Abstract
Background
The aim of this study was to investigate whether needles introduce skin plugs into joints during arthrocentesis.
Material/Methods
In the first part of this study, the arthrocentesis site was scrubbed with a fluorescein sodium swab, and 90 needles were inserted through the joint tissue and collected for examination under a fluorescence microscope. In the second part of this study, the joints were injected using 720 needles of different gauges. Two different randomly assigned needle insertion techniques were used: needle insertion straight through the joint capsule (subgroup 1) or insertion of the needle into the subcutaneous tissue followed by flushing of the needle with 0.5 mL of 0.9% normal saline prior to advancing the needle through the joint capsule (subgroup 2).
Results
Of the 90 needle tips examined in the first part of this study, 21 had high-grade fluorescein contamination. In the second part of this study, the incidence of tissue, epidermis, and dermis contamination in subgroup 1 was 57.2%, 43.1%, and 25.0%, respectively. There was no significant difference in the incidence among different gauge needles, except for a difference in epidermis contamination between the 21-gauge and 23-gauge needles. Compared to subgroup 1, subgroup 2 had a significantly lower OR for tissue contamination.
Conclusions
It is common to introduce tissue coring with epidermis and dermis into the joint during arthrocentesis, which poses a potential risk for septic arthritis. However, tissue contamination of the joint may be reduced by flushing saline through the needle into the subcutaneous tissues prior to entering the joint capsule.
MeSH Keywords: Arthritis, Infectious; Injections, Intra-Articular; Needles
Background
Arthrocentesis is a commonly performed medical procedure that is invaluable for the diagnosis and treatment of joint disease. Unsuccessful arthrocentesis may contribute to diagnostic and treatment delays and potentially exposes the patient to complications from the procedure [1]. Septic arthritis is one of the most serious complications that can result from arthrocentesis. While post-arthrocentesis infection is considered to be rare, it does occur, with reported incidences ranging from 1 in 2700 to 1 in 50 000 [2–4]; it is unclear if low-grade joint infection may occur more frequently. Nicholas et al. revealed that there was an increased risk of postoperative total knee arthroplasty (TKA) infection when patients had undergone an ipsilateral knee injection before undergoing primary TKA [5].
Appropriate aseptic technique prior to arthrocentesis is critical to decrease the number of cutaneous microorganisms. However, up to 20% of skin bacteria are in the deeper layers of the skin and the pilo-sebaceous units, and these areas are untouched by antiseptics [6,7]. Additionally, contamination of the surface of the arthrocentesis site can occur immediately after disinfection, or an antiseptic may insufficiently eradicate certain pathogens from the arthrocentesis site [8,9]. Therefore, despite adherence to the most stringent aseptic skin preparation techniques, a core of skin tissue with microbial flora may be introduced into the joint as the needle cuts through the skin and passes through the subcutaneous tissue and joint capsule. This tissue coring could result in an intra-articular infection following the injection or could contaminate aspirated synovial fluid, leading to a false-positive bacterial culture.
There is limited literature regarding arthrocentesis and tissue coring. Thus, we conducted this study using amputation specimens to investigate the incidence, nature, and prevention of tissue coring during arthrocentesis.
Material and Methods
In the present study, all arthrocenteses were performed on 12 above-the-knee-amputation specimens. The specimens were obtained from patients who underwent amputation due to peripheral arterial disease or diabetes-related lower-limb chronic critical ischemia. The specimens were free of any evidence of intra-articular infection. There were no skin lesions on the knee area, such as wound, rashes, or ulcers. The arthrocenteses were performed on fresh specimens, within 30 min of the limb amputation. The knee joint arthrocentesis was performed via either the superolateral or the superomedial approach [10]. The arthrocentesis site was aseptically prepared using the same technique as for a joint aspiration or injection of the knee [11]. The degree of potential tissue contamination of the joints was studied using 2 different techniques: fluorescence and histologic examination.
Study part 1
The arthrocentesis site was scrubbed with a fluorescein sodium swab. The fluorescein agent (Fluorescite 10%; BioDee BioTech Corporation Ltd., Beijing, China) was diluted in 0.9% normal saline to a concentration of 0.01% (weight/volume) before use. Ninety needles were inserted through the skin and joint capsule after the scrubbing solution had dried. All needle tips were then carefully cut off and collected on clean microscope slides, which were placed away from light and examined by a fluorescence microscope (Vert.A1; Carl Zeiss AG, Oberkochen, Germany) within 1 h. Different slides were used for each needle tip to avoid cross-contamination. The amount of fluorescein on the needle tips was classified into 3 grades: Grade 1, no noticeable fluorescein; Grade 2, fluorescein only on the needle tips; and Grade 3, obvious and large fluorescein of tissue particles in the needle core.
Study part 2
Arthrocentesis is most commonly performed with 19-gauge, 21-gauge, and 23-gauge open-ended sharp disposable needles [11]. In the present study, 720 hollow needles were used to perform the arthrocenteses. The needles were 38 mm long and 19-gauge, 21-gauge, or 23-gauge in size. Two different needle insertion techniques were used at random: insertion straight (90°) through the skin and joint capsule with a rapid thrust (subgroup 1), or the flushing of the needle with 0.5 mL of 0.9% normal saline into the subcutaneous tissue prior to advancing the needle through the joint capsule (subgroup 2). Following insertion, all needles (both subgroup 1 and subgroup 2) were removed from the joint and flushed with 2 mL of 0.9% normal saline onto clean glass plates. An equal number of needles was used to perform the arthrocentesis via the superolateral or the superomedial approaches on each specimen. Each needle passed through a separate route. Needles were used only once and then discarded. The skin was prepared only once before the arthrocenteses, and all arthrocenteses were performed within 90 min for each specimen.
Using a surgical microscope (Leica, German) with a 10-power lens, all glass plates were checked by 2 senior pathologists to identify whether tissue coring samples were present. Coring samples identified on the glass plates were fixed in 10% formalin for 3 days. Each sample was then made into a paraffin block. Given the small size of the tissue coring, the collection process was performed on filter paper using a funnel. The sample embedding in paraffin, cutting, and staining with hematoxylin-eosin (H&E) were performed in accordance with conventional pathological techniques. Two senior pathologists independently assessed the sample slides under light microscopy (Olympus Corp, Japan) to identify any kind of skin tissue. The pathologists who performed the histological evaluation were blinded to the study protocol.
Statistical analysis
Bivariate associations were assessed using the Pearson χ2 test for categorical data. The difference in incidence of tissue coring is presented by odds ratios (ORs) and 95% confidence intervals (95% CI). The reference group was a 19-gauge needle inserted straight (90°) through joint tissue. An OR>1 suggested an increased incidence of tissue coring whereas OR<1 suggested a reduced incidence. If the CI included 1, it indicated no significant difference in incidence compared with the reference group. Data were analyzed using SPSS software (SPSS for Windows version 21). For all statistical comparisons, P<0.05 was considered significant.
Ethics
The Ethics Committee of the hospital approved this study. The procedures involving human participants were performed in accordance with the World Medical Association Declaration of Helsinki. Participating patients provided informed consent regarding data collected from the study and publication of these data.
Results
We reviewed the records of 38 patients who developed septic arthritis after intra-articular injection between October 2013 and May 2017. The criterion standard for diagnosing septic arthritis is a positive bacterial culture from synovial fluid within 3 months of an intra-articular injection. The culture results of our 38 patients are shown in Table 1. Staphylococcus aureus was the primary pathogen (39.47%). Other pathogens included coagulase-negative Staphylococcus, Escherichia coli, Streptococcus, Pseudomonas aeruginosa, and fungus. Staphylococcus aureus is a transient microorganism on the skin, which is not consistently present and is easily transmitted between individuals. While the majority of transient skin organisms are easily removed, some reports have shown that these pathogens are difficult to remove entirely, suggesting that these organisms may be the source of bacteria that can lead to septic arthritis following intra-articular injections [9,12].
Table 1.
Pathogen cultures.
| N (%) | |
|---|---|
| Staphylococcus aureus | 15 (39.47) |
| Coagulase-negative Staphylococcus | 8 (21.05) |
| Escherichia coli | 5 (13.16) |
| Streptococcus | 4 (10.53) |
| Pseudomonas aeruginosa | 3 (7.89) |
| Fungus | 3 (7.89) |
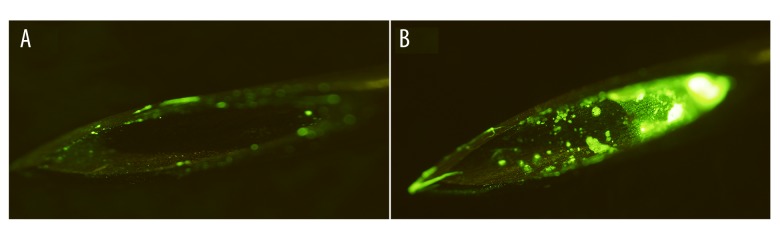
In the first part of the study, different amounts of fluorescein were detected on the needle tips (Figure 1). According to the fluorescent classification, 27 tips were Grade 1 (30.0%), 42 tips were Grade 2 (46.7%), and 21 tips were Grade 3 (23.3%).
Figure 1.
Fluorescein on needle tips. (A) Grade 2 fluorescein with a few fluorescein particles on the edge of the needle tip. (B) Grade 3 fluorescein with fluorescein particles was in the needle cores. (×10 magnification)
The incidence of tissue coring and histologic results are shown in Table 2. The incidence of tissue coring in subgroup 1 was 57.2% (206/360), without a significant difference between 19-gauge, 21-gauge, and 23-gauge needles. The subgroup 2 incidence of tissue coring was significantly less than the incidence in subgroup 1 (OR=0.02; 95% confidence interval [CI], 0.01–0.04; P<0.001) (Table 3). Additionally, we found visually larger debris caused by the larger needle gauge. Due to the irregular shape and spatial structure, it was difficult to measure the size of the tissue coring.
Table 2.
Coring rate and histologic results.
| Groups | Times | Incidence of tissue coring (%) | Histologic results | |
|---|---|---|---|---|
| Epidermis (%) | Dermis (%) | |||
| Subgroup 1 | ||||
| 19-G | 120 | 69 (57.50) | 55 (45.83) | 27 (22.50) |
| 21-G | 120 | 65 (54.17) | 41 (34.17) | 29 (24.17) |
| 23-G | 120 | 72 (60.00) | 59 (49.17) | 34 (28.33) |
| Subgroup 2 | ||||
| 19-G | 120 | 3 (2.50) | None | None |
| 21-G | 120 | 5 (4.17) | None | None |
| 23-G | 120 | 2 (1.67) | None | None |
Table 3.
Odds ratios, 95% confidence intervals, and P-values for tissue coring after needle insertion through joint tissues.
| Group | Odds ratio | 95% Confidence interval | P-value |
|---|---|---|---|
| Subgroup 1 | |||
| 19-G | 1.00 | Reference | – |
| 21-G | 1.00 | 0.36–2.78 | 1.00 |
| 23-G | 1.15 | 0.41–3.20 | 0.79 |
| Subgroup 2 | |||
| 19-G | 0.03 | 0.03–0.22 | P<0.001 |
| 21-G | 0.09 | 0.02–0.34 | P<0.001 |
| 23-G | 0.03 | 0.03–0.22 | P<0.001 |
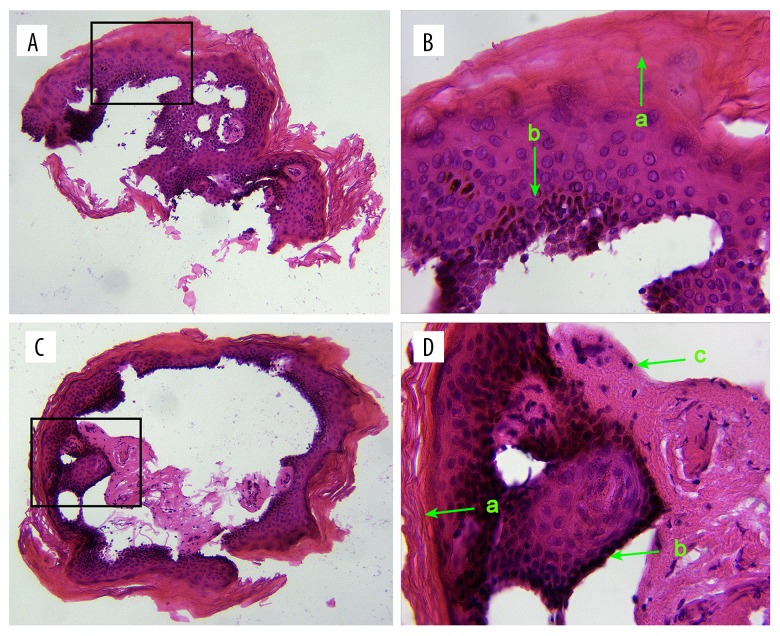
The epidermis, including the stratum basale, stratum spinosum, stratum granulosum, and stratum corneum, was identified on most blocks in subgroup 1. The dermal superficial papillary layer was also found in subgroup 1 (Figure 2). The incidence of epidermis and dermis contamination was 43.1% (155/360) and 25.0% (90/360), respectively. Compared to 21-guage needles, 23-guage needles had a significantly greater OR for epidermis contamination (OR=1.86; 95% CI, 1.11–3.13; P=0.018). No significant differences in ORs were identified between different needle gauges for dermis contamination (P=0.561). No epidermal or dermal structure was found in subgroup 2. The pilo-sebaceous unit was not identified using light microscopy in subgroup 1 or subgroup 2.
Figure 2.
The epidermal and dermal structure of subgroup 1 is shown in (A, C) (×10 magnification) and (B, D) (×40 magnification). a) stratum corneum, b) stratum basale and c) dermal superficial papillary layer.
We mathematically calculated the theoretical incidence of tissue coring containing hair follicles. The schematic diagram of the calculation method is shown in Figure 3 and described as follows: the average calf region hair follicle density from people of Chinese decent is 26 per cm2. In other words, there is 1 hair follicle per 3.85 mm2 (Square 4). The average diameter of a follicular orifice on the calf region is 0.35 mm. In Figure 3, Circle 1 represents a follicular orifice and AB is the semi-diameter. The inner diameter of a 21-gauge needle is 0.5 mm. Circle 2 represents the cross-section of a 21-gauge needle tip, and BC is its semi-diameter. The center of Circle 3 is depicted as A, and AC (the sum of AB and BC) is the semi-diameter of Circle 3. If the center of Circle 2 is inside Circle 3, the 2 circles will overlap, which indicates the needle cut through the hair follicle. Therefore, the theoretical incidence of tissue coring is the area ratio of Circle 3 to Square 4 (14.7%).
Figure 3.
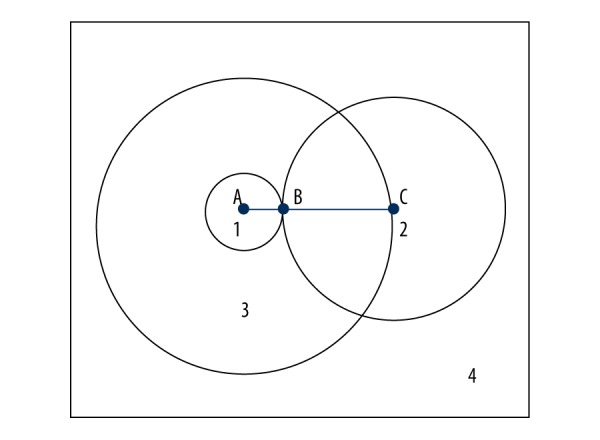
Theoretic incidence of coring rate.
Discussion
Intra-articular injections can be performed to inject a corticosteroid into a joint for the treatment of a non-infectious inflammatory process or for the injection of a viscosupplement [13,14]. While septic arthritis has been reported to occur in less than 1 out of 2700 cases following intra-articular injection, it is unclear if low-grade joint infections occur more frequently [4]. Furthermore, as the number of TKAs performed each year increases, so does the number of patients with periprosthetic joint infections (PJI) [15,16]. Synovial fluid aspiration is one of the most valuable procedures for the diagnosis of PJI. Therefore, we believe the results of this study have clinical significance for both rheumatologists and orthopedic surgeons.
It is essential to aseptically prepare the patient’s skin before joint aspiration/injection. The cutaneous flora cannot be eliminated completely, especially bacteria located in the pilo-sebaceous units. The types of cutaneous microbial flora include Staphylococcus aureus, coagulase-negative Staphylococcus, Enterococcus, Escherichia coli, group A Streptococci, and Pseudomonas aeruginosa. If these cutaneous flora multiply, they have been proven to be the cause of harmful infections despite the application of skin antiseptics [9,12,17]. Therefore, tissue coring caused by needles may carry cutaneous bacteria into the joint, which may result in intra-articular infection or a false-positive bacterial culture in joint fluid. There is limited research in the literature on tissue coring following joint injection/aspiration.
As a needle passes through tissue, a piece of the tissue is removed by the needle, resulting in tissue coring. This coring can either be removed from the needle bore by suction, or it can be carried deeper into the tissue by the needle and deposited, which could result in septic or chemical contamination. Tissue coring has been demonstrated using different needle designs in lumber punctures [18–20]. Some research suggests that epidermoid tumors may develop due to the deposition of epithelial tissue coring into the subarachnoid space during a lumbar puncture. These studies used cytological methods to identify whether the coring was composed of proliferative potential cells which could produce an epidermal tumor.
In the present study, the average incidence of coring due to arthrocentesis was similar to that reported in other studies on coring and lumber puncture. Tissue contamination of the joint seems be common, while septic arthritis after arthrocentesis is rare. One reason is that that even a small amount of highly virulent bacteria (e.g., Staphylococcus aureus) could result in intra-articular infection. Another reason is that patients with immunocompromise or immunosuppression are more vulnerable to intra-articular infection than patients without.
This is the first study to use a pathological method to analyze the nature of the coring. Both dermis and epidermis structures were identified in the coring tissue in the present study. The typical epidermis contains epithelial cells arranged in 4 layers: the stratum basale, the stratum spinosum, the stratum granulosum, and the stratum corneum. The dermis is the layer of skin between the epidermis and subcutaneous tissues, and it is divided into 2 layers: the superficial papillary layer and the deeper reticular layer. Pilo-sebaceous units, located mainly in the dermis, are an important and complex epidermal appendage of the skin. Bacteria resides in the infundibulum of hair follicles and in the subcutaneous ducts and glands where the bacteria reproduce [21]. In the present study, although there were no hair follicles in the tissue, dermis was identified, which verified the possibility of coring tissue with pilo-sebaceous units.
To avoid tissue coring, 0.5 mL of 0.9% normal saline was injected into the subcutaneous tissue in subgroup 2. There was an obvious difference in the coring rate between the 2 subgroups. Therefore, it could be effective to avoid tissue coring by flushing saline into the subcutaneous tissue before entering the joint cavity with the needle. Furthermore, although the differences in the incidence of tissue coring were not significant among the different needle gauges, we observed larger coring debris when larger-gauge needles were used. Thus, a smaller-gauge needle may result in less tissue coring.
This study has several limitations. The most important limitation was that we failed to identify pilo-sebaceous units in the tissue coring. This may be associated with incomplete coring collection, a small sample size, and structural damage caused by the needle tips. Additionally, although our study could confirm the existence of tissue coring following joint injection/aspiration, no research has proven that this coring carries pathogens. We could culture the tissue coring for bacteria in a further study. One minor limitation of the study was the contamination caused by foreign skin debris. A future study could be performed using laminar airflow, reducing the foreign skin debris contamination.
Conclusions
Despite the low risk of joint infection following arthrocentesis, the present study has clinical significance. This present study suggests that tissue coring commonly occurs during arthrocentesis, posing a potential risk for the development of septic arthritis. Flushing the arthrocentesis needle into the subcutaneous tissues prior to entering the joint capsule could be a way to reduce joint contamination from tissue coring.
Acknowledgments
We thank the patients who participated in this study and the staff involved in this work.
Footnotes
Source of support: This work was supported by the National Natural Science Foundation of China (grant number 81672192)
References
- 1.Berona K, Abdi A, Menchine M, et al. Success of ultrasound-guided versus landmark-guided arthrocentesis of hip, ankle, and wrist in a cadaver model. Am J Emerg Med. 2017;35(2):240–44. doi: 10.1016/j.ajem.2016.10.056. [DOI] [PubMed] [Google Scholar]
- 2.Charalambous CP, Tryfonidis M, Sadiq S, et al. Septic arthritis following intra-articular steroid injection of the knee – a survey of current practice regarding antiseptic technique used during intra-articular steroid injection of the knee. Clin Rheumatol. 2003;22(6):386–90. doi: 10.1007/s10067-003-0757-7. [DOI] [PubMed] [Google Scholar]
- 3.Shemesh S, Heller S, Salai M, Velkes S. Septic arthritis of the knee following intraarticular injections in elderly patients: Report of six patients. Isr Med Assoc J. 2011;13(12):757–60. [PubMed] [Google Scholar]
- 4.Geirsson AJ, Statkevicius S, Vikingsson A. Septic arthritis in Iceland 1990–2002: Increasing incidence due to iatrogenic infections. Ann Rheum Dis. 2008;67(5):638–43. doi: 10.1136/ard.2007.077131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bedard NA, Pugely AJ, Elkins JM, et al. The John N. Insall Award: Do intraarticular injections increase the risk of infection after TKA? Clin Orthop Relat Res. 2017;475(1):45–52. doi: 10.1007/s11999-016-4757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selwyn S, Ellis H. Skin bacteria and skin disinfection reconsidered. Br Med J. 1972;1(5793):136–40. doi: 10.1136/bmj.1.5793.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caldeira D, David C, Sampaio C. Skin antiseptics in venous puncture-site disinfection for prevention of blood culture contamination: Systematic review with meta-analysis. J Hosp Infect. 2011;77(3):223–32. doi: 10.1016/j.jhin.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 8.Zubrod CJ, Farnsworth KD, Oaks JL. Evaluation of arthrocentesis site bacterial flora before and after 4 methods of preparation in horses with and without evidence of skin contamination. Vet Surg. 2004;33(5):525–30. doi: 10.1111/j.1532-950X.2004.04074.x. [DOI] [PubMed] [Google Scholar]
- 9.Dumville JC, McFarlane E, Edwards P, et al. Preoperative skin antiseptics for preventing surgical wound infections after clean surgery. Cochrane Database Syst Rev. 2015;(4):CD003949. doi: 10.1002/14651858.CD003949.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Douglas RJ. Aspiration and injection of the knee joint: Approach portal. Knee Surg Relat Res. 2014;26(1):1–6. doi: 10.5792/ksrr.2014.26.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Courtney P, Doherty M. Joint aspiration and injection and synovial fluid analysis. Best Pract Res Clin Rheumatol. 2013;27(2):137–69. doi: 10.1016/j.berh.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Echols K, Graves M, LeBlanc KG, et al. Role of antiseptics in the prevention of surgical site infections. Dermatol Surg. 2015;41(6):667–76. doi: 10.1097/DSS.0000000000000375. [DOI] [PubMed] [Google Scholar]
- 13.Kokubun BA, Manista GC, Courtney PM, et al. Intra-articular knee injections before total knee arthroplasty: Outcomes and complication rates. J Arthroplasty. 2017;32(6):1798–802. doi: 10.1016/j.arth.2017.01.041. [DOI] [PubMed] [Google Scholar]
- 14.Wang Q, Jiang X, Tian W. Does previous intra-articular steroid injection increase the risk of joint infection following total hip arthroplasty or total knee arthroplasty? A meta-analysis. Med Sci Monit. 2014;20:1878–83. doi: 10.12659/MSM.890750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang C, Li R, Wang Q, et al. Leukocyte esterase as a biomarker in the diagnosis of periprosthetic joint infection. Med Sci Monit. 2017;23:353–58. doi: 10.12659/MSM.899368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cardone DA, Tallia AF. Diagnostic and therapeutic injection of the hip and knee. Am Fam Physician. 2003;67(10):2147–52. [PubMed] [Google Scholar]
- 17.Shortt CP, Morrison WB, Roberts CC, et al. Shoulder, hip, and knee arthrography needle placement using fluoroscopic guidance: Practice patterns of musculoskeletal radiologists in North America. Skeletal Radiol. 2009;38(4):377–85. doi: 10.1007/s00256-009-0648-3. [DOI] [PubMed] [Google Scholar]
- 18.Ting NT, Della Valle CJ. Diagnosis of periprosthetic joint infection-an algorithm-based approach. J Arthroplasty. 2017;32(7):2047–50. doi: 10.1016/j.arth.2017.02.070. [DOI] [PubMed] [Google Scholar]
- 19.Shepherd A. Contamination of injection sites by landmark palpation after skin antisepsis. J Hosp Infect. 2009;71(1):97–98. doi: 10.1016/j.jhin.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Puolakka R, Andersson LC, Rosenberg PH. Microscopic analysis of three different spinal needle tips after experimental subarachnoid puncture. Reg Anesth Pain Med. 2000;25(2):163–69. doi: 10.1053/rapm.2000.0250163. [DOI] [PubMed] [Google Scholar]
- 21.Guldogus F, Baris YS, Baris S, et al. Comparing tissue coring potentials of hollow needles without stylet and caudal needles with stylet: An experimental study. Eur J Anaesthesiol. 2008;25(6):498–501. doi: 10.1017/S0265021508003906. [DOI] [PubMed] [Google Scholar]
- 22.Ferraz IL, Barros GA, Ferreira Neto PG, et al. Does spinal block through tattooed skin cause histological changes in nervous tissue and meninges?: An experimental model in rabbits. Reg Anesth Pain Med. 2015;40(5):533–38. doi: 10.1097/AAP.0000000000000282. [DOI] [PubMed] [Google Scholar]
- 23.Richardson M. Understanding the structure and function of the skin. Nurs Times. 2003;99(31):46–48. [PubMed] [Google Scholar]