21 days prior to blood donation, and less than 100 mL of intraoperatively harvested blood.
Blood collection
For volunteers of group A, intraoperative blood was harvested by gentle aspiration in the presence of acid-citrate dextrose solution A (ACD-A). Then, the harvested blood was filtered with a 70-mm cell strainer to remove bone debris. For volunteers of group B, venous blood was collected by venipuncture and anticoagulated with ACD-A at the same ratio.
PRP preparation
PRP was prepared with WEGO PRP preparation system (WEGO, Weihai, China), which is the only commercial PRP preparation system approved by the China Food and Drug Administration, and PRP was prepared according to manufacturer’s instructions. In brief, 35 mL of anticoagulated whole blood was spun at 400 g for 10 minutes. After the first-spin, the anticoagulated whole blood was separated into three components: platelet-containing plasma at the top, buffy coat in the middle, and erythrocytes at the bottom. Then, erythrocytes were removed from the centrifuge tube and discarded, and platelet-containing plasma and buffy coat were spun again at 400 g for 10 minutes. After the second-spin, the supernatant platelet-poor plasma was removed, and the remaining plasma and precipitated platelets were blended evenly to obtain a total of 4 mL of PRP.
Whole blood analysis
Platelet concentrations in whole blood and PRP samples were evaluated by whole blood analysis using an automatic hematology analyzer (XS-800i, Sysmex, Kobe, Japan) in the clinical laboratory within 30 minutes after collection.
Enzyme-linked immunosorbent assay (ELISA)
ELISA was performed to quantify PDGF-AB and TGF-β1 concentrations in activated PRP samples as described elsewhere [14]. Briefly, PRP samples were incubated in 10% calcium chloride (final concentration 22.8 mM) for seven days at 37°C in a humidified atmosphere with 5% CO2. Then, the formulations were spun at 2800 g for 15 minutes to collect the supernatant. PDGF-AB and TGF-β1 concentrations in the supernatant were determined using the Quantikine Human Immunoassay kits (R&D Systems, Minneapolis, MN, USA) according to manufacturer’s instructions.
Isolation and expansion of cells
Human bone marrow-derived mesenchymal stem cells (HBMSCs) were isolated according to the protocols described previously [15]. Briefly, bone marrow aspirates were harvested during femur fracture surgery. After removing bone debris by filtering, the cells were cultured in 75-cm2 flasks at the density of 5.0×105 cells/flask in α-modification of minimum essential medium (α-MEM; Sigma-Aldrich, St Louis, MO, USA) containing 10% fetal bovine serum (FBS) (Gibco, Carlsbad, CA, USA) and 1% antibiotics (penicillin G and streptomycin) (Gibco) at 37°C in a humidified atmosphere with 5% CO2. The medium was changed after 48 hours to remove non-adherent cells, and thereafter changed every three days. Cells passages between three and five were used in this study.
CCK-8 assay
HBMSCs were seeded in 96-well plates at a density of 5,000 cells/well and serum-starved for 24 hours. Then, cells were cultured in medium supplemented with 10% of FBS or PRP prepared from harvested intraoperatively blood or venous blood for seven days. At the end of the incubation period, 10 μL of CCK-8 solution (Dojindo, Kumamoto, Japan) was added into each well containing 100 μL of medium and incubated for three hours. The absorbance value at 450 nm was measured using a microplate reader (Bio-Rad, Hercules, CA, USA).
Live/dead staining
HBMSCs were seeded in 6-well plates at a density of 1.0×105 cells/well, serum-starved for 24 hours, and cultured in medium supplemented with 10% of FBS or PRP for seven days. Then, cells were stained with a Cell Viability Imaging Kit (Life Technologies, Gaithersburg, MD, USA) according to manufacturer’s instructions. The percentages of viable cells were counted and calculated on five randomly selected fields per well.
Statistical analysis
The data were analyzed with SPSS 22.0 (IBM, Chicago, IL, USA) and presented as means ± standard deviation or numbers of volunteers. Chi-square test or independent-samples Student’s t-test was performed to analyze difference between groups as appropriate, and one-way analysis of variance (ANOVA) and Bonferroni post-hoc test were performed to analyze difference among groups. Correlations between platelet concentrations and growth factor concentrations were evaluated by Pearson correlation analysis. Significance was set a p<0.05.
Results
General information
General information of grouped volunteers is shown in Table 1; there was no significant difference in gender and age between groups.
Table 1.
General information of grouped volunteers.
| Group A | Group B | p value | |
|---|---|---|---|
| Number of volunteers | 20 | 20 | |
| Gender (Male: Female) | 11: 9 | 13: 7 | 0.748 |
| Age (years) | 51.35±7.26 | 49.95±9.54 | 0.604 |
Platelet concentrations in whole blood and PRP
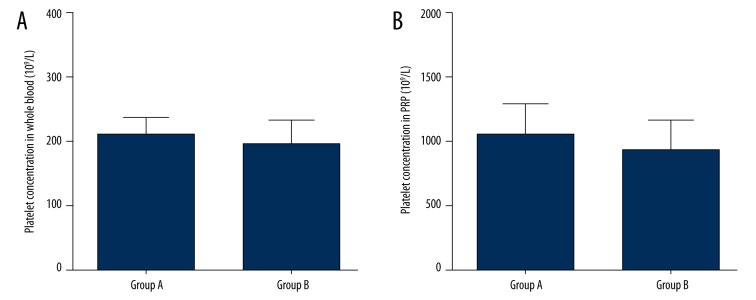
The mean whole blood platelet concentrations of group A and group B were 209.15±27.88×109/L and 195.60±38.64, respectively, and the mean PRP platelet concentrations of group A and group B were 1046.90±237.28×109/L and 932.55±234.1×109/L, respectively. Group A and group B had similar platelet concentrations in whole blood (p=0.211, Figure 1A) and PRP (p=0.133, Figure 1B).
Figure 1.
Platelet concentrations in whole blood and PRP. There was no significant difference in whole blood platelet concentration (A) and PRP platelet concentration (B) between group A and group B.
Growth factors concentrations in PRP
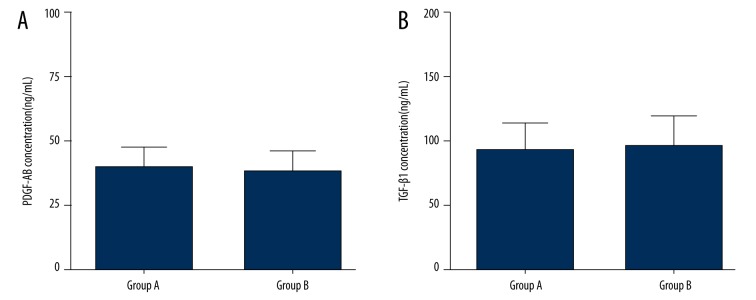
The mean PDGF-AB and TGF-β1 concentrations in PRP were 40.23±8.17 ng/mL and 92.82±20.84 ng/mL, respectively, in group A; and 38.80±7.43 ng/mL and 96.40±23.86 ng/mL, respectively, in group B. Also, there was no significant difference between groups with regard to PDGF-AB concentrations (p=0.566, Figure 2A) and TGF-β1 concentrations (p=0.616, Figure 2B).
Figure 2.
PDGF-AB and TGF-β1 concentrations in PRP. There was no significant difference in PDGF-AB concentration (A) and TGF-β1 concentration (B) in PRP between group A and group B.
Correlations between platelet and growth factors concentrations
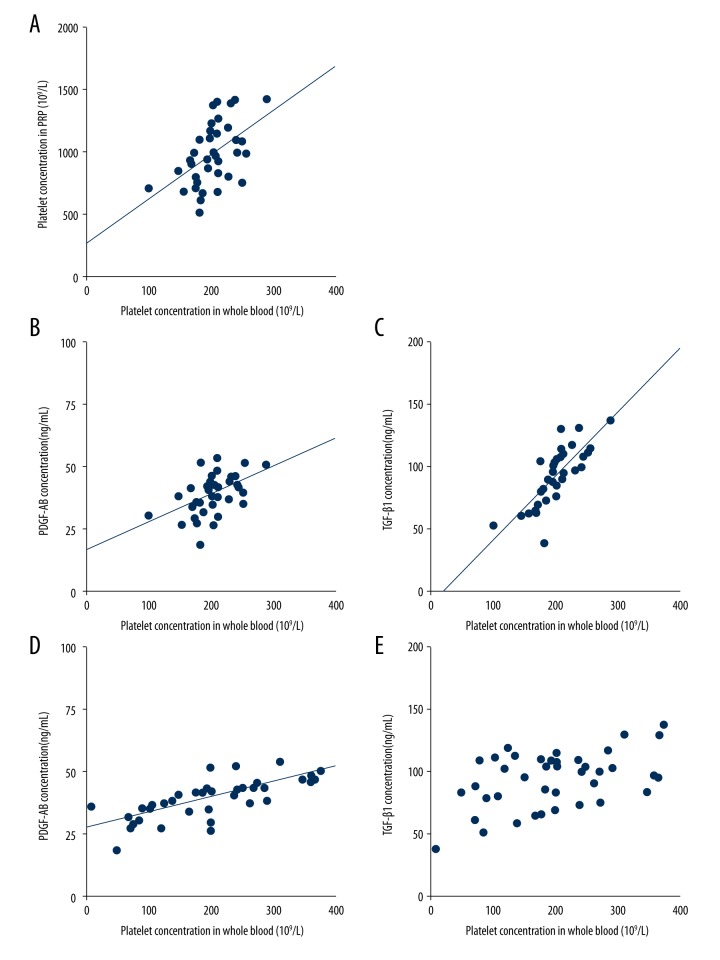
There were significantly positive correlations between whole blood platelet concentration and PRP platelet concentration (r=0.504, p=0.001, Figure 3A), PDGF-AB concentration (r=0.492, p=0.001, Figure 3B) and TGF-β1 concentration (r=0.789, p<0.001, Figure 3C). Also, there were significantly positive correlations between PRP platelet concentration and PDGF-AB concentration (r=0.729, p<0.001, Figure 3D) and TGF-β1 concentration (r=0.476, p=0.002, Figure 3E).
Figure 3.
Significantly positive correlations between concentrations of platelets and growth factors. (A) There was significantly positive correlation between whole blood platelet concentration and PRP platelet concentration. (B, C) There were significantly positive correlations between whole blood platelet concentration and PDGF-AB concentration (B) and TGF-β1 concentration (C). (D, E) There were significantly positive correlations between PRP platelet concentration and PDGF-AB concentration (D) and TGF-β1 concentration (E).
Effects of PRP on cell proliferation and viability
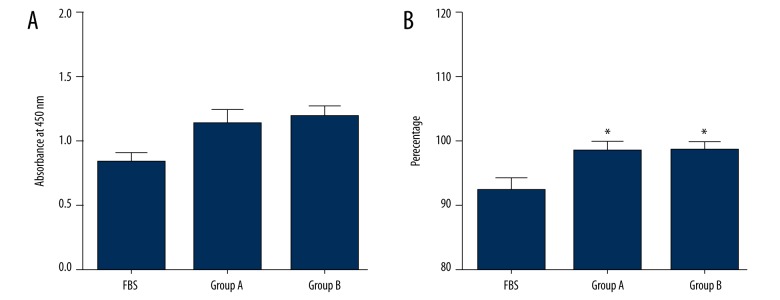
As shown in Figure 4A, results of the CCK-8 assay demonstrated that both PRP formulations promoted cell proliferation compared with FBS, but there was no significant difference between PRP formulations of group A and group B.
Figure 4.
Effects of PRP on cell proliferation and viability. (A) Results of CCK-8 assay demonstrated that both PRP formulations promoted cell proliferation compared with FBS, but there was no significant difference between PRP formulations of group A and group B. (B) Quantified results of live/dead staining demonstrated that both PRP formulations enhanced cell viability compared with FBS, but no significant difference was observed between PRP formulations of group A and group B.
Quantified results of live/dead staining are shown in Figure 4B. Similar to the results of cell proliferation, both PRP formulations enhanced cell viability compared with FBS, but no significant difference was observed between PRP formulations of group A and group B.
Discussion
Intraoperative blood harvest is part of routine management in patients undergoing major surgery with anticipated considerable blood loss. Multiple commercial systems for intraoperative blood harvest are available today. However, blood harvested by these systems barely has active platelets, and therefore, is not qualified for PRP preparation [16]. Thus, we harvested intraoperative blood directly by a syringe prefilled with anticoagulant, rather than using a commercial intraoperative blood harvest system. Our results showed that blood harvested intraoperatively in this study had comparable platelet concentration compared with venous blood collected from healthy volunteers by venipuncture. Moreover, PRP prepared from harvested blood also had comparable platelet concentration compared with PRP prepared from venous blood, and there was a close correlation between platelet concentrations in whole blood and PRP. Therefore, it is plausible to conclude that the harvested blood potentially qualifies for PRP preparation.
The basis of PRP therapy is believed to be growth factors released from platelets [17–19]. Thus, the concentrations of growth factors in PRP were quantified in this study to clarify whether PRP formulations that were similar in platelet concentration were also similar in concentration of growth factors. PDGF-AB is a potent chemokine and regulator of cell proliferation and extracellular matrix deposition [20,21] while TGF-β1 is involved in cell proliferation and apoptosis, as well as extracellular matrix deposition [22,23]. Moreover, PDGF-AB and TGF-β1 have been shown to promote bone regeneration through enhanced osteogenesis [6,7]. Although other growth factors, such as IGF, FGF, and EGF, also have been detected consistently in PRP and demonstrated to have beneficial effects on cell proliferation and bone matrix synthesis [23], they were found to have much more variable concentrations in whole blood, and therefore in PRP [24]. As a result, only PDGF-AB and TGF-β1 were selected to characterize PRP formulations prepared from whole blood harvested intraoperatively or by venipuncture. Our findings showed that PRP prepared from the harvested blood and venous blood also had similar concentrations for growth factors. Additional analysis revealed that the similar platelet concentrations in whole blood and PRP between groups resulted in the similar concentrations of growth factors in PRP. These findings indicated that the harvested blood could be used to prepare a PRP that has similar cellular and growth factor components compared with PRP prepared from venous blood, and therefore, the blood harvested intraoperatively may qualify for PRP preparation, as long as enough active platelets are also harvested.
However, similar therapeutic effects may not be the direct result of these similar platelet and growth factors concentrations, as the correlations between components and therapeutic effects of PRP might be not linear [25–28]. Therefore, the effects of PRP prepared from harvested blood and venous blood on cells were evaluated in this study. Our results demonstrated that PRP prepared from harvested blood and venous blood had similar effects on promoting proliferation and enhancing viability of HBMSCs. It is universally known that HBMSCs proliferation and viability are beneficial for tissue regeneration in bone or osteochondral lesions [29,30]. Therefore, PRP prepared from harvested blood and venous blood may have similar outcomes in spinal fusion. Further studies are needed to substantiate this in vivo and in vitro.
Conclusions
In this study, we demonstrated that intraoperatively harvested whole blood and whole blood collected by venipuncture were similar in platelet concentrations. Besides that, PRP formulations prepared from both kinds of whole blood were similar in concentrations of platelets and growth factors. Additional analysis showed that the similar concentrations of growth factors resulted from the similar platelet concentrations of whole blood and PRP between the two groups. Moreover, these two kinds of PRP formulations had similar effects on promoting cell proliferation and enhancing cell viability. Therefore, intraoperatively harvested whole blood may be a potential option for preparing PRP for spinal fusion. However, considering the small sample included in this study, further study with a larger sample size is needed to substantiate this in vivo and in vitro.
Footnotes
Conflict of interest
None.
Source of support: This work was supported by grants from the Innovation Fund and Science and Technology Development Foundation of Pudong District (PKJ2012-Y10) and Key Discipline Construction Project of the Pudong Health Bureau of Shanghai (PWZx2014-02)
References
- 1.Boden SD. Biology of lumbar spine fusion and use of bone graft substitutes: Present, future, and next generation. Tissue Eng. 2000;6:383–99. doi: 10.1089/107632700418092. [DOI] [PubMed] [Google Scholar]
- 2.Oryan A, Alidadi S, Moshiri A, et al. Bone regenerative medicine: Classic options, novel strategies, and future directions. J Orthop Surg Res. 2014;9:18. doi: 10.1186/1749-799X-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castillo TN, Pouliot MA, Kim HJ, et al. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39:266–71. doi: 10.1177/0363546510387517. [DOI] [PubMed] [Google Scholar]
- 4.Magalon J, Bausset O, Serratrice N, et al. Characterization and comparison of 5 platelet-rich plasma preparations in a single-donor model. Arthroscopy. 2014;30:629–38. doi: 10.1016/j.arthro.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 5.Appel TR, Potzsch B, Muller J, et al. Comparison of three different preparations of platelet concentrates for growth factor enrichment. Clin Oral Implants Res. 2002;13:522–28. doi: 10.1034/j.1600-0501.2002.130512.x. [DOI] [PubMed] [Google Scholar]
- 6.Joyce ME, Roberts AB, Sporn MB, et al. Transforming growth factor-beta and the initiation of chondrogenesis and osteogenesis in the rat femur. J Cell Biol. 1990;110:2195–207. doi: 10.1083/jcb.110.6.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centrella M, Massague J, Canalis E. Human platelet-derived transforming growth factor-beta stimulates parameters of bone growth in fetal rat calvariae. Endocrinology. 1986;119:2306–12. doi: 10.1210/endo-119-5-2306. [DOI] [PubMed] [Google Scholar]
- 8.Hock JM, Centrella M, Canalis E. Insulin-like growth factor I has independent effects on bone matrix formation and cell replication. Endocrinology. 1988;122:254–60. doi: 10.1210/endo-122-1-254. [DOI] [PubMed] [Google Scholar]
- 9.Kanczler JM, Oreffo RO. Osteogenesis and angiogenesis: The potential for engineering bone. Eur Cell Mater. 2008;15:100–14. doi: 10.22203/ecm.v015a08. [DOI] [PubMed] [Google Scholar]
- 10.Wozney JM, Rosen V, Celeste AJ, et al. Novel regulators of bone formation: Molecular clones and activities. Science. 1988;242:1528–34. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 11.Okamoto S, Ikeda T, Sawamura K, et al. Positive effect on bone fusion by the combination of platelet-rich plasma and a gelatin beta-tricalcium phosphate sponge: A study using a posterolateral fusion model of lumbar vertebrae in rats. Tissue Eng Part A. 2012;18:157–66. doi: 10.1089/ten.TEA.2011.0283. [DOI] [PubMed] [Google Scholar]
- 12.Wu WC, Trivedi A, Friedmann PD, et al. Association between hospital intraoperative blood transfusion practices for surgical blood loss and hospital surgical mortality rates. Ann Surg. 2012;255:708–14. doi: 10.1097/SLA.0b013e31824a55b9. [DOI] [PubMed] [Google Scholar]
- 13.Bross MH, Soch K, Smith-Knuppel T. Anemia in older persons. Am Fam Physician. 2010;82:480–87. [PubMed] [Google Scholar]
- 14.Cavallo C, Filardo G, Mariani E, et al. Comparison of platelet-rich plasma formulations for cartilage healing: An in vitro study. J Bone Joint Surg Am. 2014;96:423–29. doi: 10.2106/JBJS.M.00726. [DOI] [PubMed] [Google Scholar]
- 15.Soleimani M, Nadri S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc. 2009;4:102–6. doi: 10.1038/nprot.2008.221. [DOI] [PubMed] [Google Scholar]
- 16.Oswald E, Streif W, Hermann M, et al. Intraoperatively salvaged red blood cells contain nearly no functionally active platelets, but exhibit formation of microparticles: Results of a pilot study in orthopedic patients. Transfusion. 2010;50:400–6. doi: 10.1111/j.1537-2995.2009.02393.x. [DOI] [PubMed] [Google Scholar]
- 17.Torricelli P, Fini M, Filardo G, et al. Regenerative medicine for the treatment of musculoskeletal overuse injuries in competition horses. Int Orthop. 2011;35:1569–76. doi: 10.1007/s00264-011-1237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weibrich G, Hansen T, Kleis W, et al. Effect of platelet concentration in platelet-rich plasma on peri-implant bone regeneration. Bone. 2004;34:665–71. doi: 10.1016/j.bone.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 19.Anitua E, Sanchez M, Zalduendo MM, et al. Fibroblastic response to treatment with different preparations rich in growth factors. Cell Prolif. 2009;42:162–70. doi: 10.1111/j.1365-2184.2009.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heldin CH, Eriksson U, Ostman A. New members of the platelet-derived growth factor family of mitogens. Arch Biochem Biophys. 2002;398:284–90. doi: 10.1006/abbi.2001.2707. [DOI] [PubMed] [Google Scholar]
- 21.Xie X, Wang Y, Zhao C, et al. Comparative evaluation of MSCs from bone marrow and adipose tissue seeded in PRP-derived scaffold for cartilage regeneration. Biomaterials. 2012;33:7008–18. doi: 10.1016/j.biomaterials.2012.06.058. [DOI] [PubMed] [Google Scholar]
- 22.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342:1350–58. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 23.Foster TE, Puskas BL, et al. Platelet-rich plasma: From basic science to clinical applications. Am J Sports Med. 2009;37:2259–72. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- 24.Berg U, Gustafsson T, Sundberg CJ, et al. Local changes in the insulin-like growth factor system in human skeletal muscle assessed by microdialysis and arterio-venous differences technique. Growth Horm IGF Res. 2006;16:217–23. doi: 10.1016/j.ghir.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Marx RE. Platelet-rich plasma (PRP): What is PRP and what is not PRP? Implant Dent. 2001;10:225–28. doi: 10.1097/00008505-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Anitua E, Zalduendo MM, Alkhraisat MH, et al. Release kinetics of platelet-derived and plasma-derived growth factors from autologous plasma rich in growth factors. Ann Anat. 2013;195:461–66. doi: 10.1016/j.aanat.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Arnoczky SP, Sheibani-Rad S. The basic science of platelet-rich plasma (PRP): What clinicians need to know. Sports Med Arthrosc. 2013;21:180–85. doi: 10.1097/JSA.0b013e3182999712. [DOI] [PubMed] [Google Scholar]
- 28.Boswell SG, Schnabel LV, Mohammed HO, et al. Increasing platelet concentrations in leukocyte-reduced platelet-rich plasma decrease collagen gene synthesis in tendons. Am J Sports Med. 2014;42:42–49. doi: 10.1177/0363546513507566. [DOI] [PubMed] [Google Scholar]
- 29.Andersen RK, Zaher W, Larsen KH, et al. Association between in vivo bone formation and ex vivo migratory capacity of human bone marrow stromal cells. Stem Cell Res Ther. 2015;6:196. doi: 10.1186/s13287-015-0188-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J, Nie H, Xu Z, et al. Construction of PRP-containing nanofibrous scaffolds for controlled release and their application to cartilage regeneration. Journal of Materials Chemistry B. 2015;3:581–91. doi: 10.1039/c4tb00515e. [DOI] [PubMed] [Google Scholar]